Abstract
Multipotent mesenchymal stem cells (MSCs) are an attractive tool for cell therapy and regenerative medicine. Being applied in vivo, allogeneic MSCs are faced with both activated and unstimulated immune cells. The effects of MSCs on activated immune cells are well described and are mainly suppressive. Less is known about the interaction of MSCs with unstimulated immune cells. We evaluated the contribution of tissue-related O2 level (“physiological” hypoxia—5% O2) and cell-to-cell contact to the interaction between allogeneic adipose tissue-derived MSCs (ASCs) and unstimulated peripheral blood mononuclear cells (PBMCs). Under both O2 levels, ASCs affected the immune response by elevating the proportion of CD69+ T cells and modifying the functional activity of unstimulated PBMCs, providing a significant reduction of ROS level and activation of lysosome compartment. “Physiological” hypoxia partially attenuated the ASC modulation of PBMC function, reducing CD69+ cell activation and more significantly supressing ROS. In direct co-culture, the ASC effects were more pronounced. PBMC viability was preferentially maintained, and the lymphocyte subset ratio was altered in favour of B cells. Our findings demonstrate that allogeneic ASCs do not enhance the activation of unstimulated immune cells and can provide supportive functions. The “hypoxic” phenotype of ASCs may be more “desirable” for the interaction with allogeneic immune cells that may be required in cell therapy protocols.
Keywords: MSC, Lymphocytes, Immunosuppression, Cell-to-cell interaction, Hypoxia, Immune response
Introduction
Multipotent mesenchymal stromal cells (MSCs) are considered a promising tool for regenerative medicine due to their multilineage differentiation potential, as well as their high paracrine and proliferative activity. For a while, MSCs were thought to be hypoimmunogenic or immune privileged, providing many possibilities for the application of allogeneic MSCs in cell therapy and transplantation (Kassem et al. 2004; Le Blanc et al. 2008; Gonzalo-Daganzo et al. 2009; Kaplan et al. 2011; Caplan and Sorrell 2015; Consentius et al. 2015; Tremp et al. 2015; Wang et al. 2016). Meanwhile, recent studies have found that MSCs are capable of acting as antigen-presenting cells that express HLA-II molecules on their surfaces under certain conditions, which may be a trigger for the initiation of allogeneic lymphocyte activation (Chan et al. 2006; Ren et al. 2008; Yoo et al. 2009; Ankrum et al. 2014; Najar et al. 2016). When applied in vivo, allogeneic MSCs will be faced with not only activated but also unstimulated immune cells. Thus, the elucidation of both of these immune set responses is of great importance.
To estimate the impact of different factors on the immune response, several parameters are commonly evaluated: T cell activation (CD25, CD69, HLA-DR expression), T cell proliferation, and cytokine production (Le Blanc et al. 2004; Puissant et al. 2005; Nauta and Fibbe 2007; Cappellesso-Fleury et al. 2010; Kronsteiner et al. 2011). Using these indicators, the suppressive effects of MSCs on activated leukocytes have been well demonstrated (Madrigal et al. 2014; Gornostaeva et al. 2016).
Less in known about the interaction of MSCs with non-activated lymphocytes as with activated ones. A few studies have shown that allogeneic MSCs did not stimulate the proliferation of PBMCs (Puissant et al. 2005; Suva et al. 2008; Yang et al. 2009). The available data on lymphocyte activation are quite contradictory. It was demonstrated that MSCs did not provoke the expression of CD69 (Le Blanc et al. 2004) and CD25 (Le Blanc et al. 2004; Magin et al. 2009), which are markers of lymphocyte activation. In contrast, other papers have described the triggering of CD69 (Magin et al. 2009) or both CD69 and CD25 (Crop et al. 2010) expression by MSCs.
The influence of allogeneic MSCs on leukocyte function may be governed by different factors such as direct heterotypic cell contact or paracrine regulation. Furthermore, the local microenvironment and tissue oxygen level, in particular, are known to play an important role in the realization of MSC function (Malladi et al. 2006; Grayson et al. 2007; Fehrer et al. 2007; Buravkova et al. 2009; Gornostaeva et al. 2013, 2016; Bobyleva et al. 2016; Murabayashi et al. 2017; Sisakhtnezhad et al. 2017). Recently, we demonstrated that the interaction of mitogen-activated PBMCs with adipose tissue-derived MSCs (ASCs) under “physiological” hypoxia (5% O2) resulted in significant suppression of PBMC proliferation, increased IL-10 production and reduced proinflammatory cytokine secretion compared to ambient O2 (20%) (Gornostaeva et al. 2013). The impact of tissue-related oxygen level on the MSC/unstimulated lymphocyte interaction remains unexplored.
In the present study, we evaluated the direct cell-to-cell and paracrine effects of allogeneic ASCs on unstimulated PBMCs and elucidated the impact of O2 level in the microenvironment [“physiological” hypoxia (5% O2) vs the ambient level (20% O2)] on the ASC/PBMC interaction. The criteria mentioned above (i.e., T cell activation, proliferation, and cytokine profile) were applied to evaluate the immune response of nonactivated PBMCs. Moreover, we extended the analysis of ASC-mediated alterations to include functional activity, such as viability, lymphocyte subpopulation distribution, ROS level and the activity of organelles.
Materials and methods
Peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated by density gradient centrifugation (Histopaque 1077, Sigma-Aldrich, St. Louis, MO, USA) from the whole blood obtained from healthy volunteers, after informed consent, according to a standard protocol. Isolated cells were resuspended in RPMI-1640 (31870-025, Gibco, Grand Island, NY, USA) with 1% penicillin–streptomycin (PanEco, Moscow, Russia), 2 mM glutamine (MP Biomedicals, Santa Ana, CA, USA), and 5% heat-inactivated foetal bovine serum (FBS) (HyClone, Logan, UT, USA). For each donor, the immune profile was determined by flow cytometry (Accuri C6, Becton–Dickinson, San Diego, CA, USA). The ratio of lymphocyte populations, and T cell subsets in PBMCs was demonstrated to be within the reference range of human peripheral blood (T cells: 65–85%, B cells: 8–14%, T helpers: 32–50%, T cytotoxic: 14–16%, NKs: 8–16%, and NK-Ts: 1–5%). PBMC viability after isolation was 98% on average.
