Abstract
The present study was aimed to investigate the effect of 28-homobrassinolide on leukotriene synthesis in the 2H3 cells. Cell viability was determined by using sulforhodamine B (SRB) assay. Leukotriene C4 (LTC4) and Leukotriene B4 (LTB4) were determined in the cell and cell lysates. Intracellular Ca2+ was determined in the intact cells. Phospholipases A2 (PLA2) expression was determined in the cells using immunofluorescence. LTC4 and LTB4 were tremendously increased in IgE-loaded 2H3 cells. However, these levels were significantly reduced following treatment with 28-homobrassinolide. The intracellular Ca2+ level was not altered by treatment. Expression of PLA2 was significantly reduced following treatment with 28-homobrassinolide. Taking all these data together, it is suggested that the 28-homobrassinolide may be a potential therapeutic agent to inhibit leukotriene synthesis in 2H3 cells.
Keywords: 28-homobrassinolide, Leukotrienes, Phospholipases A2, 2H3 cells
Introduction
Phytohormones are regarded as chemicals that control plant growth and regulation. A few phytohormones are released from the plants. Glands are absent in plant. However, each cell is can able to produce several hormones (Vob et al. 2014). Phytohormones regulate cellular processes in target cells and determine the flower formation, stem formation, leave formation, fruit ripening and development. Plant hormones shape the plant, time of flowering, affecting seed growth, senescence of leaves, the sex of flowers and fruits (Kazan 2015; Li et al. 2010). They affect the direction of tissue growth, leaf formation and stem growth, fruit development and ripening, plant longevity and plant death (Muthuraman and Srikumar 2009, 2010; Muthuraman et al. 2010). Phytohormones are critical to growth and development of the plant, and in their absence, plants would be undifferentiated cells (Kumar et al. 2014).
Phytohormones affect gene expression and transcription levels, which are essential for cell division and growth. Phytohormones are naturally produced in the plants, whereas similar chemicals are produced by fungi and bacteria that affect plant growth and development (Srivastava 2002). A number of similar compounds have been synthesized chemically for the growth and development of plants.
Leukotriene B4 (LTB4), Leukotriene D4 (LTD4), and Leukotriene C4 (LTC4) are well-known arachidonate’s metabolites. These metabolites are produced during an allergic reaction from mast cells (Koshihara et al. 1984). Inflammatory stimulations and eosinophils are major factors for the release of these metabolites (Sharma and Mohammed 2006). Bronchial asthma is one of the chronic inflammatory diseases with symptoms of wheezing and coughing. Bronchial asthma disease progress and development is regulated by these leukotrienes (Ukena et al. 2008). Marom et al. (1982) have reported that the promotion of airway vasculature permeability, stimulation mucus secretion, and bronchial smooth muscle constriction are regulated by LTD4 and LTC4. Borgeat and Samuelsson (1980) have reported that the allergic inflammation and the late allergic response are regulated by LTB4. Therefore, the present study was aimed to investigate the effect of 28-homobrassinolide on leukotriene level in 2H3 cells.
Materials and methods
Antibiotics (penicillin–streptomycin), fetal bovine serum (FBS), and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Santa Crutz Biotech (Shangai, China). 28-homobrassinolide (> 99%), LTC4, LTA4, LTB4, arachidonic acid, mouse monoclonal Phospholipase A2 (PLA2) and donkey anti-rabbit IgG H&L (FITC) were obtained Sigma-Aldrich (St. Louis, MO, USA). Paraformaldehyde, Triton X-100 and bovine serum albumin (BSA) were purchased from Thermo Fisher Scientific (Yeongdong-daero, Gangnam-gu, Seoul, Korea).
Cell culture
2H3 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were grown under standard conditions (37 °C and 5% CO2) with standard growth medium (10% FBS and 1% penicillin–streptomycin).
