Abstract
The phenotypic transformation and dysfunctions of vascular smooth muscle cells (SMCs) such as abnormality proliferation and apoptosis are key pathological basis of atherosclerosis. The recent study aimed to detect the role of miR-29b in phenotypic transformation of SMCs. In this study, we investigated the expression level of miR-29b and MMP-2 in acute coronary syndrome (ACS) patients, verified whether MMP-2 is the target gene of miR-29b by luciferase reporter gene system, and explored the role of miR-29b in the viability and apoptosis of SMCs. We found that the plasma level of miR-29b was significantly downregulated to 56% of controls (p < 0.01). The plasma level of MMP-2 in health controls was 34.9 ± 6.9 ng/mL, and that it significantly increased to 46.2 ± 13.2 ng/mL in ACS patients. MMP-2 is a target gene of miR-29b. The overexpression of miR-29b significantly downregulated the expression of MMP-2 mRNA and protein. miR-29b mimics inhibited the cell viability of SMCs, and cell apoptosis was significantly enhanced compared with the NC group, especially in the early stage. In the presence of MMP-2 inhibitor SB-3CT, the cell viability and apoptosis of SMC cells were significantly reduced and enhanced, respectively, while the miR-29b -inhibited cell viability and -induced cell apoptosis were not significantly changed. Taken together, miR-29b was downregulated in ACS patients. MiR-29 mimics inhibits cell viability and promotes cell apoptosis via directly targeting on MMP-2, which could be a potentially promising therapy target for cardiovascular diseases.
Keywords: MiR-29b, Cell viability, Apoptosis, Smooth muscle cells, MMP-2
Introduction
Atherosclerosis as a chronic inflammatory disease, is one of the most serious cardio- and cerebro-vascular diseases (Tunstall-Pedoe et al. 2000; Tunstall-Pedoe et al. 1999). At present, it is believed that the migration and proliferation of vascular smooth muscle cells (SMCs) into the intima is the key pathological basis of atherosclerosis (Sobue et al. 1999). The migration of SMCs is restricted by the extracellular matrix at rest. During atherosclerosis, the changes in platelet-derived growth factor, oxidized low density lipoprotein (ox-LDL) and inflammatory mediators result in induction of matrix metalloproteinases (MMPs) that enhanced cell proliferation and migration capacity, and in decrease of contraction-related markers such as alpha actin, smooth muscle 22α, smooth muscle cell myosin heavy chain and calmodulin, so as to induce modulation of SMC from contractile phenotype to synthetic phenotype (Zeng et al. 2014, 2015; Di Pietro et al. 2016). On one hand, SMCs with synthetic phenotype migrate to the intima and accumulate the ox-LDL transforming to foam cells (Di Pietro et al. 2016; Viola et al. 2016; Jiang et al. 2017). On the other hand, SMCs with synthetic phenotype secret a variety of cytokines to activate the mitogen response and degrade the extracellular matrix, which result in proliferation of SMCs and eventually lead to arterial stenosis, forming of atherosclerotic plaque and fibrous cap (Tang et al. 2010; Owens 2007). However, the molecular mechanism of SMCs proliferation is still unclear.
It was demonstrated that MMPs are involved in the proliferation and apoptosis of SMCs during atherosclerosis. MMPs are a kind of zinc-dependent protein endopeptidase, which is secreted as zymogen and is actived after degradation by serine proteins such as plasmin (Ferrans 2002). Under physiological circumstances, only a very small number of active MMPs exist in the arterial wall. In the atherosclerotic plaque, the SMCs with synthetic phenotype express a variety of MMPs, such as MMP-1, -2, -3, -9 and -14 (Handorf et al. 2015; Botham and Wheeler-Jones 2013; Belo et al. 2015). Numerous studies have demonstrated that increased activity of MMPs leads to imbalance of synthesis and degradation of extracellular matrix, which is an important factor leading to instability of atherosclerosis plaque. The plasma levels of MMP-3 and MMP-9 were elevated in patients with hyperlipidemia, and there is a significant correlation between MMP-3 and carotid plaques. The instability of atherosclerosis plaque is closely associated with acute coronary syndrome (ACS). Recently, it was demonstrated that MMPs are related to the phenotype transformation of SMCs. The migration capacity of SMCs was depended on the expression levels of MMP-2 and -9 (Delbosc et al. 2008). MMP-1, -2, -3, and -9 could accelerate the modulation of SMC from the contractile phenotype to the synthetic phenotype (Hopps and Caimi 2015; Cifani et al. 2015; Amin et al. 2016).
