Abstract
Cells are often characterized by their gene expression profile. However, commonly used methods to detect mRNA require cell pooling and could therefore mask differences in gene expression within heterogeneous cell populations. q2PISH allows for the analysis of both qualitative and quantitative (q2) gene expression on cultured cells for quality control measures with single cell resolution. q2PISH was optimized for the subsequent use of two alkaline phosphatase substrates in combination with a cell nucleus count to allow for accurate quantification of gene expression per cell and simultaneously qualitative assessment of potential culture population drift or heterogeneity. As proof of principle the assay was applied to cell lines derived from different areas of the bovine intervertebral disc, showing significant difference in the expression of Col1a1, Col2a1, Acan and Sox9. Furthermore, the assay served to explore a potential impact on cultured cells when substituting a critical media component, fetal bovine serum (FBS), suggesting no significant difference in gene expression for the biomarkers analyzed. As a tool, q2PISH serves as an accurate quality control with single cell resolution for cultured cells.
Keywords: Alkaline phosphatase, Bovine, NBT/BCIP, PISH, pNPP, RNA in situ hybridization, Single cell gene expression
Introduction
Accurate in situ gene expression analysis on cells, tissues and organs was traditionally performed with radioactive labeled nucleic acid probes. DNA or RNA probes were synthesized using P32 or S35 and detected using light sensitive x-ray film or photographic emulsion (Chen et al. 1996; Holtke and Kessler 1990; Tribioli and Lufkin 1997). Advanced technologies combine digoxigenin (DIG) labeling of nucleic acids with immunohistochemistry using fluorescent tagged or enzyme conjugated antibodies; for example, an alkaline phosphatase (AP) (E.C.3.1.3.1) conjugated anti-DIG antibody that recognizes DIG-labeled nucleic acid probes has been used extensively (Holtke and Kessler 1990; Kraus and Lufkin 1999; Schaeren-Wiemers and Gerfin-Moser 1993; Wang et al. 2000). For in situ gene expression analysis, after hybridization between the DIG-labeled RNA antisense probe and the respective target messenger RNA (mRNA), the anti-DIG AP conjugated antibody recognizes the DIG epitope (Holtke and Kessler 1990). Typically the antibody bound AP enzyme converts 5-Bromo-4-Chloro-3-Indolyl-Phosphate (BCIP) into an intermediate that releases hydrogen ions upon dimerization. The hydrogen ions reduce the chromogenic substrate Nitroblue Tetrazolium (NBT) into an insoluble intracellular deposition of purple NBT Diformazan, which can be assessed qualitatively (Holtke and Kessler 1990; Zhao et al. 2003). This allows the monitoring of the heterogeneity of cultured cells, an important quality control aspect when using cells for regenerative medicine based therapies (Kraus et al. 2017). This qualitative assessment has proven very helpful to identify non-homogeneous gene expression in cell cultures (Kraus and Lufkin 2016), however, a need to quantify gene expression levels frequently arises. Commonly, quantification is achieved by quantitative image analysis, qRT-PCR or microarray analysis (Tan et al. 2013). These procedures for RNA quantification can suffer from lack of accuracy, are time-consuming or require cell pooling and therefore bear the risk of masking any variation in gene expression levels between cells within a heterogeneous population (Lufkin 1996). To facilitate a precise quantification of the cell population as well as qualitative assessment at a single cell level we investigated the serial application of different AP substrates culminating in a highly improved protocol termed “qualitative and quantitative PISH” (q2PISH). Owing to the robustness of the AP enzyme, typically inactivated through prolonged heating and fixation, the serial application of several substrates to the anti-DIG conjugated commercial AP appeared feasible. Here, we explored chemiluminescent and chromogenic substrates for the quantitative assessment of mRNA expression in cell culture. The AP enzyme is known to dephosphorylate the chemiluminescent substrate CDP-Star to a transient intermediate, releasing energy in the form of light measurable at 475 nm upon decomposition (O’Connor and Culp 1994). The AP enzyme is also capable of converting the chromogenic substrate p-Nitrophenyl Phosphate (pNPP) into a yellow soluble product with measurable absorbance in the supernatant at 405 nm. Comparing these different substrates resulted in the inclusion of a pNPP substrate step preceding the NBT/BCIP staining into our previously published PISH protocol (Kraus et al. 2015). To increase accuracy and allow for comparable “per cell” data acquisition, a total cell count procedure was included after NBT/BCIP staining; To-PRO-3, a DNA intercalating nuclear dye (Bink et al. 2001), was employed to identify individual cell nuclei and derive an accurate total cell count per well.
