ABSTRACT
Amino sugars are good sources of both ammonia and fructose-6-phosphate, produced by the glucosamine 6-phosphate deaminase, NagB. NagB is known to be allosterically regulated by N-acetylglucosamine 6-phosphate (GlcNAc-6P) and the phosphocarrier protein of the bacterial phosphotransferase system, HPr, in Escherichia coli. We provide evidence that NanE, GlcNAc-6P epimerase, and the uridylylated PII protein (U-PII) also allosterically activate NagB by direct protein-protein interactions. NanE is essential for neuraminic acid (NANA) and N-acetylmannosamine (ManNAc) utilization, and PII is known to be a central metabolic nitrogen regulator. We demonstrate that uridylylated PII (but not underivatized PII) activates NagB >10-fold at low concentrations of substrate, whereas NanE increases NagB activity >2-fold. NanE activates NagB in the absence or presence of GlcNAc-6P, but HPr and U-PII activation requires the presence of GlcNAc-6P. Activation of NagB by HPr and uridylylated PII, as well as by NanE and HPr (but not by NanE and U-PII), is synergistic, and the modeling, which suggests specific residues involved in complex formation, provides possible explanations. Specific physiological functions for the regulation of NagB by its three protein activators are proposed. Each regulatory agent is suggested to mediate signal transduction in response to a different stimulus.
IMPORTANCE The regulation of amino sugar utilization is important for the survival of bacteria in a competitive environment. NagB, a glucosamine 6-phosphate deaminase in Escherichia coli, is essential for amino sugar utilization and is known to be allosterically regulated by N-acetylglucosamine 6-phosphate (GlcNAc-6P) and the histidine-phosphorylatable phosphocarrier protein, HPr. We provide evidence here that NanE, GlcNAc-6P epimerase, and the uridylylated PII protein allosterically activate NagB by direct protein-protein interactions. NanE is essential for N-acetylneuraminic acid (NANA) and N-acetylmannosamine (ManNAc) utilization, and the PII protein is known to be a central metabolic nitrogen regulator. Regulatory links between carbon and nitrogen metabolism are important for adaptation of metabolism to different growth conditions.
KEYWORDS: glucosamine 6-phosphate deaminase/isomerase, NagB, allosteric regulation, protein-protein interactions, nitrogen regulator, PII, N-acetylglucosamine 6-phosphate epimerase, NanE, signal transduction, N-acetylglucosamine 6-phosphate epimerase, NanE, nitrogen regulator, PII, signal transduction
INTRODUCTION
Amino sugars, including N-acetylglucosamine (GlcNAc) and N-acetylneuraminic acid (NANA) (1), are present in many glycans in all organisms from bacteria to animals, and in mammals, for example, they are found in human milk (2) and cell surface mucus (3). The microbiome member and model organism for Gram-negative bacteria, Escherichia coli, is an important human pathogen and is used in numerous biotechnological applications. E. coli tightly controls the utilization of amino sugars, which are excellent sources of both carbon and ammonia.
NANA is essential for the synthesis of some polysaccharides and the glycosylation of certain proteins and lipids in both eukaryotes and prokaryotes. Exogenous NANA is utilized by E. coli via the transporter, NanT, and further hydrolyzed by a lyase, NanA, to produce pyruvate and N-acetylmannosamine (ManNAc). ManNAc and mannosamine (ManN), as well as glucosamine (GlcN), are taken up from the growth medium by the ManXYZ enzyme complex of the bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) and concomitantly phosphorylated to ManNAc-6P, ManN-6P, and GlcN-6P, respectively, and N-acetylglucosamine is taken up via a distinct sugar phosphorylating PTS permease, NagE (4–6) (Fig. 1). In contrast, ManNAc, produced from NANA hydrolysis, is phosphorylated in the cell by an ATP-dependent ManNAc kinase: NanK, regulated by the NanR regulator (7).
FIG 1.
