Abstract
Leukotrienes (LTs), chemical mediators produced by mast cells, play an important role in allergic symptoms such as food allergies and hay fever. We tried to construct an evaluation method for the anti-LTB4 activity of chemical substances using a mast cell line, PB-3c. PB-3c pre-cultured with or without arachidonic acid (AA) was stimulated by calcium ionophore (A23187) for 20 min, and LTB4 production by the cells was determined by HPLC with UV detection. LTB4 was not detected when PB-3c was pre-cultured without AA. On the other hand, LTB4 production by PB-3c pre-cultured with AA was detectable by HPLC, and the optimal conditions of PB-3c for LTB4 detection were to utilize the cells pre-cultured with 50 µM AA for 48 h. MK-886 (5-lipoxygenase inhibitor) completely inhibited LTB4 production, but AACOCF3 (phospholipase A2 inhibitor) slightly increased LTB4 production, suggesting that LTB4 was generated from exogenous free AA through 5-lipoxygenase pathway. We applied this technique to the evaluation of the anti-LTB4 activity of food components. PB-3c pre-cultured with 50 µM AA for 48 h was stimulated with A23187 in the presence of 50 µM soybean isoflavones (daidzin, genistin, daidzein, and genistein), equol, quercetin, or kaempferol. Genistein, equol, quercetin, and kaempferol strongly inhibited LTB4 production without cytotoxicity. These results suggest that a new assay system using PB-3c is convenient to evaluate LTB4 inhibition activity by food components. This method could be utilized for elucidation of the mechanisms of LTB4 release suppression by food components such as flavonoids and the structure–activity relationship.
Keywords: Allergy, Mast cells, Leukotriene, Flavonoid, Isoflavone
Introduction
Allergies are disorders of immunoresponse that are classified into five types by mechanism. Immediate hypersensitivities, such as food allergies and hay fever, are classified as type I allergies (Gell and Coombs 1963; Rajan 2003), in which mast cells, a kind of granulocyte, play an important role in the allergic reaction. The cross-link of IgEs by the specifically binding allergen on the surface of mast cells triggers signal transduction followed by Ca2+ influx into the cells (Siraganian 2003). This induces arachidonic acid (AA) release from phospholipids of the cell membrane by phospholipase A2, and leukotrienes (LTs) are produced from the AA by 5-lipoxygenase (5-LOX), LTA4 hydrolase, and LTC4 synthase. Then, the LTs are secreted into the extracellular space and act as chemical mediators. The LTs facilitate mucus secretion, smooth muscle contraction, and leukocyte chemotaxis, which cause allergic symptoms such as sneezing and prolong the inflammatory reactions.
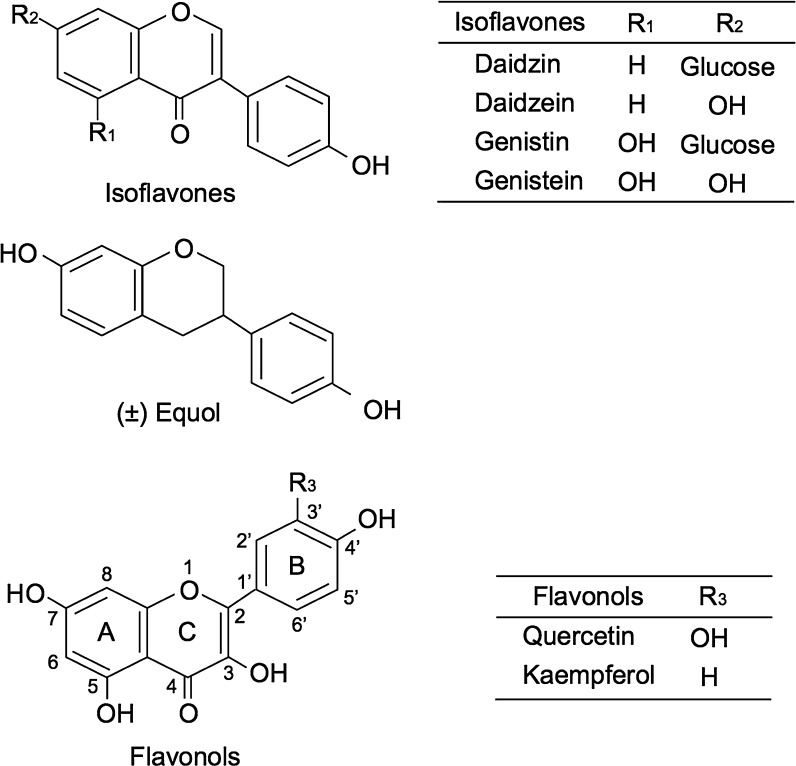