SRB assay for cell viability
2H3 cells were cultured at a density of 2.5 × 104 cells/well in 96-well plates and allowed to adhere for 24 h at 37 °C. The cells were treated with different concentration of 28-homobrassinolide (20, 30, 40, 50, 60, 70 and 80 µg/mL) for 24 h. At the end of treatment, the cell viability was determined (Muthuraman et al. 2016).
Determination of LTC4 and LTB4 levels in cells
LTC4 and LTB4 synthesis was determined in 2H3 cells within 30 min of stimulation. Stimulation was performed by loading IgE into the 2H3 cells for 30 min with 0.1 µl of anti-rat IgE sheep serum. Cells were cultured at a density of 104 cells/well in a standard cell culture dish. Cells were incubated with IgE at 37 °C for 5 h. After incubation, cells were washed with PBS and treated with 28-homobrassinolide (20, 40 and 80 µg/mL) for 15 min at 37 °C (Matsumoto et al. 1995).
Determination of LTC4 and LTB4 level in cell lysate
LTC4 and LTB4 levels were determined using radioimmunoassay (RIA) techniques according to a standard method (Matsumoto et al. 1995; Hamasaki et al. 1994). Stimulation was performed by loading IgE into the 2H3 cells for 30 min with 0.1 µl of anti-rat IgE sheep serum. Gamma-globulin, 0.9% NaC1, anti-LTB4 anti-serum and [3H]LTB4 (Santa Cruz Biotechnology (Shanghai) Co., Ltd. Shanghai, China) were incubated with 100 µL of sample. Gamma globulin-coated charcoal (0.4 mL) was added to the reaction volume and frees [3H]LTB4 was removed from the tubes. A liquid scintillation counter was used to measure the radioactivity in the reaction mixture. A standard curve was used to determine LTB4 content. LTC4 was determined in the reaction mixture with a similar procedure.
Determination of intracellular Ca2+ level
The potential of 28-homobrassinolide on the intracellular Ca2+ level was determined. Stimulation was performed by loading IgE into the 2H3 cells for 30 min with 0.1 µl of anti-rat IgE sheep serum. Cells were grown at 104/mL and washed with PBS. Cells were incubated with immunoglobulin E (IgE) 37 °C for 1 h and treated with fura-2 AM (Santa Cruz Biotechnology (Shanghai) Co., Ltd. Shanghai, China) for 25 min at room temperature. Then, cells were treated with different concentrations of 28-homobrassinolide. Fluorescence was measured at spectrofluorophotometer (Hojo et al. 1994).
Immunofluorescence
Cells were cultured in a confocal dish at a density of 2.5 × 104 cells/well, and treated with 40 and 80 µg/mL of 28-homobrassinolide for 24 h. Cells were fixed with 3% paraformaldehyde and permeabilized in 0.1% Triton X-100. Then, cells were blocked with 3% BSA and incubated with mouse monoclonal Phospholipase A2 (PLA2) antibody for overnight. Then, cells were incubated with a donkey anti-rabbit IgG H&L (FITC) conjugated antibody for 1 h. Coverslips were mounted with a fluorescent mounting medium and viewed under Confocal Laser Scanning Microscope (CLSM) (1X81R Motorized Inverted Microscope, Olympus, Tokyo, Japan) (Muthuraman et al. 2014; Nagajyothi et al. 2016).
Statistical analysis
All experimental data are presented as mean ± standard error of the mean (SEM). Experimental values are analyzed by using ANOVA test. A P < 0.05 was taken statistically significant.
