Abstract
In this study, we developed a new purification method using chondroitin sulfate C (CSC) and protamine sulfate (PS) to concentrate lentivirus. To evaluate the efficiency of this new method, we compared it with several previously described purification protocols, including virus concentrated by ultracentrifugation (Ultra), precipitated by polyethylene glycol (PEG), and sedimented by CSC combined with polybrene (PB). After using the different methods to purify and concentrate equivalent amounts of lentivirus supernatant, the virus pellets precipitated by the different methods were resuspended using the equivalent volumes of DMEM. Subsequently, 10 μl of each lentivirus stock carrying EGFP gene was used to transduce two types of cells, human embryonic kidney 293T (HEK293T) cells and mouse mesenchymal stem cells (mMSC). It was obvious that HEK293T and mMSC appeared much intensiver green fluorescence through virus transduction from PS method than from other methods. To quantitate the transduction efficiency of the viruses, we examined virus titer in the cells after transduction using a real-time PCR-based analysis. Accordingly, we verified that PS precipitation could generate virus with a higher titer (4.39 × 108 IU/ml) than PB (2.43 × 108 IU/ml), Ultra (1.16 × 108 IU/ml), and PEG (0.56 × 108 IU/ml) in HEK293T cells. As for HEK293T cells in mMSC, the PS method also generated virus with a higher titer (4.66 × 108 IU/ml) than the Ultra method (2.36 × 108 IU/ml), and a much higher titer than those of the other chemical-based precipitation methods using PB (4.82 × 106 IU/ml) and PEG (8.98 × 104 IU/ml). Furthermore, the HEK293T cells and mMSC transduced by PS(1X)-virus appeared to have higher cell growth ratios, respectively, than the HEK293T cells and mMSC transduced by lentivirus using the other methods. We conclude that our new method for purifying lentivirus is cost-effective, time-saving, and highly efficient, and that lentivirus purification by this means could possibly be used to transduce a variety of cells, including stem cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0133-0) contains supplementary material, which is available to authorized users.
Keywords: Lentivirus, Concentration, Protamine sulfate, Transduction
Introduction
Traditionally, lentivirus particles can be packaged in human embryonic kidney 293T (HEK293T) cells and then be released into a culture medium with titers ranging from 106 to 107 transducing units per milliliter. However, for some specific purposes, the original virus supernatant needs to be further treated. For example, it may be necessary to replace the virus pool with a different medium for gene transduction in specific cell types, or it may be necessary to concentrate the virus pool for gene therapy in vivo. Currently, many manipulations have been developed to purify and concentrate virus. Among the available methods, virus purification by ultracentrifugation is the most commonly used method (Tiscornia et al. 2006). However, purification by this means can easily result in the virus envelope being damaged because of the shearing force caused by high-speed centrifugation, unless, that is, the virus is pseudotyped with VSV-G or RD114 envelope proteins (Burns et al. 1993; Gatlin et al. 2001). Moreover, using this protocol to purify lentivirus is time-consuming, labor-intensive, and, most importantly, cost-ineffective due to the necessity of using and maintaining an expensive machine. Ultrafiltration represents another physical approach by which to concentrate virus with high efficiency and reduced toxicity (Reiser 2000; Papanikolaou et al. 2013). However, this procedure may also be overly time-consuming because clogs can form that require the filter to be replaced after a certain amount of culture medium has passed through it (Sun et al. 2014). In contrast to these physical methods, chemical-based methods for concentrating lentivirus provide alternative approaches that do not require the use of a specific apparatus. Rather, simple protocols of chemical supplementation and low-speed centrifugation are adopted to precipitate virus. Thus, such methods constitute easier means of virus concentration, and are potentially suitable for most laboratories. Chemicals are employed in these approaches in order to precipitate virus by using different mechanisms; for example, virus can be precipitated due to decreased solubility in medium caused by the exclusionary effect of polyethylene glycol (PEG) (Kohno et al. 2002; Fontes et al. 2005) or by the salting-out effect of ammonium sulfate (Armon et al. 1988). Virus can also be aggregated and precipitated through the use of a charged molecule such as poly-lysine (Zhang et al. 2001), or by combining anionic and cationic polymers such as chondroitin sulfate C (CSC) and polybrene (PB) (McMillin et al. 2005; Landazuri and Le Doux 2006). Nevertheless, the residual chemicals along with precipitated virus possibly hamper the virus application. Thus, it is necessary to assess the influence of residual chemicals prior to advance application. In this study, we present a method involving the use of a new set of reagents, CSC and protamine sulfate (PS), to concentrate lentivirus. Virus concentration by this means is compared to virus concentration via three other methods, namely ultracentrifugation (Ultra), PEG precipitation (PEG), and precipitation using CSC combined with PB (PB). The influences of all four different methods on cell survival, gene transfer rate, and titer in cells were evaluated.