The generation of soluble and insoluble AP reaction products depends on the concentration of the anti-DIG conjugated AP enzyme, which itself correlates with mRNA sense transcript levels in a cell for a given probe. Applying this principle, we developed a method termed q2PISH, as a sensitive, rapid and reproducible way of quantifying gene expression for a variety of RNA probes and facilitating simultaneously the assessment of culture heterogeneity, an important quality control measure, typically not taken into consideration in quantitative assays relying on PCR amplification, cell-pooling or total population averaging for mRNA extraction.
Materials and methods
Cell lines
Four cell lines (I, II III and IV) were independently derived from structurally different areas of fresh adult (I, II) and fetal (III, IV) bovine intervertebral discs (IVD) and maintained with D10 medium as previously described (Kraus and Lufkin 2016; Kraus et al. 2017), containing 10% heat-inactivated fetal bovine serum (HI-FBS) from GIBCO (Waltham, MA, USA) (A) or Seradigm (Radnor, PA, USA) (B), as indicated. For quantitative PISH (qPISH) optimization, cells were plated as serial dilutions from a confluent well and counted using a hemocytometer. Confluence was achieved when plating ~5 × 105 cells/well of a 96-well plate (Greiner Bio-one Cellstar, Radnor, PA, USA). Five replicates of each cell dilutions were seeded on 0.1% (w/v) gelatin coated 96-well plates (Greiner Bio-one Cellstar) and cultured for 24 h, fixed with 4% (w/v) paraformaldehyde (PFA) for at least 20 min at room temperature and washed 3× with nuclease-free (nf) phosphate buffered saline (PBS). For cell line comparison or fetal bovine serum (FBS) comparison, cells were seeded at confluence as quintuplicate (GIBCO) A, and triplicate (Seradigm) B, data points. Bovine tails were retrieved from local abattoirs. All procedures were in accordance with ethical standards of Clarkson University.
Probe generation and immunohistochemistry
All probe templates were generated by polymerase chain reaction (PCR) from bovine genomic DNA with gene specific primers (Fig. 1a). Col1a1, Col2a1, Acan, Sox9 and Oct4 primers were described previously (Kraus et al. 2017). The following base primers were employed for Endoglin (5′-tcctcggagagcagtagca-3′; 5′-atataggggaggacccaggac-3′), Gli3 (5′-cgaacagccgcgacctgtct-3′; 5′-tcggatttactggcatcggg-3′) and T (5′-gcagtgtttgagcggcagtc-3′;
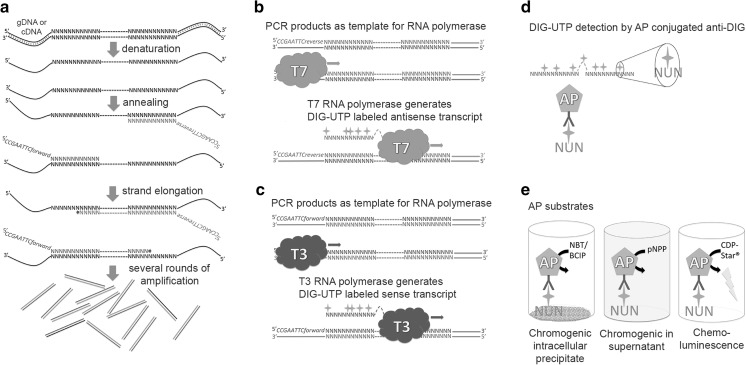
Fig. 1.
Schematic representing the basic principle leading to development of the PISH and qPISH protocols. a Probe template is generated by PCR amplification with gene specific primers containing the 5′ T3 or T7 RNA polymerase recognition site. DIG-UTP is incorporated into the anti-sense and sense RNA probe employing T7 b or T3 RNA polymerases c, respectively. d The DIG-UTP label is recognized by an AP-conjugated anti-DIG antibody. e Different AP substrates were investigated to develop the qPISH assay
5′-agcaaacattctagcaggcagag-3′). Primers also contained recognition sequence as a 5′ addition to the base primers for the T7 (5′-gtaatacgactcactatagggc-3′) and T3 (5′-gtaatacgactcactatagggc-3′) RNA polymerase to generate antisense (Fig. 1b) and sense (Fig. 1c) probes through in vitro transcription, respectively. RNA probes were labeled through digoxigenin (DIG) UTP incorporation as described (Kraus et al. 2017). An AP conjugated anti-DIG antibody specifically recognized the DIG epitope (Fig. 1d), facilitating subsequent AP catalyzed substrate conversion by cells expressing the target RNA (Fig. 1e).