Anti-allergic medicines such as LT receptor antagonists and 5-LOX inhibitors have been used to mitigate the allergic symptoms. On the other hand, it has been expected that the intake of natural food components improves the condition of patients because it should have few side effects. Genistin and daidzin are the major isoflavone glycosides in soybeans (Fig. 1), and they are hydrolyzed by endogenous and intestinal bacteria β-glucosidase to the aglycones, genistein and daidzein, respectively, in the human gastrointestinal tract (Setchell et al. 2002a). Daidzein is further metabolized to equol by specific intestinal bacteria, which are distributed to 30 to 60% of populations consuming Western or soybean-rich Asian diets (Hodgson et al. 1996; Rafii 2015). We have previously reported that genistein and equol suppress LTB4 release from peritoneal exudate cells (PEC) (Takasugi et al. 2014). Some flavonols, such as quercetin and kaempferol (Fig. 1) in fruits and vegetables, also suppress LTB4 release from PEC (Yamada et al. 1999). However, it is difficult to elucidate the mechanisms using PEC because PEC is neither a mast cell line nor a homogeneous cell population (Bos et al. 1989; Matsuo et al. 2000). We found that LTB4 release was inhibited by quercetin, kaempferol, genistein, and equol but histamine release was not inhibited (Yamada et al. 1999; Takasugi et al. 2014). This means the effect of food components on LTB4 production and histamine release may differ due to difference of mechanisms of degranulation and arachidonate cascade. Therefore, a method for evaluation of anti-LTB4 production by mast cells would be beneficial. Rat basophilic leukemia cell lines, such as RBL-2H3, are used for the evaluation of degranulation, which are other chemical mediators related to type I allergies, but LTs produced by rat basophilic leukemia cells are undetectable by high-performance liquid chromatography (HPLC) with UV detection. Shimizu et al. (1986) reported that AA conversion through the 5-LOX pathway in a mouse mast cell line (PB-3c) is more prominent than that of rat basophilic leukemia cells.
Fig. 1.
Structures of soybean isoflavones and flavonols
The objective of this study was to construct an evaluation method of anti-LTB4 activity of chemical substances using PB-3c. Then, the new technique was applied to measure anti-LTB4 activities of some dietary phenolic compounds.
Materials and methods
Materials
Soybean isoflavones were purchased from Funakoshi (Tokyo, Japan). (±) Equol was obtained from Extrasynthese (Genay, France). MK-886 (5-LOX inhibitor), AACOCF3 (phospholipase inhibitor), quercetin, and kaempferol were supplied from Calbiochem (Darmstadt, Germany), Tocris (Minneapolis, MN, USA), Nacalai Tesque (Kyoto, Japan), and Wako Pure Chemical Industries, Ltd. (Osaka, Japan), respectively. All other chemicals were of reagent grade.
Cell culture
PB-3c, a mouse mast cell line, was obtained from the Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan). The cells were cultured in RPMI-1640 medium (Wako Pure Chemical Industries, Ltd.) containing 10% fetal bovine serum (Hyclone Laboratories, South Logan, UT, USA), 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma, St. Louis, MO, USA), 1% MEM non-essential amino acids (Gibco, Grand Island, NY, USA), 1 mM sodium pyruvate (Wako Pure Chemimcal Industries, Ltd.), 25 mM HEPES buffer, 0.0035 µl/ml 2-mercaptoethanol (Sigma), and 2 ng/ml IL-3 (PeproTech, London, UK).