Materials and methods
Cell culture
HEK293T cells were cultured in DMEM (Invitrogen, Life Technologies Corp., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (SAFC Biosciences, Lenexa, KS, USA), penicillin G (Sigma-Aldrich Co., St. Louis, MO, USA) (100 U/ml), and streptomycin (Sigma-Aldrich Co., St. Louis, MO, USA) (100 μg/ml) at 37 °C in a water-saturated atmosphere containing 5% CO2 in air. HEK293T cells were seeded at a density of 5 × 104/ml per well in 12-well tissue culture plates. 10 μl of concentrated viruses were then added to the cells for gene transduction. After 24 h, the culture medium was replaced with fresh DMEM medium supplemented with 10% FBS (SAFC Biosciences., Lenexa, Kansas, USA). After 4 days of incubation, the cells were harvested and their DNA was isolated.
Isolation and culture of mesenchymal stem cells (MSC)
Mouse MSC (mMSC) cultures were obtained according to the protocols described by Soleimani and Nadri (2009) with slight modifications. Briefly, 8 ICR mice aged 6–8 weeks old were sacrificed, and their bone marrow cells were pooled. After filtration through a 70-μm filter mesh to remove any bone spicules or cell clumps, the bone marrow cells were centrifuged at 400×g for 5 min and planted in 10-cm dishes at a density of 107 cells/cm2. They were then incubated at 37 °C with 5% CO2 in a humidified chamber. After 3 h, non-adherent cells were removed by changing the medium. The medium was replaced with fresh medium every 8 h for 3 days. After 3 days, the cells were trypsinized and replated in alpha-MEM (Gibco., Carlsbad, CA, USA). After 10 days, the cells were trypsinized again and frozen in alpha-MEM with 5% DMSO and 30% FBS. For experiments, the mMSC were thawed and maintained in alpha-MEM medium supplemented with 10% FBS, and were seeded at a density of 5 × 103/ml per well in 12-well tissue culture plates. Ten μl of concentrated virus were added to the cells, and after 24 h, the culture medium was replaced with fresh alpha-MEM medium supplemented with 10% FBS. After 7 days of incubation, the cells were harvested and their DNA was isolated.
Preparation of VSV-G pseudotyped lentivirus supernatants
HEK293T cells were cultured in 60-mm plates for transfection of the lentiviral vectors. The pCMV-deltaR8.91 packing plasmid, the pMD.G envelope plasmid, and the pLAS3w.PeGFP-I2-Bsd lentiviral vector plasmid (National RNAi Core Facility, Academia Sinica, Taiwan) were cotransfected into HEK293T cells using the X-tremeGENE HP DNA Transfection Reagent (Roche, Mannheim, Germany). The transfection medium was replaced with fresh medium containing 1% bovine serum albumin (BSA) (Sigma-Aldrich Co., St. Louis, MO, USA) at 24 h post-transfection. The viral supernatant was harvested at 24, 48, and 72 h after the high-BSA medium exchange, filtered through a 0.45-μm filter (Millipore, Billerica, MA, USA), and stored at 4 °C for later use.
Concentration of lentivirus
To concentrate lentivirus using PEG, PEG 8000 (Sigma) and NaCl were added to the virus supernatant until final concentrations of 10% and 0.15 M, respectively, were reached. The mixtures were incubated for 16–20 h on a shaker at 4 °C, and then were centrifuged for 30 min at 10,000×g and 4 °C. To purify lentivirus using ultracentrifugation, virus particles were collected by ultracentrifugation at 50,000×g and 4 °C for 2 h. To concentrate lentivirus using PB and CSC, virus was precipitated with 80 μg/ml of PB (Sigma) and 80 μg/ml of CSC (Sigma). The mixtures were incubated for 16–20 h on a shaker at 4 °C, and were centrifuged for 30 min at 10,000×g and 4 °C. To concentrate lentivirus using PS and CSC, virus supernatant was mixed with 80 μg/ml of CSC and various concentrations of PS (Sigma) separately, including 16 μg/ml (0.2X), 40 μg/ml (0.5X), 80 μg/ml (1X), 160 μg/ml (2X), and 320 μg/ml (4X). The mixtures were incubated for 16–20 h on a shaker at 4 °C, and were centrifuged for 30 min at 10,000×g and 4 °C. The virus pellets acquired using the above four methods were resuspended at 1/100 of the original volume using DMEM complete medium and stored at 4 °C. Virus samples were prepared three times by different operators. In each time, an operator prepared one set of viruses using the different methods, including Ultracentrifugation, PEG precipitation, PB precipitation, and PS precipitation (0.2X, 0.5X, 1X, 2X, 4X).