A recent study showed that noncoding small RNA (miRNA) plays an important role in phenotypic transformation of SMCs (Ma et al. 2017; Laffont and Rayner 2017; Gabunia et al. 2017) and cardiovascular diseases (Zeng et al. 2015). Urinary miR-29b correlated with carotid intima-media thickness (cIMT) in patients with type 2 diabetes, which might serve as biomarker for atherosclerosis (Peng et al. 2013). Moreover, antagonizing miR-29 may promote beneficial plaque remodeling as an independent approach to stabilize vulnerable atherosclerotic lesions (Ulrich et al. 2016). By using bioinformatics tools, we predict that MMP-2 might be the target gene of miR-29b, playing an important role in the proliferation and apoptosis regulated by miR-29b. In this study, we investigated the expression level of miR-29b and MMP-2 in ACS patients, verified whether MMP-2 is the target gene of miR-29b by luciferase reporter gene system, and explored the role of miR-29b in the proliferation and apoptosis of SMCs. The findings provide novel mechanism of VSCM proliferation and apoptosis from the perspective of miRNA, and provide potential therapeutic targets for atherosclerosis and ACS.
Materials and methods
ACS patients and plasma collection
ACS is defined as acute myocardial infarction or unstable angina. Patients with previously known history of diabetes, kidney disease (creatinine > 2.5 mg/dL), and severe left ventricular (LV) systolic dysfunction (LV ejection fraction < 30%) were excluded from the study. The Ethical Committee of the Institution approved the study protocol. Informed consent of the study subjects was obtained. Plasma was collected from 30 cases of ACS and 30 normal health subjects. Serum was prepared and immediately stored at −80 °C prior to RNA extraction.
Cell culture and transfection
Human umbilical aortic smooth muscle cells (SMC) (Lonza, Walkersville, USA) were cultured in MCDB-131 Medium supplemented with 5% Fetal Bovine Serum, 2 mM l-Glutamine, 100 U/mL Penicillin, 0.1 mg/mL Streptomycin and 12.5 U/mL Nystatin, 2 ng/mL Insulin (Sigma Aldrich, St Louis, MO, USA), 0.5 ng/mL Epithelial Growth Factor (Sigma Aldrich), 3 ng/mL Basic-Fibroblasts Growth Factor (bFGF) (PeproTech Inc., Rocky Hill, New Jersey, USA) and 1 µg/mL Hydrocortisone (Sigma Aldrich) at 37 °C in a humidified atmosphere containing 5% CO2. All experiments were done using 70–80% confluent cultures. Only passages 4–8 were used for this study.
The miR-29b mimics and miRNA negative control (NC) were purchased from GenePharm, Shanghai, China. SMCs were seeded in a 6-well plate (2 × 105/well) for 24 h before transfection, and then were transfected with miR-29b mimics (50 nM) or NC (50 Nm) using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) in accordance with the manufacture’s instruction. After transfection, the cells were grown in medium without antibiotics for 48 h and then were used for further experiments. The expression of miR-29b and MMP-2 in SMCs were determined.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extract from the serum and cell cultures by using miRNaesy Serum/Plasma Kit (Qiagen, Hilden, Germany) and TRIZOL (Invitrogen), respectively. The RNA concentration was measured by GeneQuant II (Pharmacia, Uppsala, Sweden) at 260 nm. Reverse transcription reaction and cDNA synthesis was performed according to the manufacturer’s instructions (Invitrogen). PCR analysis was performed on Applied Biosystems 7500 Sequence Detection system (ABI, Foster City, CA, USA) using SYBR Premix Ex Taq GC kit (Takara, Kusatsu, Japan). The stem-loop primers used for the PCR amplification were synthesized by RiboBio (Guangzhou, China). The relative expression level of miR-29b was normalized against U6 expression level. The primers were as follows: for the MMP-2: 5′-ACACTCCAGCTGGGTAGCACCATTTGAAATC-3′ (forward) and 5′-TGGTGTCGTGGAGTCG-3′ (reverse); for U6: 5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-TGGTGTCGTGGAGTCG-3′ (reverse); for the MMP-2: 5′-GGATCCGCAAGTGGTCCGTGTGAA GTAT-3′ (forward) and 5′-AAGCTTGCTGTACCCTT GGTCAGGGCAGAA-3′ (reverse); for β-actin: 5′-GAAACTACCTTCAACTCCATC-3′ (forward) and 5′-CGAGGCCAGGATGGAGCCGCC-3′ (reverse). Gene expression of MMP-2 was normalized to the level of β-actin within each sample using the relative ΔΔCT method. Gene expression is shown as relative expression to control. The data shown are representative of three independent experiments.