Adipose tissue-derived MSCs
Adipose tissue samples were obtained from patients in accordance with the Scientific Agreement from the multidisciplinary clinic “Soyuz” (Moscow, Russia) after elective aesthetic liposuction procedures under local anaesthesia, and with written informed consent; patients were healthy females without co-morbidities who were 30–45 years old. Adipose stromal cells (ASCs) were isolated from adipose tissue using method previously described (Zuk et al. 2001), with some modifications (Buravkova et al. 2009). Briefly, tissue samples were treated with 0.075% collagenase IA (Sigma-Aldrich). After the cells were washed with PBS, they were resuspended in α-MEM (Gibco) supplemented with 10% FBS, 1% penicillin–streptomycin, and 2 mM glutamine.
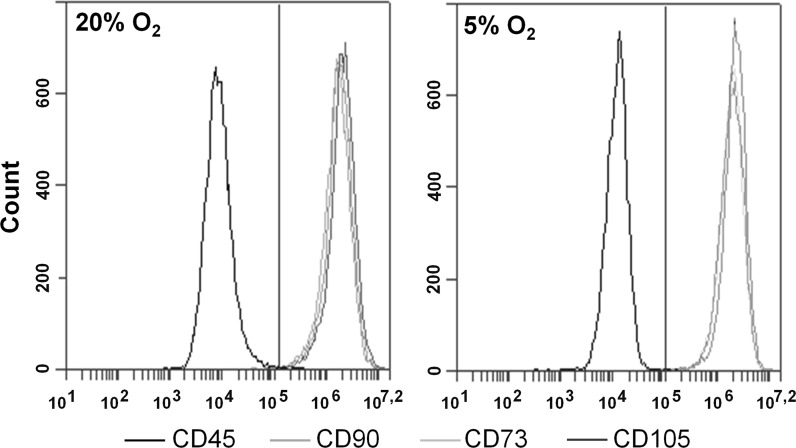
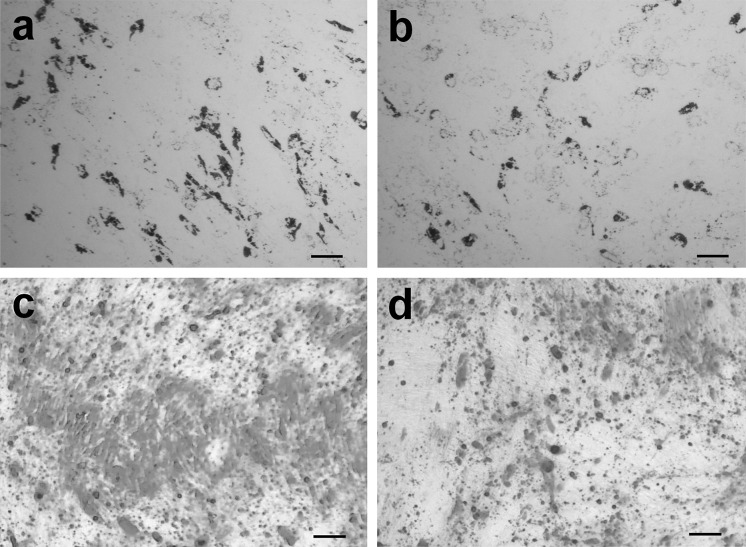
ASCs were divided into two portions after isolation. One portion was further expanded in a standard laboratory CO2-incubator (Sanyo, Osaka, Japan) with 5% CO2 and 95% air (20% O2, normoxia); the other portion was propagated in a multigas incubator (Sanyo) at 5% O2, 5% CO2, 90% N2 (“physiological” hypoxia). After the cells reached 70–80% confluence, they were sub-cultured at a seeding density of 2000/cm2. ASCs at passages 2–4 were used in the experiments. ASC immunophenotype and differentiation capacity were evaluated for each passage. ASCs were positive for CD90, CD73, and CD105 but negative for CD45 antigens (Fig. 1), and they were undergoing osteo- and adipogenic differentiation in the presence of the appropriate stimuli in the medium (Fig. 2), which satisfies the minimal criteria for MSC (Dominici et al. 2006; Bourin et al. 2013).
Fig. 1.
Flow cytometric analysis of the ASC phenotype under 20 and 5% O2. ASCs were divided into two portions and permanently expanded under 20 or 5% O2. The ASC immunophenotype was evaluated after each passage. Representative histograms of ASCs stained with antibodies against positive (CD90-FITC, CD73-PE, CD105-PE) and negative (CD45-PE) markers (n = 7). The gate (marked with vertical red line) is selected by the maximum value of the fluorescence intensity of cells incubated with Ig-FITC and Ig-PE. The histograms were created by BD Accuri C6 Software (BD Biosciences)
Fig. 2.
Osteogenic and adipogenic differentiation of ASCs. ASCs were divided into two portions and permanently expanded under 20 and 5% O2. The induced osteo- and adipo-differentiation was histochemically evaluated in each passage. Cells were cultured in full medium with osteogenic stimuli for 21 days or with adipogenic stimulation for 7 days. After the end of the cultivation period, the cells were fixed. The osteogenic differentiation of ASCs was assessed by staining mineralized matrix components with the alizarin red dye. Adipogenic differentiation potential was evaluated by accumulation of Oil Red O positive cytoplasmic lipid droplets. Representative images of cells of the 3rd passage (n = 7). a, c ASCs at 20% O2; b, d ASCs at 5% O2. a, b Lipid droplets in ASCs (Oil Red O staining). Bar: 100 µm. c, d ASC mineralized matrix (alizarin red staining). Bar: 100 µm
All cell culture experiments were approved by the Biomedicine Ethics Committee of the Institute of Biomedical Problems, Russian Academy of Sciences.
