Abstract
The success of stem cell application in regenerative medicine, usually require a stable source of stem or progenitor cells. Fat tissue represents a good source of stem cells because it is rich in stem cells and there are fewer ethical issues related to the use of such stem cells, unlike embryonic stem cells. Therefore, there has been increased interest in adipose-derived stem cells (ADSCs) for tissue engineering applications. Here, we aim to provide an easy processing method for isolating adult stem cells from human adipose tissue harvested from the subcutaneous fat of the abdominal wall during gynecologic surgery. We used a homogenizer to mince fat and compared the results with those obtained from the traditional cut method involving a sterile scalpel and forceps. Our results showed that our method provides another stable and quality source of stem cells that could be used in cases with a large quantity of fat. Furthermore, we found that pregnancy adipose-derived stem cells (P-ADSCs) could be maintained in vitro for extended periods with a stable population doubling and low senescence levels. P-ADSCs could also differentiate in vitro into adipogenic, osteogenic, chondrogenic, and insulin-producing cells in the presence of lineage-specific induction factors. In conclusion, like human lipoaspirates, adipose tissues obtained from pregnant women contain multipotent cells with better proliferation and showed great promise for use in both stem cell banking studies as well as in stem cell therapy.
Keywords: Adipose-derived stem cells, Homogenizer, Cell therapy, Regenerative medicine
Introduction
Gestational diabetes mellitus (GDM) is a condition commonly encountered during mid to late pregnancy with pathologic manifestations including hyperglycemia, hyperinsulinemia, insulin resistance, fatty liver, and fetal development (Friedman 2015). The deficit and dysfunction of insulin secreting β-cells are signature symptoms for GDM. Stem cells offer promise to those with diabetes, including GDM.
Mesenchymal stem cells have been widely used in experimental and clinical research because of their unique biological characteristics and advantages (Dave et al. 2013, 2014). In a previous study, we have developed a method to rescue liver fibrosis by several kinds of mesenchymal stem cells (Chang et al. 2009; Lin et al. 2010; Harn et al. 2012). Adipose-derived stem cells (ADSC) have a similar endodermal hepatogenic potential to bone marrow derived stem cells (BMSC) (Talens-Visconti et al. 2006; Ruiz et al. 2010; Lee et al. 2012); moreover, pancreatic progenitors derived from ADSC were shown to be able to effectively treat diabetes in mice recently (Ji et al. 2015). The approach was successfully performed in the studied patients, and the first-in-human clinical trial revealed that ADSC therapy was safe and did not affect the pregnancy or embryonic development (Sanz-Baro et al. 2015). However, a longer culture period and higher proliferation capacity may be an ideal source of large amounts of autologous stem cells (Schaffler and Buchler 2007).
Characteristics distinct to ADSC include long-term viability, multipotential differentiation, inhibition of apoptosis, immune privilege, and suppression of inflammation (Pachon-Pena et al. 2011; Technau et al. 2011; Luo et al. 2012). Recent reports have shown that the advantages of adipose tissue-derived stromal cells are better than for BMSC, including lower harvest site morbidity (Neupane et al. 2008; Vieira et al. 2010), less affected by donor age, easier cell isolation, and greater stromal cell density (Sen et al. 2001; Schaffler and Buchler 2007). The biggest challenges of regenerative medicinal applications are the creation of stromal cell banks with characterized cell stores and GMP approved (Gimble et al. 2007; Ra et al. 2011). To ensure successful cell therapies, the consistency of stem cell performance across donors is critical. However, the effect of the donor’s reproductive status on ADSC proliferation rates and differentiation capacity is limited (Ng et al. 2009).
The adipose tissue from abdominal surgery or liposuction is usually rich in stem cells that can meet the needs of regenerative medicine (Ng et al. 2009; Kuhbier et al. 2010). Meanwhile, these stem cells have a high ability for proliferation and multi-lineage differentiation. Therefore, ADSC is becoming a potential source for stem cell banks and an ideal source of seeding cells for tissue engineering. Although some groups have successfully isolated ADSCs from adipose tissues, there is still not a widely-accepted efficient method for mincing lump fat (Kuhbier et al. 2010). In order to successfully apply ADSCs in clinical therapies, the consistency of their performance across different donors must be ensured. The goal of current research is to provide the efficiency method to isolate and identify pregnancy adipose-derived stem cells (P-ADSCs) from the fat tissue of pregnant women by using a commercial homogenizer, ULTRA-TURRAX® Tube Drive.
