ABSTRACT
Transmembrane bacterial chemoreceptors are extended, rod-shaped homodimers with ligand-binding sites at one end and interaction sites for signaling complex formation and histidine kinase control at the other. There are atomic-resolution structures of chemoreceptor fragments but not of intact, membrane-inserted receptors. Electron tomography of in vivo signaling complex arrays lack distinct densities for chemoreceptor rods away from the well-ordered base plate region, implying structural heterogeneity. We used negative staining, transmission electron microscopy, and image analysis to characterize the molecular shapes of intact homodimers of the Escherichia coli aspartate receptor Tar rendered functional by insertion into nanodisc-provided E. coli lipid bilayers. Single-particle analysis plus tomography of particles in a three-dimensional matrix revealed two bend loci in the chemoreceptor cytoplasmic domain, (i) a short, two-strand gap between the membrane-proximal, four-helix-bundle HAMP (histidine kinases, adenylyl cyclases, methyl-accepting chemoreceptors, and phosphatases) domain and the membrane-distal, four-helix coiled coil and (ii) aligned glycines in the extended, four-helix coiled coil, the position of a bend noted in the previous X-ray structure of a receptor fragment. Our images showed HAMP bends from 0° to ∼13° and glycine bends from 0° to ∼20°, suggesting that the loci are flexible hinges. Variable hinge bending explains indistinct densities for receptor rods outside the base plate region in subvolume averages of chemotaxis arrays. Bending at flexible hinges was not correlated with the chemoreceptor signaling state. However, our analyses showed that chemoreceptor bending avoided what would otherwise be steric clashes between neighboring receptors that would block the formation of core signaling complexes and chemoreceptor arrays.
IMPORTANCE This work provides new information about the shape of transmembrane bacterial chemoreceptors, crucial components in the molecular machinery of bacterial chemotaxis. We found that intact, lipid-bilayer-inserted, and thus functional homodimers of the Escherichia coli chemoreceptor Tar exhibited bends at two flexible hinges along their ∼200-Å, rod-like, cytoplasmic domains. One hinge was at the short, two-strand gap between the membrane-proximal, four-helix-bundle HAMP (histidine kinases, adenylyl cyclases, methyl-accepting chemoreceptors, and phosphatases) domain and the membrane-distal, four-helix coiled coil. The other hinge was at aligned glycines in the extended, four-helix coiled coil, where a bend had been identified in the X-ray structure of a chemoreceptor fragment. Our analyses showed that flexible hinge bending avoided structural clashes in chemotaxis core complexes and their arrays.
KEYWORDS: helical coiled coils, transmembrane proteins, protein arrays, transmembrane signaling, bacterial chemotaxis
INTRODUCTION
Transmembrane chemoreceptors are crucial components of the molecular machinery that mediates bacterial chemotaxis, i.e., the movement of cells in favorable directions in chemical gradients (for reviews, see references 1–3). These receptors bind ligands at sites in their periplasmic domains and interact with each other at their membrane-distal cytoplasmic tips to form trimers of dimers, which in turn interact with the autophosphorylating chemotaxis histidine kinase and a coupling protein to form core signaling complexes (4–7). In vivo, core signaling complexes polymerize to form large arrays that can contain hundreds to thousands of molecules. The receptors are extended rod-like homodimers, and their cytoplasmic domains are composed of helical bundles and helical coiled coils (Fig. 1). Three-dimensional, atomic-resolution structures have been determined for chemoreceptor fragments (8–10) but not for intact receptors. Lower-resolution views of intact chemoreceptors are provided by electron tomographic reconstructions of in vivo arrays of signaling complexes (5, 6). These views are incomplete, however, because receptor densities become diffuse membrane proximal from the well-ordered hexagonal base plate of kinase and coupling protein. This loss of receptor resolution implies variable receptor orientation membrane proximal to the base plate. To investigate this implication, we assessed the shape of isolated functional chemoreceptor homodimers inserted into native lipid bilayers (11–13) by using negative-stain electron microscopy combined with image analysis.
FIG 1.
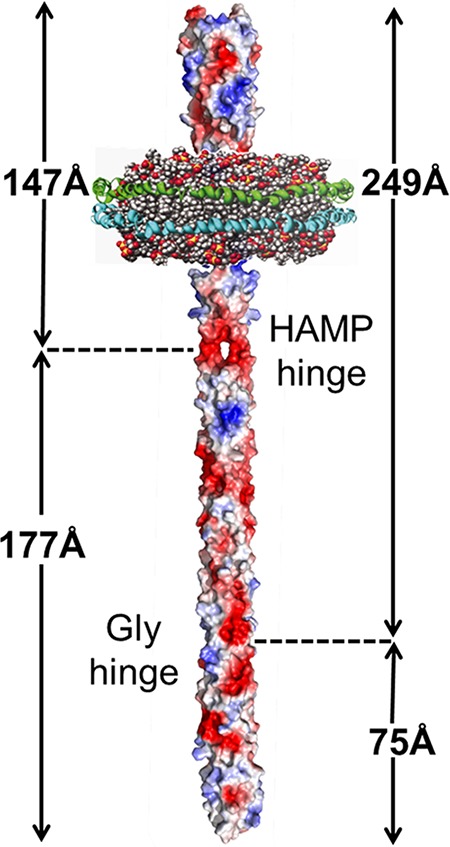
Cartoon of a chemoreceptor dimer inserted in the lipid bilayer of a nanodisc. Basic and acidic side chains are colored blue and red, respectively. Two membrane scaffold proteins (blue and green) encircle a plug of phospholipid bilayer (head groups are red, and hydrophobic chains are gray). Receptor dimensions and the positions of the flexible hinges that are documented in this work are indicated.
Chemotaxis signaling complexes, as well as individual chemoreceptor dimers, are in equilibrium between two functional and conformational states, one kinase on/methylation off and the other kinase off/methylation on (1). The kinase-on/methylation-off state is favored by methylation of specific glutamyl residues in the receptor cytoplasmic domain and by unoccupied ligand-binding sites. The kinase-off/methylation-on state is favored by demethylation and ligand occupancy.
Chemoreceptor homodimers are sufficiently destabilized by detergent solubilization (14) that they do not perform transmembrane signaling or serve as the substrates for physiologically crucial adaptational modifications (11, 15, 16). Fortunately, insertion into lipid bilayers provided by nanodiscs stabilizes chemoreceptor structure (14) and restores function (11–13). Therefore, we characterized chemoreceptor shape using functional receptor homodimers reconstituted into bilayers of native lipids contained in individual nanodiscs.
RESULTS
Chemoreceptor purification and sample preparation.
We purified detergent-solubilized Escherichia coli aspartate chemoreceptor Tar utilizing a six-histidine tag on its carboxyl terminus (17), and we reconstituted single receptor homodimers into individual nanodiscs assembled with native E. coli membrane lipids (11–13, 18). The resulting particles were adsorbed onto continuous carbon-coated grids, treated with negative stain, and examined using a transmission electron microscope, at a nominal magnification of ×39,000. Tar and related chemoreceptor dimers are elongated rods on the order of 300 Å long but only ∼25 Å in diameter over most of that length (19). Effective imaging of these dimers required optimization so that there was sufficient negative stain to reveal the thin molecules but not so much that they were obscured (see Materials and Methods). In addition, optimization was necessary to identify conditions under which nanodiscs adsorbed to the grid in an edge-on orientation with high frequency, thus providing views of the inserted receptor dimers along their long axes. With optimized conditions, many nanodisc-contained receptor dimers were visible in a field (Fig. 2).