Experimental design
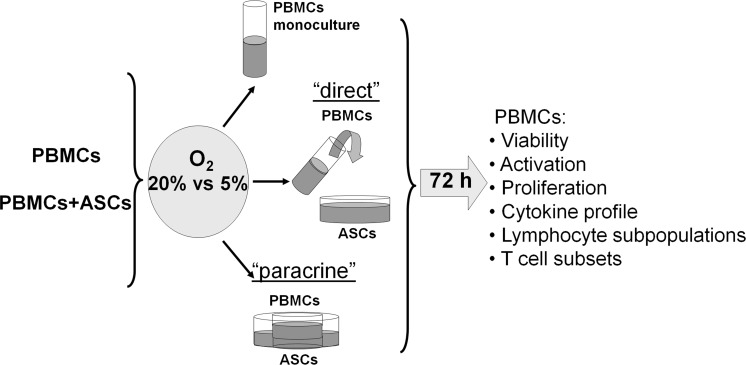
ASCs were continuously cultured at 20 and 5% O2 in α-MEM. For co-culture 60 * 103 ASCs were seeded in each well (9.6 cm2) of a 6-well Transwell plate (Corning, Corning, NY, USA) and grown to 70–80% confluence (approximately 100 * 103 cells/well). Before co-culture, α-MEM was removed, and ASCs were washed with PBS (Sigma). Then, 106 freshly isolated PBMCs in 2 ml of RPMI 1640 were added to each well. The cell ratio in the co-cultures was 1:10 (106 PBMCs were added to 105 ASCs) in all experimental settings. ASC/PBMC interaction was examined in two settings (Fig. 3): “direct” and “paracrine”. In the “direct” setting, PBMCs were inoculated directly into an ASC monolayer. For the “paracrine” setting, PBMCs were inoculated in Transwells with semipermeable membrane inserts (0.4 μm pore diameter) to exclude direct cell-to-cell contacts. ASC and PBMC monocultures were used as controls. Cells were co-cultivated in the full RPMI 1640 medium for 72 h. Each experiment was reproduced 4–7 times, with analytical measurement duplication. Co-culture was performed for 72 h. Then, floating PBMCs were collected and prepared for flow cytometric analysis.
Fig. 3.
Experimental design. ASCs were continuously cultured at 20 and 5% O2 in α-MEM. For co-culture 60 * 103 ASCs were seeded in each well (9.6 cm2) of a 6-well Transwell plate (Corning) and grown to 70–80% confluence (approximately 100 * 103 cells/well). Before the co-culture, α-MEM was removed, and ASCs were washed with PBS (Sigma). Then, 106 freshly isolated PBMCs in 2 ml of RPMI 1640 were added to each well. The cell ratio in the co-cultures was 1:10 (106 PBMCs were added to 105 ASCs) in all experimental settings. ASC/PBMC interaction was examined in two settings (Fig. 3): “direct” and “paracrine”. In the “direct” setting, PBMCs were inoculated directly into an ASC monolayer. For the “paracrine” setting, PBMCs were inoculated in Transwells with semipermeable membrane inserts (0.4 μm pore diameter) to exclude direct cell-to-cell contacts. ASC and PBMC monocultures were used as controls. Cells were co-cultivated in the full RPMI 1640 medium (Gibco) for 72 h. Each experiment was reproduced 4–7 times, with analytical measurement duplication. Co-culture was performed for 72 h. Then, floating PBMCs were collected and prepared for flow cytometric analysis
Cellular organelles and reactive oxygen species (ROS) evaluation
Mitochondria, lysosomes and ROS in PBMCs were labelled with Mito-Tracker Red FM, Lyso-Tracker Green, and H2DCFDA (Molecular Probes, Invitrogen, Carlsbad, CA, USA), respectively, according to the manufacturer’s protocol.
Flow cytometry
Monoclonal antibodies labelled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE), including CD90-FITC, CD73-PE, CD105-PE, and CD45-PE (Immunotec, Marseille, France), were used for ASC phenotyping. IgG-PE or IgG-FITC was used as the negative control (Immunotec).
Apoptotic and necrotic cells were detected with an Annexin V-FITC-PI Kit according to the manufacturer’s protocol (Immunotech).
To measure proliferative activity, PBMCs were stained with intracellular 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) prior to cultivation. A two-fold reduction in per cell fluorescence intensity accompanied each cell division (Parish 1999).
To determine lymphocyte subpopulations and assess T cell activation, the CD3-FITC/CD(56 + 16)-PE/CD45-PerCP/CD19-APC, CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC, CD3-FITC/CD25-PE/CD45-PerCP/HLA-DR-APC, and CD3-FITC/CD69-PE/CD45-PerCP (BD Biosciences) antibodies were used, and IgG-PE/IgG-FITC/IgG-PerCP/IgG-APC (BD Biosciences) served as the negative control antibodies.
Flow cytometric analysis was performed using an Accuri C6 with BD Accuri C6 Software (BD Biosciences).
The cytokine profile in the conditioned medium was measured using a FlowCytomix Human Th1/Th2 11 Plex Kit (Bender MedSystems, Vienna, Austria), which provides the simultaneous identification of 11 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IFN-γ, TNF-α, and TNF-β), on a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, USA). CELLquest software (BD) was used for data acquisition. The concentration of each cytokine was linearly dependent on fluorescence intensity and was calculated using standard curves that were generated for each cytokine using Flow Cytomix Pro software (eBioscience, San Diego, CA, USA). The IL-8 concentration in the conditioned medium was determined using a Human IL-8 ELISA Set (BD Biosciences) and BD OptEIA™ Reagent Set B (BD Biosciences) according to the manufacturer’s instructions.
ASC differentiation
After co-culture experiments ASCs were cultured in full medium with osteogenic stimuli (10−8 M dexamethasone, 10 mM glycerol-2-phosphate, and 0.2 mM L-ascorbic acid 2-phosphate (Sigma)) for 21 days or with adipogenic stimulation (0.5 mM isobutyl methylxanthine, 1 μM dexamethasone, 10 μg/ml insulin, and 200 μM indomethacin (Sigma)) for 7 days. After the end of the cultivation period, the cells were fixed. The osteogenic differentiation of ASCs was assessed by staining mineralized matrix components with the alizarin red dye (Millipore, Billerica, MA, USA). Adipogenic differentiation potential was evaluated by accumulation of Oil Red O positive cytoplasmic lipid droplets (Millipore).
Statistics
Statistically significant differences were assessed using the nonparametric Mann–Whitney test (for small and medium-sized samples, n ≤ 30) with the selected significance level of p = 0.05. Statistical analysis was performed using SPSS 14.0 software.