DNA extraction and real-time PCR
DNAs from cultured HEK 293T cells and mouse MSC were extracted using the simplified mammalian DNA isolation procedure (Laird et al. 1991). The DNA was used as the template for real-time PCR analyses, for which gene specific primers were designed using the PerlPrimer software. The real-time PCR procedure was performed using an ABI 7500 instrument (Applied Biosystems, Carlsbad, CA, USA). The qPCR Lentivirus Titration Kit (LV900) purchased from abm (Applied Biological Materials Inc, Richmond, British Columbia, CA) that provides a standard lentivirus concentration was used to estimate the virus titers from different treatments. The concentration of lentiviral vector was assessed with CAG promoter primer set. The primer sequences were as follows: 5′-TCCGCGTTACATAACTTACGG-3′ and 5′-GGGCGTACTTGGCATATGAT-3′. The ALB gene sequence was used to normalize the amount of template for each sample extracted from the HEK293T cells, and the Alb gene sequence was used to normalize the amount of template for each sample extracted from the mouse MSC. The primer sequences were as follows: ALB, 5′-TGTGGGCTGTAATCATCGTC-3′, and 5′-ATCCAAACTCATGGGAGCTG-3′; Alb, 5′-ATGGTGAACTGGCTGACTGC-3′, and 5-TGTCCCATAAAGGTGGTTGG -3′. Each reaction was run in triplicate with the results averaged.
Cell growth ratio measurement and calculation
HEK 293T cells and mMSC were gene transduced by viruses that were prepared using different methods as described above. The HEK 293T cells and mMSC were then cultured for 4 and 7 days, respectively, and were harvested for cell counting by the standard hemocytometer method. To represent the relative cell growth ratio, the number of cells produced using each or the other methods was divided by the number of cells produced using the PS(1X) method, with the result shown as a percentage. Each relative growth ratio was measured in triplicate, and the results were averaged.
Statistical analysis
Each experiment was carried out in duplicate and repeated three times. The results are expressed as a mean ± standard deviation. The data from the various treatments were compared using a t test, and the differences with a P value of <0.05 were considered to be statistically significantly different.
Results
Lentivirus purified and concentrated by PS achieved higher transduction efficiency
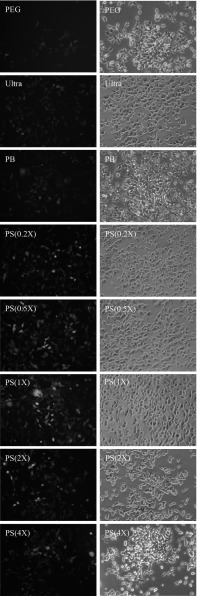
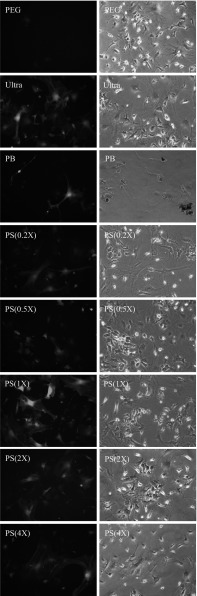
Physical methods, such as ultracentrifugation or ultrafiltration, can be used to purify functional viruses with high titers, but they are also time-consuming. Chemical-based approaches, such as PEG and PB precipitation, can be used to concentrate viruses much more conveniently, but the residual chemicals accompanying the precipitated viruses can sometimes interfere with virus transduction. Therefore, a purification method that can avoid these disadvantages would be beneficial in terms of virus-related applications. In the present study, we examined the use of several chemicals for virus purification, and one set of chemicals, PS combined with CSC, appeared to exhibit excellent performance. In order to compare the effectiveness of different methods, HEK293T cells and mMSC were gene transduced by various concentrated viruses, and EGFP expressions were examined. As shown in Fig. 1, although all purification methods provided satisfactory results in the transduction of HEK293T cells, the PS(1X) transduced cells exhibited significantly higher green fluorescence intensity than cells transduced using the other methods. We then questioned whether the PS method is appropriate for the transduction of mMSC, because the residual chemicals in chemical-based virus stock may influence the virus’s access to the primary cells, such as mMSC. After virus transduction, we found that all the chemical-based virus stocks except those produced with the PS-virus method were unable to transduce mMSC with high efficiency (Fig. 2). Ultra-virus remained consistent in transduction to mMSC (76%), yet PEG-virus and PB-virus’s transduction activity were dramatically lower (22.2 and 48.1%) to mMSC than their transduction to HEK293T. PS-virus still exhibited the highest performance in terms of transduction to mMSC (87%), even in comparison to Ultra-virus.
Fig. 1.
