Abstract
The procedure of obtaining qualified endothelial progenitor cells (EPCs) is still unclear and there has been some controversy on their biological properties and time of emergence. In this study, we used long-term culture of Adipose Derived Stem Cells (ADSCs) in an endothelial induction medium to obtain endothelial progenitor-like cells, and investigated the features of a few surface markers and the physiologic functions of the cells produced. In order to achieve our aim, rat ADSCs were isolated and cultured in an endothelial basal medium (EBM2), supplemented with an endothelial growth medium (EGM2). The cells were cultured 1 week for short-time, 2 weeks for mid-time, and 3 weeks for long-time cultures. Morphological changes were monitored by phase contrast and electron microscopy. The expressions of a few endothelial progenitor cells markers were analyzed by real-time RT-PCR. Low-density lipoprotein uptake and lectin binding assay were also performed for functional characterization. After induction, ADSCs showed changes in morphology from spindle-shaped in the first week to cobblestone-shaped during the next 2 weeks. Then, endothelial cell phenotype was defined by the presence of Weibel–Palade bodies in the cytoplasm and tube formation, without the use of Matrigel in the third week. In keeping with gene expression analysis, VEGFR-2 showed significant expression during early stages of endothelial differentiation for up to 3 weeks. A significantly increased expression of Tie2 was observed on day 21. Likewise, VE-Cadherin, CD34, CD133, WVF and CD31 were not significantly expressed within the same period of time. Endothelial differentiated cells also showed little LDL uptake and little to no lectin binding during the first 2 weeks of induction. However, high LDL uptake and lectin binding were observed in the third week. It appears that long term culture of ADSCs in EGM2 leads to significantly increased expression of some endothelial progenitor cells markers, strong DiI-ac-LDL uptake, lectin binding and tube-like structure formation in endothelial differentiated cells. Therefore, selection of an appropriate culture time and culture medium is crucial for establishing an efficient route to obtain sufficient numbers of EPCs with optimized quantity and quality.
Keywords: Adipose derived stem cells, Endothelial progenitor like cells, Endothelial cells differentiation
Introduction
Endothelial progenitor cells (EPCs) are immature cells (Kawamoto and Losordo 2008) that are present in the bone marrow (Martinez-Estrada et al. 2005; Santos et al. 2007), peripheral blood (Song et al. 2010) and vessel wall (Ranjan et al. 2009; Sun et al. 2014). Furthermore, they are isolated from sources such as the umbilical cord blood (Ingram et al. 2004). EPCs induce angiogenesis and promote vascular repair by proliferation, differentiation and also have angiogenic factors (Guan et al. 2013). They are understood to have immense potential to improve tissue function after ischemia (Tongers and Losordo 2007) and vascular disease (Guan et al. 2013).
Two phenotypes of EPCs have been characterized at different points of time during in vitro differentiation, including early and late EPCs. Early EPCs appear in short-term cultures while late EPCs appear in long-term cultures (Lee and Poh 2014). EPCs can take up acetylated low density lipoprotein (ac-LDL), bind to lectin from Ulex europaeus agglutinin1 (UEA1) (George et al. 2011), and express various combinations of surface markers of the endothelial cell lineage (Yang et al. 2011).
In recent years, various studies have been focused on the differentiation of mesenchymal stem cells (MSC) derived from different sources such as amniotic fluid (Benavides et al. 2012) and bone marrow (Janeczek Portalska et al. 2012) to endothelial cells. Adipose-derived stem cells (ADSCs) contain progenitor cells that can be easily obtained; these cells can differentiate into several cell lineages such as endothelial precursor cells (Cao et al. 2005; Fischer et al. 2009; Guan et al. 2006; Kang et al. 2014) and cooperate in blood vessel formation (George et al. 2011).
Despite numerous studies on the different protocols in cell culture, i.e., culture media (Oshima-Sudo et al. 2011), culture period (Igreja et al. 2008; Werling et al. 2013) and sources of EPCs in humans (Feng et al. 2005; Liu et al. 2007; Oswald et al. 2004) and animals (Alphonse et al. 2015; Kang et al. 2014), controversies related to cell surface markers and the times of their emergence during differentiation still remain.
Guan et al. (2006) differentiated human ADSCs into endothelial-like cells and showed expression of the Von Willebrand factor (VWF) and CD34 after 6 days of induction (Guan et al. 2006). However, in more recent years, Kang et al. (2014) were not able to observe the expression of VWF in Canine ADSCs after 2 weeks culture (Kang et al. 2014).
Bellik et al. (2005) isolated and differentiated peripheral blood mononuclear cells into EPC by 1 week culture and found an increase in expression of VEGF-R2, VE-cadherin, PECAM-1 and CD45 after 2 days and a decreased expression of VEGF-R2 after 7 days (Bellik et al. 2005). On the other hand, Eggermann et al. isolated unselected and selected CD34+ mononuclear cells from human umbilical cord blood and differentiated them within 9 days (short-term culture) and 4 weeks (long-term culture); they found that selected mononuclear cells during short-term and long-term culture can express endothelial markers such as VEGFR2 and VE-cadherin. Concerning unselected mononuclear cells during short-term culture, CD133 and CD34 were not expressed compared to long-term culture, these cells expressed progenitor and mature endothelial cell markers including VEGFR-2, CD133, CD34 and VWF (Eggermann et al. 2003).
There are numerous conflicting reports for EPCs culture times, gene expression and correlation between gene expression and EPC appearance during culture period. The main purpose of this study is to evaluate the characterization and gene expression of EPC derived from rat ADSCs during 1 week as a short-time, 2 weeks as a mid-time, and 3 weeks as a long-time culture.
Method and materials
Isolation and culture of adipose derived mesenchymal stem cells
In this experimental study, animal procedures related to tissue isolation were in accordance with the ethical standards for research on laboratory animals of Tarbiat Modares University. To achieve ADSCs, adipose tissues of rats (Wistar albino, female, 250–300 g, Pasteur Institute, Tehran, Iran) were isolated from the inguinal region and tissues were cut into small pieces using a pair of sterile surgical scissors. Then, minced tissues were placed in 15 ml tubes containing 0.05% collagenase type 1 (Sigma, Steinheim, Germany), incubated at 37° for an hour and shaken every 10 min.
After filtering, performing centrifugation at 1200 rpm for 5 min and discarding the supernatant, the pellet was suspended in medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Glasgow, UK) and supplemented with Fetal Bovine Serum (FBS, Invitrogen, Carlsbad, CA, USA) 15%, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) (Niyaz et al. 2012). The culture media were changed every 2 days and when cells reached approximately 90% confluence, they were detached using trypsin (Invitrogen) and sub-cultured. At passage five, the cells were used for the following experiments.
Flow cytometry analysis of ADSCs surface markers
In order to confirm the presence of MSCs derived from adipose tissue, superficial markers of the cultured cells were analyzed using flow cytometry. The cells were harvested with trypsin and washed with PBS at the fifth passage. The population of cells at a concentration of 105–106 were stained using antibodies against CD90/Thy1 (554894, BD, San Diego, CA, USA), CD29/Itgb1 (555005, BD), CD44 (554869, BD) and CD73 (551123, BD) as positive cell surface markers, CD45/PTPRC (554878, BD) as a common hematopoietic lineage, and CD11b (554862, BD) as an endothelial lineage marker was used as a negative control for mesenchymal stem cells. All antibodies were from BD Biosciences (Pharmingen, USA). Appropriate fluorescent labeled isotype control antibodies were also used. Flow cytometric assays results were analyzed by the FlowJo software (version 10.2).