LTB4 assay
PB-3c (5 × 105 cells/ml) was pre-cultured in the medium supplemented with AA (Sigma) before the LTB4 assay. After washing the cells twice with phosphate buffered saline (PBS), 2 × 106 cells were suspended in Tyrode buffer (137 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1 mM MgCl2, 12 mM NaHCO3, 1.8 mM CaCl2, 5.6 mM glucose, pH 7.2) containing 0.05% bovine serum albumin (Sigma). When the anti-LTB4 effects of inhibitors or flavonoids were examined, the samples, dissolved in ethanol, were added to Tyrode buffer, and the final concentration of ethanol in Tyrode buffer was 0.5%. After the incubation at 37 °C for 10 min in the absence or presence of the samples, the cells were stimulated with 5 µM calcium ionophore (A23187, Sigma) at 37 °C for 20 min. The stimulation was terminated by the addition of 50 µl of acetonitrile:methanol (30:25, v/v), including 250 ng of prostaglandin B2 (Cayman, Ann Arbor, MI, USA) as an internal standard (Matsuo et al. 1996). The LTB4 produced by PB-3c was determined by HPLC on an ODS-A column (150 × 6.0 mm I.D., 5 µm particle size; YMC, Kyoto, Japan) at room temperature. The injected samples were eluted with 5 mM ammonium acetate:acetonitrile:methanol (9:6:5, v/v/v) at a flow rate of 1.0 ml/min. LTB4 and prostaglandin B2 were detected by the absorbance at 280 nm.
Statistical analysis
Data are expressed as mean ± standard error (SE). The statistical significance of differences was analyzed by Tukey–Kramer test. The differences with p values of less than 0.01 were considered significant.
Results
Optimization of experimental conditions for LTB4 assay using PB-3c
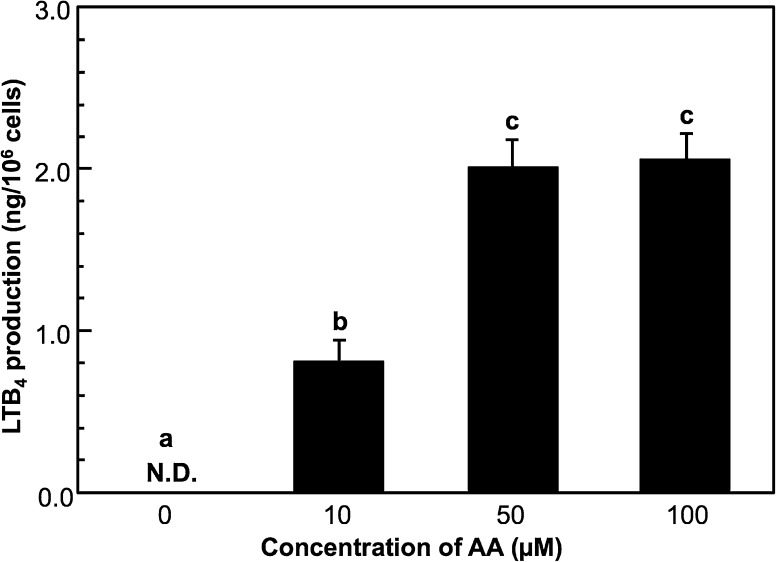
PB-3c was pre-cultured with various concentrations of AA for 48 h, and the cells were stimulated with calcium ionophore (Fig. 2). LTB4 was produced when the cells were pre-cultured with AA (10–100 µM), but LTB4 was not observed when the cells were pre-cultured without AA. LTB4 production by PB-3c pre-cultured with 10 µM AA was 0.81 ng/106 cells and the production was significantly higher than that without AA. LTB4 production at 50 and 100 µM was almost the same and seemed to be reached a plateau (2.0 ng LTB4/106 cells) at 50 µM AA.
Fig. 2.
