Abstract
Ceramide is found to be involved in inhibition of cell division and induction of apoptosis in certain tumour cells. Ceranib-2 is an agent that increases ceramide levels by inhibiting ceramidase in cancer cells. Therefore, we aimed to investigate the effects of ceranib-2 on cell survival, apoptosis and interaction with carboplatin in human non-small cell lung cancer cells. The cytotoxic effect of ceranib-2 (1–100 µM) was determined by MTT assay in human lung adenocarcinoma (A549) and large cell lung carcinoma (H460) cells. Carboplatin (1–100 µM) and lung bronchial epithelial cells (BEAS-2B) were used as positive controls. Morphological and ultrastructural changes were analysed by light microscope and TEM. Apoptotic/necrotic cell death and acid ceramidase activity were analysed by ELISA. Combination effects of ceranib-2 and carboplatin were investigated by MTT. The expression levels of CASP3, CASP9, BAX and BCL-2 were examined by qRT-PCR. The IC50 of ceranib-2 was determined as 22 μM in A549 cells and 8 μM in H460 cells for 24 h. Morphological changes and induction of DNA fragmentation have revealed apoptotic effects of ceranib-2 in both cell lines. Ceranib-2 and carboplatin has shown synergism in combined treatment at 10 and 25 μM doses in H460 cells for 24 h. Ceranib-2 inhibited acid ceramidase activity by 44% at 25 µM in H460 cells. Finally, CASP3, CASP9 and BAX expressions were increased while BCL-2 expression was reduced in both cells. Our results obtained some preliminary results about the cytotoxic and apoptotic effects of ceranib-2 for the first time in NSCLC cell lines.
Keywords: Ceranib-2, Ceramidase inhibitor, Cytotoxicity, Apoptosis, Combined therapy
Introduction
Lung cancer is the most common cause of cancer-related mortality (Spiro and Porter 2002) and has two major subtypes; small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Approximately 80% of lung cancers occurs as NSCLC (Siegel et al. 2016), proliferates rapidly and is therefore generally diagnosed in late stages (Dela Cruz et al. 2011). Surgery and/or chemoradiation therapy can be a curative option in early stages, in metastatic setting the therapy is given with palliative intent. Chemotherapy is frequently used for therapeutic purposes. Various side effects and primary or secondary resistance to therapy limits the clinical activity of drugs (Lin and Shaw 2016). New effective systemic treatment options are needed to improve the outcomes of NSCLC.
The effects of ceramide and sphingosine-1-phosphate (S1P) as secondary messengers in sphingolipid metabolism, have been identified on cell cycle and apoptosis (Obeid et al. 1993). It was previously reported that ceramide induced cell death while S1P caused survival and drug resistance in tumours (Ogretmen and Hannun 2004; Taha et al. 2006). So the investigation of the effects of these molecules on cancer cell proliferation has become a current issue (Pettus et al. 2002).
Ceramidase is the enzyme which hydrolysis ceramide. Studies showed that increased activation of ceramidase leads to reduced ceramide levels and elevated levels of S1P. Therefore, intracellular ceramidase level is important to keep balance between ceramide and S1P in cancer treatment (Obeid et al. 1993). Ceranib-2 is a novel cellular ceramidase inhibitor (Draper et al. 2011). Several studies have shown that ceranib-2 caused inhibition of ceramidase activity, elevated endogenous ceramide level, reduced S1P level and inhibited cell proliferation and tumor growth. Previous studies have demonstrated that the cytotoxic effect of ceranib-2 has been associated with cell cycle arrest and apoptosis in human ovarian carcinoma (SKOV3) (Draper et al. 2011), H-ras transformed rat fibroblast (5RP7) (Vejselova et al. 2014) and human prostate cancer (DU145, LNCaP) cells (Kus et al. 2015) in vitro. Additionally, researchers have considered ceramidase enzyme inhibition as important for prevention of chemotherapeutic drug resistance and induction of apoptosis (Draper et al. 2011; Vejselova et al. 2014; Kus et al. 2015).
In this study, we investigated the effects of ceranib-2 on cell viability, morphology, cell death and apoptosis related CASP3, CASP9, BAX and BCL-2 expressions in NSCLC cell lines. Furthermore, we examined antagonistic/synergistic interaction of ceranib-2 in combination with carboplatin which is a commonly used chemotherapeutic agent in lung cancer treatment.
