ABSTRACT
The BCL11B gene encodes a Krüppel-like, sequence-specific zinc finger (ZF) transcription factor that acts as either a repressor or an activator, depending on its posttranslational modifications. The importance of BCL11B in numerous biological processes in multiple organs has been well established in mouse knockout models. The phenotype of the first de novo monoallelic germ line missense mutation in the BCL11B gene (encoding N441K) strongly implies that the mutant protein acts in a dominant-negative manner by neutralizing the unaffected protein through the formation of a nonfunctional dimer. Using a Förster resonance energy transfer-assisted fluorescence-activated cell sorting (FACS-FRET) assay and affinity purification followed by mass spectrometry (AP-MS), we show that the N-terminal CCHC zinc finger motif is necessary and sufficient for the formation of the BCL11B dimer. Mutation of the CCHC ZF in BCL11B abolishes its transcription-regulatory activity. In addition, unlike wild-type BCL11B, this mutant is incapable of inducing cell cycle arrest and protecting against DNA damage-driven apoptosis. Our results confirm the BCL11B dimerization hypothesis and prove its importance for BCL11B function. By mapping the relevant regions to the CCHC domain, we describe a previously unidentified mechanism of transcription factor homodimerization.
KEYWORDS: FRET, apoptosis, cell cycle, chromatin remodeling, mass spectrometry, protein-protein interactions, transcription factors, transcriptional regulation
INTRODUCTION
Since its discovery as an interacting partner of chicken ovalbumin upstream promoter transcription factor (COUP-TF) in neurons (1), B-cell lymphoma/leukemia 11B (BCL11B) has been proven to be important for the differentiation of a variety of tissues and organs. Normal development of the central nervous system, T cells, skin, teeth, and mammary glands is severely disturbed in the absence of BCL11B, as shown by multiple studies applying mouse knockout models (2–7). Hence, dysregulated BCL11B expression and genetic aberrations involving the BCL11B gene are associated with a variety of pathologies, ranging from leukemia and cancer to neurodegenerative disorders. Interestingly, BCL11B may play opposing roles in the initiation or maintenance of different diseases. About 10% of T-cell acute lymphoblastic leukemia cases carry deletions or missense mutations within the BCL11B gene (8, 9), suggesting that loss of function contributes to the process of malignant transformation. In contrast, an aggressive subtype of adult T-cell leukemia/lymphoma (ATLL) correlates with abnormally high BCL11B expression resulting from chromosomal insertions in the BCL11B genetic region but also in cases with a normal gene copy number (10). Elevated BCL11B mRNA and protein levels are also associated with progressing cutaneous T-cell lymphomas (CTCLs) (11). Moreover, BCL11B depletion acts synergistically with histone deacetylase (HDAC) inhibitors against a fraction of cutaneous lymphomas with high BCL11B expression (12). Hence, although initially described as a tumor suppressor in an experimental model of T-cell leukemia (13), BCL11B may also display oncogenic potential, even in the same type of tissue.
The BCL11B gene encodes a Krüppel-like transcription factor equipped with six CCHH zinc finger (ZF) domains. Apart from its bona fide transcription repressor properties (14), the protein interacts in its target promoter regions with a variety of proteins or protein complexes (15–17), the recruitment of which depends on posttranslational modifications. As a consequence, BCL11B acts as a transcriptional repressor that, upon triggering of signaling-cascade-like T-cell receptor engagement, converts into a potent activator of gene expression. Two such derepression switches have been characterized to date. In mouse thymocytes, activation of the mitogen-activated protein kinase (MAPK) pathway leads to rapid phosphorylation of the BCL11B protein at a total of 23 serine and threonine residues. Phosphorylated BCL11B becomes a substrate of the SUMO-specific protease SENP and undergoes prompt desumoylation, followed by dephosphorylation and resumoylation. The ultimate outcome of this complex sequence of modifications is an increased affinity for histone acetyltransferase p300 and derepression of target genes, such as those encoding interleukin 2 (IL-2) and Id-2 (18). A similar but independent mechanism has been identified in human CD4+ lymphocytes. Here, upon activation of the protein kinase C (PKC) signaling pathway, BCL11B undergoes rapid and transient phosphorylation at Ser-2, which abolishes its interaction with the NuRD chromatin-remodeling complex and increases p300 binding, resulting in strong transcriptional activation of IL-2 and Id-2 (19).
Another intriguing feature of BCL11B has been revealed recently. A screening study of the genetic background of immunodeficiency identified the first germ line de novo mutation within BCL11B (20). In addition to lacking T cells, the patient carrying the mutation (encoding N441K) suffered from multiple organ defects, including neurological, dermal, and craniofacial abnormalities, and mental retardation. These defects mirror those found previously in mouse knockout models. This mutation was present in only one of the BCL11B alleles yet mimicked the “null” phenotype, strongly suggesting that BCL11B functions only as a dimer and is unable to bind and regulate its target genomic regions in the presence of a defective variant. In support of this hypothesis, the formation of wild-type (wt) BCL11B/N441K heterodimers was confirmed in cells transfected with these proteins. This finding prompted us to investigate which domain(s) within the BCL11B protein is involved in homomultimer formation. Previously, we observed an abnormal (cytoplasmic) localization of C-terminally truncated BCL11B derived from a T cell acute lymphoblastic leukemia (T-ALL) sample that suggested formation of homomultimeric complexes that were unable to cross the nuclear envelope. Therefore, we hypothesized the presence of a dimerizing domain within the N-terminal region of the protein.
This study investigates whether the N-terminal CCHC zinc finger motif is necessary and sufficient for the formation of BCL11B dimers, using fluorescence microscopy and Förster resonance energy transfer-assisted fluorescence-activated cell sorting (FACS-FRET). The role of the CCHC motif in homodimer assembly was further investigated using affinity purification followed by mass spectrometry (AP-MS). Functional studies investigated the role of dimerization in the induction of cell cycle arrest and protection against DNA damage-induced apoptosis.
RESULTS
The dimerization domain is located at the amino terminus of BCL11B.
We previously identified a chromosomal translocation carried by a T-ALL patient that resulted in the expression of a fusion protein consisting of the N terminus of BCL11B and the constant region of T-cell receptor delta (TRDC) (21). Cellular localization studies of BCL11B-negative cells revealed a nonphysiological cytoplasmic location of the fusion, while the wild-type protein was present exclusively in the nucleus. Interestingly, given the size of the enhanced green fluorescent protein (EGFP)-tagged fusion gene, the resulting protein (<60 kDa) should be able to pass through the nuclear envelope and distribute equally throughout the whole cell (22). The cytoplasmic sequestration indicated that the fusion protein either interacted with a cytoplasmic protein or formed a multimeric complex too big to passively cross the nuclear envelope. These findings encouraged us to investigate which part of the N-terminal BCL11B (NTB) might be involved in its aberrant localization.
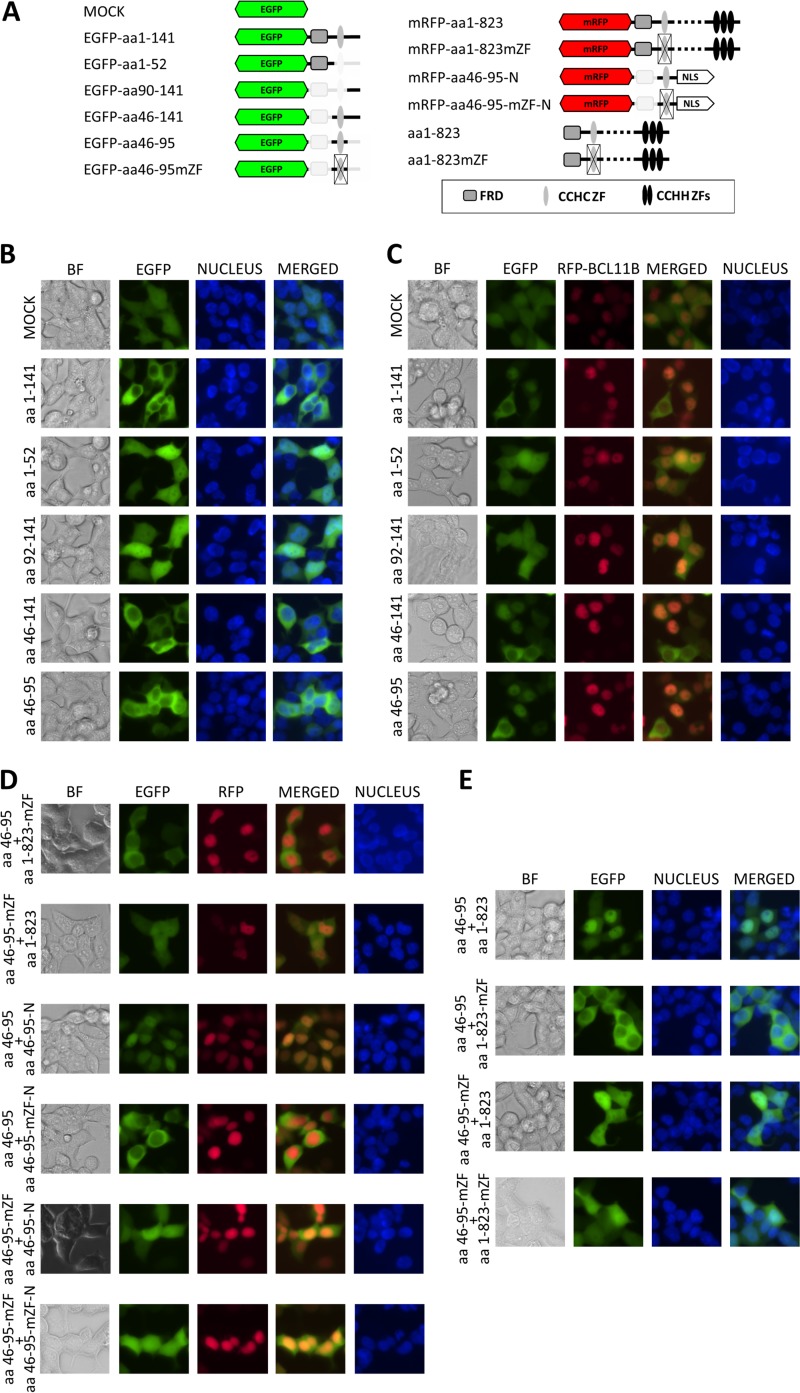
First, we tagged the N-terminal BCL11B peptide (amino acids [aa] 1 to 141) by cloning it downstream of EGFP (Fig. 1A) and transfecting it into the BCL11B-deficient HEK293 cell line (see Fig. S1F in the supplemental material). As shown previously for a BCL11B-TRDC fusion, the fluorescent reporter signal was highly enriched in the cytoplasm (Fig. 1B). Further reduction of the BCL11B sequence to the first 52 amino acids containing the Fog repressor domain (FRD) allowed free transfer of the tagged peptide across the nuclear envelope and even distribution of the fluorescent signal in the cytoplasm and the nucleus. Similarly, the distal region alone, covering amino acids 90 to 141, was unable to prevent EGFP from penetrating the entire cell. In contrast, two other fragments (aa 46 to 141 and 46 to 95), which contained an atypical CCHC zinc finger motif, reproduced the cytoplasmic localization observed for the parental peptide. As depicted in Fig. S1A in the supplemental material, the same BCL11B fragments labeled at their C termini localized in the same manner as their N-tagged analogues.