Osteogenic and adipogenic differentiation of ADSCs
The cultured cells at passage five were seeded at a density of 2 × 104 cells per 6 wells plate and cultured in appropriate medium for 3 weeks. The osteogenic medium contained DMEM with 10% FBS, 0.1 µM dexamethasone, 10 µM β-glycerophosphate, and 50 µM ascorbate-phosphate. Meanwhile, the adipogenic medium contained DMEM supplemented with 10% FBS, 1 µM dexamethasone, 200 µM indomethacin, 1.7 µM insulin, 500 µM isobutyl-methylxanthine, 0.05 U/ml penicillin, and 0.05 µg/ml streptomycin.
The control cells were cultured in DMEM with 10% FBS in absence of the osteogenic and adipogenic differentiation stimuli for 3 weeks. The medium was changed every 2 days. Then, the cells from both differentiated and control undifferentiated groups were washed with PBS and fixed in 4% paraformaldehyde (Sigma). After 20 min, cells were washed with PBS and stained by Alizarin red and Oil red to confirm osteogenesis and adipogenesis, respectively (Gregory et al. 2004; Yu et al. 2011).
Expressions of adipocyte and bone-related genes
Following differentiation of ADSCs to adipocyte and osteocyte, the total RNA was extracted using Trizol (Invitrogen), as described by the manufacturer. The quantity and quality of RNA were analyzed by Nanodrop (Thermo Fisher, Waltham, MA, USA) and the integrity of RNA was determined by agarose gel electrophoresis, then 2 µg of total RNA was used as a template for single stranded cDNA synthesis using Prime-Script TM RT reagent Kit (RR037A, Takara, Shiga, Japan). The PCR cycles were as follows: 42 °C for 25 min, 85 °C for 7 s. Standard curves for expression of each gene were generated by serial dilution of known concentration of the respective cDNA gene template. Then, the qPCR assay was performed using specific primers for adipocyte genes (LPL and PPAR-gamma) and osteocyte genes (Osteocalcin and Runx2) with the following cycling: 10 min at 95 °C; 15 s at 95 °C; 1 min at 60 °C for 40 cycles with a final infinite 4 °C hold. A no-template control (containing water) was used as a negative control for every different primer pair. The primer sequences were designed by Perl Primer software (version 1.1.20) as shown in Table 1. To assess gene expression level, the normality was calculated by using housekeeping gene (GAPDH) through the 2−ΔΔCt formula. The data were then calibrated with undifferentiated ADSCs that have been cultivated without induction for the same time period. A melting curve analysis was performed after each run to confirm product specificity.
Table 1.
Primer sequence for adipocyte and osteocyte related genes
| Gene | Forward primer | Reverse primer |
|---|---|---|
| LPL | CCCTAAGGACCCCTGAAGAC | GCCCGATACAACCAGTCTA |
| PPAR-gamma | ACCACGGTTGATTTCTCCAG | GCTTTATCCCCACAGACTCG |
| Osteocalcin | GAGGGCAGTAAGGTGGTGAA | GTCCGCTAGCTCGTCACAAT |
| Runx2 | GGACGAGGCAAGAGTTTCAC | GAGGCGGTCAGAGAACAAAC |
| GAPDH | TGCTGAGTATGTCGTGGAGT | CGGAGATGATGACCCTTTTG |
Cell cycle analysis
Cell cycle analysis of ADSCs was determined by measuring DNA content using PI (Propidium Iodide) dye. Cells at passage 5 were cultured in 25 cm2 flasks. After 3 weeks, cells were harvested and centrifuged in 1000 rpm for 3 min. The cell pellet was washed with 1X PBS and fixed with ice-cold 70% ethanol and subjected to overnight incubation at − 20 °C. Fixed cells were washed with 1xPBS and incubated with 0.5 ml staining buffer including 50 µg/ml PI (Molecular Probes, Eugene, OR, USA) and 100 µg/ml RNase (Sigma-Aldrich, Hamburg, Germany) for 30 min at room temperature. The analysis was performed with the BD FACSCalibur flow cytometry instrument (BD Bioscience) and FlowJo software (version 10.2).
Apoptosis assay
Apoptosis assay was performed using the Annexin-V/Dead Cell Apoptosis kit (Cell Signaling Technology, Inc. Danvers, MA, USA). Cells at passage 5 were cultured in 25 cm2 flasks for 3 weeks and were prepared based on the protocol provided by the kit. Briefly, the cells were harvested, centrifuged and washed with ice-cold 1X PBS. The cell pellet was then re-suspended with 1X Annexin-V binding buffer. 1 µl of Annexin V-FITC Conjugate and 12.5 µl of PI solution were added to 100 µl of cell suspension and incubated for 10 min on ice in the dark. Cell suspension was diluted to a final volume of 250 µl with ice-cold 1X Annexin-V Binding Buffer and analyzed immediately with the BD FACSCalibur flow cytometry instrument (BD Bioscience). The data were analyzed by means of The FlowJo software (version 10.2).
Endothelial cell differentiation of ADSCs
For differentiation of ADSCs into endothelial progenitor-like cells, ADSCs at identical density (3000/T25 flask or 400/6-well plates) were cultured in an endothelial basal medium (EBM2, Lonza Group, Basel, Switzerland) supplemented with an endothelial cell growth medium (EGM2, singleQuots, Lonza Group, Basel, Switzerland) containing: 10 ml fetal bovine serum (FBS), 2 ml human Fibroblastic Growth Factor (hFGF-B), 0.2 ml hydrocortisone, 0.5 ml ascorbic acid, 0.5 ml Vascular Endothelial Growth Factor (VEGF), 0.5 ml Insulin-like Growth Factor-1 (IGF-1) and 0.5 ml human Epidermal Growth Factor (hEGF), 0.5 ml gentamicin amphotericin (GA-1000) and 0.5 ml heparin. The medium was changed every 2 days. Then the morphological appearances of endothelial progenitor-like cells were observed after 1 week for short-time, 2 weeks for mid-time, and 3 weeks for long-time cultures using phase contrast microscopy (Nikon, Tokyo, Japan). Detailed studies of endothelial progenitor-like cells, ultra-structure, gene and protein expression and functional characteristic were studied using electron microscopy, qRT-PCR, immunostaining, DiI-ac-LDL uptake and binding lectin, respectively.
Electron microscopy
To preserve endothelial progenitor-like cells from ADSCs during scanning electron microscopy, the cells were grown on coverslips for 21 days and then fixed with glutaraldehyde 2.5% for 30 min at room temperature. After two PBS washes for 20 min, the differentiated cells were post-fixed in Osmium Tetroxid 1% (OsO4) for 2 h, and dehydrated with increasing graded series of ethanol. The dried section was then coated with gold nanoparticles and visualized with Scanning Electron Microscope (TESCAN/VEGA LMU, Brno, Czech Republic). For Transmission Electronic Microscopy (TEM), a monolayer of differentiated cells was also selected after 21 days’ induction, fixed with Glutaraldehyde 2.5%, post-fixed with OsO4 1%, dehydrated with increasing ethanol concentrations, and embedded in resin. The ultrathin sections with the thickness of 80 nm were prepared and placed on copper grids, stained with uranyl acetate and lead citrate, and examined using a Transmission Electron Microscope (LEO 906, Zeiss, Oberkochen, Germany). ADSCs at passage 5 were also prepared for TEM as mentioned above, and used as a negative control.