Materials and methods
Cell lines and drug preparation
Human NSCLC lung adenocarcinoma (A549), large cell lung carcinoma (H460) and human lung bronchial epithelial (BEAS-2B) cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma, St. Louis, MO, USA) containing 10% foetal bovine serum (FBS, Sigma) and 1% penicillin–streptomycin (Sigma) at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Ceranib-2 (3-[3-(4-methoxyphenyl)-1-oxo-2-propen-1-yl]-4-phenyl-2(1H)-quinolinone) (Cayman Chemical, Ann Arbor, MI, USA, CAS 1402830-75-4) was dissolved in dimethyl sulfoxide (DMSO) as 10 mM stock solution. Carboplatin (Carbodex, 50 mg/5 ml, Deva Holding A.S., Istanbul, Turkey) was purchased as vial and the stock solution’s molarity was rearranged to 10 mM using sterile distilled water. Stock solutions were diluted with DMEM to various concentrations.
Cytotoxicity assay
A549, H460 and BEAS-2B cells (1 × 104 cells/well) were seeded in 96 well plates and incubated for 24 h. Then 100 µl of medium (as control) or 1, 5, 10, 25, 50, 75 and 100 µM of ceranib-2 or carboplatin were added to the wells and cells were incubated for 24 h. Treatment doses of ceranib-2 for this study were selected according to the previous studies (Draper et al. 2011; Vejselova et al. 2014; Kus et al. 2015). We used same doses of carboplatin to compare the effectiveness of each drug. Solvent control group for 75 and 100 µM doses were also used for ceranib-2 treatment. Each experiment was performed for three times. The cytotoxic effects of each drug on cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983; Oztopcu-Vatan et al. 2015) at 550 nm wavelength with a microplate reader (BioTek, Powerwave XS, Winooski, VT, USA). The optical density read from treated wells were converted to a percentage of living cells against the control by using the following formula:
The half-maximal inhibitory concentration (IC50) was calculated as 50% cell death causing dose compared to the control group. All data are given as the mean percent fraction of control ± SEM. Statistical analysis was done by one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests. IBM SPSS Statistics 22 was used to utilize statistical analysis. A p value of less than 0.05 was considered to be significant.
Cell morphology and ultrastructural analyses
A549 and H460 cells were treated with 5, 10 and 25 µM of ceranib-2 for 24 h and observed with an inverted light microscope (Nikon Eclipse, TS100, Melville, NY, USA) to determine morphological changes.
Ultrastructural analyses were examined by transmission electron microscopy (TEM) for H460 cells. Cells (1 × 106) were seeded into 25 cm2 flasks and incubated overnight. Next day cells were treated with 1 or 10 µM ceranib-2 for 24 h. After treatment, cells were trypsinized and washed with phosphate buffer saline (PBS), and samples were directly fixed at 4 °C in PBS with 2.5% glutaraldehyde for 16 h. After washing steps with PBS, cells were post-fixed with osmium tetroxide at 4 °C for 1 h. Then, samples were washed with PBS, stained with 1% uranyl acetate for 15 min, dehydrated with graded series of ethanol at room temperature. Subsequently, samples were embedded in araldite which was allowed to polymerize by incubation at 60 °C for 48 h (Harhaji-Trajkovic et al. 2009). Thin sections were cut with ultra-microtome and stained with uranyl acetate-lead citrate for observation under TEM (JEOL JEM 1220, Tokyo, Japan).
Combination therapy
Antagonistic/synergistic interactions between ceranib-2 and carboplatin were investigated by MTT assay. The same concentrations (1, 5, 10 and 25 µM) of ceranib-2 and carboplatin were applied as combined at 1:1 ratio for 24 h. The effects were determined by MTT assay. One-way ANOVA followed by Tukey’s multiple comparison test was used for statistical analysis. Results were considered as significant for p < 0.05.