FIG 1.
The N-terminal part of BCL11B contains a dimerization domain. (A) Schematic drawing of BCL11B-derived EGFP and mRFP fusion peptides. (B) HEK293 cells were transfected with vectors encoding N-terminal fusions of EGFP and the indicated N-terminal fragments of BCL11B. The cells were visualized by fluorescence microscopy 24 h after transfection (original magnification, ×400). (C) The same fusion constructs as in panel B were cotransfected with mRFP-labeled full-length BCL11B. (D) Nonmutated CCHC motifs are required for nuclear uptake. (Top two rows) The CCHC domain fused to EGFP remains in the cytoplasm in the presence of full-length BCL11B containing a mutated CCHC ZF; EGFP fused to a mutated CCHC domain shows a diffuse pattern when cotransfected with wt BCL11B. (Bottom four rows) The mRFP-labeled and nucleus-directed (NLS-containing) CCHC motif transfers the EGFP-labeled CCHC peptides to the nucleus. Mutation of CCHC residues in at least one of the cotransfected fusion proteins abolished the colocalization. (E) The intact EGFP-tagged CCHC motif relocates to the nucleus in the presence of nonlabeled wt BCL11B but remains in the cytoplasm when coexpressed with a CCHC-mutated full-length variant. Impairment of CCHC in the EGFP-labeled construct results in a dispersed fluorescence pattern regardless of the CCHC status of the full-length BCL11B. The CCHC zinc finger structure was disordered by replacement of the zinc-coordinating residues with alanine (CCHC to AAAA). Nuclei were stained with the DNA-interacting stain Hoechst 33342. A representative of five independent experiments is shown. BF, bright-field microscopy.
Despite the lack of canonical nuclear localization sites (NLS), full-length BCL11B localizes in the nucleus, suggesting the presence of an unidentified nuclear signal downstream of the studied region. If the cytoplasmic accumulation of the CCHC-containing fragments resulted from reciprocal interaction between the regions included in these variants, the ectopically expressed full-length BCL11B might compete for binding and draw them into the nucleus. To test this possibility, a red fluorescent protein (RFP)-labeled BCL11B was cotransfected with the EGFP-tagged fragments described above. As expected, the full-length protein migrated to the nucleus (Fig. 1C) but also attracted and colocalized with the EGFP-labeled N-terminal fragments containing the CCHC zinc finger motif (aa 1 to 141, 46 to 141, and 46 to 95). The short fragments preceding or following the ZF structure remained dispersed throughout the cell. Similar effects were observed for N-terminal fragments labeled at the C termini (see Fig. S1C in the supplemental material). Mutation of the CCHC residues in the full-length BCL11B prevented the nuclear transfer of the CCHC-containing N-terminal fragments in cotransfected cells (Fig. 1D). In addition, mutation of CCHC in the EGFP-fused constructs resulted in a diffuse localization pattern in the presence of the nonmutated full-length protein. To exclude the possibility of the involvement of BCL11B regions located beyond the CCHC domain, we replaced the full-length BCL11B with the isolated CCHC motif fused to a heterologous NLS of simian virus 40 (SV40). The construct migrated to the nucleus and attracted the CCHC-containing EGFP fusion proteins, provided the CCHC ZFs were intact in both. The location of the tag had no effect on the outcome, as the same results were obtained with C-terminally labeled CCHC constructs (see Fig. S1D in the supplemental material). To further rule out the influence of the fluorescent tag on the observed protein trafficking, the EGFP-labeled CCHC was cotransfected with the nonlabeled full-length BCL11B (Fig. 1E). As in the previous experiment, the CCHC motif showed a nuclear localization pattern when combined with wt BCL11B but stayed in the cytoplasm when coupled with a CCHC-mutated variant of BCL11B. Conversely, the EGFP signal dispersed when the zinc finger structure was disrupted, regardless of the CCHC ZF status of the full-length component. Comparable observations were made with C-terminally labeled variants of the isolated CCHC domain (see Fig. S1E in the supplemental material). Taken together, these data indicate that strong reciprocal interaction between the N-terminal CCHC motifs in BCL11B molecules is crucial for the formation of functional dimers.
BCL11B CCHC zinc fingers self-associate.
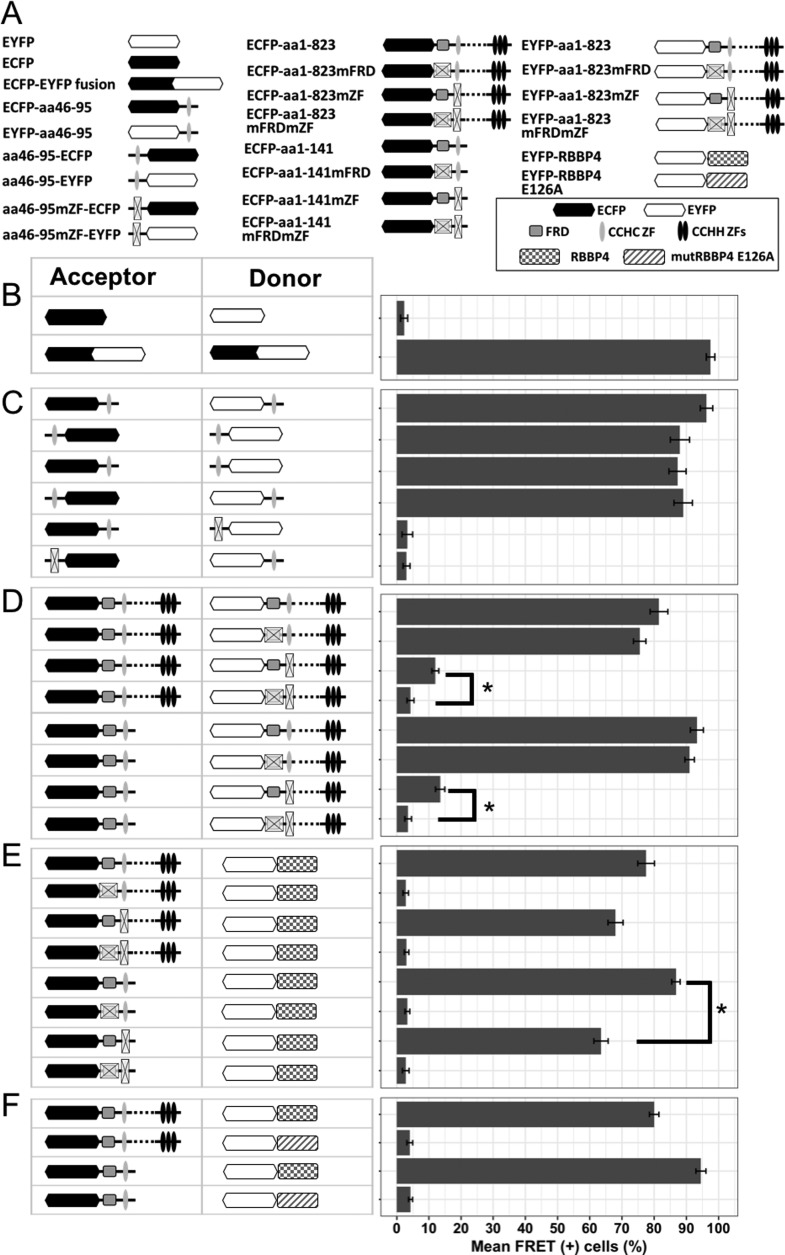
To determine whether the observed colocalization of CCHC ZF-containing peptides is the consequence of direct protein-protein interactions, we used a flow cytometry-based Förster resonance energy transfer assay (FACS-FRET). First, we validated the assay by expressing either two FRET fluorescence proteins (enhanced cyan fluorescent protein [ECFP] and enhanced yellow fluorescent protein [EYFP]) from separate plasmid vectors (negative FRET) or one FRET fluorescent protein, ECFP-EYFP, from a fusion gene to serve as a positive control (Fig. 2B). The CCHC fragments cloned in frame as either N- or C-terminal fusions with FRET donor (ECFP) or FRET acceptor (EYFP) proteins (Fig. 2A) delivered a strong FRET signal that was detectable in over 90% of the double-positive cells (Fig. 2C). In contrast, the signal was undetectable in cells bearing a mutation in the CCHC motif on either the donor or acceptor side. The position of the tag did not influence this interaction. Next, we confirmed this direct association in the contexts of the N-terminal region and full-length proteins. The nontruncated proteins labeled with ECFP and EYFP shifted over 70% of cotransfected cells into the FRET gate, but only when CCHC ZF domains on both sides were intact (Fig. 2D). Analogous observations were made using the N-terminal region instead of full-length BCL11B as the FRET donor. Interestingly, the variant with mutated CCHC and an intact FRD domain showed weak but significant FRET. One can speculate that the nondimerizing mutant might move into proximity to the wt BCL11B counterpart indirectly, e.g., through attraction to the NuRD complexes bound to wt BCL11B.