qPISH for quantitative gene expression analysis
The PISH aspect of qPISH was carried out essentially as described until the addition of the AP-substrate (Kraus et al. 2015). Prehybridization and hybridization were performed at 62 °C. A 1:2000 dilution of anti-DIG antibody (Roche) in blocking solution (Roche) was applied and cells were washed extensively to eliminate non-specific binding. Quantitative detection of mRNA transcripts was optimized for the chromogenic substrate pNPP (Thermo Fisher) and the chemiluminescence generating substrate CDP-Star (AB). Substrates were prepared according to Table 1 and 100 μL solution per well of a 96 well plate was applied with a 12-channel pipet for accuracy of timing. Chemiluminescence and absorbance measurements were performed using a SpectraMax i3× (Molecular Devices) multi-mode microplate reader. Luminescence at 475 nm was recorded 60 min post substrate application for CDP-Star and every 10 min thereafter for a total of 120 min. Absorbance readings were taken at 405 nm wavelength every 24 h after addition of substrate for pNPP for a total of 192 h.
Table 1.
Composition and origin of substrate solutions employed in qPISH and q2PISH. Typically 0.1 ml per well of a 96-well plate was applied
| Substrate | NBT/BCIP | pNPP | CDP-Star |
|---|---|---|---|
| Supplier | Roche | Thermo Scientific | AB |
| Product number | 11,681,451,001 | 34047 | T2304 |
| Type | Chromogenic | Chromogenic | Chemiluminescent |
| Substrate | 1:50 dilution of stock | 1 tablet in 5 ml | 1:10 dilution of stock |
| Tris–HCl pH9.5 | 0.1 M | 0.1 M | 0.1 M |
| NaCl | 0.1 M | 0.1 M | 0.1 M |
After completion of absorbance/luminescence data collection, each well was rinsed three times with PBS for 5 min each, fixed with 4% (w/v) PFA for at least 20 min, followed by another three washes with PBS. Subsequently fluorescent nuclear dye To-PRO-3 (Thermo Fisher) was added at 1:1000 dilution in PBS for an accurate cell count. To-PRO-3 stained cells from the entire well were imaged using a Minimax imaging cytometer attached to SpectraMax i3× with excitation and emission filters set at 625 and 713 nm, respectively.
Statistical analysis of data
Unpaired Student’s t-test was used to assess gene expression of cultured cells derived from different areas of bovine IVDs. The values considered for statistical analysis after background correction as described above were arbitrary units (a.u.) reflecting absorbance at 405 nm for the pNPP substrate and relative light units (rlu) reflecting chemiluminescence measurement for CDP-Star. Graphs were generated for these values with Prism GraphPad (Figs. 3, 4) and Excel (Figs. 2, 4b). No significant difference was considered for a p value of p > 0.05. Error bars indicate the standard error.
Fig. 3.
Quantitative and qualitative gene expression profiling using q2PISH. a Quantitative differences in gene expression illustrated as total gene expression and gene expression per cell between two different cell lines as shown for the extracellular matrix (ECM) and structural proteins encoding genes Col1a1, Col2a1 and Acan; Sox9, encoding a transcription factor (TF) crucial for chondrogenic development; Oct4 encoding a TF indicating pluripotency and T encoding brachyury, a notochordal cell marker. Arbitrary units (a.u.) are indicated on the y-axis. b Post pNPP staining qualitative NBT/BCIP assessment of gene expression as shown for Col1a1, Col2a1, Sox9 and Oct4 (Scale bars 100 μM). c, d To-PRO-3 nuclear staining was performed to estimate the total cell number to adjust pNPP quantification to a per cell value as shown here for a well with high (c) and low (d) cell count (Scale bars 1 mm). qPISH expression data per cell was subjected to unpaired student’s t-test with no significant difference (ns) indicating a p value of p > 0.05, *indicating a p value of p ≤ 0.05; **p value of p ≤ 0.01 and ***p value of p ≤ 0.001. Error bar is indicating the standard error
Fig. 4.