FIG 2.

Representative electron micrograph of nanodisc-inserted Tar dimers. The figure shows a field of nanodisc-inserted Tar-4Q particles negatively stained with 0.75% uranyl formate and imaged at a magnification of ×39,000 with a FEI Tecnai F30 transmission electron microscope.
Single-particle, two-dimensional image analysis.
Optimization and initial characterization were performed using Tar in an intermediate signaling state, the one generated by its native-gene-encoded form of two glutamyl residues and two glutaminyl residues at its four sites of adaptational covalent modification, a form designated Tar-QEQE. Images of this receptor displayed heterogeneity of receptor shape, with both apparently straight and apparently bent receptor rods (20). It was possible that this heterogeneity reflected an intermediate signaling state. Thus, we pursued detailed characterization of receptor shape using two forms of Tar, namely, Tar-4Q, which is shifted strongly to a kinase-on/methylation-off state by all glutamyl residues, mimics of methyl glutamates (21, 22), at the four sites of adaptational modification, and Tar-4E, which is shifted strongly to a kinase-off/methylation-on state by all glutamyl residues at those sites (12). From fields like the one shown in Fig. 2, we selected ∼2,000 particles with clearly defined rods of Tar-4Q and ∼1,500 with clearly defined rods of Tar-4E. The iterative stable alignment and clustering (ISAC) algorithm of the SPARX software suite (23) generated 18 stable class averages for images of Tar-4Q and 12 stable class averages for Tar-4E (see Tables S1 and S2 and Fig. S1 in the supplemental material). These classes had improved signal-to-noise ratios, relative to individual particles. For each signaling state, some receptor class averages appeared essentially straight (Fig. 3a and d; also see Fig. S1) and others exhibited a bend at a hinge point in the cytoplasmic domain near the nanodisc-enclosed lipid bilayer (Fig. 3b and e; also see Fig. S1). Figure 3c and f illustrate a second flexible hinge near the membrane-distal tip of the cytoplasmic domain. Class averages with bends at this location were revealed only with partial masking of individual particles and are discussed below.
FIG 3.

Representative two-dimensional class averages illustrating receptor shapes. Class averages, generated with the ISAC program, show examples of Tar-4Q (a to c) and Tar-4E (d to f) particles with no apparent bends (a and d), a bend at the HAMP hinge (b and e), or a bend at the Gly hinge (c and f). Class averages with bends at the Gly hinge were generated by applying an external mask to the nanodisc and periplasmic regions of each primary image in the data set (see the text). See Fig. S1 in the supplemental material for images of all unmasked and masked two-dimensional class averages. The shaded regions in panels c and f show the regions of the individual particles that were masked prior to determination of class averages, thus allowing detection of bends at the glycine hinge (see the text). Straight dashed reference lines are next to each subvolume to aid the eye in distinguishing straight from bent receptors.
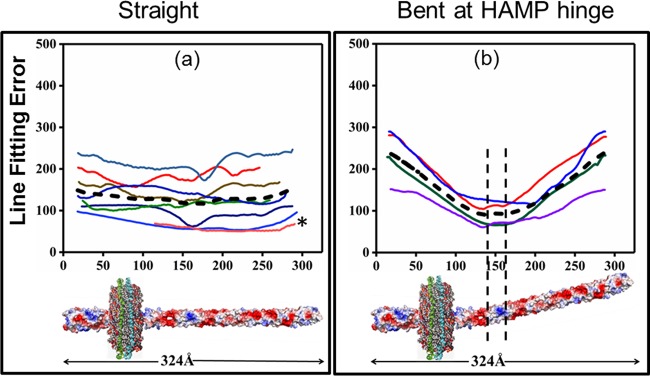
We developed an objective automated procedure for image analysis of chemoreceptor shape for two-dimensional class averages. First, we segmented the receptor from the background and produced a binary mask; next, we determined the medial axis (24) along the ∼300-Å receptor rod of each class average by tracing the center of a circle that occupied the maximal area of receptor density at each point along the receptor long axis. As illustrated by alignment of all of these medial axes by their periplasmic ends (Fig. 4), receptor class averages exhibited a range of orientations along their long axes, from essentially straight to bent. The bends were distributed over a small range of angles, with an apparently common hinge point in the receptor cytoplasmic domain, near the nanodisc-enclosed lipid bilayer. Bending was quantified by fitting each medial axis with one line or two lines and determining a line-fitting error. Briefly, two-line fits were performed for a continuum of intersections of those lines along the receptor long axis at points from the periplasmic tip to the cytoplasmic tip (see Materials and Methods) (Fig. S2). At the two receptor tips, one line would be of zero length and thus those fits were actually to single lines. Deviations of each fit from the medial axis were expressed as line-fitting errors (see Materials and Methods) (Fig. S2e) and plotted as a function of the position of intersection of the two fitting lines along the normalized length of the receptor (Fig. 5; also see Fig. S2f). Bending angles ranged from 0° to a maximum of ∼13° (Fig. 4).
FIG 4.

Aligned medial axes of two-dimensional class averages of chemoreceptors. A plot of medial axes (see the text) for unmasked, two-dimensional, class averages of negatively stained Tar-4Q (blue lines) and Tar-4E (red lines) particles aligned by their respective periplasmic tips is shown above a cartoon representation of nanodisc-inserted chemoreceptor dimers bending at the HAMP flexible hinge. The ordinate scale is expanded relative to the abscissa scale for effective display of the range of bending angles. The position of the HAMP flexible hinge along the receptor long axis is indicated below the cartoon by the intersection of the two dimension bars.
FIG 5.
Analysis of chemoreceptor shapes by line-fitting errors for nanodisc-inserted, surface-adsorbed, negatively stained chemoreceptors. Errors in approximating medial axes by two-line fits (see the text) for each two-dimensional, negatively stained, class average for Tar-4Q (a to c) and Tar-4E (d to f) are plotted as a function of the position on the long axis from which the two-line fit was performed. Using the criteria described in the text, class averages were grouped as essentially straight (a and d), bent at the HAMP hinge (b and e), or bent at the glycine hinge (c and f). For each group, line-fitting errors were averaged and plotted as dashed lines. In panels b, c, e, and f, vertical dashed lines mark ±10 Å from the minimum of the average line-fitting-error curves. Cartoons of the respective receptor shapes are shown below the plots. The shaded regions in panels c and f show the regions of the individual particles that were masked prior to determination of class averages, thus allowing detection of bends at the glycine hinge (see the text).