Evaluating lentivirus transduction efficiency to HEK293T cells by EGFP expression. Lentivirus carrying EGFP was purified and concentrated by different methods, including PEG precipitation, ultracentrifugation (Ultra), PB precipitation, PS precipitation (0.2X, 0.5X, 1X, 2X, 4X). The actual chemical concentrations used are described in the "Materials and methods” section. All fluorescence images (left panel) were photographed under the same lighting
Fig. 2.
Evaluating lentivirus transduction efficiency to mMSC by EGFP expression. Lentivirus carrying EGFP was purified and concentrated by different methods, including PEG precipitation, ultracentrifugation (Ultra), PB precipitation, PS precipitation (0.2X, 0.5X, 1X, 2X, 4X). The actual chemical concentrations used are described in the “Materials and methods” section. All fluorescence images (left panel) were photographed under the same lighting
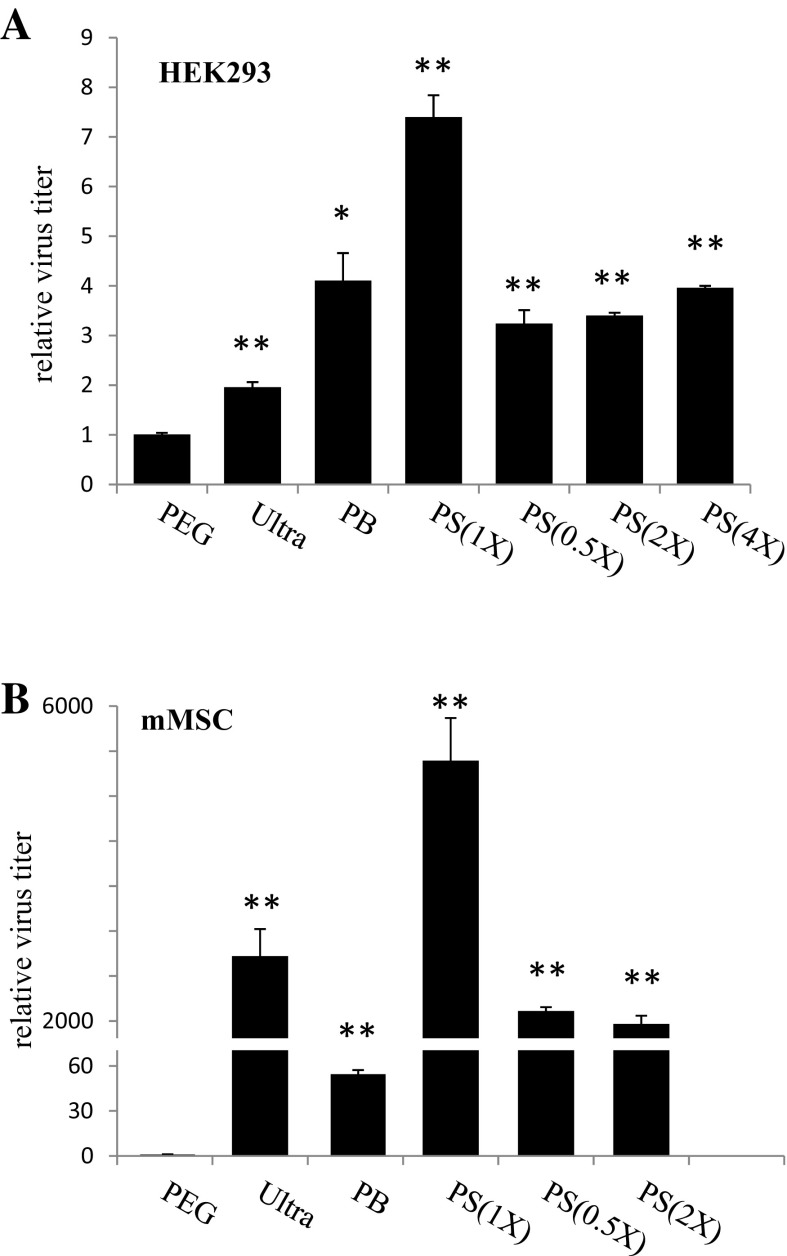
The lentivirus titer purified by PS was higher than those purified by using other methods, particularly in transduction of mMSC
To reflect the authentic status of transduction efficiency, we used real-time PCR to measure the virus DNA extracted from cells after transduction. Also, since different cells exhibit diverse responses to virus transduction, we performed PCR reactions separately with HEK293T cells and mMSC. For the results of PCR detection with the HEK293T cells (Fig. 3a), the virus titers were determined in accordance with the EGFP expression; the PS(1X)-virus was found to have the highest titer (4.39 × 108 IU/ml), followed by PB-virus (2.43 × 108 IU/ml), Ultra-virus (1.16 × 108 IU/ml), and PEG-virus (0.56 × 108 IU/ml). It is noteworthy that in addition to PS, other two chemical-based, PEG and PB-virus were also detected a high titer in HEK293T cells. Unlike the HEK293T cell line, the mMSC appeared to exhibit rather different cell behavior after being isolated from the live animal tissue, insofar as it was apparently ready to respond to extracellular signals for growth or differentiation. Therefore, it is rational to assume that a virus stock with complicated components, such as the chemical-based virus stocks used in this study, may interfere with their transduction into mMSC. As a matter of fact, we detected the titers of the viruses precipitated with PB and PEG (Fig. 3b) were dramatically reduced to 4.82 × 106 and 8.98 × 104 IU/ml, respectively. Intriguingly, PS-virus still contributed the highest efficiency of transduction in response to its higher titer (4.66 × 108 IU/ml), which was higher than that of Ultra-virus (2.36 × 108 IU/ml). This result is interesting because the PS-method maintains the convenience of manipulating virus concentrations offered by the other chemical-based methods, but avoids the disadvantage of low transduction efficiency to certain cells.