FIG 2.
Detection of direct protein-protein interactions by FACS-FRET assay. (A) Schematic representation of ECFP and EYFP fusions used for FRET assay. (B) HEK293 cells were transfected with either separate ECFP and EYFP FRET vectors (negative control) or a fused ECFP-EYFP construct (positive FRET control) and analyzed for FRET signal 24 h after transfection. (C) The isolated CCHC domain was linked with either ECFP or EYFP at its N or C terminus, and all possible combinations of labeled variants were cotransfected and checked for FRET signal. This excludes the position effect of the tags on the interaction. Mutation of the CCHC motif at the donor or acceptor site abrogated FRET. (D) The full-length BCL11B (top) or N-terminal deletion mutant (bottom) was used as a FRET donor, in combination with acceptor-labeled full-length BCL11B containing wt or mutated FRD and/or CCHC domains. (E) The coding sequence of RBBP4 fused with a FRET acceptor (EYFP) was cotransfected with full-length or N-terminal fragments of BCL11B tagged with ECFP, and variants with different statuses of FRD and/or CCHC were used as donors. (F) Wild-type RBBP4 or its mutant unable to bind the FOG-derived FRD domain (E126A) was fused to EYFP and cotransfected with ECFP-labeled full-length BCL11B or the fragment containing FRD and CCHC domains. The data represent mean values of four independent experiments with standard deviations (SD). *, P < 0,01.
The N-terminal region of the BCL11B protein has been shown to be crucial for recruiting the NuRD complex (15) through binding of the FRD domain to MTA and RBBP proteins. To investigate the putative role of the adjacent CCHC zinc finger in this interaction, we fused the FRET acceptor (EYFP) to the coding sequence of RBBP4 and cotransfected it with either the full-length or N-terminal region of BCL11B. In both cases, direct association was confirmed for all constructs encoding the wt FRD domain. However, in variants encoding wt FRD with a mutated CCHC, the FRET signal was weaker, indicating that dimerization is not necessary for NuRD-BCL11B interaction but has a strengthening effect. Replacement of wild-type RBBP4 with the E126A mutant that is unable to bind the FRD domain of Friend of Gata 1 (FOG1) (23) prevented interaction, reducing the FRET signal to background level for both the full-length and N-terminal variants (Fig. 2F). This result suggests that the FRD domains of the FOG and BCL11 proteins, in addition to being highly homologous, attract the NuRD complexes via the same mechanisms.
In conclusion, the FRET assay demonstrated that the N-terminal region of BCL11B is involved in both homodimerization and NuRD binding. Despite being mediated by separate domains, the two processes seem to enhance each other and occur independently of the C-terminal DNA binding region of BCL11B.
The CCHC zinc finger of BCL11B is not directly involved in interactions with the endogenous NuRD chromatin-remodeling complex.
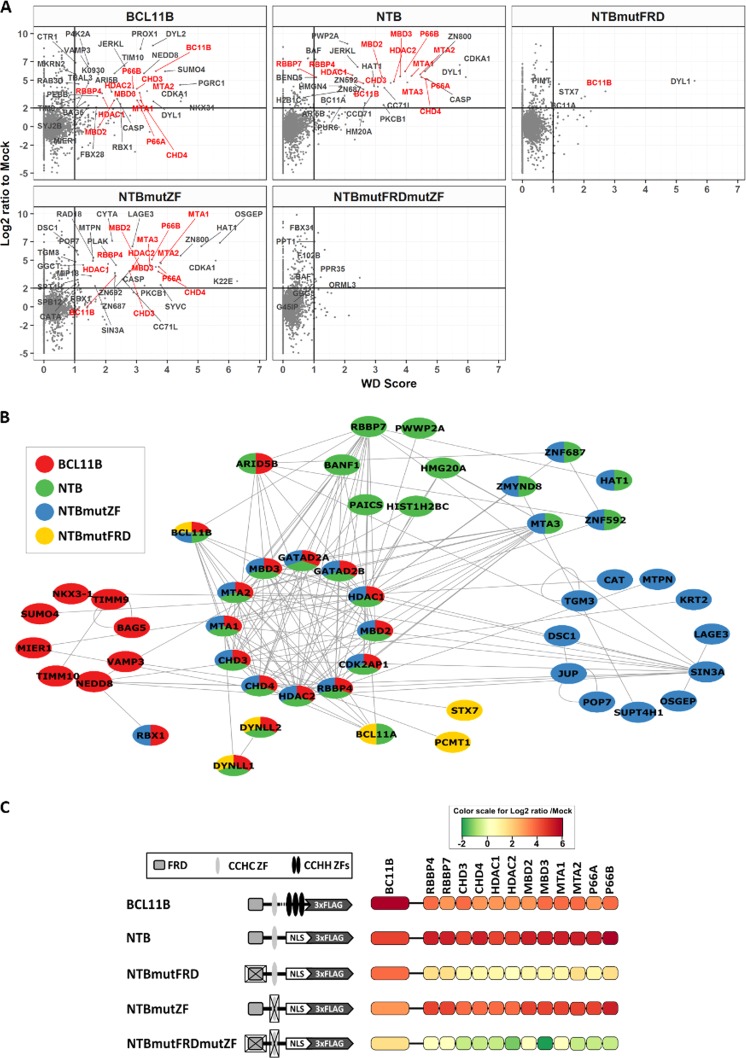
Having established the significance of the CCHC zinc finger for BCL11B dimerization in BCL11B-deficient HEK293 cells, we next switched to the T-cell line huT-78, which endogenously expresses BCL11B. First, using full-length protein or the N-terminal fragments labeled with the FLAG epitope as bait, we immunoprecipitated the native proteins associated with FLAG-tagged peptides. Mass spectrometry analysis of the affinity-purified complexes revealed approximately 4-fold enrichment of 12 different proteins known to form the NuRD complex when full-length BCL11B was used as bait (Fig. 3A). The nonmutated N-terminal region of BCL11B coimmunoprecipitated an even higher number of NuRD-derived peptides. Using this shortened sequence as bait, we detected a second member of the protein family, BCL11A. Mutations in the FRD domain had no major influence on the level of endogenous BCL11B or BCL11A identified by mass spectrometry but decreased the number of associated NuRD peptides dramatically. In contrast, destruction of the CCHC motif within the N-terminal region resulted in the immunoprecipitation of increased numbers of NuRD-forming peptides but only slightly elevated native BCL11B. As expected, the double NTB mutant appeared incapable of binding either NuRD proteins or endogenous BCL11B. The identified interactions and the relative enrichment of NuRD members compared to a mock-transduced sample are summarized and displayed in Fig. 3B and C. A complete list of identified proteins is provided in Table S1 in the supplemental material.
FIG 3.
Proteins identified via affinity purification followed by mass spectrometry (AP-MS) analysis of nuclear protein lysates acquired from huT-78 cells transduced with FLAG-tagged BCL11B or the N-terminal domain. (A) AP-MS data from discovery experiments (DDA) was used to identify high-confidence interacting proteins by combined stringent filters of a log2 ratio of 2 (in comparison with mock) and a WD score of 1. BCL11B and NuRD components are in red and other proteins in gray. (B) The list of candidate confidently copurified proteins with the corresponding baits was submitted and visualized using the PINA4MS application in Cytoscape. PINA4MS uses an existing ensemble of protein-protein interaction databases to connect two proteins, thereby establishing a network. Coloring is according the bait used. (C) Relative quantitative values in comparison with mock obtained from discovery experiments using log2 ratio-based relative coloring of BCL11B and the proteins belonging to the NuRD complex that were copurified, along with the corresponding baits.