Results
ASCs support PBMC viability
The number of apoptotic cells is one of the key parameters in assessing the functional state of lymphocytes since these cells tend to go into apoptosis upon activation or an alteration of the microenvironment. Therefore, it could be assumed that ASCs with high paracrine activity as well as reduced oxygen level could also affect immune cell survival.
The percentages of viable, necrotic and apoptotic cells were assessed among unstimulated PBMCs in monoculture and ASC co-culture at 20 and 5% O2. The effect of ASCs on PBMC viability depended on the interaction setting. The proportion of viable unstimulated PBMCs in “paracrine” setting was similar to that of PBMCs in the monoculture and was significantly lower than that in “direct” setting (p < 0.05). Herewith, the share of necrotic PBMCs in “direct” co-cultures was three-fold lower and the percentage of apoptotic cells was two-fold lower than PBMCs in “paracrine” setting or in the monoculture (p < 0.05). Reduced oxygen level did not affect PBMC viability (Table 1). Thus, direct communication with allogeneic ASCs enhanced the viability of unstimulated PBMCs. These data are evidence that ASCs may play not only a suppressive but also a supportive role in immunomodulation.
Table 1.
The PBMC viability in monoculture and in co-culture with ASCs
| Viable (%) | Necrotic (%) | Apoptotic (%) | ||||
|---|---|---|---|---|---|---|
| 20% O2 | 5% O2 | 20% O2 | 5% O2 | 20% O2 | 5% O2 | |
| PBMCs | 81 ± 2 | 81 ± 2 | 12 ± 1.7 | 11 ± 1.1 | 6.8 ± 0.7 | 7.5 ± 1 |
| PBMCs/ASCs direct | 94 ± 1.5* | 95 ± 1* | 3 ± 1* | 2.5 ± 0.7* | 2.5 ± 1* | 2.5 ± 0.7* |
| PBMCs/ASCs paracrine | 83 ± 1 | 82 ± 2 | 10 ± 0.7 | 11 ± 0.9 | 6 ± 1 | 7 ± 1 |
PBMC viability was evaluated after 72 h of co-culture with ASCs using AnnexinV-FITC-PI kit with flow cytometry. PBMCs were added directly onto ASC monolayer, or into upper chamber of transwells with ASC monolayer in the inner chamber. The ASC/PBMCs ratio was 1:10 in all experimental settings
Data are presented as the mean ± SEM of 7 independent experiments
* Significance level (p < 0.05) versus PBMC monoculture
ASCs provoke early lymphocyte activation
An increase in the proportion of lymphocytes that express activation markers is an essential manifestation of the immune response. It is important that the immune system response be minimal after the introduction of MSCs in vivo. To assess spontaneous and ASC-provoked T cell activation, we used antibodies against the following marker molecules: the early activation markers CD69 (C-type membrane glycoprotein) and CD25 (low-affinity interleukin-2 receptor) and the late activation marker HLA-DR, a major histocompatibility class II (MHC II) antigen.
There were no signs of T cell activation in the PBMC monoculture after 72 h at either 20 or 5% O2 (Table 2). After co-culture, the proportion of CD25- and HLA-DR-positive T cells was unchanged, while the share of CD69-positive cells was increased (Table 2). At 20% O2, the share of CD69-positive cells was significantly higher in the “direct” setting (p < 0.05). This effect was attenuated at “physiological” hypoxia (p < 0.05).
Table 2.
T-cell activation in PBMC monoculture and co-culture with ASCs
| CD3+/CD69+ (%) | CD3+/CD25+ (%) | CD3+/HLA-DR+ (%) | ||||
|---|---|---|---|---|---|---|
| 20% O2 | 5% O2 | 20% O2 | 5% O2 | 20% O2 | 5% O2 | |
| PBMCs | 4 ± 2 | 3 ± 1 | 6 ± 1 | 6 ± 0.6 | 1.6 ± 0.6 | 1.6 ± 0.7 |
| PBMCs/ASCs direct | 26 ± 1* | 20 ± 0.1*, # | 6 ± 0.1 | 6 ± 0.7 | 1.2 ± 0.5 | 0.9 ± 0.4 |
| PBMCs/ASCs paracrine | 11 ± 1*,** | 10 ± 3.5*,** | 5 ± 0.3 | 6 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.6 |
After 72 h of co-culture with ASCs, PBMCs were labeled with CD3-FITC/CD25-PE/CD45-PerCP/HLA-DR-APC, CD3-FITC/CD69-PE/CD45-PerCP antibody and the share of CD69/CD25/HLA-DR-positive T-cell (CD3+) was evaluated by flow cytometry
Data are presented as the mean ± SEM of 6 independent experiments
* Significance level (p < 0.05) versus PBMC monoculture
** Significance level (p < 0.05) versus “direct” settings
#Significance level (p < 0.05) versus 20% O2
Thus, allogeneic ASCs only slightly activated T cells mainly through direct cell–to-cell interaction. Low O2 in the milieu partially attenuated the CD69 expression elevation probably due to the more pronounced anti-inflammatory activity of ASCs at hypoxia. We did not detect an increase of HLA-DR expression related to initiation of graft-versus-host disease.
ASCs do not cause lymphocyte proliferation
Proliferation is the qualitative reaction of lymphocytes in case of a pronounced immune response. This is a key parameter for the evaluation of the lymphocyte response to external stimulation (in this case, ASCs).