Fig. 3.
A real-time PCR-based analysis was adopted to measure the virus titers after lentivirus transduction to HEK293T cells (a) and mMSC (b). Results indicated that virus purified by PS(1X) produced the highest concentration in both HEK293T cells and mMSC. The relative virus titers were acquired by comparison to the PEG control, which was designated as 1. The Student’s t test was adopted for the statistical analysis. *p < 0.05 and **p < 0.01 were considered significantly different from the PEG controls
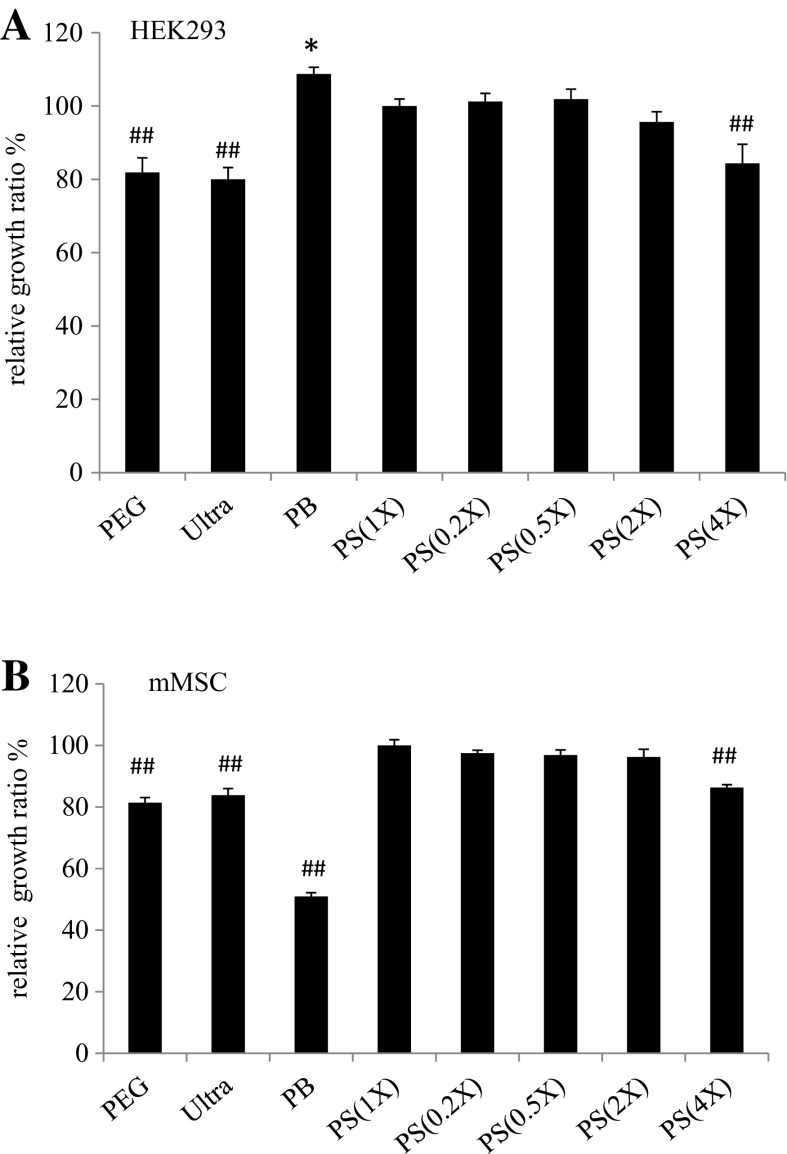
The relative cell growth ratios of HEK293T cells and mMSC after gene transduction were divergent
Residuals, such as chemicals or virus debris accompanying the precipitated virus, may affect cell behaviors, such that the growth rates of cells in response to virus precipitated with different purification methods may be remarkably different. We therefore examined and compared the growth rates of cells subjected to virus produced with the different methods. After virus transduction, HEK293T cells and mMSC were further cultured for 4 and 7 days, respectively. The cells were then harvested and their numbers were counted. We compared the growth ratios of each treatment with the PS(1X) treated HEK293T cell ratio which was designated as 100%. As shown in Fig. 4a, HEK293T cells seemed relatively tolerant to different conditions. The relative growth ratios of the HEK293T cells subjected to different virus transductions ranged from 80 to 110%, among which the cells subjected to PB-virus transduction had a slightly higher growth ratio than the PS(1X) controls (108.75 ± 1.82 vs. 100 ± 1.92%), while the cells transduced by PEG-virus and Ultra-virus had significantly lower growth ratios (81.88 ± 3.97 and 80 ± 3.21%, respectively). On the other hand, although the HEK293T cells subjected to PB-virus transduction acquired the highest growth ratio in comparison to those subjected to the other treatments, PB-virus transduction significantly affected mMSC cell growth (Fig. 4b); specifically, it appeared to result in the lowest growth ratio (50.93 ± 1.25%). mMSC subjected to PEG-virus and Ultra-virus transduction again exhibited significantly lower growth ratios (81.37 ± 1.7 and 83.85 ± 2.16%, respectively). In all, cells subjected to PS-virus transduction were relatively stable in terms of their growth. In addition to the high concentration of PS(4X)-virus, which lowered the growth ratio of HEK293T cells (84.38 ± 5.17%) and mMSC (86.33 ± 0.94%), the PS-virus (0.2X, 0.5X, 1X, 2X) transduced cells were grown at higher rates than other physical- or chemical-virus treated cells.
Fig. 4.
The relative cell growth ratios of HEK293T cells (a) and mMSC (b) after gene transduction by various precipitated lentiviruses were measured in comparison to the PS(1X) control (100%). Results indicated that HEK293T cells after PB virus transduction appeared to exhibit a slightly higher growth ratio than the PB(1X) virus transduced cells. On the other hand, the HEK293T cell growth ratio was significantly decreased after gene transduction by PEG and Ultra precipitated viruses in comparison to gene transduction by PB(1X) virus. In gene transduction to mMSC, the PEG-, Ultra-, and PB-precipitated viruses resulted in significantly lower cell growth ratios than the PB(1X)-precipitated virus. *p < 0.05 were considered to indicate significantly higher growth ratios than the PS(1X) controls, and ## p < 0.01 were considered to indicate significantly lower growth ratios than the PS(1X) controls
Discussion