Cell viability assay
Cell viability was determined by using a cell counting kit-8 (CCK-8, Beyotime, Shanghai, China). For CCK-8, cell was seeded in 96-well plates and then incubated for 24, 48 and 72 h in the presence or absence of MMP-2 inhibitor SB-3CT (100 nM). Then, the absorbance of solution was measured at 450 nm using a microplate reader (Rayto Life, Shenzhen, China). Trypan blue exclusion assay was also performed. Cells were treated with trypan blue dye (0.2%) and counted with a hemocytometer.
Cell apoptosis assay
Cell apoptosis was determined by Annexin V assay. After transfection for 48 h and in the presence or absence of MMP-2 inhibitor SB-3CT (100 nM) for 10 h, cells were harvested by trypsinization and washed with PBS, and suspended in Annexin V binding buffer. FITC-conjugated Annexin V and propidium iodide (PI; Beyotime) were added to the cells successively. After incubation, Annexin V binding buffer was added, and cells were analyzed by a FACScan (Becton–Dickinson, Lincoln Park, NJ, USA) flow cytometry. Annexin V(+)/PI(−) and Annexin V(+)/PI(+) represent the cells in early apoptosis and late apoptosis/necrosis, respectively.
Luciferase reporter assay
The 3′UTR of MMP-2 harboring either the miR-29b binding site (MMP-2-3′UTR-WT) or a mutant (MMP-2-3′UTR-MUT), was cloned into the psiCHECK-2 vectors (Promega, USA), immediately downstream of the stop codon of the luciferase gene to generate the psiCHECK-MMP-2-3′UTR luciferase reporter plasmid. Mutagenesis was performed when the seed region was mutated to remove all complementarity to nucleotides of miR-29b. Plasmid DNA/vector and mimics were co-transfected into 293T cells by using Lipofectamine 2000 (Invitrogen).
Western blotting
Cells were washed twice in cold-PBS and lysed in ice-cold radioimmune precipitation (RIPA) buffer. Supernatant was collected and protein concentrations were determined using the Bio-Rad kit (Bio-Rad, Hercules, CA, USA). Cell lysates were separated by SDS–PAGE gel, transferred to PVDF (Millipore, Billerica, MA, USA). Membranes were blotted with 10% non-fat milk, washed in TBS-Tween and incubated with primary anti-MMP-2 polyclonal antibody (1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. After washing with TBS-Tween, membranes were incubated with HPC conjugated secondary antibody for 60 min at room temperature. Then, they were washed again with TBS-Tween before detection using the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Polyclonal anti-GAPDH (1:800; Bioss, Coburn, MA, USA) was used as an internal control.
Statistical analysis
All data were presented as the mean ± SD from three independent experiments. Statistical analysis was performed by the SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) using analysis of variance (ANOVA) followed by the Tukey’s t test. P < 0.05 was considered statistically significant.
Results
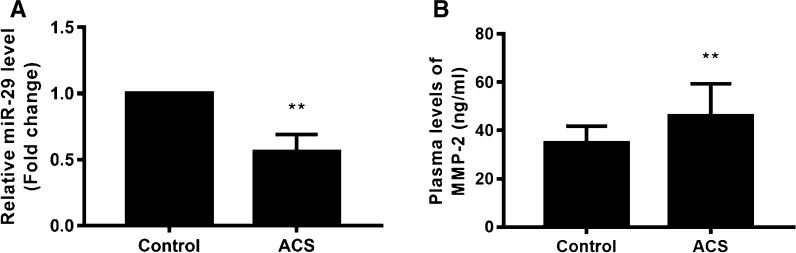
miR-29b was downregulated in ACS patients, and MMP-2 upregulated in ACS patients
To investigate the levels of miR-29b and MMP-2 in ACS patients, plasma was collected from 30 cases of ACS and 30 normal health subjects, and qRT-PCR and ELISA were performed (Fig. 1). Results reveled that miR-29b was significantly downregulated to 56% of controls (p < 0.01). The MMP-2 level in health controls was 34.9 ± 6.9 ng/mL, and it significantly increased to 46.2 ± 13.2 ng/mL in ACS patients (p < 0.01), indicating that the downregulation of miR-29b and upregulation of MMP-2 play critical role in ACS.