Application of q2PISH to assess the impact of different FBS on biomarker expression. Fetal bovine serum (FBS) received from two different suppliers (A and B) was tested for the expression of a subset of biomarkers for two different cell lines (I and II) in culture. a qPISH expression data per cell was subjected to paired student t-test, which did not reveal any significant difference in gene expression between FBS of supplier A an B for the genes analyzed. Arbitrary units (a.u.) are reflected on the y-axis. b Nuclear cell count at the experimental endpoint indicated a significant reduction in cell survival for cells cultured with FBS from supplier B. c Qualitative assessment of gene expression post quantification as shown here exemplary for Col1a1 expression by cell line I clearly indicates heterogeneity of the culture, showing cells with high (red arrow), medium (green arrow) and low (blue arrow) level of Col1a1 expression for FBS from both suppliers. qPISH expression data per cell and cell count was subjected to unpaired student’s t-test with no significant difference (ns) indicating a p value of p > 0.05, **indicating a p value of p ≤ 0.01 and ***indicating a p value of p ≤ 0.001. Error bar is indicating the standard error
Fig. 2.
Protocol optimization for quantification of gene expression using chemiluminescence (CDP-Star) and absorbance (pNPP) assays. a IVD derived cells expressing Col1a1and Endoglin were used for the assay. Images show NBT/BCIP staining from qualitative PISH (Scale bars 50 μM). b Graphs display data collected to determine the optimal time points for luminescence. c Absorbance measurement at high and low cell concentrations for these genes. Cell numbers (#) are indicated on the x-axis and relative light units (rlu) for luminescence data or arbitrary units (a.u.) for absorbance data are indicated on the y-axis: min minutes; h hours
Quantitative and qualitative gene expression analysis with q2PISH
q2PISH employed the optimized conditions for the pNPP substrate, which was removed from all wells after collecting absorbance data. Wells were washed 3× for 5 min each with NTMT buffer (100 mM Tris–HCl pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.1% (v/v) Tween 20) before adding the NBT/BCIP substrate. After four days of NBT/BCIP exposure the wells were rinsed 3× with PBS for 5 min each, fixed with 4% (w/v) PFA for at least 20 min, followed by another three rinses with PBS prior to the To-PRO-3 based nuclear staining as described above.
Results and discussion
qPISH for quantification of gene expression
While we have previously demonstrated the need for qualitative assessment of gene expression in cell cultures to identify potential heterogeneity between individual cells (Kraus et al. 2015, 2017), quantification of gene expression is often required. The AP component of the AP-conjugated anti-DIG antibody is a phosphate-transferring enzyme capable of catalyzing this reaction on a variety of substrates (Sergienko and Millan 2010). qPISH was adapted from the original PISH protocol (Kraus et al. 2015) to enable quantification of gene expression in cell culture. We have investigated here two different candidate substrates, namely CDP-Star, a chemiluminescence generating compound and p-Nitrophenyl phosphate (pNPP), a soluble chromogenic compound. Protocols for both substrates were optimized for time of data collection at a range of cell concentrations (Fig. 2), employing a high (Col1a1) and low (Endoglin) level expressed gene. Absorbance of the chromogen biocatalyzed from the substrate pNPP, measured at 405 nm, was determined to be sufficient at 120 h after administration (Fig. 2). Similarly, sufficient chemiluminescence from the CDP-Star substrate, was detected at 475 nm 90 min after administration for a cell number larger than 5000 cells/well of a 96 well plate. However luminescence measurement was not well suited for typical 96-well culture plates owing to light interference across transparent wells. Minimizing this interference by using opaque 96-well plates was possible, however, the material of the opaque plates negatively impacted on cell adherence during PISH, therefore CDP-Star was subsequently relinquished as a robust substrate for qPISH. However it appeared that below 5000 cells/well of a 96 well plate quantification using CDP-Star or pNPP was less informative. Attempts to quantify the intracellular deposition of purple NBT Diformazan in stained cells using photo-documentation and image analysis, indicated that this method was prone to artifacts and was therefore not further pursued for quantification of gene expression. In order to account for potential variability between individual wells during pNPP readings, a minimum of three replicates were analyzed per cell line, probe and condition. Absorbance values were normalized by subtracting the average no-cell control values from the sample data average. Furthermore, a To-PRO-3 nuclear count of each well was used to determine the average absorbance per cell. The values were then graphed as arbitrary units (a.u.). However, while qPISH alone enabled quantification of gene expression levels of different cell lines or assay conditions for a given probe, it failed to qualitatively assess heterogeneity of a cell population and would be of no advantage over methods that employ cell pooling. Hence it was of importance to also include the qualitative assessment of the same culture, which led us to develop q2PISH.