Effect of arachidonic acid (AA) concentration during pre-culture of PB-3c on LTB4 production. A mast cell line, PB-3c (2 × 106 cells) was pre-cultured in RPMI-1640 containing 0–100 µM of AA for 48 h. The cells were stimulated with 5 µM calcium ionophore (A23187) for 20 min and then LTB4 produced by the cells was determined by HPLC with UV detection. Data represent mean ± SE (n = 4). Means without a common letter are significantly different (p < 0.01). N.D. not detected
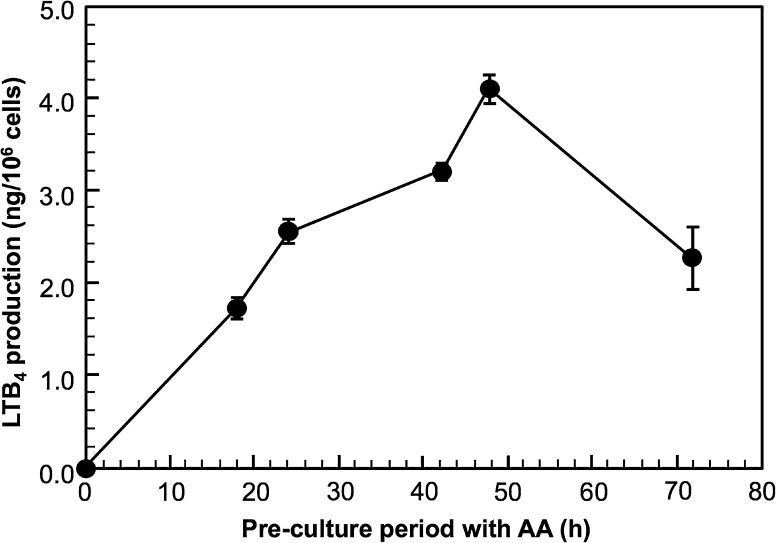
Figure 3 shows the effect of the pre-culture period with AA before the stimulation of LTB4 production. PB-3c was pre-cultured with 50 µM AA for up to 72 h, and then the cells were stimulated. LTB4 was not detected at 0 h; however, 1.7 ng/106 cells of LTB4 production was observed when PB-3c was pre-cultured with AA for 18 h and the production was gradually increased along with the pre-culture period up to 48 h. LTB4 production by PB-3c pre-cultured with 50 µM AA for 48 h was maximum (4.1 ng LTB4/106 cells) and then the production from 72 h-cultured PB-3c was decreased.
Fig. 3.
Effect of pre-culture period of PB-3c with AA before the stimulation on LTB4 production. PB-3c (2 × 106 cells) was pre-cultured in RPMI-1640 containing 50 µM AA for 0–72 h. The cells were stimulated with 5 µM A23187 for 20 min and then LTB4 produced by the cells was determined by HPLC with UV detection. Data represent mean ± SE (n = 4)
Effect of enzyme inhibitors associated with AA cascade
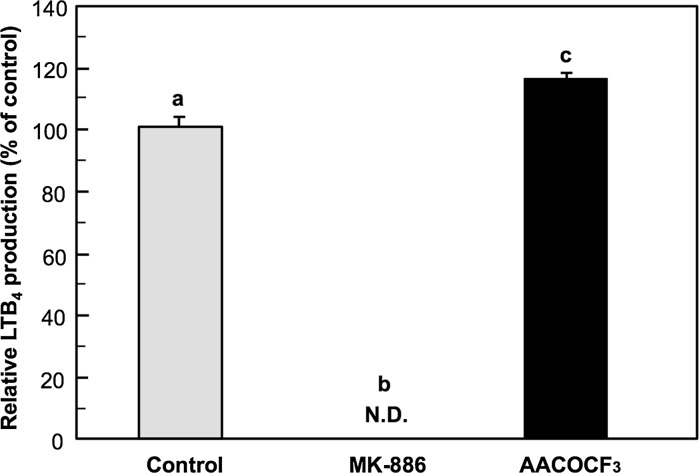
PB-3c was pre-cultured with 50 µM AA for 48 h and then stimulated with calcium ionophore in the presence of 1 µM MK-886 (5-LOX inhibitor) or AACOCF3 (phospholipase A2 inhibitor). PB-3c produced 18.3 ng/106 cells of LTB4 without the inhibitors (Fig. 4) and the production is shown as a relative value. In the presence of MK-886, LTB4 production was completely inhibited, while AACOCF3 slightly but significantly increased LTB4 production and the production was 117% of control.
Fig. 4.