We grouped receptor class averages as apparently straight or bent by assessing the patterns of two-line fitting errors along the receptor long axis. If error improved in a systematic manner from each tip and thus generated an error minimum, then the class average was considered bent, with the bend at the position of the minimum. If the two-line fitting error instead varied irregularly within a narrow range, then the receptor class average was considered straight. By these criteria, 65% of the images of Tar-4Q particles in stable, two-dimensional, class averages showed a bent receptor and 35% showed a straight receptor (Table S1). The distribution was similar for Tar-4E (71% bent and 29% straight) (Table S2). The range of bending angles (Fig. 4), from 0° to 13° with no apparently preferred angle, was reflected in plots of fitting errors that ranged from sharp through broad to essentially flat (Fig. 5a, b, d, and e). For each bent class average, the position of the error minimum identified the origin of the bend. The origins clustered in a short region along the receptor long axis, implying that these bends occurred at a common locus. To estimate the position of that locus, we calculated an average fitting error curve for bent class averages from each of the two receptor signaling states (dashed black lines in Fig. 5b and e). The minima of these curves placed the hinge ∼150 Å from the periplasmic tip, the location of the two-helix gap between the HAMP domain and the extended helical coiled coil of the cytoplasmic domain. We term this flexible locus the “HAMP hinge,” the name suggested by Swain and Falke (25) when they postulated that it might exist.
Chemoreceptors in a three-dimensional matrix.
Does bending at the HAMP hinge require interaction of nanodisc-embedded chemoreceptors with the surface of the electron microscope grid or is the hinge bent in the absence of surface interactions? We investigated this issue by examining nanodisc-embedded Tar-4Q dimers suspended in a three-dimensional matrix of trehalose containing ammonium molybdate to provide contrast (26). A representative field is shown in Fig. S3a in the supplemental material. We chose this approach, rather than the alternative of suspending unstained particles in vitreous ice, because detection of densities of the very thin (∼25-Å-diameter) chemoreceptor rods in the absence of stain is challenging and the three-dimensional matrix of trehalose served as an effective substitute for vitreous ice. Single-particle analysis, averaging, and image analysis as described for surface-adsorbed Tar-nanodisc particles generated 12 stable, two-dimensional class averages. Some of these class averages showed straight receptor rods and others bent rods (Fig. 6; also see Fig. S3b). Bends were at the HAMP hinge, ∼150 Å from the membrane-distal periplasmic tip and at angles from 0° to 10° (Fig. 6; also see Table S3). Thus, bending at the HAMP hinge occurred independent of interaction with a surface. However, only 30% of nanodisc-inserted Tar-4Q particles suspended in the three-dimensional matrix were bent (Table S3), significantly fewer than the 65% observed for such particles adsorbed to a carbon surface (Table S1). This difference could have been the result of one or more of the following: enhancement of the probability of bending by surface interactions, preference for bent receptors to settle on a surface with the bend in the plane of that surface, or obscuring of receptor bends in a three-dimensional matrix because the bend was parallel to the electron beam. In fact, it was formally possible that all receptors were bent and those that appeared straight had that appearance for one or more of these reasons. We investigated this issue by performing tilt series.
FIG 6.
Analysis of chemoreceptor shapes by line-fitting errors for nanodisc-inserted, trehalose-suspended, negatively stained Tar-4Q particles. Errors in approximating medial axes by two-line fits (see the text) for each chemoreceptor class average are plotted as a function of the position on the long axis from which the two-line fit was performed. As outlined in the legend to Fig. 5, class averages were grouped as essentially straight (a) or bent at the HAMP hinge (b). Line-fitting errors for each class average in the group were averaged and plotted as dashed lines. The plot with an asterisk shows line-fitting errors for the cytoplasmic domain alone. In panel b, vertical dashed lines mark ±10 Å from the minimum of the average line-fitting error. Cartoons of receptor shapes for the two groups are shown below the plots.
Tilt series analysis of receptor shapes.
We generated tomograms from tilt series of nanodisc-embedded Tar-4Q particles suspended in a three-dimensional layer of trehalose and stained with ammonium molybdate. Seventeen tilt series, from +50° to −50°, were collected, aligned, reconstructed, and analyzed with IMOD software (27). Nine series were aligned using patch tracking and eight using 10-nm protein A-coated gold particles as fiducial markers. Tomograms were generated from the alignments and >100 subvolumes that contained individual, nanodisc-inserted, chemoreceptor dimers were extracted. Viewed down the z axis, receptors in some subvolumes appeared straight (Fig. 7a) and others appeared bent at the HAMP hinge (Fig. 7b). We examined each subvolume by rotation around the x axis and the y axis (Fig. S6). By this analysis, 4% of apparently straight receptors were found to be bent in the plane of the z-axis view. Addition of these receptors to the 30% identified as bent at the HAMP hinge in z-axis views indicated that 34% of nanodisc-inserted chemoreceptor dimers suspended in a three-dimensional matrix were bent at the HAMP hinge. This adjusted percentage was still significantly lower than that observed for surface-adsorbed receptors. Thus, we conclude that the HAMP hinge is sufficiently flexible to bend even in the absence of forces generated by surface adsorption but such forces enhance the probability of bending.
FIG 7.

Representative subvolumes from tomographic analysis of nanodisc-inserted Tar particles suspended in a three-dimensional layer of trehalose and stained with ammonium molybdate. Representative subvolumes of Tar-4Q (upper panels) and Tar-4E (lower panels) are shown for chemoreceptors that appear straight (a), bent at the HAMP hinge (b), or bent at the glycine hinge (c). Figures S4 and S5 in the supplemental material show the subvolume slices from which these views were created. As documented by the slices shown in Fig. S4a, the periplasmic domain of the image in panel a is obscured by an interfering density but the cytoplasmic domain is distinctly straight. Straight dashed reference lines are next to each subvolume to aid the eye in distinguishing straight from bent receptors.
The subvolumes revealed a second locus of bending in the Tar cytoplasmic domain (Fig. 7c), ∼75 Å from the membrane-distal tip at a position previously identified as a “glycine hinge” (Gly hinge) (28); it was bent in 33% of subvolumes. The angles of these bends had to be measured by hand because the boundaries of the subvolumes were not sufficiently well defined to use the automated procedure we had developed to assess bending among negatively stained class averages from surface-adsorbed particles. This limitation also made it difficult to identify shallow bend angles. Thus, the bends we could identify were at least 8° (Table S4). We expect that some of the 67% of nanodisc-inserted chemoreceptor dimers classified as straight actually had bending angles of <8°. Among the glycine-hinge-bent receptors we could identify, the maximal bend angle was ∼20° (Table S4). Bends at the glycine hinge were not evident in our initial stable class averages from two-dimensional images. For many of these class averages, however, densities near the membrane-distal tip of the cytoplasmic domain were diffuse (for example, see Fig. 3 and Fig. S1), implying that class members had variable orientations in that region. We investigated this by reanalyzing our primary two-dimensional images, masking densities of the nanodisc and periplasmic domain so that the software would assess only the receptor cytoplasmic domain. In this analysis, there were four stable class averages with distinct bends ∼75 Å from the chemoreceptor membrane-distal tip, two from Tar-4E images and two from Tar-4Q images (Fig. 3c and f and 5c and f). Thus, elimination of the dominant density of the nanodisc plus the density of the periplasmic domain prior to generation of class averages yielded class averages with receptors bent at the glycine hinge. However, these class averages included only 6 to 8% of the receptor images, less than the 33% observed in tomographic analysis. This disparity likely reflects the fact that even with masking a significant proportion of glycine-hinge-bent receptors were placed into class averages of receptor dimers not bent at this locus.