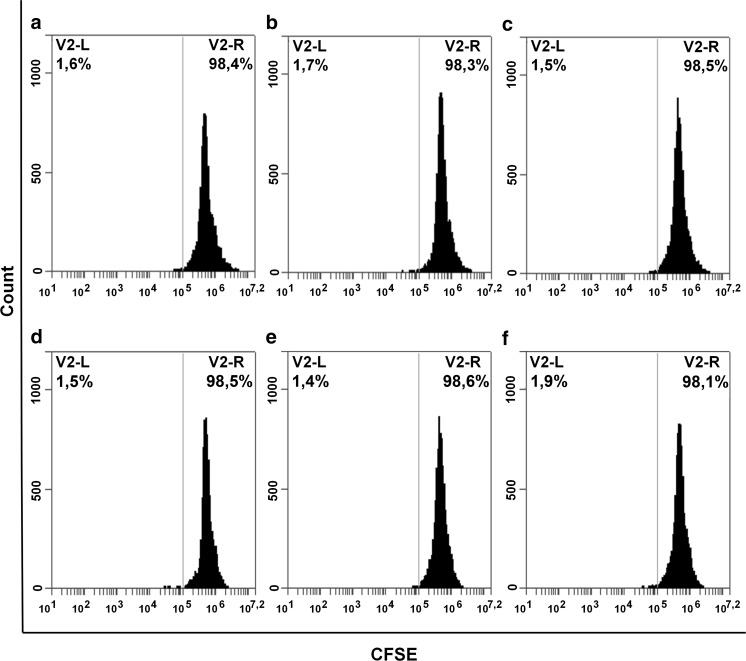
Unstimulated PBMCs in monoculture had an extremely low proliferation rate, ranging from 0.5 to 2.5%, indicating the absence of spontaneous proliferation (Fig. 4). Interaction with ASCs did not result in the stimulation of PBMC proliferation at 20 or 5% O2. These data confirm that allogeneic ASCs do not cause a significant immune response of non-activated lymphocytes.
Fig. 4.
Flow cytometric analysis of PBMC proliferation after 72 h of cultivation under 20 and 5% O2. Representative histograms of CFSE-stained PBMCs (n = 3) are shown. The histograms were created in BD Accuri C6 Software (BD Biosciences). To measure proliferative activity, PBMCs were stained intracellularly with the 5,6-carboxyfluorescein diacetate succinimidyl ester dye. A two-fold reduction in fluorescence intensity per cell should accompany each cell division. Cells were cultured in monoculture or in the presence of ASCs in a “direct” or “paracrine” settings. Mean fluorescence intensity of cells was measured by flow cytometry after 72 h of cultivation. Dividing cells are visualized by the second peak of lower fluorescence intensity, which is located left of the main peak. PBMCs cultured under 20% O2: a, d PBMC monoculture, b, e “direct” interaction, c, f “paracrine” interaction, a–c 20% O2, d–f 5% O2
ASCs cause an increase in the proportion of B cells
Examination of lymphocyte populations and T cell subsets is a routine procedure in the evaluation of donor’s immune state. There are well-defined reference boundaries for the physiological means of these parameters. Therefore, we analysed the lymphocyte populations and T cell subsets after interaction with ASCs.
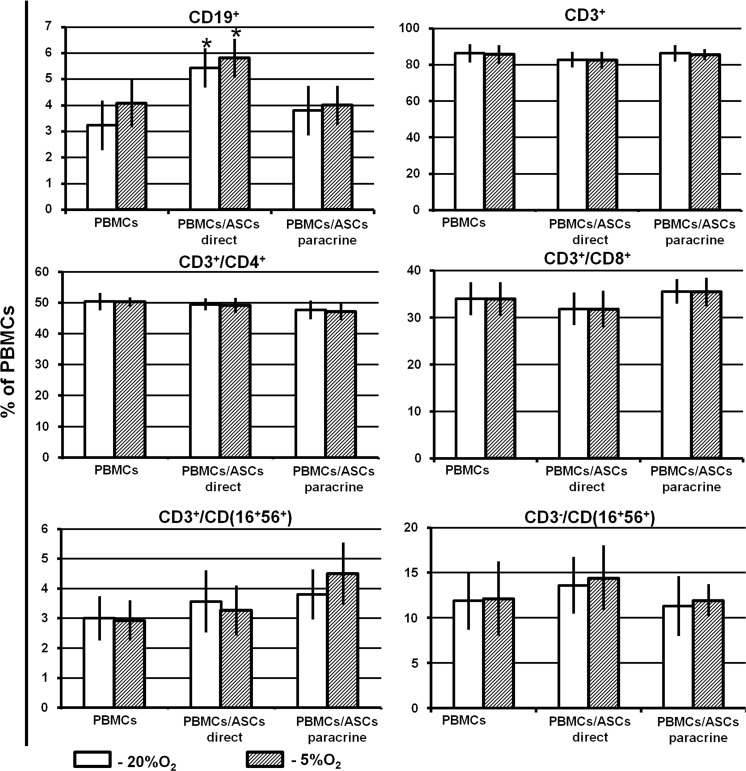
Compared to PBMC monoculture, interaction with ASCs did not affect the percentage of NK and T cells or the NK-T/T helper/T cytotoxic ratio. A slight but significant increase in B cell proportion was observed (from 3 to 5%). This effect was detected in the “direct” setting only (Fig. 5). The ratio of lymphocyte populations and T cell subsets was similar at both O2 concentrations used.
Fig. 5.
Lymphocyte populations and T cell subsets. PBMCs were cultured alone and co-cultured with ASC monolayers in a “direct” or “paracrine” settings under 20 or 5% O2. After 72 h of cultivation, PBMCs were labelled with CD3-FITC/CD(56 + 16)-PE/CD45-PerCP/CD19-APC or CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC antibody, and the proportions of B cells (CD19+), T cells (CD3+), NK cells (CD3−/CD(16 + 56)+), NK-T cells (CD3+/CD(16 + 56)+), T helpers (CD3+/CD4+), and T cytotoxic (CD3+/CD8+) cells were evaluated by flow cytometry. Data are presented as the mean ± SEM of 6 independent experiments. *Significance level (p < 0.05) versus PBMC monoculture
It was demonstrated that T cells were able to adhere to MSCs (Suva et al. 2008), which in turn may cause a decrease in the number of floating T cells in suspension. The increase in the proportion of B cells after direct cell-to-cell interaction may be a consequence of the preferential adhesion of T cells to ASCs. More importantly, we did not observe an increase in the NK and NK-T cell populations, which are known to participate in the “friend or foe” reaction and to trigger graft rejection (Fig. 5).
PBMCs retain cellular organelle activity and reduce ROS level upon contact with ASCs
The lysosomal and mitochondrial compartment state, and the level of reactive oxygen species (ROS) reflect the functional activity of cells.