A variety of physical- and chemical-based methods for concentrating lentivirus have been developed in order to increase the effective rate by which it infects cells in vitro and, for gene therapy, in vivo. Several factors should be considered before choosing one of these methods. These include whether a given method is time-saving, cost-effective, non-laborious, and safe to infected cells. Technically, chemical-based methods for concentrating lentivirus are advantageous in terms of time, cost, and labor issues, but also consistently pose challenges with regard to the safety to the target cells. After searching for several chemicals, we have successfully used a cationic molecule, protamine sulfate (PS) which could concentrate lentivirus with high efficiency and low toxicity to cells.
In this study, we adopted identical procedures except with regard to the concentration of virus used to produce virus stock. Equivalent volumes of dispersed virus produced by different schemes were then used to transduce HEK293T cells and mMSC. As shown by the EGFP expression results, PS(1X)-virus retained relatively consistent activity in terms of its capacity to transduce either HEK293T cells or mMSC, that displayed the highest fluorescence intensity. On the other hand, although PEG- and PB-virus exhibited sufficient EGFP expression in HEK293T cells, those results were not reproducible in mMSC. This variation in transduction efficiency may be a result of three factors. Firstly, the virus titers produced by different methods varied. Secondly, the infectivity levels of virus samples produced with different purification methods are not identical. Thirdly, cells respond to different samples of precipitated virus in different ways. To evaluate the titers of viruses produced using different purification strategies, we quantitated the various virus samples using real-time PCR. The results of virus titers are able to partly interpret the difference of EGFP expression, in which we acquired the PS(1X) virus titer is approximately 1.8 fold higher than PB-virus titer, threefold than Ultra-virus titer, and sevenfold than PEG-virus titer in HEK293T cells. Moreover, in mMSC, the PS(1X) virus titer was remarkably higher than the titers of viruses from other sources, with the PS(1X) virus titer being approximately 2X higher than that of the Ultra-virus, 100X higher than that of the PB-virus, and 5000X higher than that of PEG-virus. Because we adopted the intracellular virus DNA as the PCR template, those DNA quantities can directly reflect the amount of virus entering the cells and the reverse transcription occurring in the cells. The residual PB or PS combined with CSC apparently enhances virus transduction and activity in HEK293T cells. As a matter of fact, PB has been commonly used to promote virus transduction in various types of cells (Costello et al. 2000; Landazuri and Le Doux 2004; Li and Lu 2009; Kim et al. 2012; Nasri et al. 2014). In spite of these satisfactory results, a few studies have also suggested that virus transduction supplemented with certain amounts of PB negatively affects the proliferation of human MSC (Lin et al. 2011, 2012), cochlear hair cells (Han et al. 2015), and keratinocyte stem/progenitor cells (Nanba et al. 2013). Intriguingly, in our study we observed that unlike PEG-virus and Ultra-virus, which decreased the growth potentials of both types of cells, and PS-virus, which maintained a high growth ratio for the two types of cells, PB-virus exerted completely distinct influences on HEK293T cells and mMSC. Specifically, PB-virus transduction resulted in the highest growth potential in HEK293T cells but the lowest growth potential in mMSC. These findings again suggest, as the previous reports, that PB may produce a growth stress and be less tolerable to some cell types (Lin et al. 2012). The lower transduction efficiency of PEG-virus and Ultra-virus seen in our study may result from the effect of the outer envelope protein (Villegas et al. 2002; Fontes et al. 2005) and residual inhibitors (Landazuri and Le Doux 2006) released from the damaged virus envelope after concentration procedures.