Materials and methods
Source of the fats
Human adipose tissue was obtained at caesarian section from the abdominal subcutaneous tissue of obese women who had delivered in the maternity department at Min-Sheng Hospital (age range: 22–39 years; mean = 33 years old). Institutional Review Board approval has been obtained with the fund approval no. MSIRB099015. The subjects were healthy without any regular medication. Informed consent was obtained from the subjects before the surgical procedure. The study protocol was approved by the Ethic Committee of Min-Sheng Hospital. After being removed, ~10 g adipose tissue sample was relocated in a sterilized bottle filled with 0.1 M phosphate-buffered saline (PBS) at 4 °C within 24 h prior to use.
Isolation of P-ADSCs and cell culture
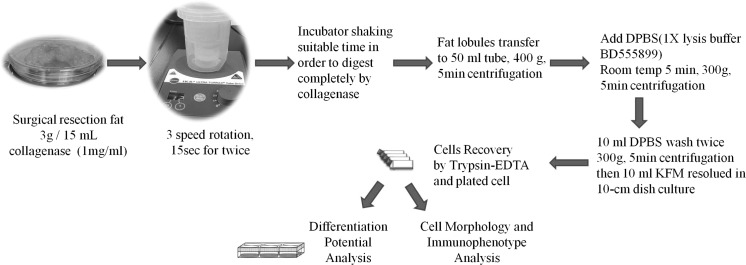
The procedure followed the description by Zuk et al. (2001) with some modifications. Human adipose tissue was harvested from the subcutaneous fat (1 cm3) of the abdominal wall during gynecologic surgery. Tissue samples were placed in Ca2+/Mg2+-free phosphate-buffered saline (PBS) and immediately transferred to the laboratory. Human adipose tissue was removed from the transport medium, placed in a Petri dish, and cut into small pieces (1–2 mm3) in the presence of Ca2+/Mg2+-free PBS. The tissues were dissociated with 0.1 mg collagenase Ia (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 60 min at 37 °C. After enzymatic digestion, the resultant cells were collected and cultured in knockout serum-free medium (Invitrogen-Gibco, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum (FBS; HyClone, Logan, UT, USA). The supernatant and debris were removed from the culture dish on day 2 of culture. The resultant P-ADSCs culture was denoted as passage 0. To prevent spontaneous differentiation, cultures were maintained at sub-confluent levels (<80% confluency). Passages of P-ADSCs cultures was performed using 2.5% trypsin in 0.23 mM ethylenediaminetetraacetic acid. Passaged cultures were deemed passage 1. The adipose tissue sample was extensively washed with sterile PBS containing 1000 U/mL penicillin and 1000 μg/mL streptomycin to remove contaminating blood cells. The 3 g specimen was homogenized by an Ultra-Turrax® tube drive systems (IKA, Staufen, Germany) or cut into 1 mm3 pieces by a knife. Then the extracellular matrix was digested with 15 mL 0.1% collagenase (Invitrogen) at 37 °C, and shaken vigorously for an appropriate time to separate the stromal cells from primary adipocytes. The collagenase activity was then neutralized by adding an equal volume of low glucose-Dulbecco’s modified Eagle’s medium (l-DMEM, Hyclone) containing 10% fetal bovine serum (FBS, Invitrogen). Dissociated tissue was filtered to remove debris and centrifuged at 1500 rpm for 10 min. The suspending portion containing lipid droplets was discarded and the cell pellet was re-suspended and washed twice. Contaminating erythrocytes were lysed with an osmotic buffer and the remaining cells were plated onto 10 cm dishes (BD-Falcon, San Jose, CA, USA) at day 5 of incubation. Figure 1 shows the flowchart. Plating and expansion medium consisted of modified MCDB 153 medium (Keratinocyte-SFM, GIBCO–Invitrogen Corporation) supplemented with N-acetyl-l-cysteine (NAC; Sigma A8199) (2 mM) and l-ascorbic acid 2-phosphate (Asc 2P; Sigma A8960) (0.2 mM). This is referred to as K-NAC medium (Lin et al. 2005). Cultures were maintained at 37 °C with 5% CO2. The medium was replaced after 5 days; and then every 3 days. Once the adherent cells were more than 80% confluent, they were detached with 0.25% trypsin–0.02% EDTA and re-plated at a dilution of 1:3.
Fig. 1.
Process flowchart for isolation of P-ADSCs using an homogenizer
Surface phenotype characterization
The 2 × 105 cells of P-ADSCs were incubated with primary antibodies against human CD34, CD44, CD45, CD73, CD90, CD117, and HLA-DR (BD Biosciences). All antibodies were diluted 1:100 and incubated with cells for 30 min at room temperature. We used same-species, same-isotype irrelevant antibody as negative controls. After two washing steps, cells were resuspended in 300 μl PBS for flow cytometric analysis and analyzed by fluorescein-activated cell sorting (FACS) Calibur (BD Biosciences).