Fig. 1.
miR-29b was downregulated and MMP-2 upregulated in ACS patients. The mRNA levels of miR-29 and MMP-2 of plasma in ACS patients (n = 30) and normal health subjects (n = 30) were detected by qRT-PCR and ELISA, respectively. a Relative expression of miR-29b. b Plasma level of MMP-2. **p < 0.01 versus control
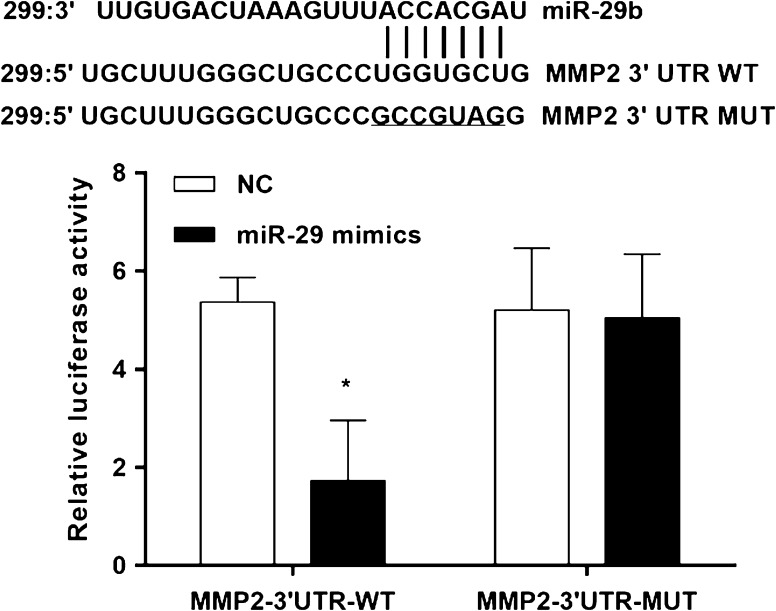
MMP-2 is target gene of miR-29
It is generally understood that miRNAs execute post-transcriptional regulation by binding to the 3′-UTR of their downstream genes. To verify whether MMP-2 is a direct target of miR-29b, a dual-luciferase reporter system was employed (Fig. 2). The 3′UTR of MMP-2 was inserted downstream of the luciferase gene and transfected into 293T cells together with miRNA-29b mimics. The results showed that miR-29b down-regulated the luciferase activity of the reporter in MMP-2-3′UTR-WT. In order to further prove its reliability, mutants of MMP-2 were constructed by deleting the miR-29b targets site and co-transfected into 293T cells together with miR-29b mimics. The luciferase expression of mutant 3′UTR of MMP-2 was no longer subject to be regulated by miR-29b. These results suggested that this site in the 3′UTR of MMP-2 was the exact regulation site for miR-29b.
Fig. 2.
miR-29 directly targets MMP-2. Sequence alignment of miR-29b and MMP-2 3′UTR using TargetScan algorithm. The 293T cells were co-transfected with miR-29b mimics and a luciferase reporter containing a fragment of the MMP-2 3′UTR harboring either the miR-29b binding site (MMP-2-3UTR-WT) or a mutant (MMP-2-3′UTR-MUT). The assay showed that luciferase activity in the MMP-2-3′UTR-WT group was significantly decreased compared to the luciferase activity of the mutant groups. *p < 0.05 versus NC
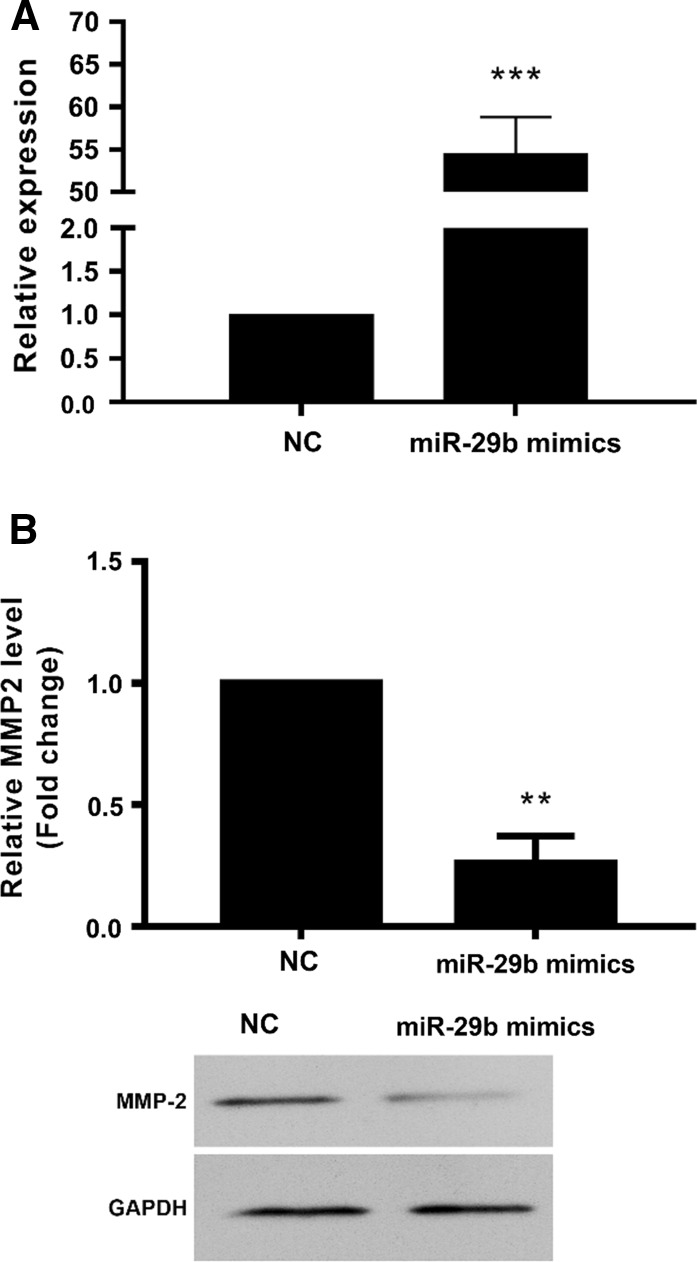
MiR-29b mimics downregulated expression of MMP-2 in SMCs