q2PISH to quantify gene expression and simultaneously assess cell to cell heterogeneity of a given cell culture
Two cell lines derived from structurally different areas of the fetal bovine IVD (lines III and IV) were subjected to quantitative mRNA expression using qPISH assessment for six different genes (Fig. 3a). Quadruplicate quantitative data points were collected for each probe and cell line. Quantitative gene expression assessment was followed by qualitative assessment, q2PISH (Fig. 3b) as shown here for Col1a1, Col2a1, Sox9 and Oct4. The final step of a To-Pro-3 nuclear stain based cell count in the same wells after exposure to the pNPP and NBT/BCIP substrates as shown for a high (Fig. 3c) and lower (Fig. 3d) cell count allows for comparable “per cell” gene expression data (Fig. 3a). Employing unpaired student t-test, a significant difference in gene expression between cell lines III and IV was detected for all genes but Oct4 and T. (Col1a1 (p < 0.0001), Col2a1 (p = 0.0170), Acan (p = 0.0296), Sox9 (p = 0.0058), Oct4 (p = 0.1175) and T (p = 0.3195). We would like to highlight here the importance of accurate per cell data acquisition since data acquired for total gene expression per well would indicate a significant difference in gene expression between cell lines III and IV for all genes but Col2a1 and Acan (Col1a1 (p = 0.003), Col2a1 (p = 0.1293), Acan (p = 0.3068), Sox9 (p = 0.0014), Oct4 (p = 0.0008) and T (p = 0.0252)) which would lead to faulty conclusions. Notably, it is the qualitative analysis step included in q2PISH (Fig. 3b) that can alert one to potential heterogeneity, an important information that could remain masked if qPISH is carried out alone. This limitation is not confined to qPISH, but applies to other known assays for gene expression quantification of pooled cells such as qRT-PCR, microarray expression profiling and even RNA sequencing.
Gene expression data per cell as obtained by q2PISH (Fig. 3), with quantitative and qualitative information gathered, allows to characterize and monitor cell lines in culture. This facilitates the awareness of a potential phenotype drift in a cell population. Noticing changes in gene expression on a single cell basis, especially for genes that have been identified as characteristic biomarkers for a cell line at an early passage or in vivo, is a helpful quality control tool for cell-based therapies.
q2PISH serves to assess culture conditions
Fetal bovine serum (FBS) is known as a critical media component, with frequent concerns that differences in FBS lots can impact on cell behavior. We employed q2PISH as quality control measure to assess in how far changing a FBS supplier would impact on the transcription of biomarkers. In this context we investigated Col1a1, Col2a1, Endoglin and Gli3 expression for the cell lines I and II derived from two anatomically different areas of the adult bovine IVD. When analyzing the per cell qPISH data with unpaired student’s t-test we found no significant difference in gene expression level of cells cultured with FBS from two different suppliers for the four biomarkers analyzed here (Fig. 4a): Cell line I (Col1a1: p = 0.7340; Col2a1: p = 0.2200; Endoglin: p = 0.7457; Gli3: p = 0.9181) and cell line II (Col1a1: p = 0.3396; Col2a1: p = 0.9427; Endoglin: p = 0.2053; Gli3: p = 0.5889). However, cell survival significantly improved for both cell lines when using FBS from supplier A over B (line I: p = 0.0009; line II: p = 0.0048) (Fig. 4b). Therefore our findings cannot exclude any potential impact of FBS on the expression of genes other than Col1a1, Col2a1, Endoglin and Gli3 that could interfere with cell survival. The importance of the qualitative aspect of q2PISH after quantification of gene expression is stressed through the obvious heterogeneity of the cultures as shown exemplary for Col1a1 expression in cell line I (Fig. 4c).
In summary, we have developed a method for consecutive qualitative and quantitative gene expression analysis including a nuclear cell count that allows single cell assessment for cells in culture. This is an important tool for the quality control of cultured cells to monitor potential drifts in cell populations.
Acknowledgements
We are grateful to Peter Braun of Woodcrest Dairy (Lisbon, NY), Willard and Sons (Heuvelton, NY) and Tritown Meat Packing (Brasher Falls, NY) for providing us with bovine tissue for cell line derivation. This work was supported by the Bayard and Virginia Clarkson Endowment Fund to Thomas Lufkin. Rachel Yerden was supported by the Collegiate Science and Technology Entry Program (CSTEP) and Community of Underrepresented Professional Opportunities (CUPO) programs.