Results
Effect of 28-homobrassinolide on cell viability
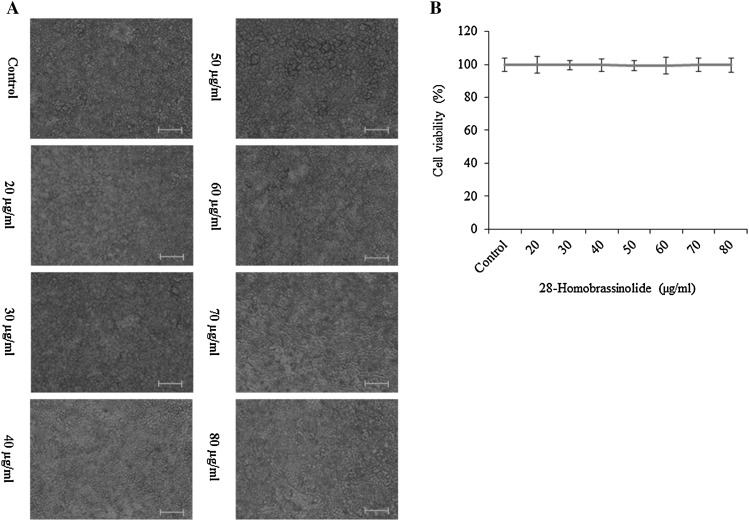
The cytotoxic effect of 28-homobrassinolide on 2H3 cells was determined. The effect of 28-homobrassinolide was observed in a dose-independent response manner (Fig. 1a). Cell growth inhibition did not appear to be significant following the treatment of 28-homobrassinolide (Fig. 1b).
Fig. 1.
Effect of 28-homobrassinolide cell viability. Results are expressed as mean ± SEM, N = 6. Images were given in ×20. Scale bar is 20 µm
Effect of 28-homobrassinolide on LTC4 level
LTC4 was increased (365 pg/105 cells) in 2H3 cells following stimulation. Cells treated with 28-homobrassinolide significantly reduced the LTC4 level. LTC4 levels were reduced to 358, 263 and 177 pg/105 at 20, 40 and 80 µg/mL of homobrassinolide treatment, respectively (Fig. 2, P < 0.05).
Fig. 2.
Effect of 28-homobrassinolide on the LTC4 level in the cells. Results are expressed as mean ± SEM, N = 6, *P < 0.05 and **P < 0.01
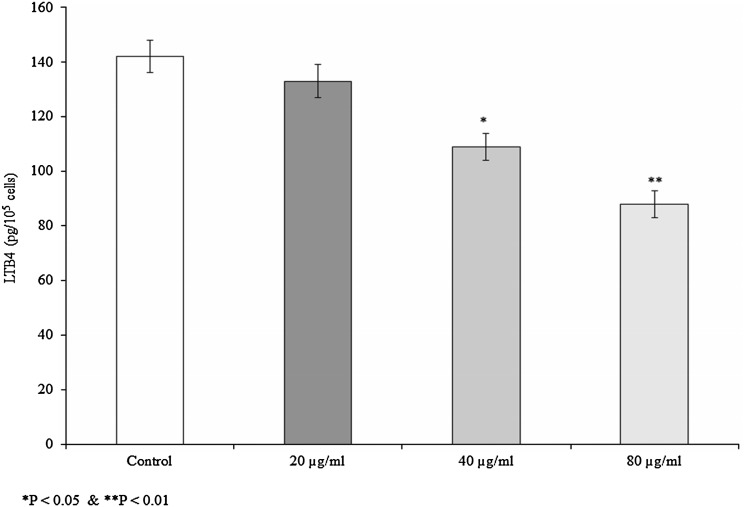
Effect of 28-homobrassinolide on LTB4 level
LTB4 was increased (142 pg/105 cells) in 2H3 cells following stimulation. Cells treated with 28-homobrassinolide significantly reduced the LTB4 level. LTB4 levels were reduced to 133, 109 and 88 pg/105 at 20, 40 and 80 µg/mL of homobrassinolide treatment, respectively (Fig. 3, P < 0.05).
Fig. 3.
