ABSTRACT
Bacterial secretins are outer membrane proteins that provide a path for secreted proteins to access the cell exterior/surface. They are one of the core components of secretion machines and are found in type II and type III secretion systems (T2SS and T3SS, respectively). The secretins comprise giant ring-shaped homo-oligomers whose precise atomic organization was only recently deciphered thanks to spectacular developments in cryo-electron microscopy (cryo-EM) imaging techniques.
KEYWORDS: T2SS, secretin, GspD
TEXT
Gram-negative bacteria have evolved highly complex molecular nanomachines which span the whole cell envelope to ensure access of proteins/enzymes to the extracellular medium or to inject toxins into other target cells. This mechanism of protein secretion is important to ensure bacterial survival and pathogenesis. The secretion systems have been classified over the years according to the nature of the different proteins that compose the building blocks. Thus, there are differences, but overall, the idea is to form a channel in the membrane that will funnel proteins to be secreted. Each set and each genuine machine were then coined a type, which now range from the type I secretion system (T1SS) to the type IX secretion system (T9SS). The nomenclature has slightly diverged, but experts in the field would recognize their favorites. The complexity of these supramolecular assemblies and their membrane localization have long been an obstacle in obtaining high-resolution structures of these nanomachines.
One of the first key structures in protein secretion systems to be resolved was the TolC trimer from the T1SS (1), which did not differ too much from classical monomeric outer membrane (OM) proteins (Fig. 1, left). However, despite forming a β-barrel, in this case the TolC barrel takes shape only through assembly of a homotrimer, while a large coiled-coil domain protrudes in the periplasm and makes a hermetic conduit connecting the inside of the cell with the outside world. Because of recent outstanding improvements in structural biology and particularly in cryo-electron microscopy (cryo-EM), acknowledged by the 2017 Nobel prize for chemistry, the pace at which larger secretion systems has dramatically increased. We can now, for example, visualize the whole T3SS (2, 3) or exquisite details of the T4SS (4). In the latter case, visualization came with a surprise in that what composes the outer membrane channel has no resemblance to our standard belief; in particular, the portion of the protein which plugs the system into the outer membrane is not a β-barrel but a series of amphipathic α-helices. This finding reminded us that we still know very little about the molecular options that bacteria have evolved to shape an outer membrane channel. Secretins are one of these options and were always suspected to form giant pores (5). They are found in the T2SS to allow the release of large folded virulence factors or in type 4 pilus assembly systems (T4PS) and T3SS for the assembly and movement of cell surface appendages. A paper by Hay et al. (6) describes the three-dimensional (3D) high-resolution structure of a T2SS secretin from enteropathogenic Escherichia coli (EPEC), which together with previously published structures is illuminating our understanding of these astounding objects.
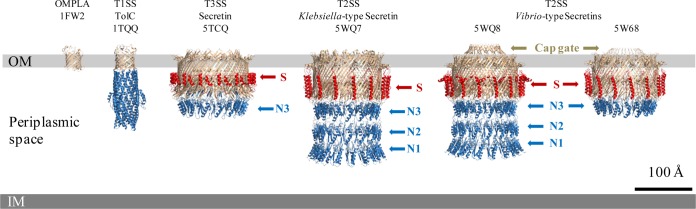
FIG 1.
3D high-resolution structures of secretion system channels and their membrane localization. Shown here to scale in the bacterial envelope, from left to right, are the typical monomeric β-barrel outer membrane protein, OMPLA (PDB accession number 1FW2); the T1SS channel, TolC (PDB 1TQQ) (1); and four bacterial secretins, the T3SS secretin (5TCQ) (3), the T2SS Klebsiella-type secretin (5WQ7), and the T2SS Vibrio-type secretins (5WQ8 [15] and 5W68 [6]), all sharing cylindrical structures. Such cryo-EM near-atomic-resolution 3D structures reveal the more complicated structural organization of bacterial secretins adopting a novel double-β-barrel architecture involving the N3 subdomain (N3) and a partial β-barrel transmembrane and showing the stabilizing property of the S-domain (S). Such resolution also highlights subtle differences among members of bacterial secretins, including the optional cap gate and different modes of outer membrane (OM) insertion, including a possible curvature of the OM for T3SS secretins. IM, inner membrane.