Effect of enzyme inhibitors related to arachidonate cascade on LTB4 production by PB-3c. PB-3c was pre-cultured with 50 µM AA for 48 h and then stimulated with 5 µM A23187 in the presence of MK-886 (5-LOX inhibitor) and AACOCF3 (phospholipase A2 inhibitor). LTB4 was determined by HPLC with UV detection. Data represent mean ± SE (n = 4). Means without a common letter are significantly different (p < 0.01). N.D. not detected
Effect of flavonoids on LTB4 production by PB-3c
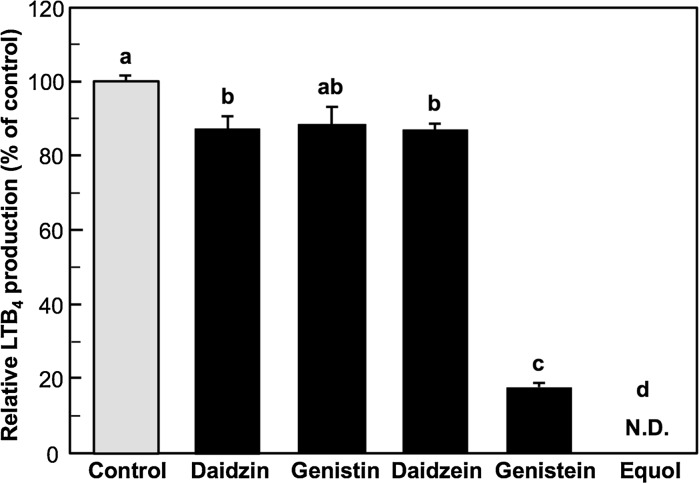
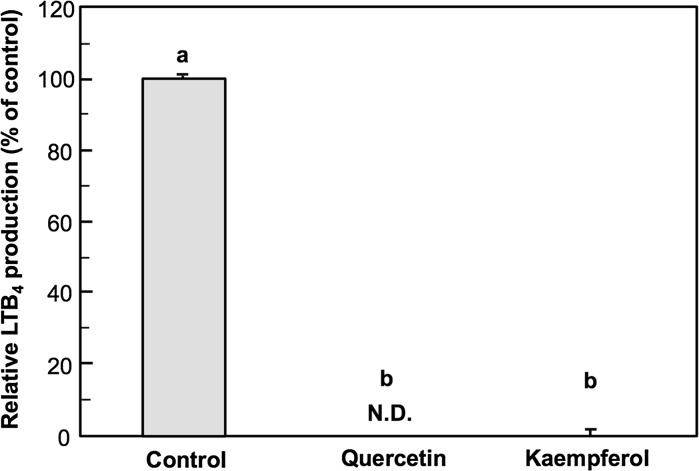
We evaluated the inhibitory activity of soybean isoflavones on LTB4 production by PB-3c using the aforementioned experimental conditions (Fig. 5). PB-3c was pre-cultured with 50 µM AA for 48 h and then stimulated in the presence of 50 µM daidzin, genistin, daidzein, genistein, and equol. Daidzin and daidzein showed a week suppression of LTB4 production and the effect was significant. Genistin also showed a tendency to decrease LTB4 production, but it was not significant. In contrast, genistein, aglycon of genistin, markedly suppressed LTB4 production by 17.5% of control. Equol exhibited the strongest LTB4 suppressing activity and completely inhibited LTB4 production by PB-3c. We examined cytotoxicity of the isoflavones by Trypan blue assay and cytotoxicity was not observed at 50 µM (data not shown). Figure 6 presents the effect of quercetin and kaempferol on the LTB4 production. These flavonoids have strong anti-LTB4 activity. Quercetin completely inhibited LTB4 production at 50 µM and trace amount of LTB4 was observed in the presence of 50 µM kaempferol. They did not exert cytotoxicity at 50 µM (data not shown).
Fig. 5.
Effect of soybean isoflavones and equol on LTB4 production by PB-3c. PB-3c was pre-cultured with 50 µM AA for 48 h and then stimulated with 5 µM A23187 in the presence of 50 µM daidzin, genistin, daidzein, genistein, and equol. LTB4 was determined by HPLC with UV detection. Data represent mean ± SE (n = 4). Means without a common letter are significantly different (p < 0.01). N.D. not detected
Fig. 6.
Effect of flavonoids on LTB4 production by PB-3c. PB-3c was pre-cultured with 50 µM AA for 48 h and then stimulated with 5 µM A23187 in the presence of 50 µM quercetin and kaempferol. LTB4 was determined by HPLC with UV detection. Data represent mean ± SE (n = 4). Means without a common letter are significantly different (p < 0.01). N.D. not detected
Discussion
LTB4, a chemical mediator released from mast cells, plays an important role in type I allergy. We have reported on the anti-LTB4 effect of some food components using PEC prepared from rats (Matsuo et al. 2000; Takasugi et al. 2014; Yamada et al. 1996; Yamada and Tachibana 2000). However, rats have to be sacrificed for PEC preparation, and PEC is not appropriate for further studies to elucidate the mechanisms of the anti-LTB4 effect because PEC is neither a cell line nor a homogeneous population (Bos et al. 1989; Matsuo et al. 2000). The principal aim of this study was to develop a new method for the evaluation of anti-LTB4 activity of chemical substances using a mast cell line.