Effect of 28-homobrassinolide on the LTB4 level in the cells. Results are expressed as mean ± SEM, N = 6, *P < 0.05 and **P < 0.01
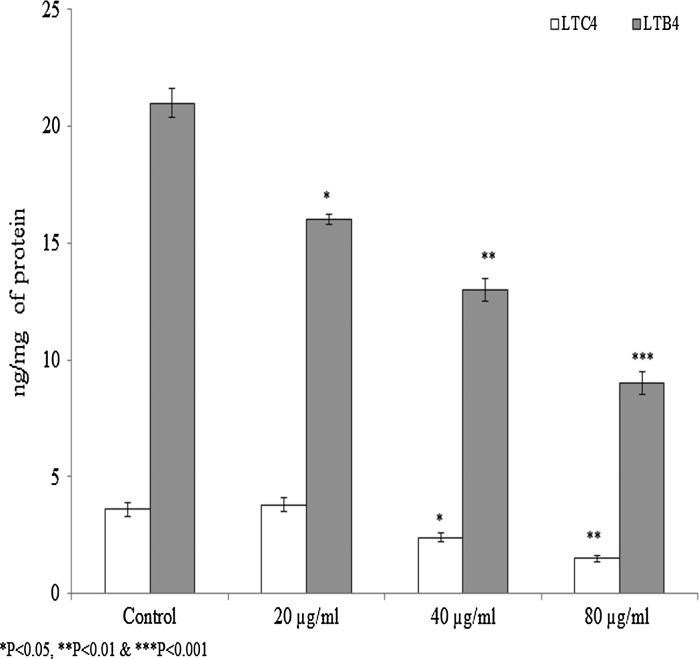
Effect of 28-homobrassinolide on the LTC4 and LTB4 levels
LTC4 and LTB4 were increased 3.6 and 21 ng/mg respectively, in the cell lysate following stimulation. LTC4 level was found 3.6 ng/mg of protein in the control group. Cells treated with 28-homobrassinolide significantly reduced the LTC4 level. LTC4 levels were reduced to 2.4 and 1.5 ng/mg of protein at 40 and 80 µg/mL of homobrassinolide treatment, respectively (Fig. 4, P < 0.05). LTB4 level was found 21 ng/mg of protein in the control group. Cells treated with 28-homobrassinolide significantly reduced the LTB4 level. LTB4 levels were reduced to 16, 13 and 9 ng/mg of protein at 20, 40 and 80 µg/mL of homobrassinolide treatment, respectively (Fig. 4, P < 0.05).
Fig. 4.
Effect of 28-homobrassinolide on LTC4 and LTB4 level in the cell lysate. Results are expressed as mean ± SEM, N = 6, *P < 0.05, **P < 0.01 and ***P < 0.001
Effect of 28-homobrassinolide on intracellular Ca2+ level
The intracellular Ca2+ level was increased (350 nM) in 2H3 cells. It was increased slightly at the beginning, and it reached at maximum within 10 min. The intracellular Ca2+ concentration was not affected by 28-homobrassinolide treatment. The massive increase (350 nM) in intracellular Ca2+ level was not reduced by 28-homobrassinolide at concentrations up to 80 µg/mL (Fig. 5, P < 0.05).
Fig. 5.
Effect of 28-homobrassinolide on the intracellular Ca2+ level. Results are expressed as mean ± SEM, N = 6
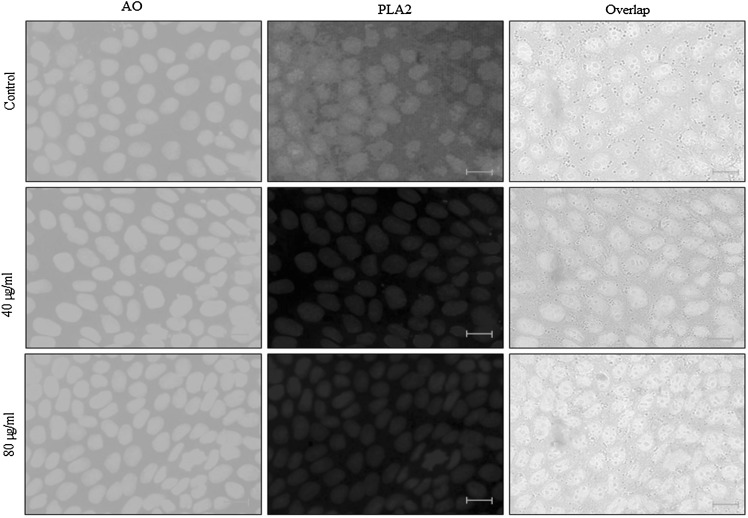
Effect of 28-homobrassinolide on PLA2 expression
PLA2 expression was monitored in the cells following 28-homobrassinolide treatment for 24 h. PLA2 expression corresponded to the appearance of immunofluorescence in the cytoplasm. PLA2 expression was increased in the control cells. However, the cells treated with 28-homobrassinolide significantly reduced PLA2 expressions (Fig. 6).