The above results were also verified by various virus transduction with the same titer. An equal virus titer of 2.0 × 108 IU/ml was used to transduce cells. As shown in the supplementary Fig. 1, we observed that the transduction efficiency to HEK293T cells with the same virus titer by various methods were relatively equal, except for slight difference in their growth ratios (Supplementary Fig. 3A). While PS-virus led to the highest fluorescence intensity after mMSC transduction, PEG- and PB-virus were inefficient to transduce mMSC (Supplementary Fig. 2), perhaps resulting from the detrimental effect of PEG and PB on mMSC (Supplementary Fig. 3B).
In general, the chemical-based methods for virus production are relatively convenient and high-yield. However, we also found that the amount of chemicals employed is critical to determining the responses of cells. Therefore, it would be preferable to use a chemical which is tolerable to cells in a wide range of quantities. Previous studies have demonstrated that PS is an effective molecule for facilitating virus transduction (Lee et al. 2001; Mizuarai et al. 2001; Yang and Hsieh 2001). Moreover, Lin et al. showed that 8 μg/ml of PS can substantially enhance virus transduction to human MSC. The transduction efficiency can even be doubled by providing up to 100 μg/ml of PS (Lin et al. 2012). These special features of PS enable us to interpret the findings of superior activity of the PS-virus detailed in this paper as spring from the fact that residual PS, but not residual PEG or PB, from virus precipitation can be both tolerable to and promote transduction to cells. As a matter of fact, in order to further investigate the influence of different amounts of PS on cells, we changed the PS concentration in the PS/CSC complex to precipitate virus. The PS concentration but not CSC was altered, ranging from 0.2X to 4X. In terms of cell growth ratios, we found that except for the 4X-virus, PS-virus (0.2X, 0.5X, 1X, 2X) treated cells retained higher growth potentials than cells treated with other virus preparations. Lastly, although the disproportional combination of PS–CSC (e.g., 0.5X PS mixed with 1X CSC) could cause reductions of virus titers, it still resulted in higher transduction efficiency in most cases than viruses produced with other methodologies.
Taken together, the results of this study indicate that PS–CSC complex appears to exhibit excellent performance in concentrating lentivirus, resulting in high titers and high transduction efficiency. Furthermore, because of its low cytotoxicity, the PS-precipitated virus can be anticipated to have relatively high utility in terms of clinical applications. Nevertheless, prior to its utilization in gene therapy, more questions should be addressed. These questions would at least include whether the PS-virus works effectively in vivo, whether PS-virus transduction frequently causes multiple integration of transgene and damages cells, and whether the residual PS is tolerable or not to test animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by grants from the National Science Council, Taiwan (NSC 104-2321-B-415-001-).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0133-0) contains supplementary material, which is available to authorized users.