Analysis of cell growth curve and kinetics
We analyzed the proliferative capacity of P-ADSCs from different passages with a hemocytometer. The cells were seeded onto 6-well culture plates with 5 × 104 cells per well and counted daily by trypan blue exclusion for one week and cell growth curves were recorded. The cell doubling numbers (CD) and cell population doubling time (DT) of P-ADSCs were calculated with the following formula (Spencer et al. 2012).
where CT = culture time, N f = final cell number, and N i = initial seeding density.
The results of the study were expressed as mean and standard error of the mean. The comparison of adipose-derived stem cells growth curve of the knife cut mince process versus the homogenizer mice process were using the two-sided Student’s t test software.
Karyotype analysis
Karyotype analysis was conducted by using standard protocols from the chromosomal Giemsa (G)-banding reference from Lorraine Faxon Meisner and Julie A. Johnson group published method (Meisner and Johnson 2008).
Adipogenic differentiation
P-ADSCs were seeded at a density of 5000 cells/cm2 to induce adipogenic differentiation. They were cultured in adipogenic medium for 2 weeks. The medium consisted of high-glucose DMEM supplemented with 0.5 mM 3-isobutyl-1-methylxan-thine (IBMX, Sigma), 10 mg/mL insulin (Sigma), 1 mM dexamethasone, 0.1 mM indomethacin (Sigma), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA), and 10% FBS. Medium changes were carried out twice a week and adipogenesis was assessed by Oil Red O staining solution to show lipid droplets in induced cells.
Osteogenic differentiation
The P-ADSCs were induced for 2 weeks in osteogenic medium containing high-glucose DMEM, 10% FBS, 0.1 μM dexamethasone, 200 μM ascorbic acid, 10 mM β-glycerol phosphate, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA). After induction, osteoblasts were confirmed by cytochemical staining with alkaline phosphatase (ALP) to detect the alkaline phosphatase activity. The ALP activity of cells was evaluated by an alkaline phosphatase histochemistry kit (Sigma-Aldrich). The reaction was performed for 60 min at 25 °C as recommended by the manufacturer. During incubation, culture dishes were protected from drying and light. Dishes were rinsed with deionized water and air-dried prior to viewing.
Chondrogenic differentiation
The 1 × 106 cells of P-ADSCs were centrifugalize to pellet and then the cell mass induced for 3 weeks in chondrogenic medium containing high-glucose DMEM, 10% FBS, TGF-β1 (Sigma T1654) 10 ng/mL, l-ascorbate-2-phosphate (Sigma A8960) 50 μM, insulin (Sigma I1882) 6.25 μg/mL, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA). Then the cells cultured in chondrogenic differentiation medium for 21 days with medium changes every 4 days. Pellets were fixed in 4% paraformaldehyde for 15 min, then stained with alcian blue for sulfated proteoglycan-rich matrix.
Differentiation of P-ADSCs into insulin-producing cells
P-ADSCs were differentiated into insulin-secreting cells using the methods described previously (Zhang et al. 2011; Dave et al. 2014; Ouyang et al. 2014) with some modifications. The first step of the method was seeding P-ADSCs into a 100 mm dish (1 x106cells/dish) containing 2% FBS/DMEM (high glucose) supplemented with 1% non-essential amino acids (NEAA) and 0.5 mM β-mercaptoethanol (Sigma) for 2 days. In the second step, the cells were cultured for 7 days in 2% FBS/DMEM (high glucose) supplemented with 200 ng/mL activin A (Prospec, Rehovot, Israel), 10 mM nicotinamide (Sigma-Aldrich, St. Louis, MO, USA), 1 mM β-mercaptoethanol, 10 ng/mL basic fibroblast growth factor (bFGF, R&D Systems, Minneapolis, MN, USA), 10 ng/mL epidermal growth factor (EGF, R&D Systems, Minneapolis, MN, USA), and 25 mM glucose for 7 days. In the last step, the cells were incubated in 5% FBS/DMEM supplemented with 200 ng/mL activin A, 10 mM nicotinamide, and 10 nM exendin 4 (Sigma-Aldrich, St. Louis, MO, USA) for 7 days. Fresh medium was supplied every 2 days during step 3. Cell morphology was observed using a phase contrast microscope (Olympus, Center Valley, PA, USA).