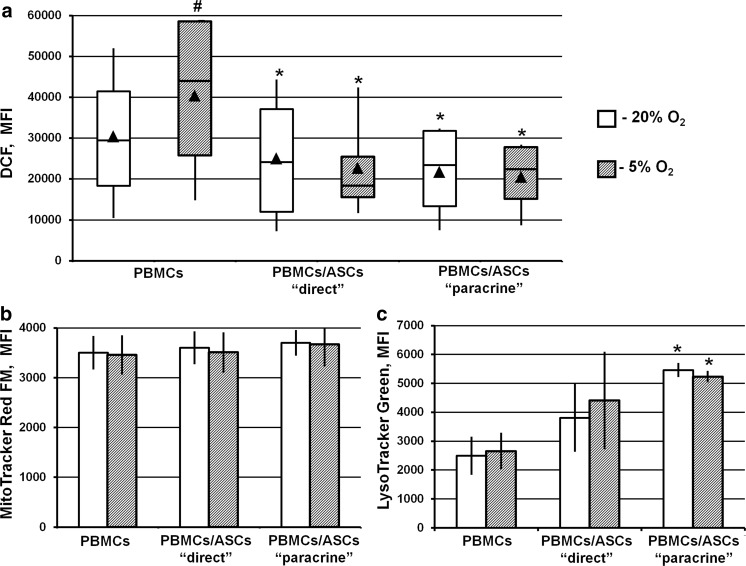
In monocultured PBMCs, DCF mean fluorescence intensity (MFI) demonstrated a 1.3-fold increase in ROS level in PBMCs at 5% compared to 20% O2 (p < 0.05) (Fig. 6a). No changes in MFI were observed after staining the mitochondria with MitoTracker FM and the lysosomes with LysoTracker Green (Fig. 6b, c).
Fig. 6.
Cellular organelle activity and ROS level. PBMCs were cultured alone or co-cultured with ASC monolayers in either a “direct” or “paracrine” settings at 20 or 5% O2. After 72 h of cultivation, PBMCs were harvested and stained with Mito-Tracker Red FM, Lyso-Tracker Green, and H2DCFDA to assess the activity of the mitochondria, lysosomes and the level of reactive oxygen species, respectively. Then, using flow cytometry, the mean fluorescence intensity (MFI) of the fluorescent dyes was estimated. a DCF, data are shown as box-and-whisker plots (n = 7), b MitoTracker FM, c LysoTracker Green. Data are shown as the mean ± SEM. Data are presented as the mean ± SEM of 7 independent experiments. *Significance level (p < 0.05) versus PBMCs monoculture. #Significance level (p < 0.05) versus 20% O2
In PBMCs after ASC co-culture, a reduction in ROS levels in both experimental settings was detected (Fig. 6a). No alteration in MitoTracker FM MFI was detected at 20 or 5% O2, indicating the membrane potential was maintained in these organelles (Fig. 6b). PBMC/ASC interaction resulted in elevated LysoTracker MFI (p < 0.05 in the “paracrine” setting), suggesting enlargement or increased acidification of the lysosome compartment (Fig. 6c).
Thus, the interaction with allogeneic ASCs stimulated lysosomal activity in PBMCs, which may be evidence of intensified catabolic processes. Meanwhile, reduced ROS level indicate the attenuation of cellular stress, confirming the supportive activity of ASCs.
ASCs do not cause a cytokine profile shift
In addition to direct cell contact, paracrine regulation plays an important role in the interaction between MSCs and immune cells. The ratio of pro- and anti-inflammatory factors may reflect the activation processes of immune cells.
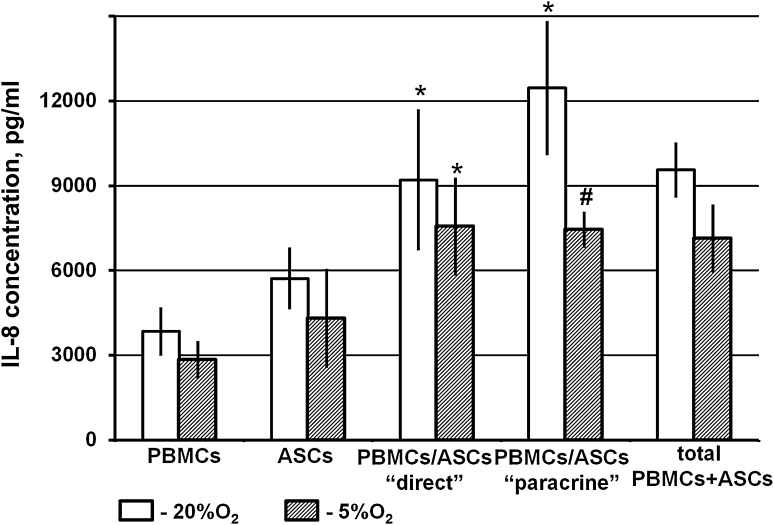
First, using a Flow Cytomix Human Th1/Th2 11 Plex Kit we conducted a preliminary screening of 11 key cytokines secreted by immune cells. Only IL-8 was detected in the conditioning medium of unstimulated PBMCs in monoculture. We further estimated the IL-8 concentration in ASC and PBMC monocultures and compared it with IL-8 production in ASC/PBMC co-cultures using ELISA. The initial level of IL-8 in PBMC and ASC monocultures was quite close with some favour to ASCs. The IL-8 level in ASC/PBMC co-cultures was significantly higher in comparison with the levels in the PBMC and the ASC monocultures (p < 0.05). However, IL-8 could be secreted by both ASCs and lymphocytes; thus, we compared the cumulative IL-8 level in the co-cultures with the summed IL-8 levels in both monocultures. The IL-8 level in the medium from co-culture did not exceed the summed production of IL-8 in the monocultures. Thus, it could be supposed that ASCs did not affect lymphocyte IL-8 production (Fig. 7).
Fig. 7.
Interleukin-8 level in conditioned medium of PBMCs, ASCs and their co-cultures. After 72 h of cultivation under 20 or 5% O2 conditioned medium was collected from the PBMC monoculture, ASC monoculture and PBMC-ASC co-culture. The IL-8 was analysed using the Human IL-8 ELISA Set (BD, USA). Data are presented as the mean ± SEM of 6 independent experiments. *Significance level (p < 0.05) versus. PBMC monoculture
Discussion
In our study, we demonstrated that allogeneic ASCs at ambient (20%) O2 and “physiological” hypoxia (5% O2) did not provoke a noticeable immune response; however, they did regulate unstimulated PBMCs through cell-to-cell contact in an O2-dependent manner.