Flexible hinge bending is independent of chemoreceptor signaling state.
Are chemoreceptor bends correlated with receptor signaling states, as has been postulated (1, 28–30)? A role in signaling implies that the extent or features of chemoreceptor bends would change as a function of signaling state. We tested this idea directly by characterizing the shapes of two different signaling conformations of chemoreceptor dimers, namely, Tar-4E, which is strongly shifted toward the kinase-off/methylation-on state by negatively charged glutamyl residues at the four methyl-accepting sites, and Tar-4Q, which is strongly shifted toward the kinase-on/methylation-off state by mimics of methylation at those sites (16). For the HAMP hinge, we observed no significant differences between the two receptor signaling states in the frequency or angle of bends for surface-adsorbed particles (Fig. 4 and 5a, b, d, and e; also see Tables S1 and S2). For the Gly hinge, tomographic analysis of ∼100 receptor-nanodisc particles suspended in a three-dimensional matrix of trehalose found no significant differences between the two receptor signaling states (Table S4). Thus, we found no indication that bends at either flexible hinge are correlated with the chemoreceptor signaling state.
Flexible hinge bending avoids structural clashes in core complexes and arrays.
Instead, flexible hinge bending could have a structural role. Thus, we investigated the geometry of chemoreceptor core signaling complexes and their extended arrays by modeling chemoreceptor placement, orientation, and shape in core signaling complexes and arrays (Fig. 8). Receptor trimers of dimers were positioned according to dimensions and angles reported in the literature or determined in this study. Each dimer was represented as a 324-Å rod (this study) with a diameter of 25 Å (9, 10) for most of its length except for the membrane-distal 50 Å of the periplasmic domain; the eight-helix structure of this region was represented as a truncated cone with a diameter of 40 Å at its membrane-proximal base and 28 Å at its membrane-distal tip (8). Symmetrical trimers of these dimers were constructed with an angle between the trimer central axis and each dimer of 13°, the angle observed in the X-ray structure of a trimer of dimers of cytoplasmic domains (9) and in the well-resolved portion of receptors near the base plate of CheA and CheW in tomographic images of native chemoreceptor arrays (5–7). Trimers of dimers were positioned in a hexagonal array constructed using information provided by electron tomographic analysis of such arrays. Thus, there was a 75-Å center-to-center separation between trimers, and neighboring trimers were positioned with two pairs of dimers facing each other (5–7). In this model, there were structural clashes between straight chemoreceptor dimers in neighboring trimers. Clashes occurred between every pair of receptor dimers that faced each other in the hexagonal array. Figure 8a and b illustrate the clashes between dimers in a core complex, but clashes also occurred between facing dimers in neighboring core complexes (D. Stalla, N. Akkaladevi, F. Bunyak, T. White, and G. L. Hazelbauer, unpublished data). All clashes could be avoided by receptor bends at the HAMP and Gly flexible hinges (see Fig. 8c and d for one example). However, there was only a narrow range of combinations of bend angles at the two flexible hinges that avoided clashes, in contrast to the many combinations that generated clashes (Fig. 9). Overall, our modeling showed that (i) straight chemoreceptor dimers generate structural clashes in core complexes and arrays, (ii) the bending angles we observed experimentally are sufficient to avoid these structural clashes, (iii) Gly hinge bending over a narrow range of experimentally observed angles can avoid clashes without bending at the HAMP hinge, (iv) bending of both flexible hinges over the range of experimentally observed angles expands the range of angles that avoid clashes, and (v) receptor orientations that avoid structural clashes are strikingly limited. Full analysis of clashes as a function of each parameter in our geometric model will be presented elsewhere (Stalla et al., unpublished data).
FIG 8.
Images from a geometric model of the positions of the six chemoreceptor dimers in a core signaling complex, within in a hexagonal array of those complexes. As described in the text, the model is based on experimentally determined distances, lengths, and angles (see panel b for their values). The two pairs of images show a top view (a and c) and a side view (b and d) of the six receptor dimers, with the positions of the two flexible hinges characterized in this study marked by circles and central axis lines above and below the circles. Panels a and b illustrate the structural clashes that would occur if the dimers did not bend at either flexible hinge angle. Panels c and d show one combination of bends at the two hinges (10° at the Gly hinge and 8° at the HAMP hinge) that would avoid structural clashes (see Fig. 9).
FIG 9.

Allowed and forbidden angles at the glycine and HAMP hinges in arrays of core chemotaxis signaling complexes. The diagram illustrates the results of analyses of bending angles for the two flexible hinges in the model illustrated in Fig. 8. To limit the complexity of the analysis, all receptors in the array were bent at the same angle and the angles explored were in the plane defined by the medial axis of the receptor rod and the central axis of each trimer of chemoreceptor dimers. Bends toward the trimer central axis were defined as positive and those away from that axis as negative. All negative bends of the glycine hinge generate structural clashes; therefore, this region of the analysis is not shown. Allowed combinations of angles (white) are those that avoid geometric structural clashes between receptor dimers. Forbidden combinations of angles are those that generate structural clashes between dimers in a trimer (red) or between dimers in neighboring trimers (blue). The white regions of allowed combinations of angles in which one or the other flexible hinge bends at an angle greater than observed experimentally are indicated by stripes.
DISCUSSION
We observed two flexible hinges in intact, bilayer-inserted and thus functional homodimers of an E. coli chemoreceptor. One, the HAMP hinge, was at the boundary between the HAMP domain and the long helical coiled coil of the chemoreceptor cytoplasmic domain (Fig. 10b). The other, the glycine hinge, was ∼75 Å from the membrane-distal tip of the cytoplasmic domain in the midst of the four-helix coiled coil (Fig. 10c). We found no evidence for a preferred angle of hinge bending (Fig. 4; also see Tables S1 to S4 in the supplemental material). Bending was limited to shallow angles, probably reflecting structural limits at these flexible loci. Many receptors were not bent at either hinge, emphasizing that the distribution of bending angles included 0° (Fig. 10a).
FIG 10.

Cartoons of the three receptor shapes documented in this study. The cartoons represent receptors that are straight (a), bending at the flexible HAMP hinge (b), or bending at the flexible glycine hinge (c).
Bending is structurally reasonable at each locus. The HAMP hinge is located in a short two-helix segment, only a few residues in length (31), between the parallel four-helix bundle of the HAMP domain and the antiparallel four-helix coiled coil of the extended receptor rod. There is a helical mismatch between the two helical bundles (3), and the region is the most protease sensitive in the entire chemoreceptor (32), implying that the two helices are not tightly packed. Loosely packed helices would result in flexibility relative to the receptor long axis, providing the basis for a flexible hinge. The glycine hinge is at a locus of aligned glycines in all four helices of the extended coiled coil (28), in the midst of a region of reduced helical packing in the four-helix coiled coil (29). The combination would create a region of reduced structural stability and a basis for a second flexible hinge. Flexible hinges at these two locations are consistent with several observations in the literature. In the following sections, we review those observations and discuss possible roles for flexible hinges in chemoreceptor structure and function.