References
- Armon R, Arella M, Payment P. A highly efficient second-step concentration technique for bacteriophages and enteric viruses using ammonium sulfate and Tween 80. Can J Microbiol. 1988;34:651–655. doi: 10.1139/m88-107. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Munoz M, Buetti E, Meylan PR, Diggelmann H, Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- Fontes LV, Campos GS, Beck PA, Brandao CF, Sardi SI. Precipitation of bovine rotavirus by polyethylene [corrected] glycol (PEG) and its application to produce polyclonal and monoclonal antibodies. J Virol Methods. 2005;123:147–153. doi: 10.1016/j.jviromet.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Gatlin J, Melkus MW, Padgett A, Kelly PF, Garcia JV. Engraftment of NOD/SCID mice with human CD34(+) cells transduced by concentrated oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. J Virol. 2001;75:9995–9999. doi: 10.1128/JVI.75.20.9995-9999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Yu D, Song Q, Wang J, Dong P, He J. Polybrene: observations on cochlear hair cell necrosis and minimal lentiviral transduction of cochlear hair cells. Neurosci Lett. 2015;600:164–170. doi: 10.1016/j.neulet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim KJ, Kim YH, Lee YA, Kim BG, Cho CM, Kang HR, Kim CG, Ryu BY (2012) Efficient enhancement of lentiviral transduction efficiency in murine spermatogonial stem cells. Mol Cells 33:449–455 [DOI] [PMC free article] [PubMed]
- Kohno T, Mohan S, Goto T, et al. A new improved method for the concentration of HIV-1 infective particles. J Virol Methods. 2002;106:167–173. doi: 10.1016/S0166-0934(02)00162-3. [DOI] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landazuri N, Le Doux JM. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. J Gene Med. 2004;6:1304–1319. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- Landazuri N, Le Doux JM. Complexation with chondroitin sulfate C and Polybrene rapidly purifies retrovirus from inhibitors of transduction and substantially enhances gene transfer. Biotechnol Bioeng. 2006;93:146–158. doi: 10.1002/bit.20697. [DOI] [PubMed] [Google Scholar]
- Lee H, Song JJ, Kim E, Yun CO, Choi J, Lee B, Kim J, Chang JW, Kim JH (2001) Efficient gene transfer of VSV-G pseudotyped retroviral vector to human brain tumor. Gene Ther 8:268–273 [DOI] [PubMed]
- Li GB, Lu GX. Gene delivery efficiency in bone marrow-derived dendritic cells: comparison of four methods and optimization for lentivirus transduction. Mol Biotechnol. 2009;43:250–256. doi: 10.1007/s12033-009-9197-1. [DOI] [PubMed] [Google Scholar]
- Lin P, Correa D, Lin Y, Caplan AI. Polybrene inhibits human mesenchymal stem cell proliferation during lentiviral transduction. PLoS ONE. 2011;6:e23891. doi: 10.1371/journal.pone.0023891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Lin Y, Lennon DP, Correa D, Schluchter M, Caplan AI. Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl Med. 2012;1:886–897. doi: 10.5966/sctm.2012-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin DW, Landazuri N, Gangadharan B, Hewes B, Archer DR, Spencer HT, Le Doux JM (2005) Highly efficient transduction of repopulating bone marrow cells using rapidly concentrated polymer-complexed retrovirus. Biochem Biophys Res Commun 330:768–775 [DOI] [PubMed]
- Mizuarai S, Ono K, You J, Kamihira M, Iijima S. Protamine-modified DDAB lipid vesicles promote gene transfer in the presence of serum. J Biochem. 2001;129:125–132. doi: 10.1093/oxfordjournals.jbchem.a002822. [DOI] [PubMed] [Google Scholar]
- Nanba D, Matsushita N, Toki F, Higashiyama S. Efficient expansion of human keratinocyte stem/progenitor cells carrying a transgene with lentiviral vector. Stem Cell Res Ther. 2013;4:127. doi: 10.1186/scrt338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri M, Karimi A, Allahbakhshian Farsani M. Production, purification and titration of a lentivirus-based vector for gene delivery purposes. Cytotechnology. 2014;66:1031–1038. doi: 10.1007/s10616-013-9652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou E, Kontostathi G, Drakopoulou E, Georgomanoli M, Stamateris E, Vougas K, Vlahou A, Maloy A, Ware M, Anagnou NP (2013) Characterization and comparative performance of lentiviral vector preparations concentrated by either one-step ultrafiltration or ultracentrifugation. Virus Res 175:1–11 [DOI] [PubMed]
- Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- Sun G, Xiao J, Wang H, Gong C, Pan Y, Yan S, Wang Y (2014) Efficient purification and concentration of viruses from a large body of high turbidity seawater. MethodsX 1:197–206 [DOI] [PMC free article] [PubMed]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- Villegas GA, Arguelles MH, Castello AA, Mas NJ, Glikmann G. A rapid method to produce high yields of purified rotavirus particles. J Virol Methods. 2002;104:9–19. doi: 10.1016/S0166-0934(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Yang YW, Hsieh YC. Protamine sulfate enhances the transduction efficiency of recombinant adeno-associated virus-mediated gene delivery. Pharm Res. 2001;18:922–927. doi: 10.1023/A:1010923924844. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xia HQ, Cleghorn G, Gobe G, West M, Wei MQ. A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Ther. 2001;8:1745–1751. doi: 10.1038/sj.gt.3301587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.