To examine the role of miR-29b and MMP-2 in atherosclerosis and ACS, overexpression of miR-29 in human umbilical aortic smooth muscle cells was performed (Fig. 3a). Results showed that expression of miR-29b was upregulated to 54.5 ± 4.3 folds of NC (p < 0.001). The overexpression of miR-29b significantly downregulated the expression of MMP-2 mRNA to 26.0 ± 11.0% of NC (p < 0.01) (Fig. 3b). Also, the overexpression of miR-29b significantly downregulated the protein levels of MMP-2 in SMCs (Fig. 3c).
Fig. 3.
miR-29 mimics downregulated MMP-2 expression in human umbilical aortic smooth muscle cells. 48h after transfection with miR-29 mimics, the expression of miR-29 and MMP-2 was detected. a miR-29 expression by qRT-PCR. b expression of MMP-2 mRNA by qRT-PCR. c expression of MMP-2 by western blot. **p < 0.01, ***p < 0.001 versus NC
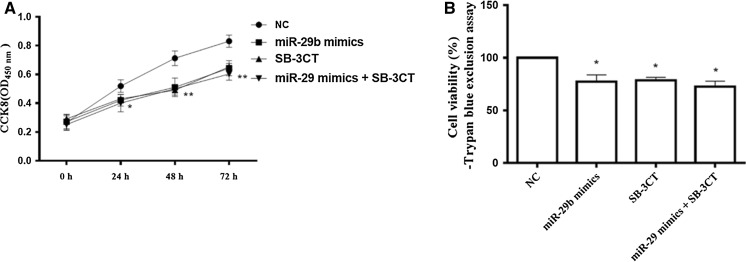
MiR-29b mimics inhibited the cell viability of SMCs
To examine the role of miR-29b and MMP-2 in atherosclerosis and ACS, cell viability in human umbilical aortic smooth muscle cells after transfected for 48 h was detected by CCK8 assay (Fig. 4a) and Trypan blue-exclusion assay (Fig. 4b) 48 h after transfection with miR-29b mimics. Cell viability at 24 h was significantly decreased compared with NC group, and further decreased at 48 and 72 h, suggesting the miR-29b reduced the viability of SMCs (Fig. 4a). Similar to CCK-8 assay, after 72 h, Trypan blue-exclusion assay showed that miR-29b significantly reduced the cell viability (Fig. 4b). In the presence of MMP-2 inhibitor SB-3CT, the cell viability of SMC cells was significantly reduced, while miR-29b mimics-reduced cell viability of SMC was not significantly changed (Fig. 4b). Thus, miR-29b mimics reduced cell viability of SMCs through downregulating of MMP-2.
Fig. 4.