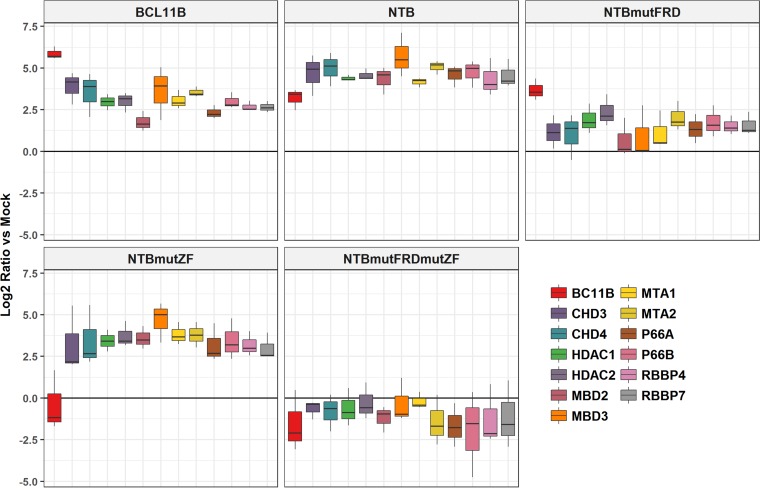
To confirm the results produced by conventional mass spectrometry, we employed selected reaction monitoring (SRM). This method is the mass spectrometric equivalent of a traditional Western blot, as it focuses on the identification and quantification of specific peptides using a targeted approach (24). In brief, for each protein to be confirmed, 2 or 3 unique tryptic peptides were synthesized and labeled with stable heavy isotopes (13C- and 15N-labeled arginine and lysine) to be used as spike-in standards. The heavy spike-in standard peptide and the native peptide of the corresponding protein coelute during liquid chromatography (LC)-tandem MS (MS-MS) because of their sequence similarity but are distinguished by MS because their masses differ (lysine, 8 Da; arginine,10 Da). Hence, adding known amounts of the labeled peptide to the sample and comparing the ratio of signals derived from native and heavy peptides allows estimation of the amount of a given protein. Furthermore, since the SRM assay allows multiplexing, all detected NuRD-related proteins could be confirmed simultaneously in each sample. The SRM results independently confirm the findings of conventional shotgun MS (Fig. 4). Compared to mock-transduced cells, the affinity-purified nuclear extracts prepared from wild-type BCL11B and nonmutated NTB cells were significantly enriched in peptides derived from all of the NuRD proteins identified using the global MS assay. Despite the disruption of the FRD domain, mutFRD (see below)-NTB appeared to attract detectable amounts of NuRD peptides, most likely due to indirect binding of native BCL11B/NuRD complexes via the CCHC domain. In contrast, mutation of the zinc-coordinating residues of the CCHC zinc finger resulted in the disappearance of the native BCL11B signal and an increase in NuRD peptides relative to those of nonmutated NTB. Neither endogenous BCL11B nor NuRD was detected (in comparison to empty vector control) in cells transduced with the NTB double mutant.
FIG 4.
SRM-based targeted validation (using stable-isotope-labeled peptides) and quantification (log2 ratio in comparison to mock) of BCL11B and NuRD complex proteins. Mock, cells transfected with empty vector control. The horizontal in each box represents the median ratio of the protein measured from three biological replicates. Whiskers indicate the spread of the ratios.
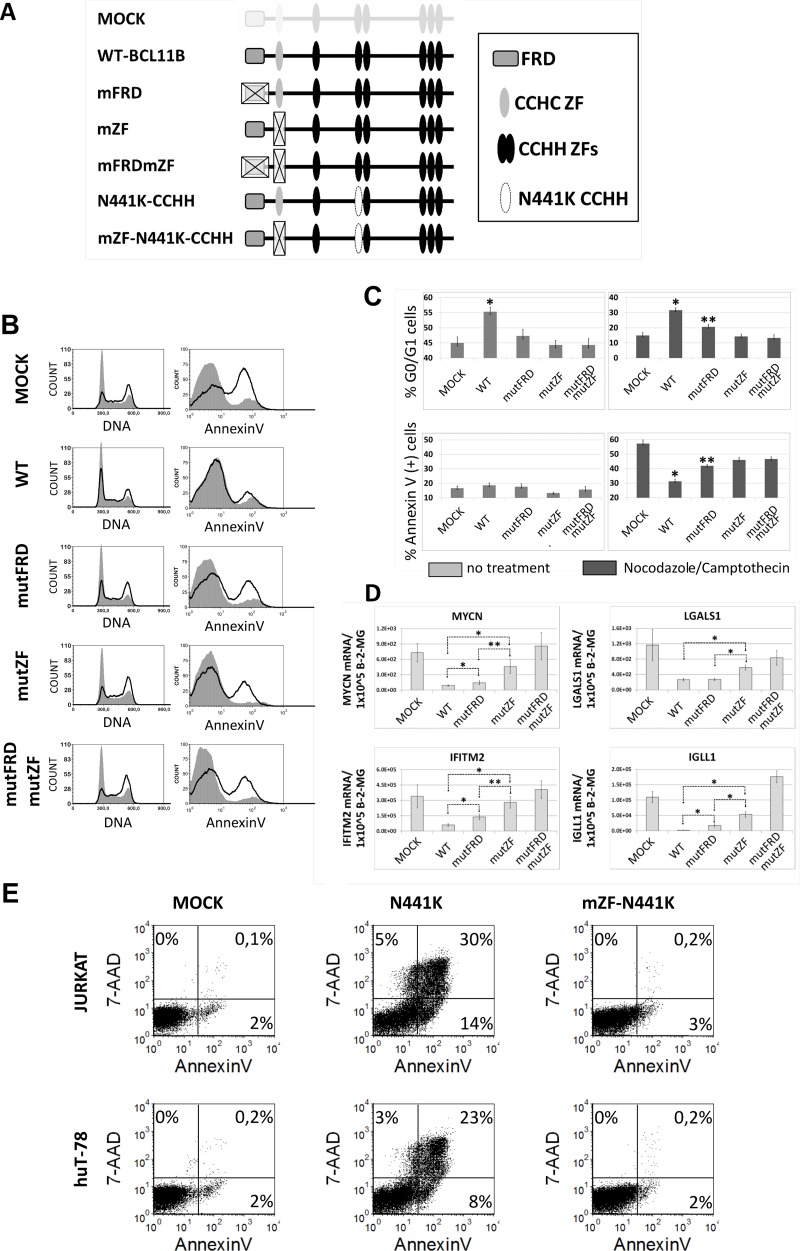
The CCHC zinc finger is essential for gene expression regulation, induction of cell cycle arrest, and chemoresistance in cells overexpressing BCL11B.
We showed previously that forced expression of BCL11B in human T-cell lines considerably delayed cell cycle entry and resulted in increased resistance to radiomimetic drugs (25). We also determined that the N-terminal region of the BCL11B protein is indispensable for this effect. Here, we engineered full-length BCL11B mutants that, instead of the deletion, carried targeted mutations within FRD (mutFR; MSRRKQS replaced by MSAAAAS), the CCHC zinc finger (mutZF; CCHC replaced with alanine), or both FRD and CCHC. Upon transduction of the human T-cell line huT-78 with wild-type BCL11B, the cell cycle partially arrested at the G0/G1 phase (Fig. 5B and C). In contrast, none of the mutants achieved these results with comparable efficiency. However, by synchronizing the cell cycle at G2/M with nocodazole, we observed a subtle but significant difference between the FRD and CCHC mutants. While loss of the zinc finger alone abolished cell cycle regulation, the BCL11B variant with mutations in the FRD domain showed an intermediate phenotype, indicating that it remained partially active. A similar observation was made regarding BCL11B-induced chemoresistance. Overexpression of BCL11B had no major influence on spontaneous apoptosis, with only marginally elevated numbers in the case of the wild-type variant (Fig. 5B and C). Interestingly, the induction of DNA damage by the topoisomerase inhibitor camptothecin was evidently less effective in all BCL11B-modified cells than in mock-transduced cells. While wt BCL11B was most potent at protecting the cells from DNA damage-induced apoptosis, the mutants were incompletely active. Analogous to the cell cycle studies, the FRD-mutated BCL11B showed modest DNA damage protection, but significantly greater than did CCHC or the double mutant. Together with the cell cycle results, this result indicates that the variant incapable of recruiting NuRD remains partially active unless it loses its ability to dimerize.
FIG 5.
The CCHC domain of BCL11B is crucial for its influence on cell cycle progression, apoptosis resistance, and transcriptional activity. (A) Schematic drawing of full-length BCL11B variants created to verify the functions of FRD and CCHC domains. (B) Representative cell cycle and apoptosis results obtained with huT-78 cells transduced with wt or mutant BCL11B. Cells were left untreated or incubated with nocodazole, which blocks the cell cycle at G2/M phase (left). Apoptosis was induced with the topoisomerase inhibitor camptothecin (right). (C) Mean percentages of G0/G1 (±SD) and annexin V-positive cells after transduction with different BCL11B variants. The cells were incubated with nocodazole (cell cycle) or camptothecin (apoptosis). (D) Relative levels of mRNAs for genes attenuated after BCL11B overexpression compared to beta-2-microglobulin housekeeping gene (B-2-MG). (E) Jurkat and huT-78 cells were transduced with vectors encoding EGFP (mock), dominant-negative BCL11B (N441K), or N441K with a mutated CCHC motif. Apoptosis was measured 48 h after transduction by annexin V/7-aminoactinomycin D (7-AAD) staining, followed by FACS. All the data were obtained from at least three independent experiments performed in triplicate. *, P < 0.01; **, P < 0.05.
Next, we investigated the effect of forced expression of BCL11B and its N-terminally mutated derivatives on the expression of several genes previously identified as repressed upon BCL11B transduction (Fig. 4C). The expression of all four selected genes, encoding immunoglobulin lambda-like polypeptide 1 (IGLL1), MYC-N proto-oncoprotein, interferon-induced transmembrane protein 2 (IFITM2), and galectin 1 (LGALS1), was markedly suppressed in cells overexpressing wt BCL11B. Inactivation of both the FRD and CCHC domains completely abrogated this repression, whereas variants with mutations in a single domain demonstrated intermediate activity. Interestingly, the inability to dimerize was more detrimental to the repression potential of BCL11B than the lack of the corepressor binding domain (FRD). In addition, the loss of repressor activity observed for the CCHC-null variant correlated well with its limited effects on cell cycle progression and apoptosis resistance. The relevance of dimerization to proper BCL11B function was ultimately confirmed using the dominant-negative variant N441K. As expected from our earlier BCL11B depletion studies, overexpression of N441K induced apoptosis in two different transformed T-cell lines (Fig. 5E). When the same variant was equipped with a mutated CCHC domain, the viability of transduced Jurkat and huT-78 cells remained comparable to that of mock-treated cells.