Expression of endothelial progenitor related genes
Total RNA was extracted from endothelial differentiated cells at days 7, 14 and 21 of culture using Trizol. The same procedure was also performed for undifferentiated ADSCs that were cultivated without induction for the same time period.
Further steps of q-PCR were performed in the same way as mentioned for adipocyte and osteocyte genes expression. The results were normalized using β-actin as a housekeeping gene and calibrated with undifferentiated ADSCs that were cultivated without induction for the same time period. Additionally, fibroblasts derived from rat lung were used as a negative control. Also, due to the fact that the wall of the blood vessels contained endothelial and endothelial progenitor cells (Kovacic and Boehm 2009; Reinisch et al. 2009), total RNA was extracted from a part of rat aorta and expression of the mentioned endothelial markers was analyzed by means of qRT-PCR and used as a positive control. The primer sequences were designed by Perl Primer Software (version 1.1.20) as shown in Table 2.
Table 2.
Primer sequence for endothelial progenitor cells related genes
| Gene | Forward primer | Reverse primer |
|---|---|---|
| CD133 | TGCTGACCCTCTGAATCTG | ATACATCCTCTGAATCCATCCTG |
| CD34 | CCACCAGAGCTATTCCCGAA | GTCTTCACCCAGCCTTTCTC |
| Tie2 | CCCTTAGTGACATTCTCCCTC | CTGGTATTGAGTGATGGTGG |
| VEGF-R2 | CCCAGAAAYGYACCAAACCA | ACTTCCTCTTCCTCCATACAG |
| VWF | AGTCAGGTTCACCCATCTTG | TCACAGATTCCACAGAGACC |
| CD31 | TACACTTATTTATGAACAGCCCT | TCTGCACCCAACATTAACA |
| VE-Cadherin | AGAATTTGCCCAGCCCTAC | GCGGTATTGTCGTGGTTG |
| β actin | ATCTGGCACCACACCTTC | AGCCAGGTCCAGACGCA |
Immunofluorescence assay
Endothelial differentiated cells at days 7, 14 and 21 were stained with KDR (abcam, ab2349, Cambridge, MA, USA), Tie2 [bioss (Woburn, MA, USA), bs-1300R-A488] and CD31 (abcam, ab64543). Briefly, cells were fixed in 4% paraformaldehyde for 20 min at 4 °C, washed with PBS, and then incubated with blocking buffer (5% normal serum in PBS) for 1 h at room temperature. Antibodies against Tie2, CD31 and KDR were used at dilution of 1:200 overnight, rinsed with PBS/tween5%, subsequently incubated with the secondary antibody Alexa Fluor 488 conjugated donkey anti-rabbit (A21206, Invitrogen) for KDR and Alexa Fluor 568-conjugated goat anti-mouse (A11004, Invitrogen) for CD31. Nuclear staining was performed with DAPI. The ADSCs at passage 5 were cultured in DMEM with 10% FBS in absence of the endothelial differentiation stimuli for 3 weeks and used as a negative control. Cells were examined by fluorescence microscopy [Olympus (Tokyo, Japan), BX-71]. Then the positive cells in the undifferentiated and differentiated cells were counted using software ImageJ. The mean percentage of positive cells is presented.
DiI-ac-LDL uptake and binding-lectin
Functional analysis of endothelial differentiated cells at days 7, 14 and 21 after induction were performed using the DiI-labeled acetylated low density lipoprotein (DiI-ac-LDL) uptake and FITC- lectin binding. In brief, the cells were incubated in 2.5 mg/L DiI-ac-LDL (Molecular Probes, Eugene, OR, USA) for 4 h at 37 °C.
Then, the cells were fixed in 4% paraformaldehyde for 20 min. The fixed cells were washed with PBS, incubated with 10 mg/L of FITC-UEA-I (Sigma, Deisenhofen, Germany) for 1 h at 37 °C and analyzed using fluorescent microscope. Cells stained positively for both markers were considered to be differentiating EPC (Ingram et al. 2004). Next, the positive cells in the undifferentiated and differentiated cells were counted using the software ImageJ and the mean percentage of positive cells was presented. In additional, functional analysis of mouse endothelial cells was considered as a positive control.
Statistical analysis
All experiments were performed at least three times. The results were presented as the mean ± SD. Statistical analysis were performed with GraphPad Prism4 (Graphpad, San Diego, CA, USA), SPSS software version 22 using one-way Anova followed by Dunnett post hoc test. P < 0.05 was considered to be significant.
Results
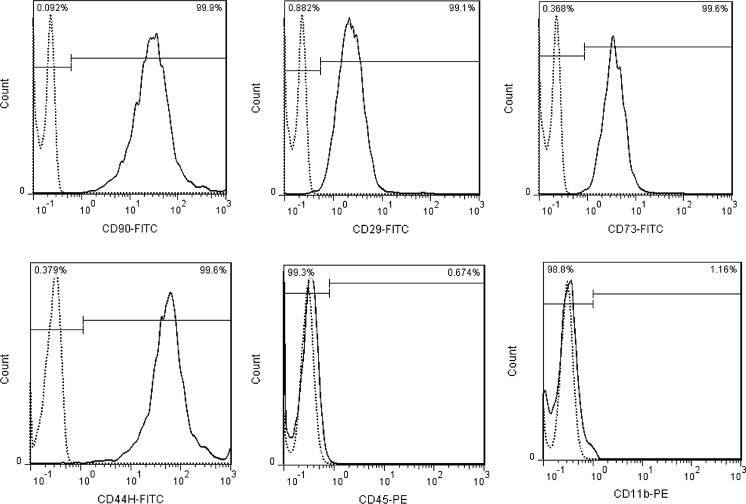
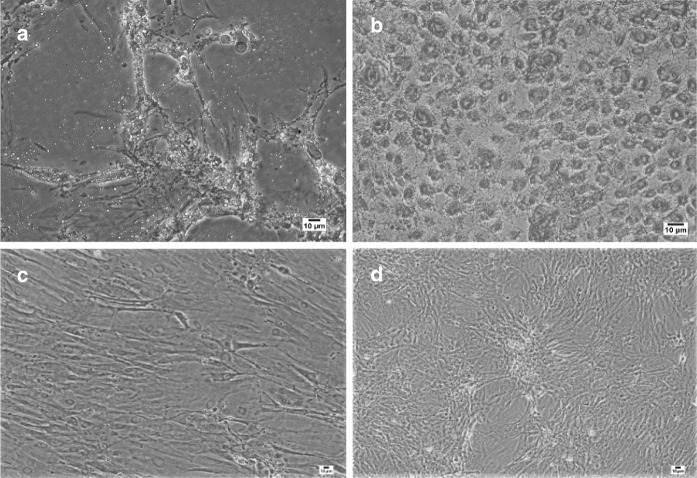
Phase contrast microscopic studies of adherent mesenchymal stem cells derived from rat adipose tissue demonstrated that spindle-shaped morphology was retained through subsequent culture passages. The phenotypes analysis of ADSCs by flow cytometry at passage 5 depicted high expressions of CD90 (99.9%), CD29 (99.1%), CD73 (99.6%), CD44 (99.6%) and low expressions of CD11b (1.16%) and CD45 (0.67%) (Fig. 1). Three weeks after using a differentiation adipogenic culture medium, numerous lipid droplets were found in the cytoplasm area of cells when stained by Oil red (Fig. 2a). Meanwhile, the cells were differentiated into osteoblast in osteogenic medium and showed red spots in extra cellular matrix, at the same period of time when stained with Alizarin red (Fig. 2b). Lipid droplet and osteoblast formation were not observed in undifferentiated ADSCs (Fig. 2c, d).