miR-29 mimics reduced cell viability in human umbilical aortic smooth muscle cells. 48 h after transfection with miR-29 mimics, cell viability was assessed at 24, 48 and 72 h in the presence or absence of MMP-2 inhibitor SB-3CT (100 nM) by a CCK-8 assay and b Trypan blue exclusion assay. *p < 0.05; **p < 0.01 versus NC
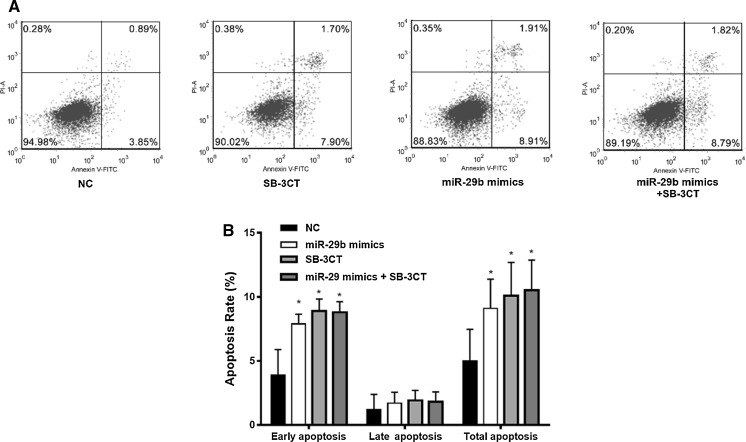
MiR-29b mimics enhanced the cell apoptosis of SMCs
To examine the role of miR-29b and MMP-2 in atherosclerosis and ACS, 48 h after transfection with miR-29b mimics and in the presence or absence of MMP-2 inhibitor SB-3CT (100 nM) for 10 h cell apoptosis was detected by flow cytometry in human umbilical aortic smooth muscle cells (Fig. 5). Cells with FITC + and PI- represent early apoptosis and cells with FITC+ and PI+ represent late apoptosis (Fig. 5a). Cell apoptosis was significantly enhanced compared with the NC group, especially in the early stage, suggesting that miR-29b promoted cell apoptosis of SMCs in the early stage (Fig. 5b). In the presence of MMP-2 inhibitor SB-3CT, cell apoptosis of SMC cells was significantly enhanced, while miR-29b mimics-enhanced cell apoptosis (especially in early stage) of SMC was not significantly changed (Fig. 5). Thus, miR-29b mimics enhanced cell apoptosis of SMCs through downregulation of MMP-2.
Fig. 5.
miR-29 mimics enhanced cell apoptosis in human umbilical aortic smooth muscle cells. 48 h after transfection with miR-29 mimics for 48 h and in presence or absence of MMP-2 inhibitor SB-3CT (100 nM) for 10 h, the cell apoptosis was detected by flow cytometry. a Flow cytometry; b quantification of the early, late, and total apoptosis. *p < 0.05 versus NC
Discussion
Atherosclerosis refers to the lipid deposition in the arterial wall and intima, with the proliferation of SMCs, which leads to intimal thickening and atherosclerotic plaque (Handorf et al. 2015; Hopps and Caimi 2015; Amin et al. 2016). Atherosclerosis was discovered in heart, brain, kidney and other organs of the body, and is closely associated with various diseases such as diabetes and hypertension and is one of the main risks of cardiovascular diseases (Di Pietro et al. 2016; Laffont and Rayner 2017). The mechanism underlying proliferation of SMCs is important to develop more effective treatment and prevention strategies of atherosclerosis. It was demonstrated that miR-29 was upregulated in diabetes serum and contributed to the development of atherosclerosis and diabetes (Nielsen et al. 2012; Arnold et al. 2014; Farr et al. 2015). In the present study, we confirmed that miR-29b is targeted to MMP-2. The overexpression of miR-29b inhibited the viability of SMCs and promoted the cell apoptosis.
It was demonstrated that miR-29b is upregualted by ox-LDL in arterial smooth muscle cells (Chen et al. 2011). ox-LDL is a major risk factor for the occurrence of atherosclerosis and can induce synthesis of MMP-2 and -9 in arterial smooth muscle cells. MMPs participate in the regulation of extracellular matrix remodeling and promote the migration of arterial smooth muscle cells, inducing the formation of neointima. Moreover, the elevated levels of MMP-2 and -9 can promote atherosclerotic rupture, triggering acute coronary syndromes (ACS) (Fernandez Machulsky et al. 2016). Low concentration of ox-LDL (100 nmol/L) could induce the upregulation of MMP-2 and -9, and miR-29b, inducing the migration of SMCs. Interleukin-3 (IL-3) has been shown to promote cell proliferation and migration in vascular disease (Kishimoto et al. 2014). Mir-9 might control the IL-3-induced proliferation and migration of SMCs (Lee et al. 2015). It seems that overexpression of miR-29b has different roles in proliferation and migration of SMCs. This should be related to the stimulus conditions. It was suggested that overexpression of miR-29b by ox-LDL regulated the methylation levels of MMP-2 and -9, whereas the IL-3 inhibited the expression of miR-29b and increased MMP-2 levels. In this study, we provide evidence that the miR-29 targeted on MMP-2, and miR-29 mimics induced downregulation of MMP-2.