There has been a long series of papers building our understanding of secretin structure that probably originated from the first observation of very-low-resolution EM images of ring-shaped structure for YscC (T3SS), XcpQ (T2SS), and PilQ (T4PS) (7, 8). Slowly but convincingly, it was shown that secretins are large homo-oligomeric assemblies of 12 to 16 monomers forming a distinctive bipartite ring-shaped and cylindrical structure on their C- and N-terminal sides, respectively. They comprise a well-conserved membrane-embedded C-terminal domain and a modular periplasmic N-terminal domain constituted by the piling up of structurally independent subdomains labeled N0 to N3, forming a cylindrical structure. In contrast to the conserved C-domain, the number of N-domains and the structural organization are variable and dependent on the nanomachine type. While four so-called N subdomains, N0, N1, N2, and N3, are found in T2SS secretins, T4PS and T3SS secretins are shorter due to the absence of N1-N2 and N2 subdomains, respectively. This might reflect a specificity associated with these domains and could depend on the kind of secretion performed. In all cases, the N-domain is thought to protrude deep into the periplasm, just as the TolC funnel does. However, it has a totally different organization, and in this case, it likely plays an important role in effector recognition in addition to connecting outer and inner membranes.
During 2 decades of extensive experimentation, the atomic organization of the C-domain (also called the pore-forming or β-domain) of the secretins was sought. Combining biochemistry, imaging, and reprojections, low-resolution cryo-EM images produced for PulD from Klebsiella (9), GspD from Vibrio (10), or PilQ from Neisseria at 12 Å (11) contributed to provide a rough but rational model of a saucer-like complex with gate properties which would sit in the outer membrane. However, the long-awaited higher resolution at 7.4 Å was first glimpsed by the impatient scientific community only in 2016 with a study on the Pseudomonas aeruginosa PilQ T4P secretin (12). A few months later, the 3.6-Å structure of the Salmonella enterica InvG T3SS secretin was published (PDB accession number 5TCQ) (Fig. 1) (3). As much as when the T4SS structure was first published, it was our pleasure to realize that many novel unpredictable features could be found in the secretin organization and to see how much ingenuity bacteria demonstrated in various structures on the same theme (Fig. 1). The secretin C-domain is indeed unique and appears to be formed by a giant pentadecameric double-layered β-barrel with an outer 60-stranded antiparallel β-sheet constituted by the 15 4-stranded β-sheets of each subunit. The high-resolution structure also showed an additional inner barrel whose β-strands extend into the channel lumen and form the periplasmic gate that separates the internal periplasmic chamber and the top lumen. This C-domain is believed to form the outer membrane (OM) portal through which secreted substrates or cell surface appendages transit to the cell surface. The C-terminal S-domain has been shown to be involved in secretin localization, assembly, and outer membrane insertion by interacting with cognate chaperones known as pilotins (13). In the high-resolution structure, the S-domain is well defined and forms a helix-turn-helix motif that extends laterally across neighboring protomers on the exterior of the β-barrel, thus providing structural arguments that, in addition to pilotin binding, S-domains have a key role in efficient secretin assembly and stability (Fig. 1).