Fig. 1.
Cell surface markers expression analysis of ADSCs by flow cytometry indicates high expression of MSC specific markers CD90, CD29, CD44, CD73 and low expression of CD45 and CD11b
Fig. 2.
Differentiation of ADSCs into adipogenic and osteogenic cells during 3 weeks’ culture in induction medium. a Adipogenic differentiated cells stained with Oil red showed lipid droplet in cytoplasm. b Osteogenic differentiated cells stained with Alizarin red showed red spots in the extra cellular matrix. c and d ADSCs cultured for 3 weeks in medium without induction, stained with Oil red and Alizarin red. The figures illustrated any lipid droplet or red spot, respectively (Scale bars: 10 µm). (Color figure online)
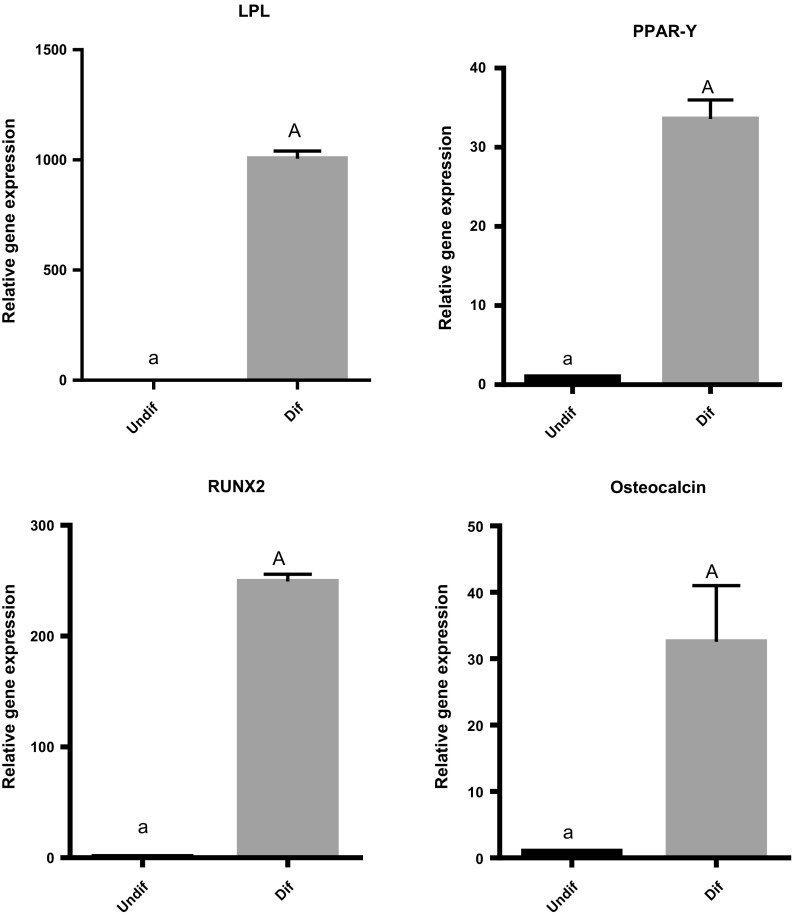
Analysis of gene expression during adipogenesis differentiation revealed significantly increased expression of LPL and PPAR-gamma genes compared to undifferentiated control cells. Also, the level of Osteocalcin and Runx2 expression significantly increased in differentiated cells during osteogenesis differentiation compared to undifferentiated ADSCs (Fig. 3).
Fig. 3.
Gene expression of differentiated ADSCs (dif) during 3 weeks of induction indicated a significant increase in expression of LPL and PPAR-Y as adipocytes and Osteocalcin and RUNX2 as osteocytes genes comparing to undifferentiated ADSCs (undif). Capital letter versus same small letter indicated significantly different at the level of P < 0.05
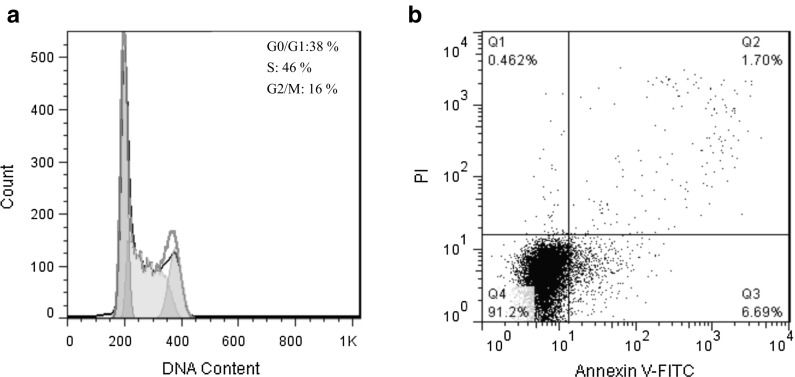
The cell cycle analysis of ADSCs at passage 5 by flow cytometry showed that 46% of the cells were in the S phase and range of cells in G0/G1 and G2/M phases were 38 and 16%, respectively. The apoptosis results during the same culture period illustrated that 91.2% of ADSCs were live in the presence of 6.69 and 1.70% for early and late apoptotic cells. These results, suggested that the ADSCs showed functional behavior without induction of cell senescence (Fig. 4).
Fig. 4.
a Cell cycle analysis of ADSCs at passage 5 by flow cytometry showed that 46% of the cells were in the S phase and range of cells in G0/G1 and G2/M phase were 38 and 16%, respectively. b The apoptosis results of ADSCs at passage 5 by flow cytometry indicated that 91.2% of ADSCs were live in the presence of 6.69 and 1.70% for early and late apoptotic cells
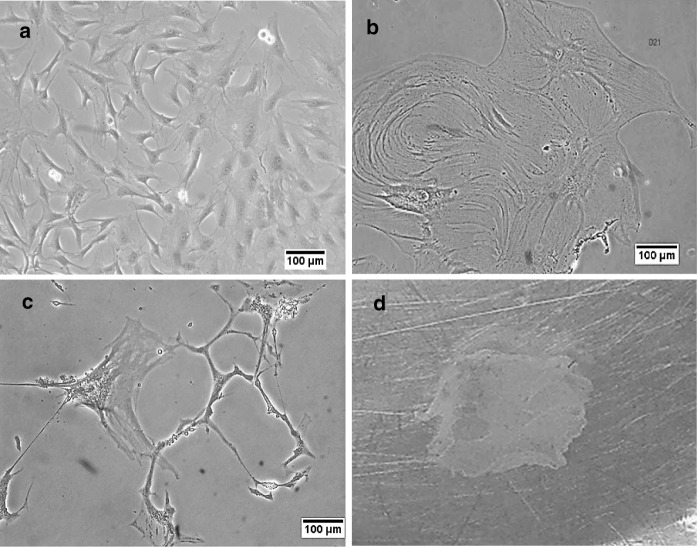
In order to differentiate ADSCs into endothelial progenitor-like cells, about 400 cells (according to our pilot study) were cultured in EBM2 with EGM2 for 3 weeks. In the early stage of differentiation (5–7 days), the cells had a fibroblast-like shape and their morphology was similar to mesenchymal stem cells whereas in the early days of the second week, cells had flat morphology and were distinct from the MSCs. At the late stages of the second week (~ 10th day), the cultured cells showed an increase in rate of proliferation and expansion. This increase in cell size continued until the end of the third week. During the third week of differentiation, the expanded cells were committed to formation of lumen-like structures. At this time, the strong connections between the cells led to assembly of a cell sheet that detached from culture plates using scrubber (Fig. 5).