Co-efficient of drug interaction (CDI) was calculated with the following formula (Daphu et al. 2014):
Determination of apoptotic and necrotic DNA fragmentation
Apoptotic and necrotic DNA fragmentations were determined by Cell Death Detection ELISA Plus kit (Roche Applied Science, Mannheim, Germany). This photometric enzyme immunoassay kit is used for the quantitative in vitro determination of cytoplasmic histone-associated DNA fragments after induced cell death. In this experiment three different doses (10, 25, 50 µM for A549 and 5, 10, 25 µM for H460 cells) were selected to apply for 24 h considering MTT results. The kit was used according to the manufacturer’s instructions. Absorbance was measured at 405 nm wavelength with a microplate reader. Unpaired t test was used for statistical analysis and results were considered as significant for p < 0.05. Enrichment factor for dose groups was calculated with the following formula (Sommer et al. 2005):
Measurement of acid ceramidase activity
Inhibitory effect of 5, 10 and 25 µM ceranib-2 doses on acid ceramidase enzyme was determined with colorimetric Enzyme-Linked Immunosorbent Assay (ELISA) Kit for Human N-Acylsphingosine Amidohydrolase 1 (ASAH1) (Cloud-Clone Corp., Katy, TX, USA) in H460 cells. The kit was applied according to the manufacturer’s instructions. Absorbance was measured at 450 nm wavelength with microplate reader. Results were converted to a percentage of ASAH1 activity against control by using the following formula:
Statistical analysis was conducted by unpaired t test and differences were considered as significant at p < 0.05.
Quantitative real-time PCR (QRT-PCR)
By QRT-PCR, CASP3, CASP9, BAX and BCL-2 expressions were analysed. Treatments were applied as 10, 25 and 50 µM for A549 cells, 5, 10 and 25 µM for H460 cells and non-treated cells were used as control.
Total RNA was isolated by using High Pure RNA Isolation Kit (Roche) and qualitative examination was performed with Nanodrop 2000c (Thermo Science, Waltham, MA, USA). 1000 ng/µl of RNA for each sample was used for reverse transcription with Transcriptor First Strand cDNA Synthesis Kit (Roche). For QRT-PCR, FastStart Essential DNA Green Master Kit (Roche) was used according to the manufacturer’s instructions. The reaction was performed in LightCycler Nano (Roche) instrument. HPRT1 gene was used for normalization. Relative expression levels of CASP3, CASP9, BAX and BCL-2 were calculated using 2ΔCt method (Schmittgen and Livak 2008). Differences between the relative mRNA expression levels of control and treatment doses were analysed with unpaired t test by GraphPad Prism V (La Jolla, CA, USA) and considered as statistically significant for p < 0.05.
Results
Cytotoxic effects of ceranib-2 and carboplatin on cell viability
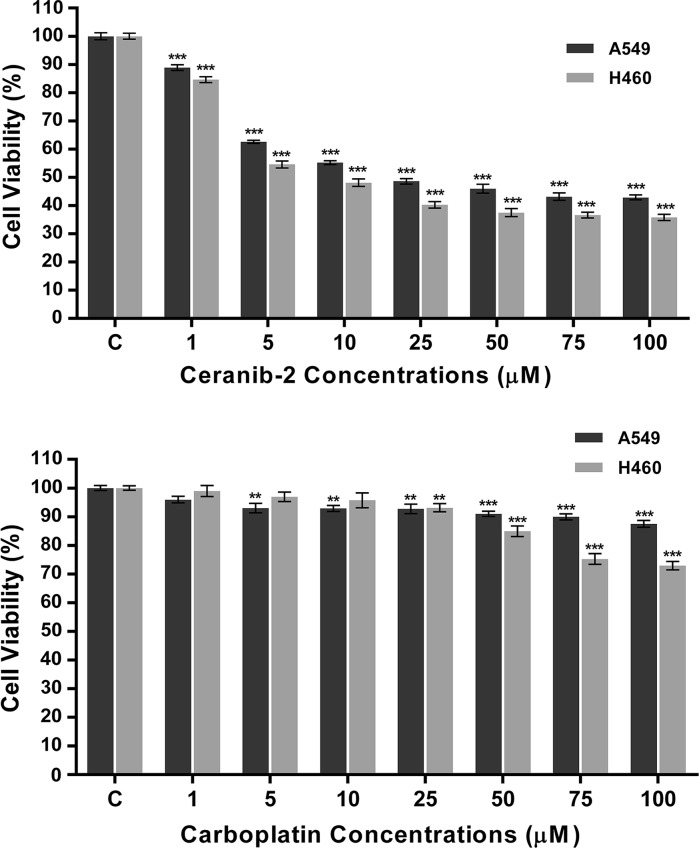
Cell viability was found decreased by ceranib-2 after treatment for 24 h in both lung cancer cells in a dose dependent manner (Fig. 1). Also treatment with the highest (0.01%) concentrations of DMSO (solvent) or untreated medium (control) did not significantly change the cell survival rate (p > 0.05) (data not shown). After 24 h treatment, the IC50 values of ceranib-2 were determined as 22 µM for A549 and as 8 µM for H460 cells. We also applied ceranib-2 to BEAS-2B cells as normal cells. Cell viability was reduced with an IC50 at 49 µM for 24 h.