It has been reported that the addition of fatty acids into the culture medium could modify the functions of mast cells (Nakano et al. 2005; Yamada et al. 1996). We checked for LTB4 production by PB-3c without AA treatment, which is a substrate for 5-LOX, but we could not detect LTB4 by HPLC with UV detection. On the other hand, LTB4 was detected when PB-3c was pre-cultured with AA for 48 h (Fig. 2). The optimal AA concentration in the medium for the maximum LTB4 production was 50–100 µM. As shown in Fig. 3, the amount of LTB4 produced by PB-3c pre-cultured with 50 µM AA increased until 48 h, although the production decreased at 72 h. From these results, we decided that PB-3c pre-cultured with 50 µM for 48 h should be used for LTB4 detection.
To confirm that LTB4 is derived from a cell response, we examined the effect of the 5-LOX inhibitor MK-886 on LTB4 production using the above-mentioned experimental conditions. MK-886 completely suppressed LTB4 production, suggesting that LTB4 was generated through the 5-LOX pathway in PB-3c (Fig. 4). On the other hand, AACOCF3, a phospholipase A2 inhibitor, did not inhibit LTB4 production. This indicates that AA esterified to phospholipids of the cell membranes during pre-culture may not be the substrate for 5-LOX, while free AA incorporated into the cells may be utilized as the substrate. In this assay system, stimulation by calcium ionophore is indispensable because LTB4 was not produced without calcium ionophore stimulation (data not shown). Calcium influx is necessary not only for phospholipase A2 activation but for signal transduction of mast cells. Nakano et al. (2005) have reported that the level of AA esterified to phospholipids in RBL-2H3 was significantly increased by incubation with 25 µM AA for 48 h, and the AA incubation enhanced β-hexosaminidase release by IgE-antigen stimulation, which is an index of degranulation. Although we have not directly determined the level of AA esterified to phospholipids in this study, our results suggest that AA may not be esterified to the phospholipids of the cell membrane. A possible reason for inconsistence in AA localization may be due to the differences of the cell lines.
We applied this technique to the evaluation of the anti-LTB4 activity of food components. As shown in Fig. 5, genistein and equol significantly inhibited LTB4 release from PB-3c, and the effect of equol was stronger than that of genistein. Our data using PB-3c were consistent with the former data using PEC. Genistein is an inhibitor of tyrosine-specific protein kinases (Akiyama et al. 1987), and the kinases play an important role in inflammatory reactions (Kinet 1999; Wong and Koh 2000; Gilfillan and Rivera 2009). There are many papers presenting that genistein inhibits inflammatory reactions in various experimental models such as murine allergic asthma (Bao et al. 2011), guinea pig asthma (Wong et al. 1997; Duan et al. 2003), and human eosinophilic inflammation (Dent et al. 2000; Kalhan et al. 2008). Kalhan et al. (2008) showed that ex vivo LTC4 synthesis by eosinophils stimulated with calcium ionophore was inhibited by a 4 week-intake of genistein in asthmatic patients, and the effect may be derived from the blockade of 5-LOX activation associated with p38 and MAPKAP-2. Genistein also inhibits LTC4 production by human eosinophils stimulated with platelet-activating factor (Dent et al. 2000) and peptidoleukotrienes (LTC4, LTD4, and LTE4) release from lung fragments of antigen challenged guinea pigs (Wong et al. 1997). While research on the anti-inflammatory effects of genistein has been cumulative, few studies have reported about the effect of equol on inflammation, but some physiological effects of equol such as antioxidant activity are stronger than that of genistein and daidzein (Setchell et al. 2002b). Recently, Tsen et al. (2016) reported that equol inhibited LTB4 production in human neutrophils, and the activity was significantly higher than that of daidzein, suggesting the effect may be due to the inhibition of 5-LOX. Here, we showed that equol might also be a potent inhibitor of LTB4 production by mast cells, with activity that is stronger than that of daidzein and genistein. The data suggest that soybean and isoflavones may contribute to the anti-inflammation and anti-allergic effects, although further studies using mast cells, such as PB-3c, are needed to elucidate the mechanisms.