Fig. 5.
The morphological changes of ADSCs during endothelial progenitor cells differentiation. a First week of differentiation: the cells still depict fibroblast-like shape. b Early in the third week of differentiation: more expansion and proliferation in differentiated cells were observed. Expanded cells were committed to promote lumen-like structures. c The end of the third week of differentiation: lumen like structure was visible. d Strong connection between the cells led to assemble sheet like structure. (Scale bars: 100 µm)
Gene expression of several markers of endothelial progenitor-like cells from ADSCs such as CD133, CD31, CD34, VEGFR2, VWF, VE-Cadherin and Tie2 genes were determined using qRT-PCR at days 7, 14 and 21 after differentiation. Furthermore, at the same time, gene expression of endothelial progenitor related markers in ADSCs in the absence of induction medium was also analyzed. VEGFR-2 showed significant expression in the early stage of endothelial differentiation, which remained stable for 3 weeks. A significant increase in expression of Tie2 was recorded on day 21 compared to undifferentiated ADSCs at the same time of culture period. Meanwhile, no significant differences in expression of VE-Cadherin, CD34, CD133, WVF and CD31 were found during 3 weeks of induction. Except for VEGFR2 and Tie2, expression of the above genes was not noticeable in fibroblast cells. Moreover, compared to fibroblast cells and EPCs derived ADSCs, rat aorta expressed considerably greater levels of the aforementioned genes (Fig. 6).
Fig. 6.

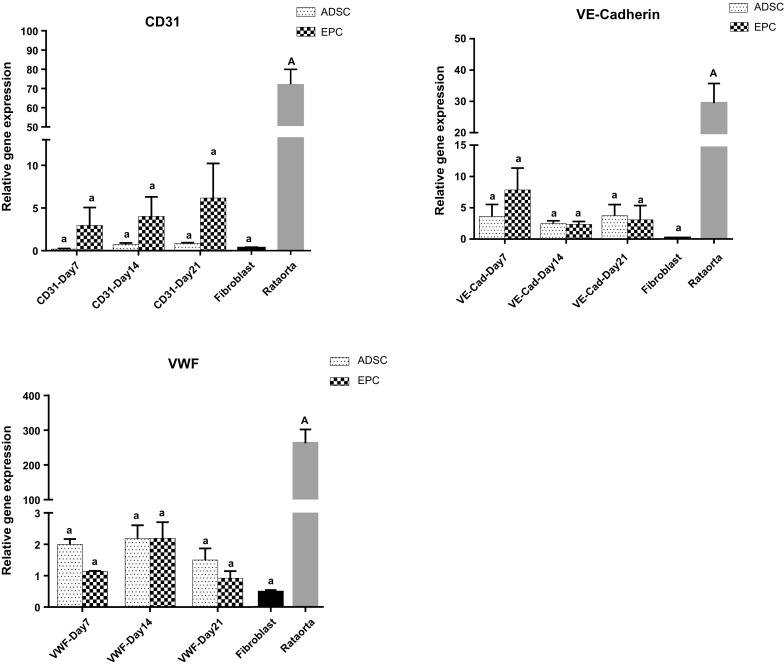
Gene expression of endothelial progenitor related markers in ADSCs cultured in induction medium compared with undifferentiated cells. Differentiated cells exhibited significant increase in expression of VEGFR-2 during 3 weeks of induction. A significant increase in expression of Tie2 was observed on day 21. The expression of these genes in lung rat fibroblast cells (negative control) was not noticeable except for VEGFR-2 on days 7, 14 and 21 and Tie2 on days 14 and 21 in EPCs. Compared to fibroblast cells and EPCs derived ADSCs, rat aorta expressed considerably greater levels of the aforementioned genes. Capital letter versus same small letters indicated significantly difference at the level of P < 0.05
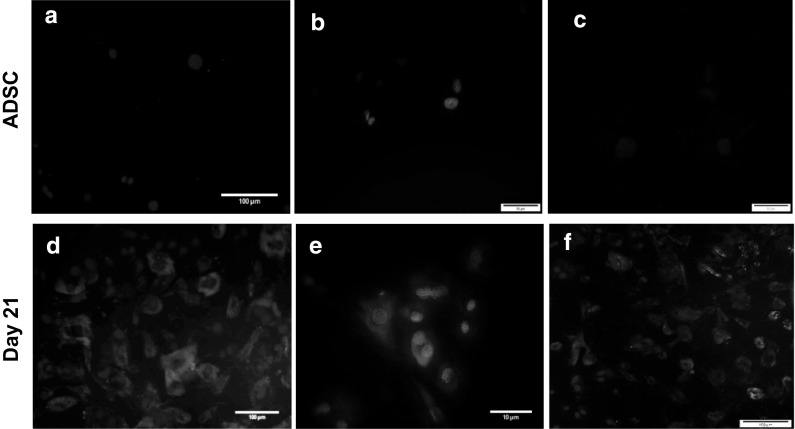
Immunofluorescence staining of differentiated cells on days 7, 14 and 21 of culture was also performed. According to the protocols, cultured cells were incubated with Tie2, CD31 and KDR antibodies. Accordingly, the obtained results showed that induced ADSCs on days 7 and 14 did not stain positively for Tie2, CD31 and KDR, whereas on day 21, expressions of Tie2 (68 ± 4.3%), CD31 (32 ± 3.6%) and KDR+/CD31+ (47 ± 3.2%) were observed. At the same time of culture period, undifferentiated ADSCs were negative for the selected markers (Fig. 7).
Fig. 7.
Immunofluorescence staining of endothelial progenitor cells differentiated from ADSCs. a–c ADSCs without induction (negative control) did not revealed any Tie2, CD31 and KDR antibodies staining. (Scale bars: 50 µm). Conversely, expression of Tie2 (d), CD31 (e) and CD31+/KDR+ (f) were only detected on day 21 after induction. Cell nuclei were counterstained with DAPI. (Scale bars: D,F: 100 µm,E: 10 µm)
Transmission Electron microscopy observation of ADSCs showed the following set of ultrastructure features. The cell membranes were irregular and numerous cytoplasmic protrusions were frequently identified. Also the presence of rounded mitochondria, rough endoplasmic reticulum and lipid granules in cytoplasm implies cell metabolic activity (Fig. 8a, b).