Indications that chemoreceptors have flexible hinges.
Chemoreceptor bending at the HAMP hinge was postulated in a study of the three-dimensional organization of the HAMP domain of Tar (25). Subsequently, the first negatively stained images of individual bilayer-inserted chemoreceptor dimers showed bends at a position consistent with a HAMP hinge (20). Soon thereafter, course-grained molecular dynamics simulations of E. coli Tsr, a chemoreceptor closely related to Tar, indicated that the simulated chemoreceptor dimer bent readily at the HAMP hinge (33). In our current study, we found that membrane-inserted, functional, chemoreceptor dimers bend at the HAMP hinge, providing direct documentation of a bend that was postulated (25), simulated (33) and initially observed in the course of a study for a different purpose (20). We observed many angles of bending (Fig. 4), which implied that there was not a preferred angle and thus the locus was best considered a flexible hinge and not a pivot point between two specific conformational states. Simulations of Tsr showed related patterns with dynamic exploration of many angles between none and a maximal limit, although the maximal bending angle in those simulations was significantly greater than that we observed for an actual chemoreceptor (33), perhaps reflecting limitations in the course-grained simulation versus observation of actual molecules. Many chemoreceptors have one or more HAMP domains connected to an extended helical coiled coil by a two-helix segment (29). Thus, flexible hinges at this interface could be a widespread feature of chemoreceptors.
Detailed analysis (28) of the crystal structure of a dimeric fragment of the Tsr cytoplasmic domain (9) detected a bend of ∼10° in its four-helix coiled coil at a locus of aligned glycines in all four helices, ∼75 Å from the hairpin tip. The authors named the locus the glycine hinge, the term we have used for the hinge at that location in intact functional chemoreceptors. Mutational analysis of Tar showed that replacement of those glycines with alanines or cysteines greatly reduced or altered receptor function, providing evidence for the functional importance of those positions (28). An evolutionary genomics study of >2,000 sequences of chemoreceptor cytoplasmic domains from >150 bacterial and archaeal species, reflecting bacterial and archaeal taxonomic diversity, identified the region surrounding the glycine hinge as a conserved flexible bundle subdomain and thus a feature likely to have an important role in chemoreceptor function and/or structure (29). Recent analysis of >22,500 chemoreceptor sequences from 5,820 species confirmed this identification (34). The observations documented in our current study demonstrate that intact functional chemoreceptors indeed bend at the glycine hinge. Thus, the bend observed initially in the crystal structure of a fragment of a chemoreceptor cytoplasmic domain reflected a property of intact functional receptor dimers. The evidence from evolutionary genomics analysis implies that such flexible hinges are likely widespread across chemoreceptor diversity.
Receptors in higher-order complexes.
We documented the existence of flexible hinges in intact isolated chemoreceptor dimers. However, receptor dimers function in the bacterial cell as trimers of dimers, and these complexes are incorporated into multicomponent core signaling complexes (4, 20), which in turn assemble into arrays (1, 3, 5, 6). Do chemoreceptors in these higher-order complexes bend at their flexible hinges? Course-grained simulations of receptor trimers of dimers did not detect bending of the constituent dimers (33). However, tomographic reconstructions of in vivo chemotaxis arrays provide indications that chemoreceptor dimers in those arrays have variable orientations, notably in the hinge regions (5, 6). Near the hexagonal base plate consisting of the kinase CheA and the coupling protein CheW, chemoreceptor cytoplasmic domains are seen as distinct densities. Those densities become diffuse for the remainder of the receptor length, beginning membrane proximal to the Gly hinge and extending to the membrane (5, 6). Our observations of bending at the HAMP and Gly hinges suggest a direct explanation for these diffuse receptor densities, i.e., positional variability generated by variable bending at the flexible HAMP and Gly hinges. In fact, subvolume classification and averaging of chemoreceptor densities in those arrays revealed multiple chemoreceptor configurations consistent with receptor bending (6). The authors of that study concluded that chemoreceptor trimers in signaling complexes have a fixed orientation near the base plate but variable orientations in the membrane-proximal region. Thus, tomographic analyses of chemotaxis arrays provide strong evidence that chemoreceptor dimers in chemotaxis arrays bend and there is no single bending angle.
Roles for flexible hinges in chemoreceptors.
Are flexible hinges involved in chemoreceptor function? Mutational analyses of the amino acyl residues at the two hinges have shown that chemotaxis ability is reduced by mutational substitutions at these loci. Substitution of cysteine or alanine for the glycines in the glycine hinge greatly reduced chemotaxis, as well as kinase activation or kinase control through ligand occupancy (28), and a recent, more extensive, mutational analysis observed similar effects of other substitutions (34). The two-helix region of the HAMP hinge corresponds to residues T262, D263, and T264 in Tar (31). Individual cysteine substitutions at these Tar positions greatly reduced the ability of the chemoreceptor to mediate chemotaxis (35), as did other mutational substitutions at the Tsr residue corresponding to Tar position 264 (J. S. Parkinson, personal communication). Overall, mutational analyses document that the side chains of the residues in the flexible hinges are important for chemotaxis function. This importance could reflect side chain requirements for hinge bending.
If bending at chemoreceptor flexible hinges were important for chemotaxis, then the importance could be functional, structural, or both. A central functional property is conformational signaling within a chemoreceptor dimer (12). We investigated whether chemoreceptor bends were correlated with the signaling state of chemoreceptor dimers by characterizing the shapes of strongly kinase-on and strongly kinase-off chemoreceptor dimers (16). We found no significant differences in the frequency or extent of hinge bending as a function of the chemoreceptor signaling state and thus no indication that chemoreceptor bending was linked to receptor conformational signaling as generated by adaptational modification (Fig. 4, 5, and 7). In contrast, we identified a structural role for the flexible hinges, a possibility suggested previously (28). We found that, in core complexes and arrays, structural clashes would occur between neighboring chemoreceptor dimers, thus prohibiting complex formation if the dimers were straight but not if they were bent at their flexible hinges (Fig. 8 and 9). Such clashes were avoided in an all-atom model of a receptor array (7, 36) by modeling receptor dimers as curved rods (C. K. Cassidy, University of Illinois Urbana-Champaign, personal communication). However, the dimer shapes we characterized were not curved rods but rather rods with two distinct flexible hinges. Thus, it appears that chemoreceptor dimers avoid structural clashes in core complexes and arrays by bending at these hinges.
Concluding comments.