Fig. 1.
Cell viability (%) after ceranib-2 and carboplatin treatment of A549 and H460 cells for 24 h. The control group was taken as 100%. Each bar represents mean value of three independent experiments and results were considered as significant for p < 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001)
Carboplatin treatment in A549 cells decreased cell viability significantly but the IC50 value could not be calculated. The survival rate of H460 cells was also reduced by carboplatin treatment (Fig. 1). IC50 was not found within 24 h of treatment.
Cell morphology and ultrastructural changes
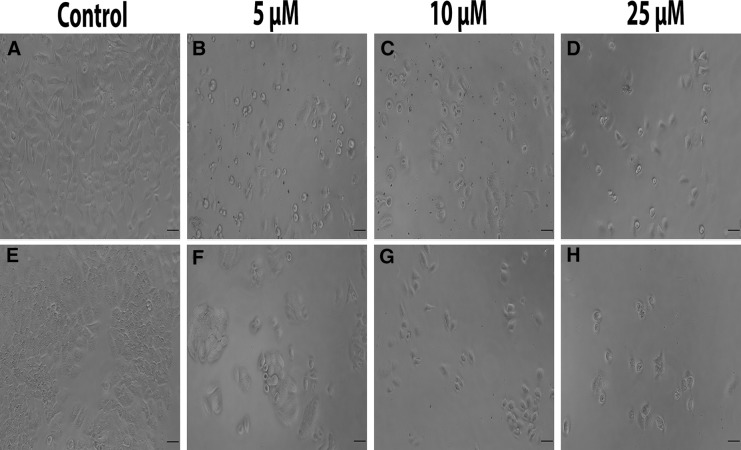
In both cell lines, 5–25 µM ceranib-2 decreased cell number compared to control cells. All treated cells were observed as rounded and shrunk. Moreover, H460 cells lost the ability to cluster and were separated from each other compared to control cells (Fig. 2).
Fig. 2.
Morphological changes in A549 (a–d) and H460 (e–h) cell lines caused by ceranib-2 for 24 h (Scale bar: 50 µM)
Ultrastructural analysis was done by TEM method. After treatment of H460 cells with 1 and 10 µM doses of ceranib-2 for 24 h, cell shrinkage, corruption in nucleus morphology, chromatin condensation, swelling in mitochondria, vacuolization and membrane blebbing were observed at 1 µM dose (Fig. 3a). Cells which were treated with 10 µM ceranib-2 showed nuclear fragmentation, disappearance of organelles and increased vacuolization (Fig. 3b).
Fig. 3.
Ultrastructural analysis of ceranib-2 treated H460 cells. a 1 µM ceranib-2, b 10 µM ceranib-2; n nucleus, v vacuole, mi mitochondrion and b blebbing
Combined drug interaction
As a result of 1–25 µM of combined drug application (ceranib-2 and carboplatin) in the A549 cells, IC50 was not found for the following 24 h of treatment. According to combined drug interaction calculation; antagonism was found between two drugs for 24 h treatment. H460 cell viability was found reduced with combined therapy and IC50 values were calculated as 7 µM for 24 h. Synergism was detected between two drugs for 24 h of treatment except 1 and 5 µM doses (Table 1).
Table 1.