Fig. 6.
Effect of 28-homobrassinolide on PLA2 expression. Representative images from six independent experiments. N = 6. Scale bar is 50 µm. AO: Acridine orange, PLA2: Phospholipases A2
Discussion
Natural products has been used as a dietary supplementation and for medicinal purposes (Phua et al. 2009). Alternate medicine system has come into existence by utilizing medicinal properties of plants and their products. These are the Ayurveda, Siddha, Unani, Homeopathy and Chinese systems of medicine (Muthuraman and Srikumar 2009). The approaches have employed either extremely concentrated preparations of plant extracts or have utilized specific plant parts in the development of medicinal concoctions or pure isolates of plant material as drug constituent. Nonetheless, understanding the specific functionality or pharmacological effect of many of the identified and available natural products remains meager to this day (Muthuraman and Srikumar 2009). Flavones, flavonoids, phenolics, terpenoids, isoflavones, plant steroids, indole acetic acid, isoprenoids, ethylene, jasmonic acids, ellagic acid and polyamines are well known natural products. The phytohormones constitute a group of compounds for plant growth, development, flowering, fruiting, pathogen resistance, and senescence (Muthuraman and Srikumar 2009; Wasfi et al. 1992). The abundance of active endocrine substances has been reported in food as natural compounds (Hughes et al. 1991).
LTC4 and LTB4 synthesis were reduced in our study and indicated that 28-homobrassinolide may be a potential therapeutic agent for bronchial asthma. Leukotriene level was reduced in 2H3 cells following incubation with 28-homobrassinolide. LTB4 and LTC4 levels were altered through the alteration of biochemical and molecular events with interaction with IgE receptors. Signals from IgE receptors initiate a series of biochemical events to the generation of LTC4 and LTB4 (Nakashima et al. 1991). Stimulation of IgE receptors activates the production of inositol 1, 4, 5-triphosphate and diacylglycerol following the initiation of inositol turnover. Intracellular Ca2+ was released into the cytosol region after receiving signals from 1, 4, 5-triphosphate. Arachidonic acid (AA) release is consistently regulated by the enzyme phospholipases A2 (PLA2) in the cytosolic region (Channon and Lesile 1990).
LTC 4 synthase converts LTA4 into LTC4, and LTA4 hydrolase converts LTA4 into LTB4. PLA2 activity was significantly reduced by 28-homobrassinolide, and arachidonate 5-lipoxygenase (5-LO) is a well-known cofactor and plays a vital role in signal transduction. PLA2 releases arachidonic acid, and arachidonic acid is oxidized into LTA4 through the activation of 5-LO (Denis et al. 1991). LTC4 synthase and 5-lipoxygenase activating protein (FLAP) are located in perinuclear space. Furthermore, Ca2+, 5-LO, and PLA2 were also translocated into perinuclear space (Schienvella et al. 1995; Penrose et al. 1995). The synthesis of LTB4 and LTC4 were reduced following treatment of 28-homobrassinolide. However, the biochemical and molecular mechanism of 28-homobrassinolide on leukotriene synthesis is not clear.