Reverse transcription polymerase chain reaction
Total cellular RNA was isolated from the P-ADSCs with an RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. The cDNA synthesis was performed using maxime RT Pre-Mix (iNtRON Biotechnology, Kyungki-Do, Korea). Primers were included for the GADPH gene, which served as the internal standard. Semiquantitative PCR parameters included 35 amplification cycles. PCR products were then separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide (Invitrogen) staining. The primer pairs used in this study are listed below:
Pdx1 (262 bp) (F): 5′-CCC ATG GAT GAA GTC TAC C-3′, (R): 5′-AAG GGC TTT ATT CCA TCT CTC TCG-3′; Insulin (263 bp) (F): 5′-AAC CAA CAC CTG TGC GGC TC-3′, (R): 5′-AAG GGC TTT ATT CCA TCT CTC TCG-3′; NeuroD (439 bp) (F): 5′-TGG TCT CCT TCG TTC AGA CGC TTT-3′, (R): 5′-AGG CTT AAC GTG GAA GAC ATG GGA-3′; α-fetoprotein (675 bp) (F): 5′-AGA ACC TGT CAC AAG CTG TG-3′, (R): 5′-GAC AGC AAG CTG AGG ATG TC-3′; GADPH (965 bp) (F): 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′, (R): 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′.
Results
Homogenizer process for fat tissue separation
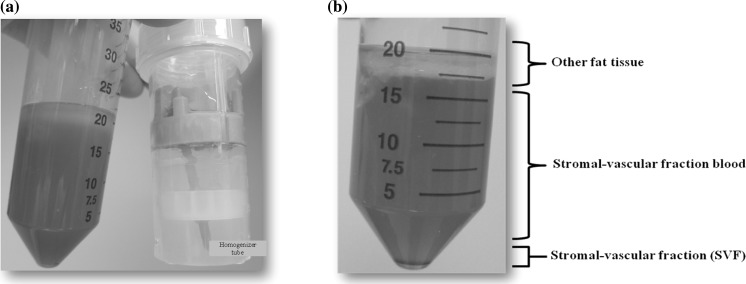
After the homogenization and the centrifugation process, the dissected fat tissues were separated into three clear layers in the tube (Fig. 1). Then we packed the fat into the Ultra-Turrax tube and added the collagenase solution and turned on the rotator twice for 15 s. After that we transferred to new 50 mL centrifuge tube and incubator in appropriated time via collagenase digestion. It was found that the fat lobules were digested completely and also separated into several layers (Fig. 2a). Finally, the three clear parts, including other fat tissues, stromal-vesicular fractioned blood, and stromal-vesicular fraction were harvested and collected through a 5 min centrifugation at 400 xg (Fig. 2b).
Fig. 2.
The fat after Ultra-Turrax® homogenizer mincing process. a The results of fat after homogenizer and collagenase processing. b The fat lobules separated into three main levels by centrifugation
Growth curves of the adipose-derived stem cells from knife cutting or homogenizer mincing
In order to compare both methods, we weighted the fat tissue (3 g) and put them into the tube with 15 mL collagenase (1 mg/mL) solution. Next, we used knife cutting or Ultra-Turrax homogenizer mincing to isolate the P-ADSCs. These P-ADSCs were passaged 1 to 4 every 3 days. Among the cells in different passages, we used trypsin–EDTA to detach the cells from the third passage and seeded them in a 6-well plate (P3 P-ADSC). Generally, we counted the cells at day 0, 3, 5, and 6 by hemocytometer. Findings suggested that the ultraturrax processed P-ADSCs presented highly active proliferation than the samples harvested from knife cutting (Fig. 3a). The images of the knife processed adipose tissue-derived stromal cells from the pregnancy P3 (upper panels) and ultra-turrax dealt P3 (lower images) showed the P3 pADSCs displayed a homogeneous phenotype of spindle-shaped morphology (Fig. 3b).
Fig. 3.
Comparison of adipose-derived stem cells growth curve of the knife cut mince process versus the homogenizer mince process. a SVF derived viable cells; b cell morphology captured daily. Scale bar 200 μm for micrograph
High yield of P-ADSCs with normal morphological characteristics via mincing method
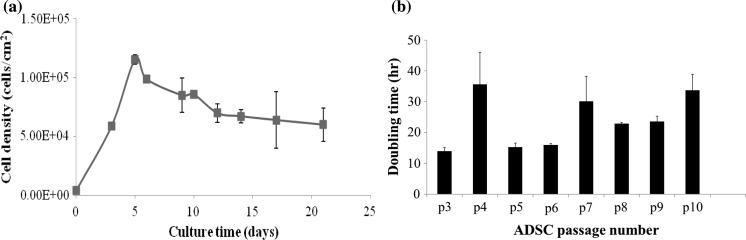
The stromal vascular fraction (SVF) was isolated from human adipose tissue by a knife or a homogenizer combined collagenase Ia digestion. We found that 1 g of adipose tissue could give yield up to 5.98 × 104 viable cells via the Ultra-Turrax homogenizer (n = 3) and 1.98 × 104 viable cells via knife homogenization (n = 3). Then these 5.98 × 104 cells in total after culture in vitro will achieve to 1.2 × 105 hADSCs/cm2 at day 5. They were passaged every 3–4 days for a maximum of 10 passages without major morphological alteration. The primary and passaged cells displayed typical fibroblast-like morphological features with fusiform shape and a normal growth curve (Fig. 5a). The following comprehensive identification and characterization illustrated pronounced features of mesenchymal stem cells (MSCs). The fibroblast-like P-ADSCs exhibited typical ultrastructure details for vigorous cell activities. We compared the proliferation of P-ADSCs derived from knife mince method and the ultra-turrax homogenizer processing by culturing these cells on a 6-well plate in vitro. A heterogenous population of primary adipose cells formed distinct colonies after 3 days and reached confluency of 80–90% by knife mince method versus confluency of 90–100% by ultra-turrax homogenizer process after 5–6 days.