In the meantime, the high-resolution cryo-EM structures of two Klebsiella- and Vibrio-type (14) T2SS secretins, from Escherichia coli K-12 (PDB accession number 5WQ7) and Vibrio cholerae (PDB accession number 5WQ8) (Vibrio type), were reported at ∼3-Å resolution (Fig. 1) (15). While confirming the global 3D structure organization observed in the T3SS secretin's β-domains, this dual presentation highlights interesting differences between the two T2SS secretins (Fig. 1). The major difference is the presence of a cap gate in the Vibrio type which is absent in the Klebsiella type, as can be clearly seen in Fig. 1. The cap gate of the Vibrio-type channel has a cone-like structure formed by 15 pairs of antiparallel β12-β13 strands. The linker loops between outer β-barrel and cap gate (β12 and β13 strands) are relatively loose, which suggests that the cap gate of the Vibrio type is probably independent of other structural parts, therefore constituting a signature distinguishing two different classes of secretins (15). The internal diameters of the cap and central gates of the secretins are not compatible with the diameters of the secreted substrates and therefore suggest that purified full-length secretins are in a “closed state” and must undergo conformational changes during the secretion process. Yan et al. (15) proposed that the flexible linker loops on the bottom of the cap gate may act as pivots to allow β-strands in the cap gate to rotate outward during substrate secretion. Regarding the central gate, formed by a four-β-strand extension from the inner β-barrel, site-directed mutagenesis of the highly conserved glycine residues located at the bend points in those β-strands revealed their involvement as pivots to allow the conformational changes of β-strands in the central gate. The authors therefore proposed that upon substrate loading into the lumen of the Vibrio-type channel, the substrate reaches the central gate and triggers the upward rotation of the β-strands around the glycine pivots. This also involves the force provided by a distinct element in the T2SS, the periplasmic pseudopilus, to allow substrate entry into the top lumen. With the uninterrupted drive of the periplasmic pseudopilus, β12 and β13 of the cap gate are forced to move outward to open the cap gate and release the substrate. After substrate release, strands of the cap and central gates revert to their closed-state conformations to start a new translocation cycle.
Altogether, the high-resolution structures of the three secretins revealed that the N3 subdomain, which is common to all secretins, structurally belongs to the C-domain (3, 15) (Fig. 1). It was, moreover, shown that the N2 domain is linked to the N3 domain through a long linker loop inducing considerable flexibility between N0-N1-N2 subdomains and the upper N3/C-domain. This bipartite structural organization is supported by previous studies showing that the N3 subdomain plays an important role in secretin prepore stabilization by forming a thermodynamic seal (16). These observations suggest that secretins are structurally organized into two independent domains, the N-domain, composed of N0, N1, and N2 subdomains, and the C-domain, constituted by the rest of the protein (N3 and former C-domain).
In the paper by Hay et al. (6), it seems obvious that secretin structures are likely to adopt slight variations that may accommodate specific functions even with secretins from the same subfamily. The study presents the cryo-EM high-resolution 3D structure of the T2SS secretin from enteropathogenic E. coli (EPEC) O127:H6 strain E2348/69 (PDB accession number 5W68) at 3.3 Å (Fig. 1) and compares the structure with the other available high-resolution secretin 3D structures. The upper chamber and inner and cap gates are clearly resolved. The N-domain was less well resolved than the upper secretin part, suggesting that there is a relatively high flexibility/movement among the N-domains. Based on sequence analysis, the authors previously proposed that the EPEC secretin belongs to the Vibrio-type subfamily (14). In agreement with sequence similarities, a similar structural architecture can be seen between the two Vibrio-type secretins, i.e., the EPEC structure from the work of Hay et al. (6) and the Vibrio structure from the work of Yan et al. (15), with loops extending from the extracellular side to form the typical cap gate (Fig. 1). It is noticeable that the EPEC and K-12 E. coli secretins diverge, and obvious differences are apparent in the cap structure, which is longer in the EPEC or Vibrio-type secretins and protrudes outside the outer membrane in a closed state. Interestingly, the electrostatic charges of the interiors of the cavities are also dissimilar: the EPEC secretin contains alternating positively and negatively charged bands, whereas the E. coli K-12 Klebsiella-type secretin is largely negatively charged. This would be in agreement with the different shapes and electrostatic properties of their respective secreted substrates, i.e., mucinases StcE and SslE for EPEC and the pullulanase PulA for Klebsiella oxytoca, thus constituting a new environment to investigate substrate specificity in the T2SS. In their study, Hay et al. clarify secretion topography by defining in the EPEC T2SS secretin the hydrophobic belt and aromatic girdle signature that they found to be highly conserved among the sequences of 581 Vibrio-type secretins.
Overall, with their structure-based BLAST experiment, Hay et al. (6) revealed that the previous T2SS secretin classification, i.e., Vibrio and Klebsiella types (14), is definitely structurally relevant. Moreover, in providing an additional high-resolution 3D structure of a secretin, these authors are giving us a reminder of the structural diversity existing among T2SS secretins and are lending support to the existence of subtle functional differences. This should be seen as an encouragement for the resolution of additional structures, which is essential to pinpointing singularities of significant common features.