It has been shown that miR-29 has the potential to be a biomarker of atrial fibrillation in patients with coronary artery disease (Dawson et al. 2013). Dawson et al. (2013) suggested that the expression level of miR-29b in plasma of patients with atrial fibrillation was decreased. The expression of miR-29b in plasma of patient with atrial fibrillation and in patients with chronic heart failure was significantly lower than that in health controls while in patients with both atrial fibrillation and chronic heart failure, plasma expression of miR-29b was lower. miR-29b was also downregulated in patients with congestive heart failure caused by dilated cardiomyopathy (Leptidis et al. 2013). In this study, we analyzed the levels of miR-29b and MMP-2 in 30 cases of ACS and 30 normal health subjects, and the results revealed that miR-29b was significantly downregulated to 56% of controls. MMP-2 in health controls was 34.9 ± 6.9 ng/mL, and that significantly increased to 46.2 ± 13.2 ng/mL in ACS patients. We further examined the association of miR-29b and MMP-2, and their potential roles in atherosclerosis and ACS. The cell viability and cell apoptosis in SMCs after transfected with miR-29b mimics for 48 h was detected by CCK8 assay and flow cytometry, respectively. We found that miR-29b mimics reduced the viability of SMCs, and that cell apoptosis was significantly enhanced compared with NC group, especially in the early stage, suggesting that miR-29b promoted cell apoptosis of SMCs in the early stage. We also determined the role of MMP-2 in cell viability and apoptosis of SMCs after miR-29b mimics transfection by using MMP-2 inhibitor SB-3CT. We found that SB-3CT significantly reduced cell viability and enhanced cell apoptosis of SMCs, but not significantly changed the cell viability and cell apoptosis of miR-29b mimics-transfected SMCs. Thus, inhibition of MMP-2 activation significantly reduced cell viability and enhanced cell apoptosis of SMCs, but inhibition of MMP-2 activation did not influent the cell viability and cell apoptosis of miR-29 mimics-transfected SMCs. As miR-29b significantly downregulated the expression of MMP-2, it implied that MiR-29 mimics reduce cell viability and promote cell apoptosis via downregulation of MMP-2. Although our results suggested the modification in cell viability is due to the induction of apoptosis, there is no evidence that miR-29b and MMP-2 directly inhibit proliferation at present. The possibility that miR-29b and MMP-2 inhibit cell proliferation by other mechanisms such as progression of cell cycle, in addition to the induction of apoptosis will be investigated in the future.
In conclusion, miR-29b was downregulated in ACS patients. MiR-29 mimics reduces cell viability and promote cell apoptosis via direct targeting MMP-2, which could be a potentially promising therapy target for cardiovascular diseases.
References
- Amin M, Pushpakumar S, Muradashvili N, Kundu S, Tyagi SC, Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front Biosci (Landmark Ed) 2016;21:89–118. doi: 10.2741/4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold N, Koppula PR, Gul R, Luck C, Pulakat L. Regulation of cardiac expression of the diabetic marker microRNA miR-29. PLoS ONE. 2014;9:e103284. doi: 10.1371/journal.pone.0103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo VA, Guimaraes DA, Castro MM. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res. 2015;52:221–231. doi: 10.1159/000441621. [DOI] [PubMed] [Google Scholar]
- Botham KM, Wheeler-Jones CP. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog Lipid Res. 2013;52:446–464. doi: 10.1016/j.plipres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–1728. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- Cifani N, Proietta M, Tritapepe L, Di Gioia C, Ferri L, Taurino M, Del Porto F. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med. 2015;47:441–446. doi: 10.3109/07853890.2015.1073346. [DOI] [PubMed] [Google Scholar]
- Dawson K, Wakili R, Ordog B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kaab S, Nattel S. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–1475. doi: 10.1161/CIRCULATIONAHA.112.001207. [DOI] [PubMed] [Google Scholar]
- Delbosc S, Glorian M, Le Port AS, Bereziat G, Andreani M, Limon I. The benefit of docosahexanoic acid on the migration of vascular smooth muscle cells is partially dependent on Notch regulation of MMP-2/-9. Am J Pathol. 2008;172:1430–1440. doi: 10.2353/ajpath.2008.070951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84:1–7. doi: 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Farr RJ, Joglekar MV, Hardikar AA. Circulating microRNAs in diabetes progression: discovery, validation, and research translation. EXS. 2015;106:215–244. doi: 10.1007/978-3-0348-0955-9_10. [DOI] [PubMed] [Google Scholar]