Fig. 8.
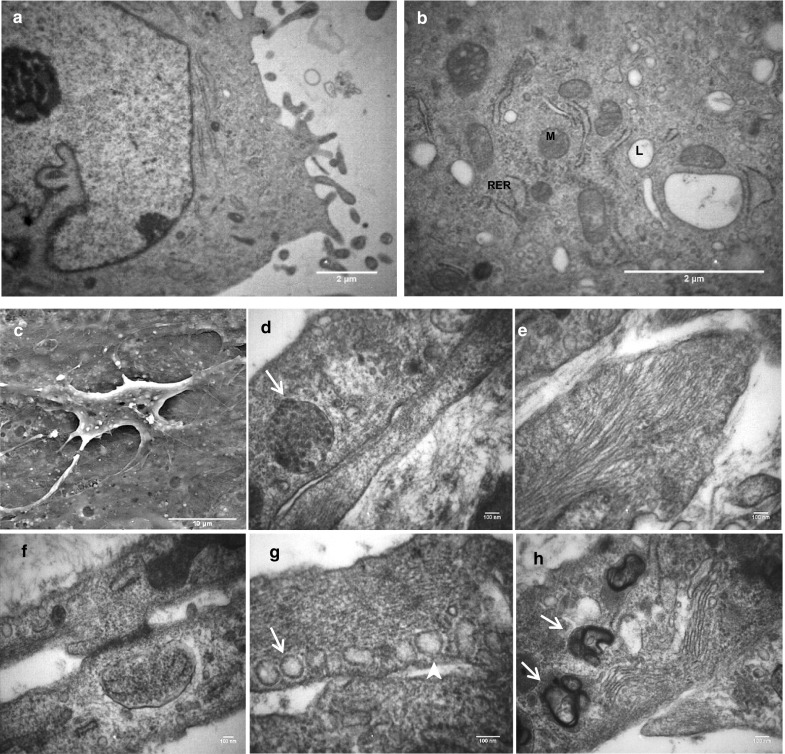
Electron micrograph of endothelial progenitor cells derived from ADSCs after 21 days of induction. a, b Transmission electron micrographs of ADSCs as a negative control. a Irregular surface of cell membrane due to numerous cytoplasmic protrusions. b In the cytoplasm, rounded mitochondria (M), rough endoplasmic reticulum (RER) and lipid granules (L) are seen. (Scale bars: 2 µm). c Scanning electron microscopy of EPC revealed unequal cellular extensions. Scale bar: 10 µm. d–h Transmission electron microscopy images: d cross-sectioned of Weibel–Palade bodies (arrow). e Endothelial cell filaments. f Junctional complex. g Caveolae (arrowhead) and vesicles (arrow). h Golgi complex (arrowhead) and lamellar body (arrow). (Scale bars: 100 nm)
Scanning electron microscopy images of endothelial progenitor-like cells after 21 days of induction, illustrated the atypical shapes with multiple cellular extensions and short scatter micro villus structures on the cell surface (Fig. 8c). Transmission electron microscopy images also showed Weibel–Palade bodies (WPBs) as a marker of endothelial cells. Many organelles such as Golgi complex and mitochondria were seen in the cytoplasm of EPCs. In addition, the observed vesicles and invaginations of cell membranes (caveolae) are presumably involved in ac-LDL uptake through endothelial cell endocytosis. Moreover, some scattered atypical laminated bodies, and numerous filaments and junctional complexes between cells were detected (Fig. 8d–h).
Functional characterization of endothelial progenitor-like cells were also investigated by assessing LDL uptake and lectin binding on days 7, 14 and 21 after induction. The results showed that, compared to no uptake in ADSCs, EPCs presented little or no LDL uptake (9.8% ± 4.1, 42.1% ± 3.8) and lectin binding (0% ± o.9, 26.7% ± 5.1) on days 7 and 14, respectively. After 21 days of culture in the EGM-2 medium, EPCs showed high levels of LDL uptake and lectin binding (74% ± 4.6). However, mouse endothelial cells as a positive control showed a maximum level of LDL uptake and UEA-I binding (100% ± 0.6) (Fig. 9).
Fig. 9.
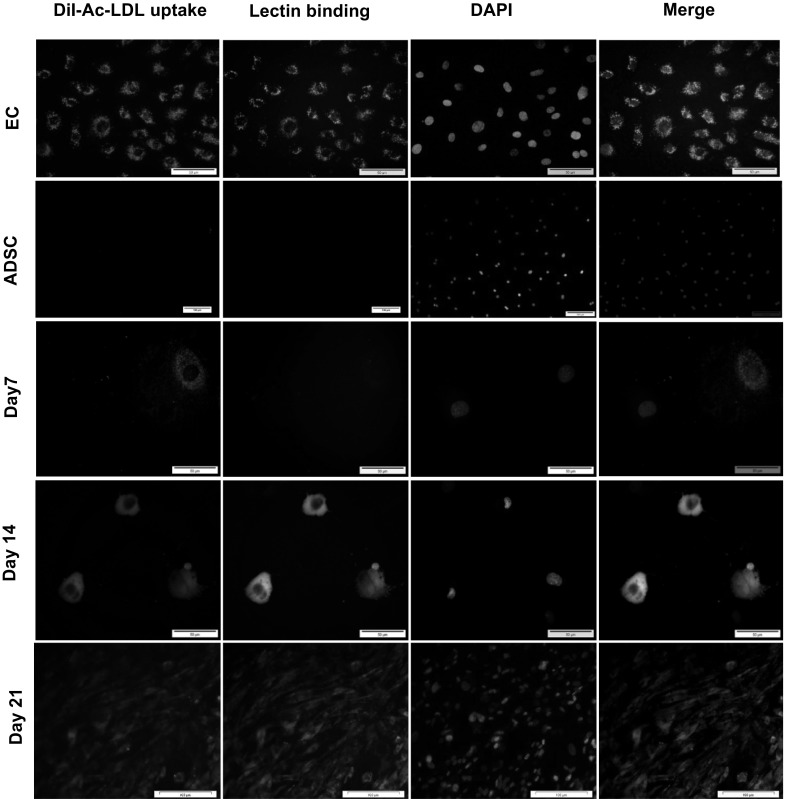
Fluorescence images of Ac-LDL uptake and lectin binding by endothelial progenitor cells during 3 weeks of induction. High Ac-LDL uptake and lectin binding on day 21 after induction were observed, comparable with ADSC and differentiated cells on days 7 and 14. However Ac-LDL uptake and lectin binding were more prominent in mouse endothelial cells as a positive control (EC). (ADSC, Day 21, scale bars: 100 µm-EC, Day 7, Day 14, scale bars: 50 µm)
Discussion
Since the first identification of a human circulating endothelial progenitor cell by Asahara et al. (1997) some 16 years ago, many investigations have been performed on EPCs. Although large number of studies have been conducted in endothelial progenitor cell culture, contradictory reports in time differences and cell markers expression during differentiation have been noticed (Cheng et al. 2013; Eggermann et al. 2003; Igreja et al. 2008; Oshima-Sudo et al. 2011). Therefore, in this study we have attempted to induce endothelial progenitor-like cells from rat ADSCs by culturing the cells in EBM2 culture medium supplemented with EGM-2. We have also specifically evaluated the effects of long-term culture of ADSCs in endothelial differentiation media. In addition to these, we have examined morphological changes, the expression levels of several EPCs related markers and functionality analysis during 1 week as a short-time culture period, 2 weeks as mid-time period, and 3 weeks as a long-time period. The obtained results of this study revealed acquisition of endothelial cell characteristics of rat ADSCs in vitro and correlations between different culture time points.