We have clarified that quercetin and kaempferol also inhibit LTB4 release from PB-3c using our new method, as shown in Fig. 5, which is consistent with our previous research using PEC (Yamada et al. 1999). It has been reported that quercetin, kaempferol, or the extracts containing them suppressed LTB4 not only in mast cells but also in other cells such as rat basophilic cells and human neutrophils (Bouriche et al. 2005; Loke et al. 2008; Kim et al. 2014; Ribeiro et al. 2014). As the mechanism of the effect, the inhibition of soybean LOX activity by quercetin (Bourche et al. 2005) and the inhibition of protein and mRNA expression of 5-LOX by quercetin and kaempferol have been proposed (Kim et al. 2014). Some researchers found a structure–activity relationship between flavonoids and their LTB4 inhibitory activity. Yamada et al. (1999) have suggested that the importance of a 4-carbonyl group on the C-ring for anti-LTB4 activity. In addition, Li et al. (2012) reported that baicalein, which has a 4-carbonyl group on the C-ring, also inhibited LTB4 production by macrophages. Furthermore, the 2, 3-double bond on the C-ring may also contribute to exert the inhibitory effect on LTB4 production (Loke et al. 2008; Ribeiro et al. 2014). Our data support the hypotheses because quercetin and kaempferol that possess 4-carbonyl groups and 2, 3-double bonds on their C-rings strongly inhibited LTB4 production by mast cells.
In conclusion, we constructed a new convenient assay system to evaluate LTB4 inhibition activity by food components using a mast cell line, PB-3c, and the results obtained by this assay system are consistent with previous studies using PEC. Furthermore, we suggest that this method could be utilized for elucidation of the mechanisms of LTB4 release suppression by food components, such as flavonoids, and the structure–activity relationship.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 17700565.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Bao ZS, Hong L, Guan Y, Dong XW, Zheng HS, Tan GL, Xie QM. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol. 2011;11:899–906. doi: 10.1016/j.intimp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Bos HJ, Meyer F, Veld JC, Beelen RHJ. Peritoneal dialysis fluid induces change of mononuclear phagocyte proportions. Kidney Int. 1989;36:20–26. doi: 10.1038/ki.1989.155. [DOI] [PubMed] [Google Scholar]
- Bouriche H, Miles EA, Selloum L, Calder PC. Effect of Cleome arabica leaf extract, rutin and quercetin on soybean lipoxygenase activity and on generation of inflammatory eicosanoids by human neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2005;72:195–201. doi: 10.1016/j.plefa.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Dent G, Munoz NM, Zhu X, Ruhlmann E, Magnussen H, Leff AR. Involvement of protein tyrosine kinases in activation of human eosinophils by platelet-activating factor. Immunology. 2000;100:231–237. doi: 10.1046/j.1365-2567.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003;167:185–192. doi: 10.1164/rccm.200205-420OC. [DOI] [PubMed] [Google Scholar]
- Gells PGH and Coombs RRA . The classification of allergic reactions underlying disease. In: Coombs RRA, Gells PGH, editors. Clinical aspects of immunology. 1. Hoboken: Blackwell Science; 1963. [Google Scholar]
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JM, Croft KD, Puddey IB, Mori TA, Beilin LJ. Soybean isoflavonoids and their metabolic products inhibit in vitro lipoprotein oxidation in serum. Nutr Biochem. 1996;7:664–669. doi: 10.1016/S0955-2863(96)00133-7. [DOI] [Google Scholar]