DISCUSSION
Zinc finger domains (ZFs) are broadly represented across eukaryotic genomes, and about 1% of all mammalian genes encode proteins containing single or multiple repeats of these motifs. Until recently, ZFs were considered solely as structures capable of binding to DNA or RNA in a sequence-specific manner. This notion was challenged when the mechanism of action of another class of ZFs was revealed. The CCHC-type ZFs, by means of spacing, resemble the most common classes of zinc fingers, such as CCHH and CCCC. They occur as multiple repeats, as in FOG transcription factors (26), or as single domains accompanied by multiple classical CCHH ZFs, as in Snail or Krüppel proteins (27, 28). Of note, none of the identified CCHC domains has been shown to mediate nucleic acid binding. Instead, CCHC ZFs have been implicated in protein-protein rather than protein-DNA interactions. For instance, in the FOG protein family, CCHC ZFs mediate interactions with the GATA transcription factor through binding to the amino-terminal CCCC ZF domain of the latter (29, 30). The single CCHC zinc finger domain of BCL11B is located at its N terminus and follows another FOG-related sequence called FRD. Its structure differs slightly from that of other CCHC ZF domains, having 2 additional amino acids separating the second cysteine and histidine. Despite this difference, our results strongly indicate that, like the other CCHC ZFs characterized thus far, the CCHC motif of BCL11B is capable of mediating protein-protein interactions, more specifically, to form a homomultimeric complex. As suggested recently, this interaction is crucial to protein function, as an inactivating mutation (N441K) in even one allele leads to the formation of inactive dimers, eliciting the “null” phenotype (20).
The dimerization of TFs is believed to have appeared evolutionarily as a consequence of symmetrical palindromic TF recognition sequences present within the promoters of some genes (31). This event was followed by the appearance of interaction interfaces between tandemly bound TFs, which strengthened the TF-DNA complexes and increased DNA binding specificity. Subsequent divergence of TFs and their heterodimerization further extended the range of regulated chromatin regions. Furthermore, the transition from monomer to dimer may represent an additional mechanism of regulation. The involvement of the BCL11B CCHC ZF in interactions with other proteins to form heterodimers is unlikely. Our coimmunoprecipitation (co-IP)/MS data obtained using constructs encoding native CCHC motifs revealed the peptides derived from BCL11B itself and its close homolog BCL11A. The two proteins share expression patterns and have been proven to be crucial for normal development of the same organs and tissues. Interestingly, the sequences of their CCHC domains differ by just one amino acid (valine replaced by isoleucine in BCL11A). Moreover, this substitution affects the hydrophobic region; due to its chemical nature, this region is not exposed, and its influence on any external interactions is rather limited. The hypothetical BCL11B-BCL11A heterodimer combines two different binding specificities, thus extending the repertoire of regulated target sequences or binding to the same promoter regions with different affinities. Thus, although not yet proven to occur between endogenously expressed proteins, the homotypic dimerization of BCL11A and BCL11B is plausible and deserves further investigation.
Eukaryotic transcription factors that require dimerization have been classified based on the mechanisms or specific domains that mediate the homotypic interaction. The bHLH (basic helix-loop-helix) family of proteins engages the four-helix bundle of the bHLH region to form a dimer at E-box elements. Leucine zippers (LZ) serve as dimerizing domains in some members of the bHLH family, but mainly in so-called bZIPs and HD-ZIPS factors (32, 33). In the NF-κB family, dimerization is made possible by the presence of C-terminal immunoglobulin-like regions (34). Moreover, homotypic interactions can be mediated by multiple domains within the same protein, as in MADS box proteins (35) or nuclear receptors (NRs), where a ligand-binding region participates in the interaction (36). From this perspective, BCL11B represents another class of dimerizing TFs in which the self-association is executed by a single CCHC structure. It is tempting to speculate that the same mechanism is employed by BCL11A and other proteins that contain the FRD-CCHC motifs, such as SALL1 (37) and the entire family of spalt-like proteins. A similar but distinct strategy for forming dimers has been identified in Ikaros TFs (38). Here, however, the interaction requires two ZFs located at the C terminus of the protein, and although they do not bind DNA, they possess typical CCHH topology. The CCHC ZFs of FOG family TFs in turn appear to play dual roles. Four out of five CCHC motifs (1, 5, 6, and 9) can independently mediate an interaction with the N-terminal CCCC ZF of GATA. Surprisingly, two out of the four zinc fingers (1 and 9) demonstrate behavior indicative of self-association (30). Since it has been suggested that multiple FOG molecules occupy promoters containing multiple GATA sites (23), one cannot exclude the possible occurrence of CCHC-mediated FOG-FOG interactions within native GATA-FOG-NuRD complexes.
Our observations confirm the earlier conclusions that FRD is a fully autonomous domain capable of mediating transcription repression through NuRD recruitment. However, how this interaction arises and which members of NuRD are directly involved, in our opinion, remains ambiguous. The first report indicated direct binding of the BCL11B N terminus exclusively to MTA1/2 proteins, based on a set of glutathione S-transferase (GST) pulldown experiments using recombinant proteins (15). Later studies of analogous interactions of FOG demonstrated that the short N-terminal FRD domain is sufficient for the recruitment of NuRD and that direct binding occurs not only between FRD and MTAs but also with RBBP4/7 proteins (23). It has been postulated that both interactions exist independently at promoter regions occupied by multiple FOG molecules. In this case, one FOG molecule would recruit NuRD via RBBP4/7 while another did so by binding to MTAs. Notably, the FRD-RBBP4/7 interaction is comprehensively documented, and the crucial residues on both sides of the interaction interface have been identified. In contrast, the binding of MTAs was concluded from pulldown experiments conducted using in vitro-translated proteins, and the exact architecture of the interaction interface remains unclear. The FACS-FRET assay we used to confirm the associations between BCL11B molecules and BCL11B and NuRD seems not to support direct BCL11B-MTA interaction. Neither full-length fragments nor any of the MTA1/2 N- or C-truncated fragments demonstrated increased florescence in the presence of N-terminal BCL11B constructs (data not shown). One cannot rule out the possibility that the addition of a FRET tag abolishes the capacity of labeled MTA1/2 to interact with FRD or to join the NuRD complex. On the other hand, our observations were made under conditions much closer to physiological than were the GST pulldown assays, which suffer from misfolding of tagged proteins and frequently yield false-positive results (39). Our failed attempts to map the BCL11B-MTA1/2 interaction interface using the N- and C-truncation approach in living cells suggest that the interaction might be nonspecific, and it requires further verification by an alternate methodology.
In addition to contributing to a better understanding of BCL11B biology and the mechanisms of transcriptional regulation, our findings may have practical applications. BCL11B depletion during embryogenesis is fatal, and a number of diseases are associated with elevated BCL11B expression (10, 11, 40, 41). Moreover, in the treatment of mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma, interfering with BCL11B expression alone induces apoptosis and exerts a synergistic effect when combined with HDAC inhibitors. Given the necessity of dimerization for BCL11B function, this interaction is an attractive target for the development of inhibitors. While long believed to be a high-hanging fruit in drug discovery (42), the inhibition of TFs by blocking protein-protein interactions, including dimerization, has been successfully achieved for numerous targets, including Stat3 (43), c-MYC/Max (44), and HDM2/p53 (45).
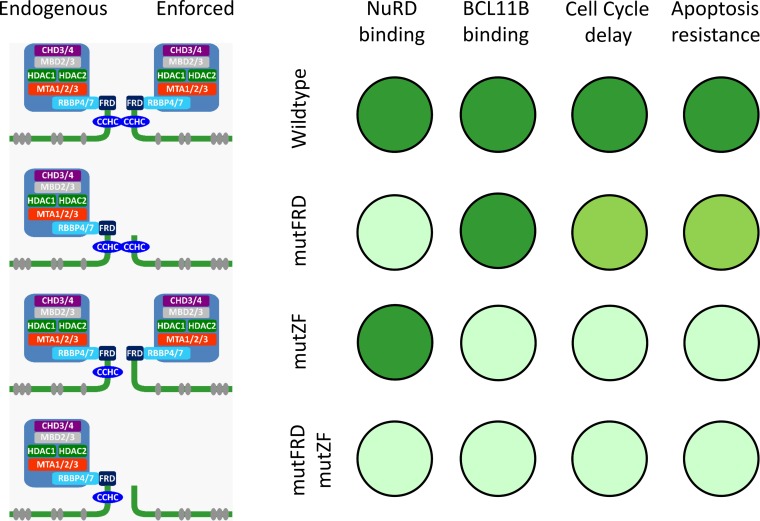
In conclusion, we revealed a previously unknown mechanism of transcription factor dimerization that occurs between noncanonical zinc finger domains and is crucial for BCL11B function (Fig. 6). Future studies will verify whether this mechanism can be generalized to other CCHC-containing proteins.
FIG 6.
Summary of interactions and functions mediated by the N-terminal part of BCL11B. The canonical DNA binding CCHH ZFs are shown as gray ellipses, and the CCHC and the FRD domains are indicated. The intensities of the green circles are indicative of and proportional to the strength of interaction or magnitude of the biological effect.
MATERIALS AND METHODS
Plasmids and chemicals.
All the BCL11B sequences and fragments used in this study correspond to isoform 2, consisting of exons 1, 2, and 4 (accession number NM_022898.2).
For fluorescence microscopy experiments, DNA fragments corresponding to the N-terminal part of the BCL11B coding sequence (aa 1 to 141, 1 to 52, 90 to 141, 46 to 141, and 46 to 95) were PCR amplified from human T cell cDNA and cloned in frame into pEGFP-C1 or pEGFP-N1 plasmid vectors (TaKaRa Bio Europe, Saint-Germain-en-Laye, France) to create N- or C-terminally labeled variants, respectively. The PCR fragments were cloned into the vectors using the InFusion ligase-free cloning system (TaKaRa Bio Europe, Saint-Germain-en-Laye, France). Similarly, the full-length BCL11B and its mutated or truncated variants were PCR amplified and fused with the monomeric red fluorescent protein (mRFP). The short fragments encoding the CCHC ZF or its mutated variant fused to mRFP were equipped with heterologous SV40-derived NLS. The nonlabeled BCL11B and its CCHC ZF-mutated version were cloned into the pCMV-3TAG3A vector (Agilent Technologies, Santa Clara, CA).