We documented the presence of two flexible hinges in the extended, rod-like cytoplasmic domain of an intact, functional bacterial chemoreceptor dimer. It is likely that these hinges are a widespread feature of bacterial chemoreceptors. Available evidence indicates that bending at these hinges occurs in receptors incorporated into chemotaxis arrays and that side chain alterations at the hinges can perturb receptor function. Bending at the flexible hinges did not correlate with the signaling state of isolated chemoreceptor dimers. However, analysis of the geometry of core signaling complexes in arrays revealed that the bends we have documented avoid the structural clashes that would otherwise occur among straight receptor dimers in core signaling complexes and in the extended arrays of those complexes. Such arrays have been observed across bacterial diversity (37). Receptor bends likely play important structural roles in many such arrays.
MATERIALS AND METHODS
Proteins and lipids.
The membrane scaffold protein MSP1D1(−) (38) and the chemoreceptor Tar with a six-histidine carboxyl-terminal extension (Tar-6H) (17), carrying zero, two, or four glutamines at the four methyl-accepting sites, were purified as described previously (18). A polar extract of total E. coli lipids was obtained from Avanti Polar Lipids (Alabaster, AL).
Reconstitution of Tar into nanodiscs.
Nanodiscs containing Tar-6H were prepared essentially as described previously (18), using membrane scaffold protein MSP1D1(−) (38) at molar ratios to E. coli lipids of 1:60 and to Tar dimers of 10:1. The latter ratio is enriched for discs containing single receptor dimers (11, 18). Receptor-containing nanodiscs were separated from empty discs by using a Ni-nitrilotriacetic acid (NTA) affinity column at room temperature (∼24°C) and then were fractionated by size exclusion chromatography at room temperature using a Tosoh Haas TSK G4000 PWXL column (7.8 mm [inner diameter] by 30 cm) equilibrated in 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.5 mM EDTA, and run with the same buffer at 0.6 ml/min. The average number of Tar dimers per disc in each fraction was determined by examination of negatively stained samples with the electron microscope and by quantification of the intensities of Coomassie brilliant blue-stained bands of Tar and MSP1D1(−) on an SDS polyacrylamide gel (18) and comparison with standards of each protein quantified by quantitative amino acid analysis. Fractions containing approximately 1 Tar dimer per disc were pooled and subjected to a second round of size exclusion chromatography and assessment, to produce material with 1 Tar dimer per disc for detailed characterization.
Sample preparation for electron microscopy.
Negative staining of nanodisc-inserted Tar dimers for two-dimensional electron microscopy with uranyl acetate or methyl amine tungstate resulted in many visible nanodiscs but few visible chemoreceptors. Staining with uranyl formate (Ted Pella, Reading, CA) maintained the visibility of nanodiscs and greatly improved contrast and thus visibility of the thin, ∼2.5-nm-diameter chemoreceptor rods inserted in those discs. We attribute improved visibility to the smaller grain size of uranyl formate (39). However, many nanodiscs were oriented en face, not edge on, the orientation in which the extended length of inserted chemoreceptors would be visible. Many more edge-on orientations were generated with the addition of a fluorinated detergent, Fos-Choline-8 (40) (Fig. 2). Specifically, 0.3 μM Fos-Choline-8 was added to nanodisc-inserted Tar dimers diluted to ∼0.05 μM in 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.5 mM EDTA, the mixture was placed immediately on a freshly glow-discharged (15 mA for 45 s at 39 Pa), carbon-coated copper, 300-mesh grid (Pelco Easiglow), the grid was blotted with Whatman no. 1 filter paper to remove excess liquid, and the mixture was stained with 0.75% uranyl formate (Ted Pella), as described previously (39).
For analysis of receptor shape in a three-dimensional matrix, a 2.5-μl droplet of ∼2 μM nanodisc-inserted Tar dimers was placed on a freshly glow-discharged, Multi-A holey carbon, 200-mesh, gold-coated (see below) grid (Quantifoil, GmbH), the grid was blotted with Whatman no. 1 filter paper after 30 s to remove excess liquid, a solution of freshly prepared 1% trehalose, 5% ammonium molybdate (pH 7.0), was added (41), and the grid was blotted after 10 s. For some preparations, the solution included 10-nm colloidal gold (DMC, Utrecht, Netherlands) added to the sample to serve as fiducial markers. The carbon film was sputter-coated on both sides (K575X; Emitech, London, UK) for 20 s at 10 mV, generating ∼5-nm gold.
Data collection.
Electron microscopic images were collected with a FEI Tecnai F30 microscope (FEI Co., Hillsboro, OR) in a low-dose mode at 200 kV (extraction voltage, 3,950 μA; gun lens, 6; spot size, 4), using a Gatan Ultrascan 4000 charge-coupled device (Gatan, Pleasanton, CA). Two-dimensional data were collected at a nominal magnification of ×39,000 (3 Å/pixel). Contrast was increased by 2-fold binning, generating 6 Å/pixel. Tilt series for nanodisc-inserted chemoreceptors suspended in a trehalose matrix and stained with ammonium molybdate and in some cases with 10-nm colloidal gold (added to provide fiducial markers) were collected using Xplore 3D (FEI Co.) between −54° and +54° in 2° increments, using a dose-distribution factor of 1.5 at a nominal magnification of ×31,000, with an underfocus of −2 to −4 μm and an effective pixel size of 3.8 Å/pixel.
Image analysis.
Raw micrographs of nanodisc-inserted Tar dimers were imported and evaluated using EMAN2.1 (42). We used a box size of 128 by 128 pixels in the EMAN2.1 boxer program to pick manually 2,109 particles of uranyl formate-stained Tar-4Q, 1,500 particles of uranyl formate-stained Tar-4E, and 1,800 particles of trehalose-suspended and ammonium molybdate-stained Tar-4Q (42). Automatic contrast transfer function (CTF) parameters generated from CTF-fitting procedures were applied to particles in each micrograph. Reference-free, two-dimensional class averages were generated using the ISAC algorithm of the SPARX software suite (23). Tilt-series data were processed using the IMOD software package (43–45) and aligned utilizing gold fiducials or patch tracking by producing 16 patches (600 by 600 pixels) that included only areas that contained the three-dimensional trehalose matrix (45). Weighted back-projections were used to reconstruct tomograms with 2-fold binning to increase contrast. Nanodisc-inserted Tar-4Q particles were identified in the reconstructed tomograms, and subvolumes were extracted with the boxstartend program in the IMOD software (27) for further inspection.
Segmentation analysis of receptor bending.
Stable, two-dimensional, class average images from the ISAC algorithm were assessed using custom-designed modules for image segmentation, shape analysis and decomposition, and bending analysis (see Fig. S2 in the supplemental material). Image segmentation separated chemoreceptor density from the background by multiscale ridge detection using a Hessian matrix (46), k-means clustering (47, 48), and marker-controlled watershed methods (49) (Fig. S2b). Shape analysis refined the boundaries of the chemoreceptor decomposed shape into three subregions, i.e., the periplasmic domain, the cytoplasmic domain, and the nanodisc (Fig. S2c), and extracted medial axes for the periplasmic and cytoplasmic domains (Fig. S2d). Coordinates of points on the medial axes were extracted and represented by parametric curves described by C[x(t),y(t),t]t = (1,T). The shape of each curve was approximated by two lines, L2a and L2b, corresponding to the sections of the curve on each side of a potential hinge position. For positions at either end of the curve, the two-line fit collapsed into a one-line fit, L1. Individual lines were fit to relevant sections of the curve by using a least-squares polynomial method. Line-fitting errors, E1, were the area between the curve and the line. Two-line fitting errors, E2(t), for positions t on the curve were the sums of fitting errors for the lines L2a(t) and L2b(t) (Fig. S2e). Fitting errors were exhaustively computed for each point t. Hinge points t* were identified as positions on the curve at which the two-line fitting error E2(t) had a distinct minimum (Fig. S2f). Straight chemoreceptor classes had low one-line fitting errors and two-line fitting errors not significantly better. In contrast, the one-line fitting errors for bent chemoreceptor class averages were high, and two-line fitting errors as a function of position t achieved distinct minima for E2(t*), the hinge position. Bending angles were computed using the difference in the slopes of the fitted lines L2a(t*) and L2b(t*).