Investigation of morphological changes over the 3 weeks since induction showed that, in the early stage of differentiation, cells appeared spindle-shaped. On the other hand, during the next 2 weeks, they gradually increased in size and showed a rapid proliferation rate. According to numerous studies on mononuclear cells from peripheral blood, it is clear that EPCs are composed of a heterogeneous population of cells in both early and late EPCs (Hur et al. 2004). However, there is no consensus on their time of appearance and some biological properties.
Asahara et al. suggested that early EPCs can be observed between 4 and 7 days in culture, representing a spindle shape and with low proliferation. Endothelial outgrowth cells, also called late EPCs, which approximately appear after 7 days culture, showed a flat morphology and a high proliferation rate (Asahara et al. 1997). In this regard, Hur et al. (2004) suggested that early EPCs show maximum growth at 2–3 weeks and disappear at 4 weeks, whereas late EPC appeared at 2–3 weeks, have an exponential growth at 4–8 weeks, and preserve for up to 12 weeks (Hur et al. 2004).
In this study, ADSCs were seeded into 6-well plates at identical density (3000/T25 flask or 400/6-well plates) and grown for 21 days in EGM2 medium. Evidence showed that ADSC grew more rapidly after 7 days culture in endothelial induction medium so that the high proliferation and strong connections between the cells led to assemble a cell sheet after 3 weeks of differentiation. Our findings are similar with studies of Asahara et al. (1997). Moreover, Kange et al. indicated that canine ADSCs could gain an endothelial phenotype, such as morphological changes and high proliferative potential, when cultured in EGM-2 for 2 weeks (Kang et al. 2014). Our results are in accord with this.
In order to assess endothelial progenitor-like cells differentiation of rat ADSCs, gene expression analysis of most related genes including VEGFR2, VWF, VE-Cadherin, CD31, CD133, Tie-2 and CD34 were performed by qRT-PCR during the 3 weeks. Significant expression of VEGFR2 in the early stage of differentiation was observed, which continued until the third week. Additionally, expression of Tie-2 significantly increased in the third week of induction. Although there was no significant difference in the expression of the rest of the genes at different time points, there was a noteworthy fluctuation pattern in the expression of several genes.
Herein, VEGFR2 showed strong expression in expanded differentiated EPCs. VEGFR2 (KDR or FLK1) by VEGF plays a key role in angiogenesis responses during wound healing (Santos et al. 2007), differentiation (Kellouche et al. 2007) and migration of EPCs (Asahara et al. 1999). In the present study, this finding could be correlated with early EPCs that secrete angiogenic factors (VEGF, HGF) promoting migration and proliferation of endothelial cells (Arnaoutova et al. 2009). Furthermore, the EGM2 culture medium containing VEGF could up-regulate VEGFR2 as its own receptor (Smadja et al. 2007).
Several levels of gene expression found in this study were inconsistent with limited earlier studies. Oswald et al. (2004) isolated human bone marrow mesenchymal stem cells and cultured them in the 2% FCS and 50 ng/ml VEGF for 7 days. They reported expression of VWF, VEGFR1 and R2, VE-Cadherin, and VCAM-1, while a lack of expression of CD31 and CD34 was observed after 1 week. They believed that expression of these markers may be up-regulated due to extension of differentiation time (Oswald et al. 2004).
This contrasts with van den Akker et al. who stated porcine bone marrow-derived mononuclear cells are unable to differentiate into endothelial phenotype, and expression of CD34, VEGFR-2 and VWF were below the detection level (van den Akker et al. 2012).
Furthermore, Benavides et al. isolated mesenchymal stem cells from amniotic fluid and cultured them in EGM2 medium with different concentrations of VEGF for 2 weeks. In their assessments, expressions of VWF, endothelial nitric oxide synthase (eNOS), CD31, VE-cadherin and VEGF receptor2 were observed, while a positive correlation between CD31 expression and VEGF concentration was also shown (Benavides et al. 2012).
These different results may be due to medium composition used for induction. In this regard, Ning and colleagues found that FGF2 is a critical growth factor for differentiation of ADSCs to endothelial cells and LDL-uptake ability. Thus, they concluded that EGM2 medium contains essential factors stimulating ADSC endothelial differentiation (Ning et al. 2009).
In the current study, in spite of the significantly increased expressions of VEGFR2 and Tie2 in differentiated cells (ADSCs), highly expressed endothelial genes (VEGFR2, VWF, VE-Cadherin, CD31, CD133, Tie-2 and CD34) were observed in rat aorta. However, different factors and signaling pathways have contributed in MSC-EC differentiation in vivo (Ghadge et al. 2011). Apparently, using different endothelial induction or growth factors did not sufficiently induce expression of some endothelial genes in MSC culture in vitro (van den Akker et al. 2012). Thus, it is possible that these stem cells are limited in their ability to express some endothelial markers. Therefore, instead of using the “Endothelial Progenitor Cell”, the term of “Endothelial Progenitor-like Cell” was used.
In this study, double positive staining of DiI-acLDL uptake and lectin binding indicates that rat ADSCs have the capability to differentiate into functional endothelial cells. On days 7 and 14, after differentiation of endothelial-like cells, a low level of LDL uptake and lectin binding occurred. In contast, after 21 days of cultivation, significant LDL uptake and lectin binding were observed. This study also showed the tube formation of endothelial-like cells at 3 weeks induction in EGM2 without using Matrigel. To the contrary, in many previous studies, Matrigel is used to induce the lumen formation in a short period of time (Alphonse et al. 2015; Benavides et al. 2012).
Fischer et al. (2009) isolated ADSCs from human and canine adipose tissue, evaluating the effects of endothelial cell growth supplement (ECGS) and the shear force on stimulated differentiation of ADSCs into endothelial-like cells for up to 3 weeks culture. They demonstrated that ECGS is unable to induce expression of some of endothelial markers such as VWF and CD31, and also failed to take up ac-LDL. After 7 days of culture in ECGS and 24 h of plated cells on Matrigel, tube formation was observed (Fischer et al. 2009). However, Matrigel has been widely used to study mechanisms of angiogenesis because of its capability to induce rapid formation of capillary-like structures.
According to the obtained results, there was a lack of ac-LDL uptake, known as a biomarker of functional endothelial cells. However, the ability of ADSCs to differentiate into functional endothelial progenitor cells prior to tube formation was shown with low ac-LDL uptake within the first 2 weeks after induction. Previous studies showed that early and late EPCs have distinct behavior in tube formation. Early EPCs’ angiogenic factors secretion, promote migration and proliferation of endothelial cells, whereas late EPCs could form blood vessels better than early EPC (Rehman 2011). It has been suggested that expansion of culture time improves function of late EPCs due to the significant increase in levels of ac-LDL uptake, lectin binding, the appearance of WPBs and lumen formation in the third week after induction (Hur et al. 2004).