- Kalhan R, Smith LJ, Nlend MC, Nair A, Hixon JL, Sporn PHS. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin Exp Allergy. 2008;38:103–112. doi: 10.1111/j.1365-2222.2007.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Lim SJ, Kang SW, Um BH, Nho CW. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J Agric Food Chem. 2014;62:3750–3758. doi: 10.1021/jf405486c. [DOI] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Li L, Zeng H, Shan L, Yuan X, Li Y, Liu R, Zhang W. The different inhibitory effects of Huang–Lian–Jie–Du–Tang on cyclooxygenase 2 and 5-lipoxygenase. J Ethnopharmacol. 2012;143:732–739. doi: 10.1016/j.jep.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, Stewart S, McKinley AJ, Needs PW, Kroon PA, Hodgson JM, Croft KD. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem Pharmacol. 2008;75:1045–1053. doi: 10.1016/j.bcp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Yamada K, Yamashita K, Shoji K, Mori M, Sugano M (1996) Inhibitory effect of tea polyphenols on histamine and leukotriene B4 release from rat peritoneal exudate cells. In Vitro Cell Dev Biol-Anim 32:340–344 [DOI] [PubMed]
- Matsuo N, Yamada K, Mori M, Shoji K, Ueyama T, Yunoki S, Yamashita K, Ozeki M, Sugano M. Inhibition by dietary tea polyphenols of chemical mediator release from rat peritoneal exudate cells. Biosci Biotechnol Biochem. 2000;64:1437–1443. doi: 10.1271/bbb.64.1437. [DOI] [PubMed] [Google Scholar]
- Nakano N, Nakao A, Uchida T, Shirasaka N, Yoshizumi H, Okumura K, Tsuboi R, Ogawa H. Effects of arachidonic acid analogs on FcεRI-mediated activation of mast cells. Biochem Biophys Acta. 2005;1738:19–28. doi: 10.1016/j.bbalip.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5:56–73. doi: 10.3390/metabo5010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan TV. The Gell–Coombs classification of hypersensitivity reactions: a re-interpretation. Trends Immunol. 2003;24:376–379. doi: 10.1016/S1471-4906(03)00142-X. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Freitas M, Tome SM, Silva AMS, Porto G, Cabrita EJ, Marques MMB, Fernandes E. Inhibition of LOX by flavonoids: a structure-activity relationship study. Eur J Med Chem. 2014;72:137–145. doi: 10.1016/j.ejmech.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Izumi T, Seyama Y, Tadokoro K, Radmark O, Samuelsson B. Characterization of leukotriene A4 synthase from murine mast cells: evidence for its identity to arachidonate 5-lipoxygenase. Proc Natl Acad Sci USA. 1986;83:4175–4179. doi: 10.1073/pnas.83.12.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Takasugi M, Shimada K, Yamada K, Arai H. Effects of soybean isoflavones on the release of chemical mediators from rat peritoneal exudate cells by allergic reaction in vitro. Food Sci Technol Res. 2014;20:725–729. doi: 10.3136/fstr.20.725. [DOI] [Google Scholar]
- Tsen SY, Tan XY, Tan YM, Yan BY, Loke WM. Relative inhibitions of 5-lipoxygenase and myeloperoxidase and free-radical scavenging activities of daidzein, dihydrodaidzein, and equol. J Med Food. 2016;19:543–548. doi: 10.1089/jmf.2015.3557. [DOI] [PubMed] [Google Scholar]
- Wong WS, Koh DS. Advances in immunopharmacology of asthma. Biochem Pharmacol. 2000;59:1323–1335. doi: 10.1016/S0006-2952(99)00378-0. [DOI] [PubMed] [Google Scholar]
- Wong WS, Koh DS, Koh AH, Ting WL, Wong PT. Effect of tyrosine kinase inhibitors on antigen challenge of guinea pig lung in vitro. J Pharmacol Exp Ther. 1997;283:131–137. [PubMed] [Google Scholar]
- Yamada K, Tachibana H. Recent topics in anti-oxidative factors. BioFactors. 2000;13:167–172. doi: 10.1002/biof.5520130127. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mori M, Matsuo N, Shoji K, Ueyama T, Sugano M. Effects of fatty acids on accumulation and secretion of histamine in RBL-2H3 cells and leukotriene release from peritoneal exudate cells isolated from Wistar rats. J Nutr Sci Vitaminol. 1996;42:311. doi: 10.3177/jnsv.42.301. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shoji K, Mori M, Ueyama T, Matsuo N, Oka S, Nishiyama K, Sugano M. Structure-activity relationship of polyphenols on inhibition of chemical mediator release from rat peritoneal exudate cells. In Vitro Cell Dev Biol Anim. 1999;35:169–174. doi: 10.1007/s11626-999-0020-x. [DOI] [PubMed] [Google Scholar]