Cell viability (%) results after combined treatment of ceranib-2 and carboplatin on A549 and H460 cells for 24 h
| A549 | H460 | |||||||
|---|---|---|---|---|---|---|---|---|
| Doses (µM) | Ceranib-2 | Carboplatin | Combined | CDI | Ceranib-2 | Carboplatin | Combined | CDI |
| 1 | 88.86 | 93.97 | 90.01 | 1.08 | 84.62 | 98.98 | 98.23 | 1.17 |
| 5 | 62.55 | 93.01 | 72.2 | 1.24 | 54.56 | 96.93 | 55.47 | 1.05 |
| 10 | 55.21 | 92.87 | 59.1 | 1.15 | 48.09 | 95.71 | 40.58 | 0.88 |
| 25 | 49.61 | 92.72 | 56.58 | 1.23 | 40.26 | 93.12 | 36.21 | 0.97 |
CDI coefficient of drug interaction (CDI < 1 = synergism, CDI > 1 = antagonism)
Determination of DNA fragmentation
According to DNA fragmentation results apoptosis was found 3.7, 5.9 and 8.9 fold increased by 10, 25 and 50 µM of ceranib-2 treatment, respectively, of A549 cells compared to the non-treated group (p < 0.05). In A549 cells, 10 and 25 µM concentrations did not show any significant necrotic effect whereas necrosis was found 2.5 fold increased at 50 µM (p < 0.05). In H460 cells treatment with 5, 10, 25 µM doses of ceranib-2 increased apoptosis by 6.5, 7.5, 8.2 fold, respectively, (p < 0.05) and necrosis by 5.4, 5.5, 5.6 fold (p < 0.05), respectively, were observed compared to non-treated group (Fig. 4).
Fig. 4.
Fold change of apoptotic and necrotic DNA fragmentation after ceranib-2 treatment of A549 and H460 cells for 24 h according to enrichment factor analyses. The enrichment factor of the non-treated group was set to 1 (EFK:1). Results were considered as significant for p < 0.05 (* p < 0.05, ** p < 0.01, *** p < 0.001)
Assessment of acid ceramidase activity
Enzyme activity was determined in H460 cells using the ASAH1 kit. Cells were treated with 5, 10 and 25 µM of ceranib-2 for 24 h and ceramidase activity was found reduced by 29, 31 and 44%, respectively, compared to untreated cells.
Relative expressions of CASP3, CASP9, BAX and BCL-2 genes
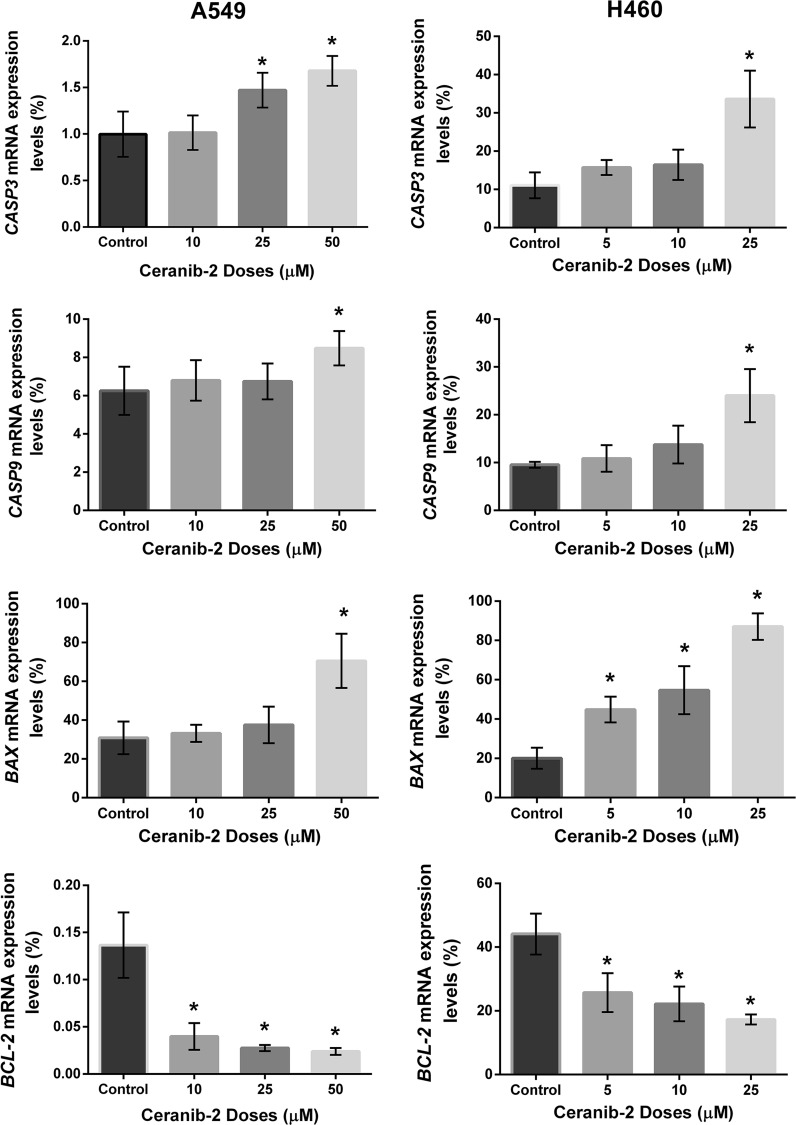
In A549 cells, CASP3 expression was found 1.5 and 1.7 fold (p < 0.05) increased at 25 and 50 µM dose applications (p < 0.05) and CASP9 expression was found increased only at 50 µM dose by 1.4 fold (p < 0.05) which was significant compared to control cells. We found significant increase for BAX expression only at 50 µM by 2.3 fold. 10, 25 and 50 µM doses decreased BCL-2 expression level significantly by 0.7, 0.8 and 0.9 fold (p < 0.05), respectively (Fig. 5).