LTA 4 hydrolase and LTC4 synthase were not altered following 28-homobrassinolide treatment, whereas 5-LO level was significantly reduced. Biochemical and therapeutic potential of 28-homobrassinolide on PLA2 and 5-LO is not clear. Ca2+ is an essential co-factor for the activation of 5-LO and PLA (Rouzer et al. 1990). It is assumed that IgE-receptor based cell activation is initiated via inhibition of intracellular Ca2+ following the treatment of 28-homobrassinolide. Cytosolic enzyme translocation into membrane was reduced through the inhibitory effect of 5-LO inhibitors (Koshihara et al. 1984). Oxidation of AA at the C-5 position has been inhibited by 5-LO inhibitors (Hamasaki and Tai 1985). Lipoxygenase has been inhibited by the glycyrrhetinic acid of Glycyrrhizin radix (Inoue et al. 1988). Honokiol of magnoliae cortex inhibits 5-LO, whereas no effect on LTA4 hydrolase, LTC4 synthase, and PLA2 (Hamasaki et al. 1996).
Conclusion
In summary, it is suggested that the 28-homobrassinolide could be a potential therapeutic agent for relaxing airway smooth muscle, and it may be considered as a potential therapeutic agent for asthma and bronchial constriction.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Borgeat P, Samuelsson B. Leukotriene A, isolation from human polymorphonuclear leukocytes. J Biol Chem. 1980;225:11828–11831. [PubMed] [Google Scholar]
- Channon JY, Lesile CC. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with the membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- Denis D, Falgueyret JP, Riendeau D, Abramovitz M. Characterization of the activity of purified recombinant human 5-lipoxygenase in the absence and presence of leukocyte factors. J Biol Chem. 1991;266:5072–5079. [PubMed] [Google Scholar]
- Hamasaki Y, Tai HH. Gossypol, a potent inhibitor of arachidonate 5- and 12-lipoxygenases. Biochim Biophys Acta. 1985;834:37–41. doi: 10.1016/0005-2760(85)90173-0. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y, Abe M, Matsumoto S, Ichimaru T, Hara N, Miyazaki S. Specific induction of LTC4 synthesis by retinoic acid in rat basophilic leukemia-1 cells. Int Arch Allergy Appl Immunol. 1994;103:260–265. doi: 10.1159/000236637. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y, Muro E, Miyanji S, Yamamoto S, Kobayashi I, Sato R, Zaitu M, Matsuo M, Ichimaru T, Tasaki H, Miyazaki S. Inhibition of leukotriene synthesis by honokiol in rat basophilic leukemia cells. Int Arch Allergy Appl Immunol. 1996;110:278–281. doi: 10.1159/000237299. [DOI] [PubMed] [Google Scholar]
- Hojo M, Fujita I, Hamasaki Y, Miyazaki M, Miyazaki S. Erythromycin does not directly affect neutrophil functions. Chest. 1994;105:520–523. doi: 10.1378/chest.105.2.520. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaldas RS, Weisinger AS. Acute and subacute effects of naturally occurring estrogens on luteinizing hormone secretion in the ovariectomized rat. Reprod Toxicol. 1991;5:127–132. doi: 10.1016/0890-6238(91)90040-M. [DOI] [PubMed] [Google Scholar]
- Inoue H, Mori T, Shibata S, Koshihara Y. Inhibitory effect of glycyrrhetinic acid derivatives on arachidonic acid-induced mouse ear oedema. J Pharm Pharmacol. 1988;40:272–277. doi: 10.1111/j.2042-7158.1988.tb05242.x. [DOI] [PubMed] [Google Scholar]
- Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Caffeic acid is a selective inhibitor of leukotriene biosynthesis. Biochem Biophys Acta. 1984;792:92–97. doi: 10.1016/0005-2760(84)90287-X. [DOI] [PubMed] [Google Scholar]
- Kumar M, Agnihotri RK, Vamil R, Sharma R. Effect of phytohormones on seed germination and seedling growth of Coriandrum sativum L. Pak J Biol Sci. 2014;17:594–596. doi: 10.3923/pjbs.2014.594.596. [DOI] [PubMed] [Google Scholar]