The FACS-FRET plasmids (pECFP, pEYFP, and ECFP-EYFP fusion) were a kind gift from H. Junker (Department of Medical Biochemistry, University Greifswald, Greifswald, Germany). To study the interactions of the N-terminal parts with full-length BCL11B and RBBP4, the relevant DNA sequences were amplified and cloned in frame as either N- or C-terminal fusions into EYFP (FRET acceptor) or ECFP (FRET donor) plasmids. To ensure proper cellular trafficking, the N-terminal BCL11B constructs were equipped with SV40-derived NLS. Mutations of the FRD or CCHC domain and within RBBP4 were generated during amplification by applying mutated PCR primers. To inactivate FRD, the conserved MSRRKQS motif was replaced by the MSAAAAS sequence. The CCHC ZF was damaged by replacement of all four zinc-coordinating residues with alanine. The glutamate residue (E126) proven to be engaged in FOG-RBBP4 binding was converted to alanine to create an RBBP4 variant unable to engage the FRD domain.
Plasmids for coimmunoprecipitation were generated by cloning the relevant fragments and their mutated versions into pCMV-3Tag, a triple-FLAG epitope-tagging mammalian expression vector (Agilent Technologies, Santa Clara, CA). The N-terminal BCL11B cDNAs were supplemented with SV40 NLS to facilitate interactions with nuclear proteins. The fusion cDNAs were recloned into the pWPXL lentiviral vector (Addgene, Cambridge, MA).
To overexpress full-length BCL11B and its FRD and/or CCHC-mutated variants, the coding sequences were produced by PCR using wt or mutated primers and cloned into the pWPXL lentiviral vector, replacing EGFP cDNA. The dominant-negative N441K mutants (N370K in isoform 2) were created using a Q5 site-directed mutagenesis kit (New England BioLabs, Ipswich, MA) by converting asparagine to lysine in a wt BCL11B vector. The same procedure was repeated for CCHC-mutated BCL11B plasmid.
The proofreading polymerase Pfu (Promega, Madison, WI) was used to generate all cloned DNA fragments. In addition, all constructs were checked by sequencing to confirm the introduced mutations and exclude the presence of undesired alterations. An endotoxin-free plasmid isolation system was used to propagate the verified plasmids (ZymoPure-EndoTero; Zymo Research, Irvine, CA). All biochemicals were purchased from Sigma (Munich, Germany) unless otherwise specified.
Cell culture and viral vector production.
The human T-cell lymphoma huT-78 (ATCC, Rockland, MD) and human acute T-cell leukemia Jurkat cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (PanBiotech Berlin, Germany), GlutaMax (Invitrogen, Carlsbad, CA), and Mycozap (Lonza Scientific, Switzerland). The lentivirus-producing cell line Lenti-X 293T (TaKaRa Bio Europe, Saint-Germain-en-Laye, France) was maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented as described above.
For recombinant-lentivirus production, the second-generation lentiviral vector system was used. Nonconfluent Lenti-X 293T cells growing on 10-cm culture dishes were cotransfected with 7 μg of purified pVSV-G envelope-expressing plasmid (Addgene, Cambridge, MA), 15 μg psPAX2 (Addgene, Cambridge, MA) vector carrying virus polymerase and packaging genes, and 20 μg of the lentiviral vector pWPXL (Addgene, Cambridge, MA) encoding EGFP (mock) or BCL11B or its variants. The transfection was performed using a CalPhos mammalian transfection kit (TaKaRa Bio Europe, Saint-Germain-en-Laye, France) according to the manufacturer's instructions. The virus-containing media were collected 48 h after transfection and used immediately or stored at 4°C and used within 7 days. To transduce the target cells, the lentiviral supernatants were supplemented with 8 mg/ml Polybrene (Sigma Chemicals, Germany) and added to the target cells, followed by 90-min spinoculation at 32°C and 2,000 × g.
Immunofluorescence and FACS-FRET assay.
For cellular localization assays, the plasmid vectors described above were transfected into HEK293 cells using the calcium-phosphate method (CalPhos mammalian transfection kit; TaKaRa Bio Europe, Saint-Germain-en-Laye, France). After 24 h of incubation, the EGFP and/or mRFP expression and localization were determined by fluorescence microscopy.
FRET assay (here combined with FACS) is a method based on measuring the fluorescence of the FRET acceptor fluorescent protein fused to the studied peptide, which increases when brought into proximity (less than 10 nm, a prerequisite for direct contact) of the FRET donor-fused protein (46). We used the standard ECFP/EYFP FRET pair. To test the interactions, our peptides were fused in frame as either N- or C-terminal fusions, as described above.
FACS-FRET measurements were performed using a FACSAria (BD Bioscience) equipped with 405-nm, 488-nm, and 633-nm lasers. To measure ECFP and FRET, cells were excited with the 405-nm laser, and fluorescence was collected in the ECFP channel with a standard 450/40 filter, while the FRET signal was measured with a 529/24 filter (Semrock). To measure EYFP, cells were excited with the 488-nm laser, while emission was also taken with a 529/24 filter (Semrock). For each sample, we evaluated a minimum of 1,000 CFP/YFP-positive cells that fell within the background-adjusted gate.
Apoptosis and cell cycle assays.
To evaluate the influence of increased expression of BCL11B or its N-terminally modified variants on apoptosis resistance, huT-78 cells transduced with lentiviral vectors were treated with camptothecin for 6 h (Sigma, Germany). Apoptosis of camptothecin-treated and control cells was measured by FACS using an annexin V-allophycocyanin (APC) binding assay (BD Pharmingen).
Cell cycle status was determined by propidium iodide DNA staining and flow cytometry analyses. Briefly, ethanol-fixed cells were incubated overnight at −20°C, washed, resuspended in propidium iodide staining solution containing RNase A (Sigma, Germany), and incubated in the dark for 30 min. Flow cytometric analysis was performed in a FACSCalibur instrument (Becton Dickinson, San Jose, CA). Cell cycle analysis was performed using ModFit LT (Verity Software House, Topsham, ME). To determine the rate of G1-to-S transition, cells were blocked in G2/M phase with 0.5 mM nocodazole, and cell cycle analysis was done at different time points.
Quantitative RT-PCR.
Total RNA was isolated with TRIzol reagent (ThermoFisher Scientific, Germany). For quantitative PCR, RNA was reverse transcribed with MultiScribe reverse transcriptase (Applied Biosysytems) using random hexamers. All the primers and probes were synthesized by TibMolBiol (Berlin, Germany). Quantification of genes regulated upon BCL11B overexpression was performed in huT-78 cells. In brief, PCR amplification was performed in a total volume of 25 μl with 2 μl of cDNA, 25 pmol of each primer, and SYBR green PCR master mix using the Applied Biosystems TaqMan 7500 real-time system (ThermoFisher Scientific). A list of the primer sequences is provided in the supplemental material.
Nuclear isolation and coimmunoprecipitation.
Human T-cell lymphoma huT-78 cells were transduced with lentiviral particles according to the protocol described above. After 48 h of incubation, the transduced cells were harvested by centrifugation at 400 × g for 10 min at 4°C. Nuclear extract preparation was performed according to the Nuclear Complex co-IP kit protocol (Active Motif Europe, Belgium; catalog no. 54001). The nuclear isolates (100 μl) were precleared with 40 μl mouse IgG-agarose (Sigma-Aldrich) for 90 min at 4°C on a roller shaker, and the agarose beads were removed by centrifugation at 8,000 × g for 30 s at 4°C. The cleared lysates were incubated with 40 μl of EZview Red anti-FLAG M2 affinity gel (Sigma-Aldrich) for 90 min at 4°C on a roller shaker. The protein-bound beads were collected by centrifugation at 8,000 × g for 30 s at 4°C and then washed seven times with 1 ml ice-cold Tris-buffered saline (TBS) with an incubation of 10 min per wash on a roller shaker. The protein-bound beads were transferred to a new tube for the 1st and 7th washing steps.
Sample preparation for mass spectrometry.
The protein-bound beads were then washed twice with 100 μl of 20 mM ammonium bicarbonate at room temperature. Next, 40 μl of trypsin (sequencing-grade modified trypsin; Promega, Mannheim, Germany) solution (10 ng/μl trypsin in 20 mM ammonium bicarbonate) was added to the protein-bound beads and incubated on a roller shaker overnight at room temperature. The supernatant containing peptides was collected by centrifugation at 8,000 × g for 30 s, and 20 μl of 5% acetic acid was added to the supernatant to stop the trypsin digestion. The resulting peptide mixtures were desalted using 5 μg C18 ZipTip columns (Millipore, Bedford, MA). The desalted peptide mixtures were lyophilized and resuspended in 50 μl of buffer A (0.1% acetic acid, 2% acetonitrile [ACN]) and subsequently used for global proteomics workflow and targeted validation proteomics workflow as described below.
Global proteome measurements: data-dependent acquisition (DDA).