Fig. 5.
Variation of the P-ADSCs growth curve and multiple cell passages doubling time derived from the homogenizer mince process. a Normal P-ADSCs growth curve. b Multiple cell passages growth curve
Effect of cell culture process on cell surface markers and multiple passages growth rate
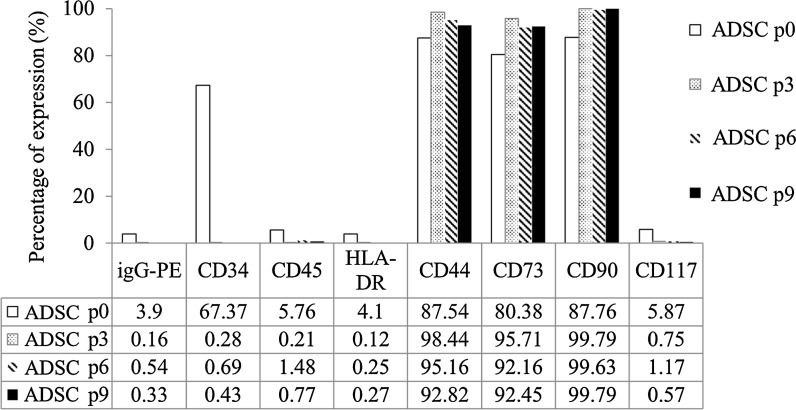
Following passages 0, 3, 6, and 9 cell surface makers of P-ADSCs from Ultra-Turrax homogenization process was analyzed. The results showed no expression of CD45, CD117, and HLA-DR and expression of CD44, CD73, and CD90 (Fig. 4). Interestingly, expression of CD34 in this case at passage 0 was higher but following passage 3 its expression became negative (Fig. 4). Also, we counted the cells following passages 3–10, the data expression cells proliferation doubling time slightly increased by as the number of passage increased (Fig. 5b). Nevertheless, the P-ADSCs obtained by ultra-turrax homogenizer process did not express hematopoietic markers CD45, CD117 and the major histocompatibility complex (MHC) marker HLA-DR. But the mesenchymal stem cell markers CD44, CD73 and CD90 have been determined on the ultra-turrax homogenizer processed P-ADSCs. The results showed that the hematopoietic stem cells marker CD34 expressed in the original P-ADSCs, but the CD34 expression could be decreased after several times cell passages.
Fig. 4.
In vitro characterization of ASCs markers. Variation of expression levels of surface markers of cultured P-ADSCs at passages 0, 3, 6, and 9
Characterization of passaged P-ADSCs maintained with normal karyotype
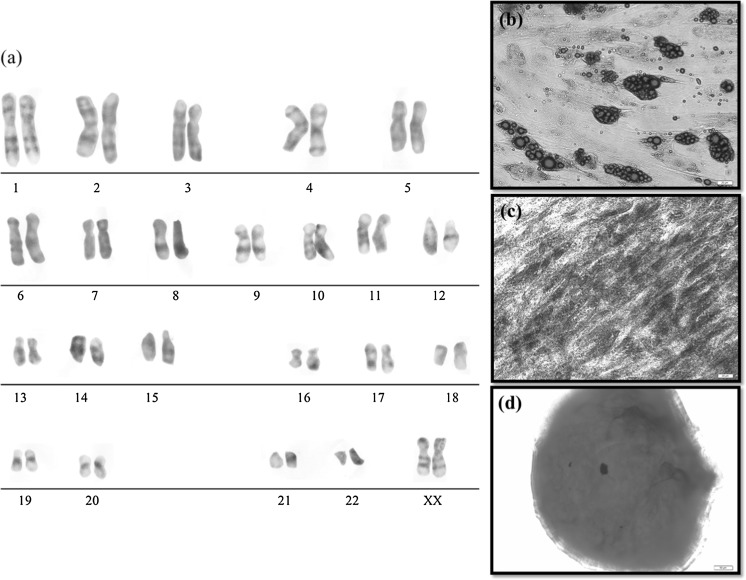
To test whether the obtained P-ADSCs by ultra-turrax homogenized process had any genetic abnormalities, after ten passage karyotype analysis was performed. Karyotype analysis confirms that stem cells generated from the fat of pregnant women maintained the normal euploid karyotype after a final of passes is P10 in vitro (Fig. 6a).