Fig. 5.
Relative expression levels of CASP3, CASP9, BAX and BCL-2 for after ceranib-2 treatment for 24 h. (* p < 0.05 by t test). Relative expressions were normalized to HPRT gene
As a result of treatment, CASP3 and CASP9 expressions were increased significantly at 25 μM by 3 and 2.5 fold (p < 0.05), respectively, in H460 cells. On the other hand, 5,10 and 25 μM ceranib-2 doses were significantly effective with 2.2, 2.7, 4.4 fold increase (p < 0.05) for BAX and 0.4, 0.5, 0.6 fold decrease (p < 0.05) for BCL-2 expression levels (Fig. 5).
Discussion
The balance between ceramide and S1P plays an important role in cancer cell fate (Taha et al. 2006; Baran et al. 2007). Ceramidase hydrolysis ceramide and regulates S1P level in tumours (Mao and Obeid 2008). Since elevation in ceramide level causes apoptosis in tumor cells, ceramidase inhibitors became an area of interest (Zeidan et al. 2008; Cheng et al. 2013). Ceranib-2 is a novel drug, which inhibits ceramidase activity and was found to be the most potent ceramidase inhibitor when its compared with ceranib-1, dimethylallyl pyrophosphate (D-MAPP) and (1R,2R)-2-N-myristoylamino-1-(4-nitrophenyl)-1,3-propandiol (D-NMAPPD) (Draper et al. 2011). Therefore we investigated cytotoxic effects of ceranib-2 as a single agent and in combination with carboplatin in two NSCLC cell lines.
The present study reveals for the first time that ceranib-2 reduces cell survival in a dose depend manner in both A549 and H460 cells. Also ceranib-2 was found to be more potent in reducing cell viability of H460 than of A549. The anti-proliferative effect level of ceranib-2 varies in different cell lines depending on the dose of treatment. Supporting our results, cell viability was decreased by ceranib-2 in human ovarian adenocarcinoma (SKOV3), H-ras transformed rat fibroblast (5RP7), human prostate cancer (DU145, LNCaP) and human breast cancer (MCF7 and MDA MB 231) cell lines in different treatment durations (Draper et al. 2011; Vejselova et al. 2014; Kus et al. 2015; Vethakanraj et al. 2015, 2016). BEAS-2B cells were less sensitive to ceranib-2 than A549 and H460 cells for 24 h. Similar to our result, ceranib-2 was detected as less potent in mouse fibroblast cells (NIH/3T3) compared to 5RP7 cells (Vejselova et al. 2014).
Carboplatin is widely used in the treatment of lung cancer (Chang 2011). Therefore it is a widely used positive control agent to compare efficiency with novel drugs for lung cancer research. According to our MTT analysis ceranib-2 was found to be more effective at suppressing cell proliferation of NSCLC cells than carboplatin.