Masking analysis.
For each of the uranyl formate-stained particles of nanodisc-inserted Tar-4Q and Tar-4E, we placed an external, square-shaped mask over the density of the nanodisc and the chemoreceptor periplasmic domain using SPIDER software (50). The masked images of only cytoplasmic domains were grouped by reference-free, two-dimensional classification with the ISAC program of SPARX (23).
Determination of bending angles at the glycine hinge.
Angles of bending at the glycine hinge were determined by hand for Tar-4Q and Tar-4E subvolumes extracted from the respective tomograms. For each subvolume, two medial axes were drawn along the receptor length, one starting at the periplasmic domain tip and the other starting at the cytoplasmic tip. The angles at the intersection point, the glycine hinge, were measured manually with a protractor.
Modeling of the geometry of chemoreceptors in core signaling complexes and arrays.
We constructed a model of the geometry of chemoreceptors in core signaling complexes and in their arrays using the Mathematica software suite. The 324-Å chemoreceptor homodimers (length determined in this study) were represented as 274-Å rods that were 25 Å in diameter (9, 10) and were capped at their periplasmic tips by a 50-Å truncated cone with a diameter of 40 Å at its membrane-proximal base and 28 Å at its membrane-distal tip (8). Trimers of these dimers were assembled as three straight dimer rods in contact at the membrane-distal tips of their cytoplasmic domains, with a 13° angle between each dimer and the central trimer axis (7, 9). The receptors in core signaling complexes were represented by two trimers placed with their centers separated by 75 Å and pairs of dimers from the adjoining trimers facing each other in a mirrored orientation (6). These trimers were arranged with a uniform symmetric geometry of a hexagonal lattice with 75-Å spacing (5, 6). Figure 8 shows two trimers, representing one core signaling complex, in that hexagonal lattice. The HAMP and Gly hinges were placed 147 Å and 249 Å, respectively, from the periplasmic tip of each receptor dimer (Fig. 1 and 5). The relationship between chemoreceptor structural clashes and hinge bending was investigated in the plane defined by the central axis of each receptor rod and the central axis of the respective trimer, with the hinges of each receptor dimer bent to the same extent. The results of different bending angles were visualized using RegionPlot in Mathematica 11.1, with 10 unique conditionals. The results of different bending angles were visualized by applying 10 unique collision conditionals to a single RegionPlot plot in Mathematica 11.1. Each combination of hinge bending angles was identified as generating or avoiding structural clashes; this yielded a map of allowed glycine hinge and HAMP hinge deflections (Fig. 9) for dimers deflected 13° from the trimer central axis (7, 9). A graphical representation of the system (composed of noninteracting volumes), according to the prescribed geometries, was rendered with Graphics3D.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the University of Missouri Electron Microscopy Core Facility for assistance; Christopher Bottoms and Scott A. Givan, University of Missouri Informatics Research Core Facility, for help using the Linux cluster; Edward Gogol, University of Missouri—Kansas City, for advice and encouragement in early stages of this work; and Wing-Cheung Lai and Mingshan Li for preparation of MSP1D1 and chemoreceptors.
This work was supported by National Institute of General Medical Sciences grant GM29963 to G.L.H.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00593-17.
REFERENCES
- 1.Hazelbauer GL, Falke JJ, Parkinson JS. 2008. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem Sci 33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazelbauer GL, Lai W-C. 2010. Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr Opin Microbiol 13:124–132. doi: 10.1016/j.mib.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkinson JS, Hazelbauer GL, Falke JJ. 2015. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol 23:257–266. doi: 10.1016/j.tim.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Hazelbauer GL. 2011. Core unit of chemotaxis signaling complexes. Proc Natl Acad Sci U S A 108:9390–9395. doi: 10.1073/pnas.1104824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briegel A, Li X, Bilwes AM, Hughes KT, Jensen GJ, Crane BR. 2012. Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc Natl Acad Sci U S A 109:3766–3771. doi: 10.1073/pnas.1115719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Hu B, Morado DR, Jani S, Manson MD, Margolin W. 2012. Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. Proc Natl Acad Sci U S A 109:E1481–E1488. doi: 10.1073/pnas.1200781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy CK, Himes BA, Alvarez FJ, Ma J, Zhao G, Perilla JR, Schulten K, Zhang P. 2015. CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling. Elife 4:e08419. doi: 10.7554/eLife.08419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milburn MV, Prive GG, Milligan DL, Scott WG, Yeh J, Jancarik J, Koshland DE Jr, Kim SH. 1991. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science 254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 9.Kim KK, Yokota H, Kim S-H. 1999. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 10.Airola MV, Watts KJ, Bilwes AM, Crane BR. 2010. Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure 18:436–448. doi: 10.1016/j.str.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boldog T, Grimme S, Li M, Sligar SG, Hazelbauer GL. 2006. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc Natl Acad Sci U S A 103:11509–11514. doi: 10.1073/pnas.0604988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin DN, Hazelbauer GL. 2010. The chemoreceptor dimer is the unit of conformational coupling and transmembrane signaling. J Bacteriol 192:1193–1200. doi: 10.1128/JB.01391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin DN, Hazelbauer GL. 2012. Influence of membrane lipid composition on a transmembrane bacterial chemoreceptor. J Biol Chem 287:41697–41705. doi: 10.1074/jbc.M112.415588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartelli NL, Hazelbauer GL. 2015. Differential backbone dynamics of companion helices in the extended helical coiled-coil domain of a bacterial chemoreceptor. Protein Sci 24:1764–1776. doi: 10.1002/pro.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogonez E, Koshland DE Jr. 1985. Solubilization of a vectorial transmembrane receptor in functional form: aspartate receptor of chemotaxis. Proc Natl Acad Sci U S A 82:4891–4895. doi: 10.1073/pnas.82.15.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin DN, Hazelbauer GL. 2010. Chemoreceptors in signalling complexes: shifted conformation and asymmetric coupling. Mol Microbiol 78:1313–1323. doi: 10.1111/j.1365-2958.2010.07408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai W-C, Hazelbauer GL. 2005. Carboxyl-terminal extensions beyond the conserved pentapeptide reduce rates of chemoreceptor adaptational modification. J Bacteriol 187:5115–5121. doi: 10.1128/JB.187.15.5115-5121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boldog T, Li M, Hazelbauer GL. 2007. Using nanodiscs to create water-soluble transmembrane chemoreceptors inserted in lipid bilayers. Methods Enzymol 423:317–335. doi: 10.1016/S0076-6879(07)23014-9. [DOI] [PubMed] [Google Scholar]