Fig. 6.
P-ADSCs maintained normal karyotype and differentiation potential in plate cultures. a The normal chromosome pattern of P-ADSCs after cultured at passage 10. b Following adipogenic induction, pADSC formed adipocytes with intracellular lipid vacuoles confirmed by Oil Red O staining. c Photomicrographs of ALP activity of P-ADSCs grown under different culture conditions as shown by ALP staining. d Extracellular matrix formation was evident in chondrogenic pellets from P-ADSCs by alcian blue staining. Scale bar 50 μm for micrograph
Characterization of passaged P-ADSCs that presented adipogenesis or osteogenesis
The growth curve revealed a high capability for self-renewal and proliferation. Moreover, these cells could be functionally induced into adipocytes, osteoblasts, and chondrocytes in the presence of an appropriate differentiation media. After 7 days of adipogenic induction lipid droplets confirmed by Oil Red O staining, indicated a high level of adipogenesis potential (Fig. 6b). During the osteogenic period, the activity of ALP-positive cells from P-ADSCs (Fig. 6c), differentiated into osteo-lineage cells. Additionally, the P-ADSCs that were cultured under chondrogenic induction, the extracellular matrix staining with Alcian blue confirmed chondrogenic pellets on day 18 (Fig. 6d).
In vitro differentiation of P-ADSCs into endoderm insulin-producing cells (IPC)
To determine whether P-ADSCs have been differentiated into IPCs, gene expression profiles of pancreatic differentiation markers were assessed by RT-PCR. Differentiation experiment was performed on p-ADSC by three steps (Fig. 7). pADSC significantly showed the increased expression of insulin, Pdx1, Insulin, Neuro D, and α-fetoprotein mRNAs after 16-days induction. After treatment with growth factors or small molecule compounds, cell morphology changed (Fig. 7a) and the cells expressed many characteristic pancreatic endocrine cell marker genes, such as pancreatic and duodenal homeobox-1 (Pdx 1), insulin, endoderm marker α-fetoprotein (Afp), and neurogenic differentiation (NeuroD) marker (Fig. 7b). The results suggest that this protocol could lead to P-ADSCs differentiated to endodermal cells such as IPC.
Fig. 7.
The morphology and RT-PCR analyses of the expression of insulin producing cells-related genes in P-ADSCs during the induced differentiation process. The step 1 decrease the FBS concentration from 10% to 2% and supplemented wtih NEAA and β-mercaptoethanol, then step 2 supplement with activin A, nicotinamide and bFGF, EGF, finally step 3 activin A, nicotinamide, and exendin 4 was supplied. a The changes in cell morphology could be observed from day 2 to day 16 after induction. b Expression analysis of islet markers using RT-PCR in pADSC after β-cell differentiation with indicated protocols. Scale bar 100 μm for micrograph
Discussion
Seeding cells is a key element in tissue engineering. Recent reports have shown that ADSC can be easily harvested from adipose tissue without ethical concern or problems of transplant rejection; and that these cells have high proliferation rates for in vitro expansion with multilineage differentiation capacity (Jiang et al. 2012). Moreover, recently reported fertility and pregnancy outcomes were not affected by ADSC therapy treatment (Sanz-Baro et al. 2015). These favorable characteristics indicate a considerable interest in applying P-ADSCs for GDM therapy in the future. Since Rodbell first isolated preadipocytes from adipose tissue (Rodbell 1966), and many methods have been developed to isolate adipose cells from fat tissue. However, as shown in the review the mincing step for surgical resection in the flowchart of fat harvesting and adipose-derived stem cells isolation process has not been fully developed (Kuhbier et al. 2010). From recently published documents, we found that the gentle MACS Dissociator can also be applied to dissociate tissue efficiently under controlled and reproducible conditions (Jungblut et al. 2008; Messier et al. 2012). Nevertheless, this cell dissociation process via gentle MACS Dissociator might have been used use to homogenize only the small mouse tissues or tumor samples. As far as we know, no reports have been published for the application of pregnancy-derived fat tissue from a surgery. In our experimental results, the viable cells derived from stromal-vascular fraction (SVF) were 74–86% (data not show). The similar result of 70~87% vitality was reported by Faustini group (Faustini et al. 2010). Further, we have established a simple and effective way to obtain high-purity P-ADSCs by using collagenase combined with homogenizer digestion and adherence screening. Isolated P-ADSCs proliferate at a high rate and maintain a multipotent differentiation capacity in vitro for up to 10 