Ceranib-2 caused significant morphological changes dose dependently in non-small cell lung cancer cells. After 24 h of treatment, cells were observed as rounded and decreased in number under inverted light microscope. The hallmarks of apoptosis, which are cell shrinkage, membrane blebbing, chromatin condensation, nuclear fragmentation and mitochondrial swelling were detected via TEM in H460 cells. Further experiments by TEM showed that DU145, LNCaP and MCF-7 cells had similar morphological changes after ceranib-2 treatment (Kus et al. 2015; Vethakanraj et al. 2015; Vejselova et al. 2016). Also vacuolization occurred in H460 cells after ceranib-2 application for 24 h. Ceramides are known to be important regulators of the crosstalk between apoptosis and autophagy (Gonzalez-Polo et al. 2005; Young et al. 2013). Thus, cytoplasmic vacuolization may be related to autophagy but further investigation is needed to determine and understand the mechanism.
The study of the effect of ceranib-2 together with other chemical drugs is rather limited. Drug synergism is crucial for successful chemotherapeutic treatment. For the first time our study is reporting the synergistic activity of ceranib-2 and carboplatin on NSCLC cells. Our results indicated that ceranib-2 increased the cytotoxic effect of carboplatin. Similarly, synergism with paclitaxel was also reported in SKOV3 cells for 72 h of combined treatment (Draper et al. 2011).
Subsequently, we evaluated ceramidase activity after ceranib-2 treatment in H460 cells to determine ceramidase inhibition level. Ceramidase activity was reduced compared to control cells after 24 h in a dose dependent manner. These findings were consistent with the decrease ceramidase activity in SKOV3 cells (Draper et al. 2011).
To explore the mechanism of cell death we assessed the DNA fragmentation in both lung carcinoma cells and we showed that DNA fragmentation occurred in both cells after ceranib-2 treatment and which was supportive of apoptosis more than of necrosis as the terminal event for the affected cells. These data support that ceranib-2 as a ceramidase inhibitor causes ceramide accumulation and apoptotic DNA fragmentation in A549 and H460 cells. Similarly to our results, apoptotic DNA fragmentation was observed in MCF-7 and MDA MB 231 cells (Vethakanraj et al. 2015).
The role of caspases and Bcl-2 family members in ceramide-induced mitochondrial apoptosis pathway has been described before in different studies (Sawada et al. 2000; von Haefen et al. 2002). Ceramide accumulation in cancer cells was found to elevate Bax and reduce Bcl-2, which caused changes in mitochondrial membrane permeabilization and cytochrome-c release followed by activation of caspase-9 and caspase-3 (Sawada et al. 2000). We investigated quantitative expression levels of pro-apoptotic CASP3, CASP9, BAX and anti-apoptotic BCL-2 via QRT-PCR method. Our results showed that CASP3, CASP9 and BAX expression levels were increased, while BCL-2 expression was decreased by ceranib-2 treatment of NSCLC. Thus, these data confirmed that ceranib-2 induces apoptotic cell death. Similarly, ceranib-2 led to elevated BAX mRNA level and diminished BCL-2 mRNA level in human breast cancer cells (Vethakanraj et al. 2015). Besides, it was demonstrated that increased levels of ceramide by sphingomyelinase caused an increase in the expression of CASP3, CASP9 and BAX while BCL-2 expression was reduced in human epithelial lung carcinoma (H1299) cells (Soans et al. 2014).
In conclusion, ceranib-2 has growth inhibitory and apoptotic effects on NSCLC cell lines. Furthermore ceranib-2 is less toxic to non-tumorigenic (BEAS-2B) cells compared to cancer cells. Also our results suggested that ceranib-2 has more potent growth inhibitory effect compared to carboplatin. Ceranib-2 enhanced cytotoxic effect of carboplatin suggesting that they might be used in combination for non-small cell lung cancer therapy. These findings support basis of the future studies and further development of this drug for lung cancer treatment.
Acknowledgements
This publications was based completely on M.Sc. thesis of the first author, Merve YILDIZ.
Funding
This study was supported by TUBITAK (The Scientific and Technical Research Council of Turkey), (Project Number: 214Z159).