These conflicting reports could be attributed to the fact that the EPC characterization methods are not standardized or uniform. Our study shows that, despite high expression of various critical endothelial cells (genes in the early stage of endothelial differentiation such as VEGFR2), there is poor functional performance during the first 2 weeks of induction (as short and mid-term culture). Presumably, expanded duration of culture is useful to achieve appropriate functional endothelial progenitor cells. Based on this idea, long-term culture of ADSCs in EGM2 not only led to more expanded proliferation of differentiated cells but also significantly increased expression of some endothelial progenitor genes (Tie2 and VEGFR2) and presence of WPBs as markers of endothelial cells. In addition, strong ac-LDL uptake, lectin binding and tube-like structures of endothelial differentiated cells were observed.
In conclusion, in order to use EPCs for clinical application, establishment of an efficient route to obtain sufficient numbers of EPCs with optimized quantity and quality is required. Therefore, selection of an appropriate culture time and culture medium is crucial.
Acknowledgements
This work was supported by Grants from Tarbiat Modares University and Royan Institute (both located in Tehran, Iran). Auteurs would like to thank Dr. Vahid Pirhajati for his kind support in preparation of EM images.
References
- Alphonse RS, Vadivel A, Zhong S, McConaghy S, Ohls R, Yoder MC, Thebaud B. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat Protoc. 2015;10:1697–1708. doi: 10.1038/nprot.2015.107. [DOI] [PubMed] [Google Scholar]
- Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellik L, Ledda F, Parenti A. Morphological and phenotypical characterization of human endothelial progenitor cells in an early stage of differentiation. FEBS Lett. 2005;579:2731–2736. doi: 10.1016/j.febslet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Benavides OM, Petsche JJ, Moise KJ, Jr, Johnson A, Jacot JG. Evaluation of endothelial cells differentiated from amniotic fluid-derived stem cells. Tissue Eng Part A. 2012;18:1123–1131. doi: 10.1089/ten.tea.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- Cheng CC, et al. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genom. 2013;14:182. doi: 10.1186/1471-2164-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann J, et al. Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res. 2003;58:478–486. doi: 10.1016/S0008-6363(03)00252-9. [DOI] [PubMed] [Google Scholar]
- Feng B, Liu YL, Feng K, Gong R, Chen H. Evaluation of endothelial cells differentiated from mesenchymal stem cells of human bone marrow with Tie-2 monoclonal antibody by immunohistochemistry in vitro. Zhongguo ying yong sheng li xue za zhi = Zhongguo yingyong shenglixue zazhi = Chin J Appl Physiol. 2005;21:340–343. [PubMed] [Google Scholar]
- Fischer LJ, et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AL, Bangalore-Prakash P, Rajoria S, Suriano R, Shanmugam A, Mittelman A, Tiwari RK. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol. 2011;4:24. doi: 10.1186/1756-8722-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Guan L, et al. In vitro differentiation of human adipose-derived mesenchymal stem cells into endothelial-like cells. Chin Sci Bull. 2006;51:1863–1868. doi: 10.1007/s11434-006-2055-7. [DOI] [Google Scholar]
- Guan XM, et al. Biological properties of bone marrow-derived early and late endothelial progenitor cells in different culture media. Mol Med Rep. 2013;8:1722–1728. doi: 10.3892/mmr.2013.1718. [DOI] [PubMed] [Google Scholar]
- Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Igreja C, et al. Detailed molecular characterization of cord blood-derived endothelial progenitors. Exp Hematol. 2008;36:193–203. doi: 10.1016/j.exphem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Ingram DA, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, de Boer J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE. 2012;7:e46842. doi: 10.1371/journal.pone.0046842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Lee SH, Kweon OK, Cho JY. Differentiation of canine adipose tissue-derived mesenchymal stem cells towards endothelial progenitor cells. Am J Vet Res. 2014;75:685–691. doi: 10.2460/ajvr.75.7.685. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellouche S, et al. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res. 2007;313:486–499. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res. 2009;2:2–15. doi: 10.1016/j.scr.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6:355–366. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JW, et al. Characterization of endothelial-like cells derived from human mesenchymal stem cells. J Thromb Haemost. 2007;5:826–834. doi: 10.1111/j.1538-7836.2007.02381.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Munoz-Santos Y, Julve J, Reina M, Vilaro S. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc Res. 2005;65:328–333. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyaz M, Gürpinar ÖA, Günaydin S, Onur MA. Isolation, culturing and characterization of rat adipose tissue-derived mesenchymal stem cells: a simple technique. Turk J Biol. 2012;36:658–664. [Google Scholar]
- Oshima-Sudo N, Li Q, Hoshino Y, Nakahama K-I, Kubota T, Morita I. Optimized method for culturing outgrowth endothelial progenitor cells . Inflamm Regen. 2011;31:219–227. doi: 10.2492/inflammregen.31.219. [DOI] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Ranjan AK, Kumar U, Hardikar AA, Poddar P, Nair PD, Hardikar AA. Human blood vessel-derived endothelial progenitors for endothelialization of small diameter vascular prosthesis. PLoS ONE. 2009;4:e7718. doi: 10.1371/journal.pone.0007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J. Chipping away at the surface of the endothelial progenitor cell (EPC) mystery. J Mol Med. 2011;89:943–945. doi: 10.1007/s00109-011-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch A, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SC, Miguel C, Domingues I, Calado A, Zhu Z, Wu Y, Dias S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res. 2007;313:1561–1574. doi: 10.1016/j.yexcr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Smadja DM, et al. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149–1161. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lu CW, Fang LJ, Yang W. Culture and identification of endothelial progenitor cells from human umbilical cord blood. Int J Ophthalmol. 2010;3:49–53. doi: 10.3980/j.issn.2222-3959.2010.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zheng L, Han P, Kang YJ. Isolation and characterization of endothelial progenitor cells from Rhesus monkeys. Regen Med Res. 2014;2:5. doi: 10.1186/2050-490X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongers J, Losordo DW. Frontiers in nephrology: the evolving therapeutic applications of endothelial progenitor cells. J Am Soc Nephrol. 2007;18:2843–2852. doi: 10.1681/ASN.2007050597. [DOI] [PubMed] [Google Scholar]
- van den Akker NM, et al. Vascular potency of Sus scrofa bone marrow-derived mesenchymal stem cells: a progenitor source of medial but not endothelial cells. Tissue Eng Part A. 2012;18:828–839. doi: 10.1089/ten.tea.2011.0284. [DOI] [PubMed] [Google Scholar]
- Werling NJ, Thorpe R, Zhao Y. A systematic approach to the establishment and characterization of endothelial progenitor cells for gene therapy. Hum Gene Ther Methods. 2013;24:171–184. doi: 10.1089/hgtb.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, et al. The characteristics of endothelial progenitor cells derived from mononuclear cells of rat bone marrow in different culture conditions. Cytotechnology. 2011;63:217–226. doi: 10.1007/s10616-010-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Floyd ZE, Wu X, Hebert T, Halvorsen YD, Buehrer BM, Gimble JM. Adipogenic differentiation of adipose-derived stem cells. Methods Mol Biol. 2011;702:193–200. doi: 10.1007/978-1-61737-960-4_14. [DOI] [PubMed] [Google Scholar]