passages. Also, we found the surface marker CD34 positive expression in an early passage of P-ADSC and the expression decreased after long-term culture. Similar results were described by other groups (Suga et al. 2009; Lin et al. 2012). Other research groups have found the adipose derived stem cells (ADSC) despite phenotypic and functional similarities to bone marrow MSC, the ADSC cells are distinguished by CD34 expression (Zimmerlin et al. 2012) and may be important to tissue maintenance and remolding (Eto et al. 2012). Besides, the study has demonstrates that human adult adipose tissue were easy accessibility, seems to be the most abundant source of MSCs, that CD34(+)/CD90(+) ADSC are extremely beneficial for regenerative medicine (Ferraro et al. 2012). Since no unique molecular markers for mesenchymal stem cells have been established, we used multiple surface markers for P-ADSCs identification. We therefore selected multiple markers, including a member of the integrin family (such as CD44), mesenchymal markers (such as CD73, CD90), the hematopoietic/leukocytic/endothelial markers (such as CD34, CD45, and CD117), and the major histocompatibility complex (MHC) class II marker (HLA-DR). These data not only excluded endothelial cells or adipose-tissue resident cell contamination, but also suggested that the clinical application of ADSC can bypass MHC restrictions. However, CD34 expression in ADSC decreases over time in culture and the implications remain unclear. Therefore, developing high efficient methods to isolate and identify ADSC is valuable and useful for regenerative medicine applications. The data presented here suggest that we have developed a highly efficient isolation and cultivation method with a processing strategy for fat mincing, identification, and characterization of P-ADSCs. These techniques will be able to provide safe and stable seed cells for research and cell banking applications.
Conclusions
Effective in vitro surgical resection fat mincing was developed in this study and could be avoided with uncertain factors for scaling-up the process or stem cell banking applications. The cells recovered maintained the potentials for CD marker expression and multiple differentiated ability. The successful easy fat mincing by Ultra-Turrax® homogenizer to provide the possibility of a larger scale for GDM patients application in the future.
Acknowledgements
This study was supported by a grant from the Guang Li Biomedicine and the Ching-Kuo Campus of Min-Sheng Hospital. The authors gratefully acknowledge Karthyayani Rajamani, Ting-Yu Lin and Tsung-Yen Ho for their technical contributions.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
References
- Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH, Chen TM, Lee RP, Lin SZ, Harn HJ, Chiou TW. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009;85:517–525. doi: 10.1016/j.lfs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Dave SD, Vanikar A, Trivedi HL, Gumber MR, Patel HV, Shah PR, Kute VB. Stem cells versus donor specific transfusions for tolerance induction in living donor renal transplantation: a single-center experience. Transplantation. 2013;95:155–160. doi: 10.1097/TP.0b013e3182752bcc. [DOI] [PubMed] [Google Scholar]
- Dave SD, Vanikar AV, Trivedi HL. In-vitro generation of human adipose tissue derived insulin secreting cells: up-regulation of Pax-6, Ipf-1 and Isl-1. Cytotechnology. 2014;66:299–307. doi: 10.1007/s10616-013-9573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura KP. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev. 2012;22:985–997. doi: 10.1089/scd.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustini M, Bucco M, Chlapanidas T, Lucconi G, Marazzi M, Tosca MC, Gaetani P, Klinger M, Villani S, Ferretti VV, Vigo D, Torre ML. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng C Methods. 2010;16:1515–1521. doi: 10.1089/ten.tec.2010.0214. [DOI] [PubMed] [Google Scholar]
- Ferraro GA, De Francesco F, Nicoletti G, Paino F, Desiderio V, Tirino V, D’Andrea F. Human adipose CD34(+) CD90(+) stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J Cell Biochem. 2012;114:1039–1049. doi: 10.1002/jcb.24443. [DOI] [PubMed] [Google Scholar]
- Friedman JE. Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man—where do we go next? The 2014 Norbert Freinkel Award Lecture. Diabetes Care. 2015;38:1402–1411. doi: 10.2337/dc15-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS, Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC, Chiou TW. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21:2753–2764. doi: 10.3727/096368912X652959. [DOI] [PubMed] [Google Scholar]