Footnotes
Petra Kraus and Rachel Yerden are equal first authors.
Contributor Information
Petra Kraus, Email: pkraus@clarkson.edu.
Rachel Yerden, Email: yerdenr@clarkson.edu.
Darren Sipes, Email: sipesdc@clarkson.edu.
Shantanu Sur, Email: ssur@clarkson.edu.
Thomas Lufkin, Phone: +1-315-268-6641, Email: tlufkin@clarkson.edu.
References
- Bink K, Walch A, Feuchtinger A, Eisenmann H, Hutzler P, Hofler H, Werner M. TO-PRO-3 is an optimal fluorescent dye for nuclear counterstaining in dual-colour FISH on paraffin sections. Histochem Cell Biol. 2001;115:293–299. doi: 10.1007/s004180100254. [DOI] [PubMed] [Google Scholar]
- Chen X, Li X, Wang W, Lufkin T. Dlx5 and Dlx6: an evolutionary conserved pair of murine homeobox genes expressed in the embryonic skeleton. Ann N Y Acad Sci. 1996;785:38–47. doi: 10.1111/j.1749-6632.1996.tb56242.x. [DOI] [PubMed] [Google Scholar]
- Holtke HJ, Kessler C. Non-radioactive labeling of RNA transcripts in vitro with the hapten digoxigenin (DIG); hybridization and ELISA-based detection. Nucleic Acids Res. 1990;18:5843–5851. doi: 10.1093/nar/18.19.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P, Lufkin T. Mammalian Dlx homeobox gene control of craniofacial and inner ear morphogenesis. J Cell Biochem. 1999;32–33:133–140. doi: 10.1002/(SICI)1097-4644(1999)75:32+<133::AID-JCB16>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kraus P, Lufkin T. Bovine annulus fibrosus cell lines isolated from intervertebral discs. Genom Data. 2016;10:83–84. doi: 10.1016/j.gdata.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P, Kocsis V, Williams C, Youngs B, Lufkin T. Plate in situ hybridization (PISH) as a time and cost effective RNA expression assay to study phenotypic heterogeneity in a population of cultured murine cells at single cell resolution. Biotechnol Lett. 2015;37:1573–1577. doi: 10.1007/s10529-015-1833-1. [DOI] [PubMed] [Google Scholar]
- Kraus P, Yerden R, Kocsis V, Lufkin T. RNA in situ hybridization characterization of non-enzymatic derived bovine intervertebral disc cell lineages suggests progenitor cell potential. Acta Histochem. 2017;119:150–160. doi: 10.1016/j.acthis.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Lufkin T. Transcriptional control of Hox genes in the vertebrate nervous system. Curr Opin Genet Dev. 1996;6:575–580. doi: 10.1016/S0959-437X(96)80086-4. [DOI] [PubMed] [Google Scholar]
- O’Connor KL, Culp LA. Quantitation of two histochemical markers in the same extract using chemiluminescent substrates. Biotechniques. 1994;17:502–509. [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Sergienko EA, Millan JL. High-throughput screening of tissue-nonspecific alkaline phosphatase for identification of effectors with diverse modes of action. Nat Protoc. 2010;5:1431–1439. doi: 10.1038/nprot.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DW, Jensen KB, Trotter MW, Connelly JT, Broad S, Watt FM. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development. 2013;140:1433–1444. doi: 10.1242/dev.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribioli C, Lufkin T. Molecular cloning, chromosomal mapping and developmental expression of BAPX1, a novel human homeobox-containing gene homologous to Drosophila bagpipe. Gene. 1997;203:225–233. doi: 10.1016/S0378-1119(97)00520-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Lo P, Frasch M, Lufkin T. Hmx: an evolutionary conserved homeobox gene family expressed in the developing nervous system in mice and Drosophila. Mech Dev. 2000;99:123–137. doi: 10.1016/S0925-4773(00)00488-3. [DOI] [PubMed] [Google Scholar]
- Zhao F, Lufkin T, Gelb BD. Expression of Tfap2d, the gene encoding the transcription factor Ap-2delta, during mouse embryogenesis. Gene Expr Patterns. 2003;3:213–217. doi: 10.1016/S1567-133X(02)00067-4. [DOI] [PubMed] [Google Scholar]