ACKNOWLEDGMENTS
We thank Badreddine Douzi for fruitful discussion and precious assistance in figure preparation.
Research in the lab of A.F. is supported by MRC program grant MR/K001930/1. T2SS research in the lab of R.V. is supported by ANR grant “4D Secretion” (ANR-14-CE09-0027-01).
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
For the article discussed, see https://doi.org/10.1128/JB.00521-17.
REFERENCES
- 1.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 2.Schraidt O, Marlovits TC. 2011. Three-dimensional model of Salmonella's needle complex at subnanometer resolution. Science 331:1192–1195. doi: 10.1126/science.1199358. [DOI] [PubMed] [Google Scholar]
- 3.Worrall LJ, Hong C, Vuckovic M, Deng W, Bergeron JR, Majewski DD, Huang RK, Spreter T, Finlay BB, Yu Z, Strynadka NC. 2016. Near-atomic-resolution cryo-EM analysis of the Salmonella T3S injectisome basal body. Nature 540:597–601. doi: 10.1038/nature20576. [DOI] [PubMed] [Google Scholar]
- 4.Low HH, Gubellini F, Rivera-Calzada A, Braun N, Connery S, Dujeancourt A, Lu F, Redzej A, Fronzes R, Orlova EV, Waksman G. 2014. Structure of a type IV secretion system. Nature 508:550–553. doi: 10.1038/nature13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korotkov KV, Gonen T, Hol WG. 2011. Secretins: dynamic channels for protein transport across membranes. Trends Biochem Sci 36:433–443. doi: 10.1016/j.tibs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay ID, Belousoff MJ, Dunstan RA, Bamert RS, Lithgow T. 2018. Structure and membrane topography of the vibrio-type secretin complex from the type 2 secretion system of enteropathogenic Escherichia coli. J Bacteriol 200:e00521-17. doi: 10.1128/JB.00521-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis GR, Tommassen J. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol 26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 8.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol 27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 9.Chami M, Guilvout I, Gregorini M, Remigy HW, Muller SA, Valerio M, Engel A, Pugsley AP, Bayan N. 2005. Structural insights into the secretin PulD and its trypsin-resistant core. J Biol Chem 280:37732–37741. doi: 10.1074/jbc.M504463200. [DOI] [PubMed] [Google Scholar]
- 10.Reichow SL, Korotkov KV, Hol WG, Gonen T. 2010. Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol 17:1226–1232. doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins RF, Frye SA, Kitmitto A, Ford RC, Tonjum T, Derrick JP. 2004. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J Biol Chem 279:39750–39756. doi: 10.1074/jbc.M405971200. [DOI] [PubMed] [Google Scholar]
- 12.Koo J, Lamers RP, Rubinstein JL, Burrows LL, Howell PL. 2016. Structure of the Pseudomonas aeruginosa type IVa pilus secretin at 7.4 A. Structure 24:1778–1787. doi: 10.1016/j.str.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Hardie KR, Lory S, Pugsley AP. 1996. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J 15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 14.Dunstan RA, Heinz E, Wijeyewickrema LC, Pike RN, Purcell AW, Evans TJ, Praszkier J, Robins-Browne RM, Strugnell RA, Korotkov KV, Lithgow T. 2013. Assembly of the type II secretion system such as found in Vibrio cholerae depends on the novel pilotin AspS. PLoS Pathog 9:e1003117. doi: 10.1371/journal.ppat.1003117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Z, Yin M, Xu D, Zhu Y, Li X. 2017. Structural insights into the secretin translocation channel in the type II secretion system. Nat Struct Mol Biol 24:177–183. doi: 10.1038/nsmb.3350. [DOI] [PubMed] [Google Scholar]
- 16.Guilvout I, Brier S, Chami M, Hourdel V, Francetic O, Pugsley AP, Chamot-Rooke J, Huysmans GH. 2017. Prepore stability controls productive folding of the BAM-independent multimeric outer membrane secretin PulD. J Biol Chem 292:328–338. doi: 10.1074/jbc.M116.759498. [DOI] [PMC free article] [PubMed] [Google Scholar]