- Fernandez Machulsky N, Gagliardi J, Fabre B, Miksztowicz V, Lombardo M, Garcia Escudero A, Gigena G, Blanco F, Gelpi RJ, Schreier L, Gidron Y, Berg G. Matrix metalloproteinases and psychosocial factors in acute coronary syndrome patients. Psychoneuroendocrinology. 2016;63:102–108. doi: 10.1016/j.psyneuen.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ. New insights into the world of matrix metalloproteinases. Circulation. 2002;105:405–407. [PubMed] [Google Scholar]
- Gabunia K, Herman AB, Ray M, Kelemen SE, England RN, Cadena RD, Foster WJ, Elliott KJ, Eguchi S, Autieri MV. Induction of MiR133a expression by IL-19 targets LDLRAP1 and reduces oxLDL uptake in VSMC. J Mol Cell Cardiol. 2017;105:38–48. doi: 10.1016/j.yjmcc.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handorf AM, Zhou Y, Halanski MA, Li WJ. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis. 2015;11:1–15. doi: 10.1080/15476278.2015.1019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopps E, Caimi G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur Rev Med Pharmacol Sci. 2015;19:2583–2589. [PubMed] [Google Scholar]
- Jiang D, Yang Y and Li D (2017) Lipopolysaccharide induced vascular smooth muscle cells proliferation: A new potential therapeutic target for proliferative vascular diseases. Cell Prolif 50 [DOI] [PMC free article] [PubMed]
- Kishimoto S, Ishihara M, Takikawa M, Takikawa M, Sumi Y, Nakamura S, Fujita M, Sato T, Kiyosawa T. Three-dimensional culture using human plasma-medium gel with fragmin/protamine microparticles for proliferation of various human cells. Cytotechnology. 2014;66:791–802. doi: 10.1007/s10616-013-9628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can J Cardiol. 2017;33:313–324. doi: 10.1016/j.cjca.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lim S, Song BW, Cha MJ, Ham O, Lee SY, Lee C, Park JH, Bae Y, Seo HH, Seung M, Choi E, Hwang KC. MicroRNA-29b inhibits migration and proliferation of vascular smooth muscle cells in neointimal formation. J Cell Biochem. 2015;116:598–608. doi: 10.1002/jcb.25011. [DOI] [PubMed] [Google Scholar]
- Leptidis S, El Azzouzi H, Lok SI, de Weger R, Olieslagers S, Kisters N, Silva GJ, Heymans S, Cuppen E, Berezikov E, De Windt LJ, da Costa Martins P. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS ONE. 2013;8:e57800. doi: 10.1371/journal.pone.0057800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yang S, Ma A, Pan X, Wang H, Li N, Liu S, Wu M. Expression of miRNA-155 in carotid atherosclerotic plaques of apolipoprotein E knockout (ApoE−/−) mice and the interventional effect of rapamycin. Int Immunopharmacol. 2017;46:70–74. doi: 10.1016/j.intimp.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Wang C, Sorensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, Juul A, Zhang CY, Pociot F, Mortensen HB. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362. doi: 10.1155/2012/896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found Symp. 2007;283:174–191. doi: 10.1002/9780470319413.ch14. [DOI] [PubMed] [Google Scholar]
- Peng H, Zhong M, Zhao W, Wang C, Zhang J, Liu X, Li Y, Paudel SD, Wang Q, Lou T. Urinary miR-29 correlates with albuminuria and carotid intima-media thickness in type 2 diabetes patients. PLoS ONE. 2013;8:e82607. doi: 10.1371/journal.pone.0082607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem. 1999;190:105–118. doi: 10.1023/A:1006941621170. [DOI] [PubMed] [Google Scholar]
- Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, Vary CP, Liaw L. Notch and transforming growth factor-beta (TGFbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem. 2010;285:17556–17563. doi: 10.1074/jbc.M109.076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557. doi: 10.1016/S0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H, Vanuzzo D, Hobbs M, Mahonen M, Cepaitis Z, Kuulasmaa K, Keil U. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000;355:688–700. doi: 10.1016/S0140-6736(99)11181-4. [DOI] [PubMed] [Google Scholar]
- Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, Perrotta P, Yin X, Bauer A, Leslie KL, Zhang P, Aryal B, Montgomery RL, Thum T, Martin K, Suarez Y, Mayr M, Fernandez-Hernando C, Sessa WC. Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol Med. 2016;8:643–653. doi: 10.15252/emmm.201506031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola M, Karousou E, D’Angelo ML, Moretto P, Caon I, Luca G, Passi A, Vigetti D. Extracellular matrix in atherosclerosis: hyaluronan and proteoglycans insights. Curr Med Chem. 2016;23:2958–2971. doi: 10.2174/0929867323666160607104602. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Liu XH, Tarbell J, Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res. 2015;339:90–95. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Liu JX, Yan ZP, Yao XH, Liu XH. Potential microRNA biomarkers for acute ischemic stroke. Int J Mol Med. 2015;36:1639–1647. doi: 10.3892/ijmm.2015.2367. [DOI] [PubMed] [Google Scholar]