- 19.Weis RM, Hirai T, Chalah A, Kessel M, Peters PJ, Subramaniam S. 2003. Electron microscopic analysis of membrane assemblies formed by the bacterial chemotaxis receptor Tsr. J Bacteriol 185:3636–3643. doi: 10.1128/JB.185.12.3636-3643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Khursigara CM, Subramaniam S, Hazelbauer GL. 2011. Chemotaxis kinase CheA is activated by three neighbouring chemoreceptor dimers as effectively as by receptor clusters. Mol Microbiol 79:677–685. doi: 10.1111/j.1365-2958.2010.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park C, Dutton DP, Hazelbauer GL. 1990. Effects of glutamines and glutamates at sites of covalent modification of a methyl-accepting transducer. J Bacteriol 172:7179–7187. doi: 10.1128/jb.172.12.7179-7187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunten P, Koshland DE Jr. 1991. Tuning the responsiveness of a sensory receptor via covalent modification. J Biol Chem 266:1491–1496. [PubMed] [Google Scholar]
- 23.Yang Z, Fang J, Chittuluru J, Asturias FJ, Penczek PA. 2012. Iterative stable alignment and clustering of 2D transmission electron microscope images. Structure 20:237–247. doi: 10.1016/j.str.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum H. 1973. Biological shape and visual science. I. J Theor Biol 38:205–287. doi: 10.1016/0022-5193(73)90175-6. [DOI] [PubMed] [Google Scholar]
- 25.Swain KE, Falke JJ. 2007. Structure of the conserved HAMP domain in an intact, membrane-bound chemoreceptor: a disulfide mapping study. Biochemistry 46:13684–13695. doi: 10.1021/bi701832b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris JR. 2008. Negative staining across holes: application to fibril and tubular structures. Micron 39:168–176. doi: 10.1016/j.micron.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Mastronarde DN. 1997. Dual-axis tomography: an approach with alignment methods that preserve resolution. J Struct Biol 120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
- 28.Coleman MD, Bass RB, Mehan RS, Falke JJ. 2005. Conserved glycine residues in the cytoplasmic domain of the aspartate receptor play essential roles in kinase coupling and on-off switching. Biochemistry 44:7687–7695. doi: 10.1021/bi0501479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander RP, Zhulin IB. 2007. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc Natl Acad Sci U S A 104:2885–2890. doi: 10.1073/pnas.0609359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson JS. 2007. Ancient chemoreceptors retain their flexibility. Proc Natl Acad Sci U S A 104:2559–2560. doi: 10.1073/pnas.0700278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ames P, Zhou Q, Parkinson JS. 2014. HAMP domain structural determinants for signalling and sensory adaptation in Tsr, the Escherichia coli serine chemoreceptor. Mol Microbiol 91:875–886. doi: 10.1111/mmi.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mowbray SL, Foster DL, Koshland DE Jr. 1985. Proteolytic fragments identified with domains of the aspartate chemoreceptor. J Biol Chem 260:11711–11718. [PubMed] [Google Scholar]
- 33.Hall BA, Armitage JP, Sansom MS. 2012. Mechanism of bacterial signal transduction revealed by molecular dynamics of Tsr dimers and trimers of dimers in lipid vesicles. PLoS Comput Biol 8:e1002685. doi: 10.1371/journal.pcbi.1002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedetta A, Massazza DA, Herrera Seitz MK, Studdert CA. 2017. Mutational replacements at the “glycine hinge” of the Escherichia coli chemoreceptor Tsr support a signaling role for the C-helix residue. Biochemistry 56:3850–3862. doi: 10.1021/acs.biochem.7b00455. [DOI] [PubMed] [Google Scholar]
- 35.Danielson MA, Bass RB, Falke JJ. 1997. Cysteine and disulfide scanning reveals a regulatory α-helix in the cytoplasmic domain of the aspartate receptor. J Biol Chem 272:32878–32888. doi: 10.1074/jbc.272.52.32878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goh BC, Hadden JA, Bernardi RC, Singharoy A, McGreevy R, Rudack T, Cassidy CK, Schulten K. 2016. Computational methodologies for real-space structural refinement of large macromolecular complexes. Annu Rev Biophys 45:253–278. doi: 10.1146/annurev-biophys-062215-011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briegel A, Wong ML, Hodges HL, Oikonomou CM, Piasta KN, Harris MJ, Fowler DJ, Thompson LK, Falke JJ, Kiessling LL, Jensen GJ. 2014. New insights into bacterial chemoreceptor array structure and assembly from electron cryotomography. Biochemistry 53:1575–1585. doi: 10.1021/bi5000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. 2004. Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J Am Chem Soc 126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 39.Ohi M, Li Y, Cheng Y, Walz T. 2004. Negative staining and image classification: powerful tools in modern electron microscopy. Biol Proced Online 6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efremov RG, Leitner A, Aebersold R, Raunser S. 2015. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517:39–43. doi: 10.1038/nature13916. [DOI] [PubMed] [Google Scholar]
- 41.Harris JR, Scheffler D. 2002. Routine preparation of air-dried negatively stained and unstained specimens on holey carbon support films: a review of applications. Micron 33:461–480. doi: 10.1016/S0968-4328(01)00039-7. [DOI] [PubMed] [Google Scholar]
- 42.Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. 2007. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Kremer JR, Mastronarde DN, McIntosh JR. 1996. Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 44.Mastronarde DN, Held SR. 2017. Automated tilt series alignment and tomographic reconstruction in IMOD. J Struct Biol 197:102–113. doi: 10.1016/j.jsb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amat F, Moussavi F, Comolli LR, Elidan G, Downing KH, Horowitz M. 2008. Markov random field based automatic image alignment for electron tomography. J Struct Biol 161:260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Frangi AF, Niessen WJ, Vincken KL, Viergever MA. 1998. Multiscale vessel enhancement filtering, p 130–137. In Wells WM, Colchester A, Delp S (ed), Medical image computing and computer-assisted intervention—MICCAI'98: first international conference, Cambridge, MA, USA, October 11–13, 1998, proceedings. Springer, Berlin, Germany. [Google Scholar]
- 47.Arthur D, Vassilvitskii S. 2007. k-means++: the advantages of careful seeding, p 1027–1035. In SODA '07: proceedings of the eighteenth annual ACM-SIAM symposium on discrete algorithms. Society for Industrial and Applied Mathematics, Philadelphia, PA. [Google Scholar]
- 48.Lioyd SP. 1982. Least squares quantization in PCM. IEEE Trans Inform Theor 28:129–137. doi: 10.1109/TIT.1982.1056489. [DOI] [Google Scholar]
- 49.Meyer F. 1994. Topographic distance and watershed lines. Signal Process 38:113–125. doi: 10.1016/0165-1684(94)90060-4. [DOI] [Google Scholar]
- 50.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. 1996. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol 116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.