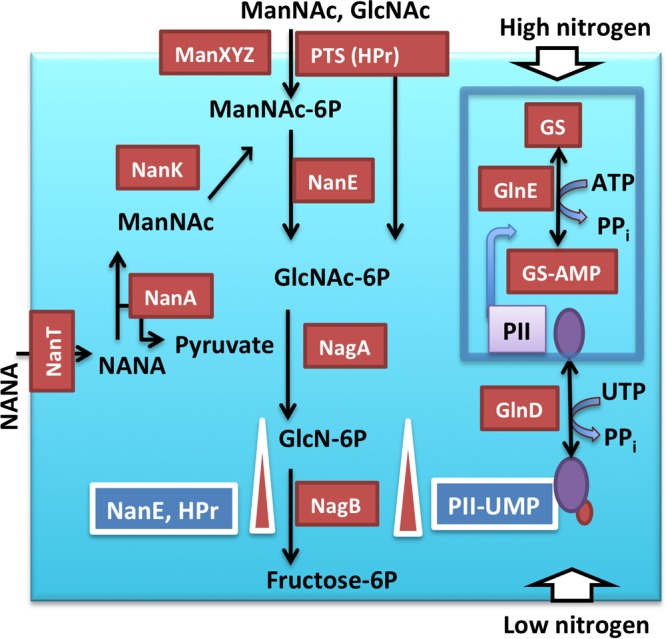
Amino sugar utilization in E. coli. Protein-protein interactions of HPr-NagB, U-PII-NagB, and NanE-NagB activate NagB by increasing the affinity of the enzyme for its substrate, GlcN-6P, and/or increasing the Vmax. NagB, glucosamine 6-phosphate deaminase. These proteins are indicated by white rectangles adjacent to the upward-pointing arrows. NagA, N-acetylglucosamine 6-phosphate deacetylase; NANA, N-acetyl-d-neuraminic acid; NanA, NANA aldolase/lyase; PTS, phosphotransferase system; ManXYZ, the mannose PTS transport/kinase system; NanT, the NANA transporter; NanK, ManNAc kinase; GS, glutamine synthetase; PII (GlnB), nitrogen regulator; GlnD, uridylyltransferase/uridylylase for the PII protein; GlnE, adenylyltransferase/adenylylase.
The pathway for N-acetylmannosamine utilization includes an epimerase, NanE, which converts ManNAc-6P to GlcNAc-6P as part of the NANA and ManNAc utilization pathways (Fig. 1). NagA further deacetylates GlcNAc-6P to GlcN-6P. Glucosamine 6-phosphate isomerase/deaminase, NagB, provides the last step in the amino sugar-specific catabolic pathway, converting GlcN-6P to NH3 and fructose 6-phosphate (Fru-6P), the first common metabolite of glycolysis. This enzyme is essential for the utilization of amino sugars in E. coli and is known to be allosterically activated by an intermediate of the GlcNAc metabolic pathway, GlcNAc-6P (8–10). Orthologs of this enzyme are present not only in bacteria but also in mammals and other organisms. E. coli NagB is encoded in an operon with the nagA gene and is regulated by NagC (11), being induced when GlcN, GlcNAc, or another amino sugar is available in the medium.
The biosynthetic pathway producing UDP-GlcNAc for incorporation into cell wall components involves the glmS, glmM, and glmU gene products, and GlmS generates the cytoplasmic GlcN-6P pool essential for peptidoglycan biosynthesis. NagB redirects GlcN-6P to the glycolytic pathway and is thus important and tightly regulated. In E. coli, nagA and nagB occur in an operon, regulated by the transcriptional regulator, NagC, and these genes are expressed only when amino sugars are present in the medium. NagB activity is regulated by two previously recognized factors, GlcNAc-6P, as noted above (allosteric regulation), and a primary protein constituent of the PTS, HPr, a sensor of the availability of extracellular PTS sugar substrates (12), including GlcNAc, ManNAc, and GlcN.
NagB interactome data (13), presented in part in Table 1, suggest that NagB interacts with several cellular proteins, including the nitrogen-related signal transduction PII protein (14, 15), the NanE epimerase described above, proline aminopeptidase PepP, nitroreductase NfsB (capable of reducing nitrofurazone and quinones), and even the riboflavin biosynthetic enzymes RibA, RibB, and RibC. The work reported here shows that NagB is activated by NanE in the presence or absence of GlcNAc-6P but not by BglA (used as a negative control) and is activated in the presence of GlcNAc-6P by the PII protein covalently modified by uridylylation, an indicator of nitrogen availability. The other interactions, suggested by the data in Table 1, have not been examined.
TABLE 1.