First, we discriminated between direct cell-to-cell and paracrine effects. We found that PBMC viability was enhanced in direct contact with the ASC monolayer, as evidenced by the reduced percentages of apoptotic and necrotic cells. In the “direct” setting, we did not detect a significant disturbance in lysosome activity, while in the “paracrine” setting, acidification of the lysosome compartment was revealed.
Further, the alteration in lymphocyte population (i.e., an increase in the B cell proportion) and the elevation of CD69+ T cell percentage were reduced significantly in the range from “direct” (ASCs + soluble factors) to “paracrine” (soluble factors only).
There are only a few papers concerning the effects of allogeneic MSCs on the activation of non-stimulated lymphocytes. Magin et al. (2009) examined the direct co-culture of MSCs/lymphocytes at ambient O2 (20%) and described an increase in the early activated CD69+ cells and no change in the proportion of CD25+ cells in the presence of MSCs. The effect was dependent on the MSC/lymphocyte ratio (Magin et al. 2009) and the duration of the interaction (Le Blanc et al. 2004; Crop et al. 2010). Our findings have expanded the data cited above with an analysis of the impact of direct/paracrine regulation on early lymphocyte activation. We determined that the absence of direct cell contact attenuated T cell stimulation, resulting in less elevation of CD69+cells.
Early activation of T cells upon interaction with ASCs did not induce lymphocyte proliferation in either the “direct” or the “paracrine” setting. Previously, similar effects were described with monolayered MSCs only (Puissant et al. 2005; Benvenuto et al. 2007; Suva et al. 2008; Yang et al. 2009). Benvenuto et al. (2007) suggested that MSCs maintained the viability of unstimulated T cells in monolayer due to the reduced expression of FAS-receptors and FAS-ligands on T cell surfaces, allowing fewer lymphocytes to undergo apoptosis.
Thus, the direct cell-to-cell contacts provided more effective PBMC maintenance than the paracrine interaction alone.
The second question we were interested in was related to the effect of low oxygen on the ASC/PBMC interaction. Microenvironmental O2 level is an important extrinsic factor governing cell fate and interactions. The tissue oxygen level is known to be approximately 1–7%. Low O2 is a niche characteristic of MSCs (Cipolleschi et al. 1993). In the body, lymphocytes are also located in areas of low oxygen (O2 in the lymphoid organs is in the range 0.5–4.5%) (Caldwell et al. 2001; Sitkovsky and Lukashev 2005).
In vitro experiments have demonstrated that cell properties at atmospheric and hypoxic O2 levels vary significantly. Under hypoxia, MSCs have higher proliferative activity, an increased number of CFU-F, and attenuated osteo- and adipogenic differentiation, but chondrogenic differentiation is accelerated (Grayson et al. 2007; Fehrer et al. 2007; Nekanti et al. 2010; Buravkova et al. 2013). Low O2 affects the properties of activated immune cells. For example, the T cell cytokine profile changes (IFN-gamma, IL-2, IL-4, and IL-1β levels increase), the percentage of cytotoxic cells and their lytic activity (Krieger et al. 1996; Caldwell et al. 2001) are reduced, and B cell immunoglobulin production decreases (Krieger et al. 1996).
However, there are a few available studies on the effects of MSCs on the viability, activation and proliferation of unstimulated lymphocytes that used a standard laboratory oxygen concentration (20% O2) (Krieger et al. 1996; Conforti et al. 2003; Puissant et al. 2005; Benvenuto et al. 2007; Suva et al. 2008; Yang et al. 2009; Magin et al. 2009). As shown earlier, hypoxia can provoke apoptosis in lymphocytes (Sun et al. 2010). The acceleration of apoptotic events revealed as induction of pro-apoptotic proteins Puma and Bim and the triggering of JNK activation, which was probably provoked by ROS (Sade and Sarin 2004; Chung et al. 2006; Yu and Zhang 2008; Lee et al. 2010). Here, we demonstrated that PBMCs in monoculture were more susceptible to “physiological” hypoxia, displaying an increased ROS level in comparison with standard 20% O2. In the ASC co-culture, a decrease in the ROS level of PBMCs was detected. The relative decrease in ROS level was more pronounced under “physiological” hypoxia, which suggests that ASCs provide enhanced protection from ROS. In addition, ASC stimulation of the CD69+ T cell ratio was attenuated under “physiological” hypoxia.
Conclusions
Our findings demonstrated that allogeneic ASCs did not provoke the exerted activation of unstimulated immune cells. Meanwhile, ASCs were able to affect PBMCs by providing supportive functions, such as enhanced viability and ROS reduction. Importantly, despite an increase in the proportion of T cells that express the early activation marker CD69, no changes in HLA-DR expression were found. HLA-DR appearance on immune cells determines their involvement in the graft-versus-host reaction. In addition, ASCs did not cause lymphocyte proliferation. The PBMC response to ASCs was less pronounced under hypoxia in vitro, supporting the hypothesis that cell interaction is governed by microenvironmental cues. Based on our findings, it could be assumed that the hypoxic phenotype of ASCs is more desirable for the interaction with allogeneic immune cells that may be required by cell therapy protocols.
Acknowledgements
The study was funded by Programme of Presidium of Russian Academy of Sciences “Integrative physiology” and Grant of the President of the Russian Federation SP-3502.2015.4.