MS analyses were performed with an on-line-coupled UltiMate 3000RSLC system (ThermoFisher Scientific, Idstein, Germany) connected to a Q Exactive Orbitrap-MS (ThermoFisher Scientific Inc.). For LC separation, peptides were enriched on an Acclaim PepMap 100 precolumn (nanoViper C18; 100 μm by 2 cm; 5-µm particle size; 100-Å pore size; ThermoFisher Scientific Inc.) and separated using an Accucore 150 C18 with a column length of 25 cm (50 cm; 150 Å; 2.6 μm; ThermoFisher Scientific Inc.) at a temperature of 40°C. For separation, a 145-min gradient was used with a solvent mixture of buffer A (5% ACN in water with 0.1% acetic acid) and increasing percentages of buffer B (ACN with 0.1% acetic acid): 2% for 10 min, 2 to 25% for 120 min, 25 to 40% for 5 min, 40 to 90% for 2 min, and 90% for 5 min. Peptides were eluted with a flow rate of 300 nl/min for the 25-cm column and 200 nl/min for the 50-cm column. Full-scan MS was carried out using a mass range of m/z 300 to 1,650. Data were acquired in a data-dependent strategy in profile mode with a resolution of 70,000 for MS at m/z 400 and 17,500 for MS-MS operated in positive-polarity mode. The method used allowed sequential isolation of the top 10 most intense ions for fragmentation using high-energy collisional dissociation (HCD) with dynamic exclusion for 30 s and disabled early expiration. An intensity threshold of 83,000 was applied, with an isolation width of 3 m/z, normalized collision energy of 27.5 eV, and a starting mass of m/z 100. The charge state screening and monoisotopic precursor selection rejected +1 and +7, +8, and >+8 charged ions.
The raw data files were searched using MaxQuant (version 1.5.3.8) (47) against the Human UniProt database with default settings using methionine oxidation as variable modification, carbamidomethylation of cysteine as static modification, and 2 missed cleavages. Spectral-count results obtained from the MaxQuant search results were converted to normalized spectral abundance factor (NSAF) values (48) using an in-house script. To efficiently distinguish biologically relevant interaction from background interactions, WD scores (48) were calculated using NSAF values, and only WD scores of >1 were considered for further data analysis.
In parallel with the WD scores, fold change values in relation to the control/mock samples were also calculated using median normalized intensity data obtained from the MaxQuant search results. Prior to fold change value calculations, missing values for each protein were imputed by the lowest value for the corresponding protein in the entire experiment divided by 8. Finally, high-confidence interactions were derived by using both WD scores of >1 and fold change of >4, similar to the method described by Hauri et al. (49). Lists of high-confidence interactions corresponding to each bait were used to generate an interaction network map based on previously known protein-protein interactions using a Cytoscape (50) plugin, PINA4MS (http://apps.cytoscape.org/apps/pina4ms). The fold change values were used for the visualization of the relative enrichment of NuRD protein complexes corresponding to each BCL11B variant. The scalable vector graphic (SVG) image used to visualize the fold change was manually drawn with Inkscape software (v0.91) (http://www.Inkscape.org), and color mapping relative to the fold change values was automatically generated in R using the SVGMapping package (v1.43) (Jean-Christophe AUDE; repository, Comprehensive R Archive Network [CRAN]) (http://svgmapping.r-forge.r-project.org/SVG_Mapping/Home.html).
Targeted protein measurements: selected reaction monitoring (SRM) data acquisition and processing.
Triple-quadrupole mass spectrometry (TSQ Vantage; ThermoFisher Scientific) interfaced with nanoAcquity Ultraperformance LC (Waters Corp., Milford, MA) was used for SRM analyses. Design of the stable-isotope-labeled peptides and SRM measurements were performed as previously described by Bhardwaj et al. (51). Thirteen selected protein targets were in silico digested with Skyline software provided with an in-house-built human peptide spectral library in the background to screen for the best-suited SRM peptides. Only fully tryptic peptides 7 to 25 amino acids in length were included, and methionine-containing peptides were excluded. The uniqueness of the peptides was confirmed by a search against the UniProtKB/Swiss-Prot human database (July 2016 release), and 2 or 3 unique peptides per protein were selected for relative quantitation of proteins. The final shortlisted peptides were synthesized by JPT (Berlin, Germany), heavy labeled with 13C and 15N Arg/Lys amino acids (referred to as “spike tides L” by the manufacturer). Lyophilized heavy peptides (20 nmol each) were reconstituted in 80% (vol/vol) ammonium bicarbonate solution (20 mM) and 20% (vol/vol) ACN, and 20-μl (approximately 4-nmol) aliquots of each peptide were stored at −80°C until further use. Heavy standard peptides were spiked into the samples after trypsin digestion, with a final concentration of approximately 10 fM for each peptide per 1 μg of protein digest.
Separation of peptides was performed by reversed-phase chromatography on a nanoAcquity C18 column (10-cm length, 100-μm inner diameter, 1.7-μm particle size; Waters Corp., United Kingdom) with a binary gradient of 0 to 25% buffer B (100% [vol/vol] ACN, 0.1% acetic acid) for 60 min, 25 to 45% B for 10 min, and 45 to 90% B for 10 min at a 300-nl/min flow rate. The eluted peptides were ionized by electrospray (spray voltage, 1,635 V; capillary temperature, 250°C). After a precursor scan in the first quadrupole, collision-induced dissociation (CID) fragmentation was performed in the second quadrupole. Collision energy was optimized for each precursor ion by the factory default method. +2 and +3 charged precursor ions were selected for each peptide, and the four most abundant transitions were chosen for SRM acquisition. Data were acquired by scheduled SRM using a 5-min scanning window. The raw data were analyzed with Skyline software version 2.5, quantification was performed by a pair of coeluting heavy and light peptides identified, and the corresponding peak areas were used to calculate the ratio between the endogenous light peptide and the spiked-in heavy peptide. Peptides that exhibited missed cleavage and bad chromatographic properties were excluded from quantification of the corresponding protein. Relative protein abundance was calculated by averaging 2 or 3 peptide ratio values for the corresponding protein. Furthermore, log2 fold change values for each protein enriched with various BCL11B variants were calculated in comparison to the mock/control.
Accession number(s).
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD008606.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by the National Science Center, Poland (decision no. DEC-2013/09/B/NZ1/01867) (G.K.P.).
We are thankful to Vishnu M. Dhople and Manuela Gesell Salazar for the LC–MS-MS measurements.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00368-17.
REFERENCES
- 1.Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. 2000. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem 275:10315–10322. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. 2005. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Mishima Y, Hitomi J, Yamamoto T, Utsuyama M, Niwa O, Aizawa S, Kominami R. 2003. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat Immunol 4:533–539. doi: 10.1038/ni927. [DOI] [PubMed] [Google Scholar]
- 4.Golonzhka O, Liang X, Messaddeq N, Bornert J-M, Campbell AL, Metzger D, Chambon P, Ganguli-Indra G, Leid M, Indra AK. 2009. Dual role of COUP-TF-interacting protein 2 in epidermal homeostasis and permeability barrier formation. J Investig Dermatol 129:1459–1470. doi: 10.1038/jid.2008.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golonzhka O, Metzger D, Bornert J-M, Bay BK, Gross MK, Kioussi C, Leid M. 2009. Ctip2/Bcl11b controls ameloblast formation during mammalian odontogenesis. Proc Natl Acad Sci U S A 106:4278–4283. doi: 10.1073/pnas.0900568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyrylkova K, Kyryachenko S, Biehs B, Klein O, Kioussi C, Leid M. 2012. BCL11B regulates epithelial proliferation and asymmetric development of the mouse mandibular incisor. PLoS One 7:e37670. doi: 10.1371/journal.pone.0037670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S, Kalisky T, Sahoo D, Dalerba P, Feng W, Lin Y, Qian D, Kong A, Yu J, Wang F, Chen EY, Scheeren FA, Kuo AH, Sikandar SS, Hisamori S, van Weele LJ, Heiser D, Sim S, Lam J, Quake S, Clarke MF. 2017. A quiescent Bcl11b high stem cell population is required for maintenance of the mammary gland. Cell Stem Cell 20:247–260. doi: 10.1016/j.stem.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez A, Kentsis A, Sanda T, Holmfeldt L, Chen S-C, Zhang J, Protopopov A, Chin L, Dahlberg SE, Neuberg DS, Silverman LB, Winter SS, Hunger SP, Sallan SE, Zha S, Alt FW, Downing JR, Mullighan CG, Look AT. 2011. The BCL11B tumor suppressor is mutated across the major molecular subtypes of T-cell acute lymphoblastic leukemia. Blood 118:4169–4173. doi: 10.1182/blood-2010-11-318873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartram I, Gokbuget N, Schlee C, Heesch S, Fransecky L, Schwartz S, Stuhlmann R, Schafer-Eckhart K, Starck M, Reichle A, Hoelzer D, Baldus CD, Neumann M. 2014. Low expression of T-cell transcription factor BCL11b predicts inferior survival in adult standard risk T-cell acute lymphoblastic leukemia patients. J Hematol Oncol 7:51. doi: 10.1186/s13045-014-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshiro A, Tagawa H, Ohshima K, Karube K, Uike N, Tashiro Y, Utsunomiya A, Masuda M, Takasu N, Nakamura S, Morishima Y, Seto M. 2006. Identification of subtype-specific genomic alterations in aggressive adult T-cell leukemia/lymphoma. Blood 107:4500–4507. doi: 10.1182/blood-2005-09-3801. [DOI] [PubMed] [Google Scholar]