- Li XJ, Yang MF, Chen H, Qu LQ, Chen F, Shen SH. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochim Biophys Acta. 2010;1804:929–940. doi: 10.1016/j.bbapap.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Marom Z, Shelhamer JH, Back MK, Morton DR, Kaliner M. Slow-reacting substance, leukotriene C4 and D4, increase the release of mucus from human airways in vitro. Am Rev Respir Dis. 1982;126:449–451. doi: 10.1164/arrd.1982.126.3.449. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hamasaki Y, lchimaru T, Miyazaki S. IL-3 and IL-5 enhance the production of LTB4 stimulated by calcium ionophore in rat basophilic leukemia cells. Prostaglandins Leukot Essent Fatty Acids. 1995;52:417–422. doi: 10.1016/0952-3278(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Srikumar K. A comparative study on the effect of homobrassinolide and gibberellic acid on lipid peroxidation and antioxidant status in normal and diabetic rats. J Enzyme Inhib Med Chem. 2009;24:1122–1127. doi: 10.1080/14756360802667563. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Srikumar K. Induction of hexokinase I expression in normal and diabetic rats by a brassinosteroid isoform. Eur J Pharm Sci. 2010;41:1–9. doi: 10.1016/j.ejps.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Ravikumar S, Srikumar K. Enhanced expression of hexokinase I mRNA in male rat tissues by homobrassinolide. Prep Biochem Biotechnol. 2010;40:256–262. doi: 10.1080/10826068.2010.488980. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Ravikumar S, Muthuviveganandavel V, Dongpil K. Effect of cortisol on calpains in the C2C12 and 3T3-L1 cells. Appl Biochem Biotechnol. 2014;172:3153–3162. doi: 10.1007/s12010-014-0753-1. [DOI] [PubMed] [Google Scholar]
- Muthuraman P, Enkhtaivan G, Kim DH. Cytotoxic effects of aspartame on human cervical carcinoma cells. Toxicol Res. 2016;5:45–52. doi: 10.1039/C5TX00269A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajyothi PC, Pandurangan M, Sreekanth TV, Shim J. In vitro anticancer potential of BaCO3 nanoparticles synthesized via the green route. J Photochem Photobiol B. 2016;156:29–34. doi: 10.1016/j.jphotobiol.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Fujimiya H, Miyata H, Nozawa Y. Antigen-induced biphasic diacylglycerol formation in RBL-2H3 cells: the late sustained phase due to phosphatidylcholine hydrolysis is dependent on protein kinase C. Biochem Biophs Res Commun. 1991;177:336–342. doi: 10.1016/0006-291X(91)91988-O. [DOI] [PubMed] [Google Scholar]
- Penrose JF, Spoctor J, Lain BK, Friend DS, Xu K, Jack RM, Austen KF. Purification of human lung leukotriene C4 synthase and preparation of polyclonal antibody. Am J Respir Crit Care Med. 1995;152:283–289. doi: 10.1164/ajrccm.152.1.7599836. [DOI] [PubMed] [Google Scholar]
- Phua DH, Zosel A, Heard K. Dietary supplements and herbal medicine toxicities—when to anticipate them and how to manage them. Int J Emerg Med. 2009;2:69–76. doi: 10.1007/s12245-009-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, Ford-Hutchinson AW, Morton HE, Gillard JW. MK-886, a potent specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- Schienvella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30479–30754. doi: 10.1074/jbc.270.51.30479. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: Is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–16. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- Srivastava LM. Plant growth and development: hormones and environment. Amsterdam: Academic Press; 2002. p. 140. [Google Scholar]
- Ukena D, Fishman L, Niebling W-B. Bronchial asthma: diagnosis and long-term treatment in adults. Dtsch Arztebl Int. 2008;105:385–394. doi: 10.3238/arztebl.2008.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vob U, Bishopp A, Farcot E, Bennett MJ. Modelling hormonal response and development. Trends Plant Sci. 2014;19:311–319. doi: 10.1016/j.tplants.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfi IA, Bashir AK, Abdalla AA. Some pharmacological studies on Teucrium mascatense. Effect on glucose homeostasis in normal and streptozotocin diabetic rats and antimicrobial activity. Arab Gulf J Sci Res. 1992;10:145–157. [Google Scholar]