References
- Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat ınvolved in the regulation of resistance to ımatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Cheng JC, Bai A, Beckham TH, Marrison ST, Yount CL, Young K, Lu P, Bartlett AM, Wu BX, Keane BJ, Armeson KE, Marshall DT, Keane TE, Smith MT, Jones EE, Drake RR, Bielawska A, Norris JS, Liu X. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J Clin Invest. 2013;123:4344–4358. doi: 10.1172/JCI64791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daphu I, Horn S, Stieber D, Varughese J, Spriet E, Dale H, Skaftnesmo K, Bjerkvig R, Thorsen F. In vitro treatment of melanoma brain metastasis by simultaneously targeting the MAPK and PI3 K signaling pathways. Int J Mol Sci. 2014;15:8773–8794. doi: 10.3390/ijms15058773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper JM, Xia Z, Smith RA, Zhuang Y, Wang W, Smith CD. Discovery and evaluation of ınhibitors of human ceramidase. Mol Cancer Ther. 2011;10:2052–2061. doi: 10.1158/1535-7163.MCT-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Polo R-A, Boya P, Pauleau A-L, Jalil A, Larochette N, Souquère S, Eskelinen E-L, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus G, Kabadere S, Uyar R, Kutlu HM. Induction of apoptosis in prostate cancer cells by the novel ceramidase inhibitor ceranib-2. In Vitro Cell Dev Biol Anim. 2015;51:1056–1063. doi: 10.1007/s11626-015-9932-9. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Shaw AT. Resisting resistance: targeted therapies in lung cancer. Trends Cancer. 2016;2:350–364. doi: 10.1016/j.trecan.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta Mol Cell Biol Lipids. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA (1993) Programmed cell death induced by ceramide. Science 259:1769–1771. http://www.ncbi.nlm.nih.gov/pubmed/8456305 [DOI] [PubMed]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Oztopcu-Vatan P, Sayitoglu M, Gunindi M, Inan E. Cytotoxic and apoptotic effects of menadione on rat hepatocellular carcinoma cells. Cytotechnology. 2015;67:1003–1009. doi: 10.1007/s10616-014-9739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585:114–125. http://www.ncbi.nlm.nih.gov/pubmed/12531544 [DOI] [PubMed]
- Sawada M, Nakashima S, Banno Y, Yamakawa H, Hayashi K, Takenaka K, Nishimura Y, Sakai N, Nozawa Y. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000;7:761–772. doi: 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Soans E, Evans SC, Cipolla C, Fernandes E. Characterizing the sphingomyelinase pathway triggered by PRIMA-1 derivatives in lung cancer cells with differing p53 status. Anticancer Res. 2014;34:3271–3283. [PubMed] [Google Scholar]
- Sommer U, Costello CE, Hayes GR, Beach DH, Gilbert RO, Lucas JJ, Singh BN. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J Biol Chem. 2005;280:23853–23860. doi: 10.1074/jbc.M501752200. [DOI] [PubMed] [Google Scholar]
- Spiro SG, Porter JC. Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med. 2002;166:1166–1196. doi: 10.1164/rccm.200202-070SO. [DOI] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM (2006a) Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol 39:113–131. http://www.ncbi.nlm.nih.gov/pubmed/16584625 [DOI] [PubMed]
- Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta Biomembr. 2006;1758:2027–2036. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejselova D, Kutlu HM, Kuş G, Kabadere S, Uyar R. Cytotoxic and apoptotic effects of ceranib-2 offering potential for a new antineoplastic agent in the treatment of cancer cells. Turkish J Biol. 2014;38:916–921. doi: 10.3906/biy-1405-36. [DOI] [Google Scholar]
- Vejselova D, Kutlu HM, Kus G. Examining impacts of ceranib-2 on the proliferation, morphology and ultrastructure of human breast cancer cells. Cytotechnology. 2016;68:2721–2728. doi: 10.1007/s10616-016-9997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethakanraj HS, Babu TA, Sudarsanan GB, Duraisamy PK, Ashok Kumar S. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem Biophys Res Commun. 2015;464:833–839. doi: 10.1016/j.bbrc.2015.07.047. [DOI] [PubMed] [Google Scholar]
- von Haefen C, Wieder T, Gillissen B, Stärck L, Graupner V, Dörken B, Daniel PT. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- Young MM, Kester M, Wang H-G. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54:5–19. doi: 10.1194/jlr.R031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan YH, Jenkins RW, Korman JB, Liu X, Obeid LM, Norris JS, Hannun YA. Molecular targeting of acid ceramidase: implications to cancer therapy. Curr Drug Targets. 2008;9:653–661. doi: 10.2174/138945008785132358. [DOI] [PMC free article] [PubMed] [Google Scholar]