- 11.Gu X, Wang Y, Zhang G, Li W, Tu P. 2013. Aberrant expression of BCL11B in mycosis fungoides and its potential role in interferon-induced apoptosis. J Dermatol 40:596–605. doi: 10.1111/1346-8138.12160. [DOI] [PubMed] [Google Scholar]
- 12.Fu W, Yi S, Qiu L, Sun J, Tu P, Wang Y. 2017. BCL11B mediated epigenetic repression is a crucial target for histone deacetylase inhibitors in cutaneous T-cell lymphoma. J Investig Dermatol 137:1523–1532. doi: 10.1016/j.jid.2017.02.980. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi Y, Inoue J, Takahashi Y, Matsuki A, Kosugi-Okano H, Shinbo T, Mishima Y, Niwa O, Kominami R. 2003. Homozygous deletions and point mutations of the Rit1/Bcl11b gene in gamma-ray induced mouse thymic lymphomas. Biochem Biophys Res Commun 301:598–603. doi: 10.1016/S0006-291X(02)03069-3. [DOI] [PubMed] [Google Scholar]
- 14.Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M. 2002. COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. Biochem J 368:555–563. doi: 10.1042/bj20020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cismasiu VB, Adamo K, Gecewicz J, Duque J, Lin Q, Avram D. 2005. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene 24:6753–6764. doi: 10.1038/sj.onc.1208904. [DOI] [PubMed] [Google Scholar]
- 16.Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. 2003. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. J Biol Chem 278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topark-Ngarm A, Golonzhka O, Peterson VJ, Barrett B, Martinez JRB, Crofoot K, Filtz TM, Leid M. 2006. CTIP2 associates with the NuRD complex on the promoter of p57KIP2, a newly identified CTIP2 target gene. J Biol Chem 281:32272–32283. doi: 10.1074/jbc.M602776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L-J, Vogel WK, Liu X, Topark-Ngarm A, Arbogast BL, Maier CS, Filtz TM, Leid M. 2012. Coordinated regulation of transcription factor Bcl11b activity in thymocytes by the mitogen-activated protein kinase (MAPK) pathways and protein sumoylation. J Biol Chem 287:26971–26988. doi: 10.1074/jbc.M112.344176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubuissez M, Loison I, Paget S, Vorng H, Ait-Yahia S, Rohr O, Tsicopoulos A, Leprince D. 2016. Protein kinase C-mediated phosphorylation of BCL11B at serine 2 negatively regulates its interaction with NuRD complexes during CD4+ T-cell activation. Mol Cell Biol 36:1881–1898. doi: 10.1128/MCB.00062-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punwani D, Zhang Y, Yu J, Cowan MJ, Rana S, Kwan A, Adhikari AN, Lizama CO, Mendelsohn BA, Fahl SP, Chellappan A, Srinivasan R, Brenner SE, Wiest DL, Puck JM. 2016. Multisystem anomalies in severe combined immunodeficiency with mutant BCL11B. N Engl J Med 375:2165–2176. doi: 10.1056/NEJMoa1509164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przybylski GK, Dik WA, Wanzeck J, Grabarczyk P, Majunke S, Martin-Subero JI, Siebert R, Dolken G, Ludwig W-D, Verhaaf B, van Dongen JJM, Schmidt CA, Langerak AW. 2005. Disruption of the BCL11B gene through inv(14)(q11.2q32.31) results in the expression of BCL11B-TRDC fusion transcripts and is associated with the absence of wild-type BCL11B transcripts in T-ALL. Leukemia 19:201–208. doi: 10.1038/sj.leu.2403619. [DOI] [PubMed] [Google Scholar]
- 22.Timney BL, Raveh B, Mironska R, Trivedi JM, Kim SJ, Russel D, Wente SR, Sali A, Rout MP. 2016. Simple rules for passive diffusion through the nuclear pore complex. J Cell Biol 215:57–76. doi: 10.1083/jcb.201601004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lejon S, Thong SY, Murthy A, AlQarni S, Murzina NV, Blobel GA, Laue ED, Mackay JP. 2011. Insights into association of the NuRD complex with FOG-1 from the crystal structure of an RbAp48.FOG-1 complex. J Biol Chem 286:1196–1203. doi: 10.1074/jbc.M110.195842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Brasier AR. 2013. Applications of selected reaction monitoring (SRM)-mass spectrometry (MS) for quantitative measurement of signaling pathways. Methods 61:313–322. doi: 10.1016/j.ymeth.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grabarczyk P, Nahse V, Delin M, Przybylski G, Depke M, Hildebrandt P, Volker U, Schmidt CA. 2010. Increased expression of bcl11b leads to chemoresistance accompanied by G1 accumulation. PLoS One 5:e12532. doi: 10.1371/journal.pone.0012532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox AH, Liew C, Holmes M, Kowalski K, Mackay J, Crossley M. 1999. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J 18:2812–2822. doi: 10.1093/emboj/18.10.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg UB, Preiss A, Seifert E, Jackle H, Knipple DC. 1985. Production of phenocopies by Kruppel antisense RNA injection into Drosophila embryos. Nature 313:703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- 28.Boulay JL, Dennefeld C, Alberga A. 1987. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature 330:395–398. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski K, Liew CK, Matthews JM, Gell DA, Crossley M, Mackay JP. 2002. Characterization of the conserved interaction between GATA and FOG family proteins. J Biol Chem 277:35720–35729. doi: 10.1074/jbc.M204663200. [DOI] [PubMed] [Google Scholar]
- 30.Matthews JM, Kowalski K, Liew CK, Sharpe BK, Fox AH, Crossley M, MacKay JP. 2000. A class of zinc fingers involved in protein-protein interactions biophysical characterization of CCHC fingers from fog and U-shaped. Eur J Biochem 267:1030–1038. doi: 10.1046/j.1432-1327.2000.01095.x. [DOI] [PubMed] [Google Scholar]
- 31.Amoutzias GD, Robertson DL, van de Peer Y, Oliver SG. 2008. Choose your partners: dimerization in eukaryotic transcription factors. Trends Biochem Sci 33:220–229. doi: 10.1016/j.tibs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Vinson C, Acharya A, Taparowsky EJ. 2006. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta 1759:4–12. doi: 10.1016/j.bbaexp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Tron AE, Welchen E, Gonzalez DH. 2004. Engineering the loop region of a homeodomain-leucine zipper protein promotes efficient binding to a monomeric DNA binding site. Biochemistry 43:15845–15851. doi: 10.1021/bi048254a. [DOI] [PubMed] [Google Scholar]
- 34.Chen FE, Ghosh G. 1999. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene 18:6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- 35.de Folter S, Immink RGH, Kieffer M, Parenicova L, Henz SR, Weigel D, Busscher M, Kooiker M, Colombo L, Kater MM, Davies B, Angenent GC. 2005. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell 17:1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, de Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. 2006. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev 58:760–772. [DOI] [PubMed] [Google Scholar]
- 37.Netzer C, Bohlander SK, Hinzke M, Chen Y, Kohlhase J. 2006. Defining the heterochromatin localization and repression domains of SALL1. Biochim Biophys Acta 1762:386–391. doi: 10.1016/j.bbadis.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.McCarty AS, Kleiger G, Eisenberg D, Smale ST. 2003. Selective dimerization of a C2H2 zinc finger subfamily. Mol Cell 11:459–470. doi: 10.1016/S1097-2765(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 39.Wissmueller S, Font J, Liew CW, Cram E, Schroeder T, Turner J, Crossley M, Mackay JP, Matthews JM. 2011. Protein-protein interactions: analysis of a false positive GST pulldown result. Proteins 79:2365–2371. doi: 10.1002/prot.23068. [DOI] [PubMed] [Google Scholar]
- 40.Ganguli-Indra G, Wasylyk C, Liang X, Millon R, Leid M, Wasylyk B, Abecassis J, Indra AK. 2009. CTIP2 expression in human head and neck squamous cell carcinoma is linked to poorly differentiated tumor status. PLoS One 4:e5367. doi: 10.1371/journal.pone.0005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiles ET, Lui-Sargent B, Bell R, Lessnick SL. 2013. BCL11B is up-regulated by EWS/FLI and contributes to the transformed phenotype in Ewing sarcoma. PLoS One 8:e59369. doi: 10.1371/journal.pone.0059369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JA, McClendon CL. 2007. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 43.Song H, Wang R, Wang S, Lin J. 2005. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A 102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiessling A, Sperl B, Hollis A, Eick D, Berg T. 2006. Selective inhibition of c-Myc/Max dimerization and DNA binding by small molecules. Chem Biol 13:745–751. doi: 10.1016/j.chembiol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Czarna A, Beck B, Srivastava S, Popowicz GM, Wolf S, Huang Y, Bista M, Holak TA, Domling A. 2010. Robust generation of lead compounds for protein-protein interactions by computational and MCR chemistry: p53/Hdm2 antagonists. Angew Chem Int Ed Engl 49:5352–5356. doi: 10.1002/anie.201001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banning C, Votteler J, Hoffmann D, Koppensteiner H, Warmer M, Reimer R, Kirchhoff F, Schubert U, Hauber J, Schindler M. 2010. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PLoS One 5:e9344. doi: 10.1371/journal.pone.0009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox J, Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 48.Paoletti AC, Parmely TJ, Tomomori-Sato C, Sato S, Zhu D, Conaway RC, Conaway JW, Florens L, Washburn MP. 2006. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc Natl Acad Sci U S A 103:18928–18933. doi: 10.1073/pnas.0606379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauri S, Comoglio F, Seimiya M, Gerstung M, Glatter T, Hansen K, Aebersold R, Paro R, Gstaiger M, Beisel C. 2016. A high-density map for navigating the human polycomb complexome. Cell Rep 17:583–595. doi: 10.1016/j.celrep.2016.08.096. [DOI] [PubMed] [Google Scholar]
- 50.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhardwaj G, Dorr M, Sappa PK, Ameling S, Dhople V, Steil L, Klingel K, Empen K, Beug D, Volker U, Felix SB, Hammer E. 2017. Endomyocardial proteomic signature corresponding to the response of patients with dilated cardiomyopathy to immunoadsorption therapy. J Proteomics 150:121–129. doi: 10.1016/j.jprot.2016.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.