Protein-protein interactome for NagB, suggesting that NagB interacts with numerous proteins in the E. coli cell
| Protein | Locationa | Score | Protein name and EC no. |
|---|---|---|---|
| DdpA | PE | 9.9 | d,d-Dipeptide-binding periplasmic protein DdpA |
| GcvT | MA | 6.1 | Aminomethyltransferase (glycine cleavage system T protein) (EC 2.1.2.10) |
| NfsB | MA | 6.1 | Nitroreductase |
| PepP | CY | 5.9 | Xaa-Pro aminopeptidase |
| GlnH | PE | 5.9 | Glutamine ABC transporter, substrate-binding protein GlnH |
| GcvP | MA | 5.8 | Glycine dehydrogenase (decarboxylating; glycine cleavage system P protein) (EC 1.4.4.2) |
| YifE | MA | 5.7 | UPF0438 protein YifE |
| CysS | CY | 5.7 | Cysteinyl-tRNA synthetase (EC 6.1.1.16) |
| HemB | CY | 5.6 | Porphobilinogen synthase (EC 4.2.1.24) |
| GpmM | CY | 5.5 | 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase (EC 5.4.2.1) |
| BglA | CY | 5.4 | 6-Phospho-β-glucosidase (EC 3.2.1.86) |
| NanE | CY | 5.4 | N-Acetylmannosamine-6-phosphate 2-epimerase (EC 5.1.3.9) |
| RibC | CY | 5.4 | Riboflavin synthase alpha chain RibC |
| Zwf | CY | 5.4 | Glucose-6-phosphate 1-dehydrogenase (EC 1.1.1.49) |
| RibB | MA | 5.4 | 3,4-Dihydroxy-2-butanone 4-phosphate synthase (EC 4.1.99.12) |
| GlnB | CY | 5.3 | Nitrogen regulatory protein P-II |
| ProC | CY | 5.3 | Pyrroline-5-carboxylate reductase (EC 1.5.1.2) |
| PpiA | PE | 5.3 | Peptidyl-prolyl cis-trans isomerase ppiA precursor (EC 5.2.1.8) |
| YbbN | CY | 5.3 | Thioredoxin domain-containing protein EC-YbbN |
| PtsH | MA | 5.3 | Phosphocarrier protein HPr |
| FumA | MA | 5.3 | Fumarate hydratase class I, aerobic (EC 4.2.1.2) |
| BioB | CY | 5.3 | Biotin synthase (EC 2.8.1.6) |
| NemA | CY | 5.3 | Flavoprotein NADH-dependent oxidoreductase |
| RibA | CY | 5.3 | GTP cyclohydrolase II |
PE, periplasm; MA, membrane; CY, cytoplasm.
The uridylylated PII protein (U-PII) is generated by posttranslational modification under nitrogen-limiting conditions involving the glutamine/α-ketoglutarate ratio-sensing uridylyltransferase/uridylyl-removing enzyme GlnD (16, 17). Adenylylation of glutamine synthetase, GlnA, is stimulated by the PII protein, GlnB, and deadenylylation is stimulated by U-PII, thus comprising a dual bicyclic cascade.
The regulatory interdependence between different metabolic pathways has been considered (18). For example, carbon metabolism is known to be controlled not only by carbon-derived signals but also by the availability of nitrogen, sulfur, and iron (19–21). Components of the PTS participate in regulatory interactions (22), resulting in the control of carbon and nitrogen metabolism (23–25), and recently, the histidine-phosphorylatable phosphocarrier protein, HPr, was shown to control the activities of glycolytic enzymes, including NagB, by direct protein-protein interactions (12).
We show here that activation of NagB by U-PII but not by PII in the presence of GlcNAc-6P leads to an increase in activity of >10-fold. The synergistic effects of HPr/U-PII and HPr/NanE but not of U-PII/NanE on NagB activation have been demonstrated. The modeling of HPr/U-PII/NagB and HPr/NanE/NagB complex formation confirmed the possibility that the two proteins (HPr/U-PII or HPr/NanE) can simultaneously interact with NagB, although U-PII and NanE cannot. These observations are rationalized from both mechanistic and physiological standpoints.
RESULTS
Effects of PII and NanE on NagB activity measured at fixed concentrations of both GlcNAc-6P and GlcN-6P.
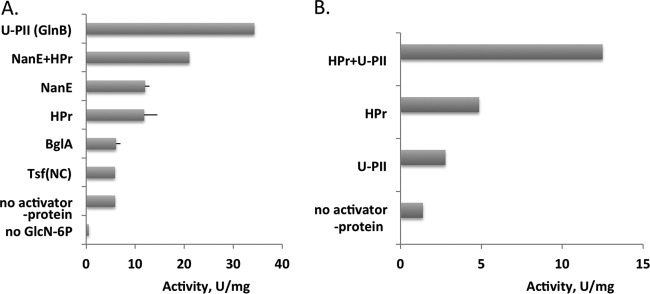
Allosteric regulation by GlcNAc-6P is known for both the E. coli NagB and the nonorthologous deaminase/isomerase, NagB-II, from Shewanella, the latter belonging to the sugar isomerase protein family (26). To demonstrate regulation of E. coli NagB, suggested by the protein-protein interactome data (Table 1), we examined the effects of purified NanE (0.7 μM), partially uridylylated PII (GlnB; 1 μM), 6-phospho-β-glucosidase (BglA; 0.2 μM), and the translation elongation factor (Tsf; 2 μM). All of these proteins are involved in carbon and nitrogen metabolism, but we have not examined all 24 of the proteins shown in Table 1, and the latter two proteins were used as negative controls for the activation of NagB. Based on activity measurements for NagB (0.01 μM), with a 0.4 mM concentration of the allosteric effector, GlcNAc-6P, and a 5 mM concentration of the substrate, GlcN-6P, NanE activated NagB as shown in Fig. 2A. There was no effect when either purified Tsf or purified BglA was added under the conditions used, in spite of the fact that an interaction of BglA with NagB has been reported (13) (Fig. 2A). The activating effect of NanE on NagB activity was ∼2-fold. However, a much greater effect was observed with purified uridylylated PII. In this experiment, the increase of NagB activity in the presence of freshly purified PII (partially uridylylated in the cell) was 7-fold compared to the negative controls when no protein or BglA or Tsf was added.