Abbreviations
- MSCs
Multipotent mesenchymal stem cells
- ASCs
Adipose stromal cells
- PBMCs
Peripheral blood mononuclear cells
- MLR
Mixed lymphocyte reaction
- CFSE
5,6-carboxyfluorescein diacetate succinimidyl ester
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- Bobyleva PI, Andreeva ER, Gornostaeva AN, Buravkova LB (2016) Tissue-related hypoxia attenuates proinflammatory effects of allogeneic pbMCS on adipose-derived stromal cells in vitro. Stem Cells Int 2016:4726267. doi: 10.1155/2016/4726267https://www.ncbi.nlm.nih.gov/pubmed/26880965 [DOI] [PMC free article] [PubMed]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buravkova LB, Grinakovskaia OS, Andreeva EP, Zhambalova AP, Kozionova MP. Characteristics of human lipoaspirate-isolated mesenchymal stromal cells cultivated under a lower oxygen tension. Cell Tiss Biol. 2009;3:23. doi: 10.1134/S1990519X09010039. [DOI] [PubMed] [Google Scholar]
- Buravkova LB, Rylova YV, Andreeva ER, Kulikov AV, Pogodina MV, Zhivotovsky B, Gogvadze V. Low ATP level is sufficient to maintain the uncommitted state of multipotent mesenchymal stem cells. Biochim Biophys Acta. 2013;1830:4418–4425. doi: 10.1016/j.bbagen.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett. 2015;168:136–139. doi: 10.1016/j.imlet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Cappellesso-Fleury S, Puissant-Lubrano B, Apoil PA, Titeux M, Winterton P, Casteilla L, Bourin P, Blancher A. Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J Clin Immunol. 2010;30:607–619. doi: 10.1007/s10875-010-9415-4. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YM, Kim JS, Yoo YD. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006;347:649–655. doi: 10.1016/j.bbrc.2006.06.140. [DOI] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of haematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- Conforti L, Petrovic M, Mohammad D, Lee S, Ma Q, Barone S, Filipovich AH. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: a possible role in T cell proliferation. J Immunol. 2003;170:695–702. doi: 10.4049/jimmunol.170.2.695. [DOI] [PubMed] [Google Scholar]
- Consentius C, Reinke P, Volk HD. Immunogenicity of allogeneic mesenchymal stromal cells: what has been seen in vitro and in vivo? Regen Med. 2015;10:305–315. doi: 10.2217/rme.15.14. [DOI] [PubMed] [Google Scholar]
- Crop M, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19:1843–1853. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gülly C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Rico MA, Bautista G, Krsnik I, Forés R, Ojeda E, Sanjuán I, García-Marco JA, Navarro B, Gil S, Sánchez R, Panadero N, Gutiérrez Y, García-Berciano M, Pérez N, Millán I, Cabrera R, Fernández MN. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11:278–288. doi: 10.1080/14653240902807018. [DOI] [PubMed] [Google Scholar]
- Gornostaeva AN, Andreeva ER, Buravkova LB. Human MMSC immunosuppressive activity at low oxygen tension: direct cell-to-cell contacts and paracrine regulation. Hum Physiol. 2013;39:136. doi: 10.1134/S0362119713020059. [DOI] [PubMed] [Google Scholar]
- Gornostaeva AN, Andreeva ER, Buravkova LB. Factors governing the immunosuppressive effects of multipotent mesenchymal stromal cells in vitro. Cytotechnology. 2016;68(4):565–577. doi: 10.1007/s10616-015-9906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358(3):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Youd ME, Lodie TA. Immunomodulatory activity of mesenchymal stem cells. Curr Stem Cell Res Ther. 2011;6:297–316. doi: 10.2174/157488811797904353. [DOI] [PubMed] [Google Scholar]
- Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- Krieger JA, Landsiedel JC, Lawrence DA. Differential in vitro effects of physiological and atmospheric oxygen tension on normal human peripheral blood mononuclear cell proliferation, cytokine and immunoglobulin production. Int J Immunopharmacol. 1996;18:545–552. doi: 10.1016/S0192-0561(96)00057-4. [DOI] [PubMed] [Google Scholar]
- Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M, Gabriel C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011;20:2115–2126. doi: 10.1089/scd.2011.0031. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (Interleukin-2 Receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O, Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS, Yoo YD. Serum deprivation-induced reactive oxygen species production is mediated by Romo1. Apoptosis. 2010;15:204–218. doi: 10.1007/s10495-009-0411-1. [DOI] [PubMed] [Google Scholar]
- Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transpl Med. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin AS, Korfer NR, Partenheimer H, Lange C, Zander A, Noll T. Primary cells as feeder cells for co-culture expansion of human hematopoetic stem cells from umbilical cord blood—a comparative study. Stem Cells Dev. 2009;18:173–186. doi: 10.1089/scd.2007.0273. [DOI] [PubMed] [Google Scholar]
- Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:1139–1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- Murabayashi D, Mochizuki M, Tamaki Y, Nakahara T. Practical methods for handling human periodontal ligament stem cells in serum-free and serum-containing culture conditions under hypoxia: implications for regenerative medicine. Hum Cell. 2017;30:169–180. doi: 10.1007/s13577-017-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6:499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999;77:499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Sade H, Sarin A. Reactive oxygen species regulate quiescent T-cell apoptosis via the BH3-only proapoptotic protein BIM. Cell Death Differ. 2004;11:416–423. doi: 10.1038/sj.cdd.4401347. [DOI] [PubMed] [Google Scholar]
- Sisakhtnezhad S, Alimoradi E, Akrami H. External factors influencing mesenchymal stem cell fate in vitro. Eur J Cell Biol. 2017;96:13–33. doi: 10.1016/j.ejcb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M, Lukashev D. Regulation of immune cells by local tissue oxygen tension: HIF1α and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang Y, Yang M, Zhang Y, Xie Q, Li Z, Dong Z, Yang Y, Deng B, Feng A, Hu W, Mao H, Qu X. Hypoxia induces T-cell apoptosis by inhibiting chemokine C receptor 7 expression: the role of adenosine receptor A(2) Cell Mol Immunol. 2010;7:77–82. doi: 10.1038/cmi.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva D, Passweg J, Arnaudeau S, Hoffmeyer P, Kindler V. In vitro activated human T lymphocytes very efficiently attach to allogenic multipotent mesenchymal stromal cells and transmigrate under them. J Cell Physiol. 2008;214:588–594. doi: 10.1002/jcp.21244. [DOI] [PubMed] [Google Scholar]
- Tremp M, Meyer Zu Schwabedissen M, Kappos EA, Engels PE, Fischmann A, Scherberich A, Schaefer DJ, Kalbermatten DF. The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transpl. 2015;24:203–211. doi: 10.3727/096368913X676934. [DOI] [PubMed] [Google Scholar]
- Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble mediators from mesenchymal stem cells supress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KH, Jang IK, Lee MW. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene Suppl. 2008;1:S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–218. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]