- Ji AT, Chang YC, Fu YJ, Lee OK, Ho JH. Niche-dependent regulations of metabolic balance in high-fat diet-induced diabetic mice by mesenchymal stromal cells. Diabetes. 2015;64:926–936. doi: 10.2337/db14-1042. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu T, Song K. Growth characteristics of human adipose-derived stem cells during long time culture regulated by cyclin A and cyclin D1. Appl Biochem Biotechnol. 2012;168:2230–2244. doi: 10.1007/s12010-012-9932-0. [DOI] [PubMed] [Google Scholar]
- Jungblut M, Oeltze K, Zehnter I, Hasselmann D, Bosio A. Preparation of single-cell suspensions from mouse spleen with the gentle MACS dissociator. J Vis Exp. 2008;pii:1029. doi: 10.3791/1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhbier JW, Weyand B, Radtke C, Vogt PM, Kasper C, Reimers K. Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol. 2010;123:55–105. doi: 10.1007/10_2009_24. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jung J, Cho KJ, Lee CK, Hwang SG, Kim GJ. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differ Res Biol Divers. 2012;84:223–231. doi: 10.1016/j.diff.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;14:92–102. doi: 10.1089/scd.2005.14.92. [DOI] [PubMed] [Google Scholar]
- Lin SZ, Chang YJ, Liu JW, Chang LF, Sun LY, Li YS, Luo GH, Liao CH, Chen PH, Chen TM, Lee RP, Yang KL, Harn HJ, Chiou TW. Transplantation of human Wharton’s Jelly-derived stem cells alleviates chemically induced liver fibrosis in rats. Cell Transplant. 2010;19:1451–1463. doi: 10.3727/096368910X514198. [DOI] [PubMed] [Google Scholar]
- Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14:1159–1163. doi: 10.3109/14653249.2012.729817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Zhang Y, Zhang Z, Jin Y. The protection of MSCs from apoptosis in nerve regeneration by TGFbeta1 through reducing inflammation and promoting VEGF-dependent angiogenesis. Biomaterials. 2012;33:4277–4287. doi: 10.1016/j.biomaterials.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Meisner LF, Johnson JA. Protocols for cytogenetic studies of human embryonic stem cells. Methods (San Diego, CA) 2008;45:133–141. doi: 10.1016/j.ymeth.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res. 2012;38:363–373. doi: 10.3109/01902148.2012.713077. [DOI] [PubMed] [Google Scholar]
- Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- Ng LW, Yip SK, Wong HK, Yam GH, Liu YM, Lui WT, Wang CC, Choy KW. Adipose-derived stem cells from pregnant women show higher proliferation rate unrelated to estrogen. Hum Reprod (Oxf) 2009;24:1164–1170. doi: 10.1093/humrep/dep001. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Huang W, Yu W, Xiong W, Mula RV, Zou H, Yu Y. Generation of insulin-producing cells from rat mesenchymal stem cells using an aminopyrrole derivative XW4.4. Chemico-Biol Interact. 2014;208:1–7. doi: 10.1016/j.cbi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Pachon-Pena G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, Gimble JM. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The metabolism of isolated fat cells. IV. Regulation of release of protein by lipolytic hormones and insulin. J Biol Chem. 1966;241:3909–3917. [PubMed] [Google Scholar]
- Ruiz JC, Ludlow JW, Sherwood S, Yu G, Wu X, Gimble JM. Differentiated human adipose-derived stem cells exhibit hepatogenic capability in vitro and in vivo. J Cell Physiol. 2010;225:429–436. doi: 10.1002/jcp.22216. [DOI] [PubMed] [Google Scholar]
- Sanz-Baro R, Garcia-Arranz M, Guadalajara H, de la Quintana P, Herreros MD, Garcia-Olmo D. First-in-human case study: pregnancy in women with Crohn’s perianal fistula treated with adipose-derived stem cells: a safety study. Stem Cells Transl Med. 2015;4:598–602. doi: 10.5966/sctm.2014-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Sen A, Lea-Currie YR, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YD, Gimble JM. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J Cell Biochem. 2001;81:312–319. doi: 10.1002/1097-4644(20010501)81:2<312::AID-JCB1046>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Spencer ND, Chun R, Vidal MA, Gimble JM, Lopez MJ. In vitro expansion and differentiation of fresh and revitalized adult canine bone marrow-derived and adipose tissue-derived stromal cells. Vet J. 2012;191:231–239. doi: 10.1016/j.tvjl.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- Talens-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gomez-Lechon MJ. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J Gastroenterol. 2006;12:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- Zhang S, Dai H, Wan N, Moore Y, Dai Z. Promoting long-term survival of insulin-producing cell grafts that differentiate from adipose tissue-derived stem cells to cure type 1 diabetes. PLoS ONE. 2011;6:e29706. doi: 10.1371/journal.pone.0029706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2012;83:134–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]