FIG 2.
Protein-protein interaction-dependent activation of NagB in the presence of a nonsaturating concentration of the allosteric effector, GlcNAc-6P (0.4 mM). (A) Activity was measured at 5 mM GlcN-6P in the presence of NanE (0.7 μM), PII (GlnB; 1 μM), and/or HPr (0.5 μM), BglA (0.2 μM), and Tsf (2 μM). The latter two proteins were included as negative controls (NC). (B) Activity was measured at 2 mM GlcN-6P in the presence of HPr (0.5 μM), U-PII (0.05 μM), or a combination of the same concentrations of HPr and U-PII at pH 7.5.
Activation of the Ser-1 mutant of NagB by GlcNAc-6P (with a Ka = 2.1 mM) has been described (12), and the concentrations of the activator used in the experiments reported here were less than required for saturation. The experiments showed that U-PII and HPr activate NagB only in the presence of GlcNAc-6P, and only NanE has an activating effect in the absence of GlcNAc-6P.
PII (GlnB)-dependent activation of NagB depends on the uridylylation state of GlnB.
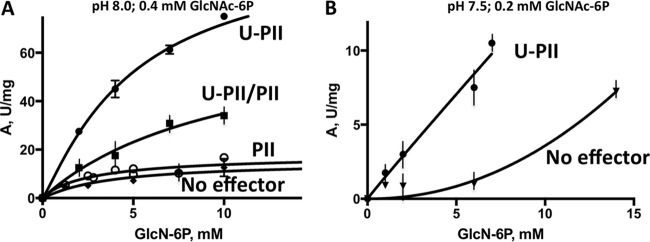
The kinetics for NagB in the presence of PII at different stages of PII modification involving uridylylation by GlnD were measured (Fig. 3A). The reaction mixture for the covalent modification of PII included 0.2 M Tris (pH 8), 0.3 mM ATP, 3 mM UTP, 1 mM DTT, 200 nM GlnD, 50 mM KCl, 1 mM α-ketoglutarate (α-KG), and 25 mM MgSO4. After incubating this reaction mixture for 20 min at 30°C, the partially uridylylated PII was collected, and after 4 h at 25°C, GlnD generated fully uridylylated PII (U-PII). For both PII forms, the kinetics for NagB activation were measured using 0.4 mM GlcNAc-6P. The assay mixture included 0.5 mM ATP and 1 mM UTP, in addition to the usual NagB assay mixture. The kinetics revealed increased activity in the presence of fully uridylylated 0.4 μM U-PII at pH 8 (Fig. 3B; Table 2). The titration was measured with U-PII (Ka = 0.14 ± 0.03 μM) (data not shown).
FIG 3.
Allosteric activation of NagB by the uridylylated PII protein (U-PII). (A) The kinetics were measured as a function of the GlcN-6P concentration (0 to 10 mM) in the presence of the allosteric effector, 0.4 mM GlcNAc-6P, in the absence or presence of 0.4 μM partially uridylylated U-PII (■), 0.4 μM fully uridylylated U-PII (●), or 0.6 μM free PII (○), all at pH 8. The assay is described in Materials and Methods. (B) Steady-state kinetics of NagB measured as a function of the GlcN-6P concentration (0 to 14 mM) in the presence of the allosteric effector, 0.2 mM GlcNAc-6P, in the absence or presence of 0.2 μM U-PII at pH 7.5. One unit of activity = 1 μmol of product formed/mg of protein/min.
TABLE 2.
Kinetic parameters of NagB measured with respect to the GlcN-6P concentrationa
| NagB conditions | Mean ± SD |
|||
|---|---|---|---|---|
| NagB activity | Vmax (U/mg) | Hill coefficient | Khalf (mM) | |
| pH 8.1, 0.4 mM GlcNAc-6P | +U-PII | 90 ± 11 | 1.3 ± 0.3 | 3.6 ± 0.8 |
| –PII | 11.3 ± 1.8 | 1.6 ± 0.8 | 2.9 ± 0.7 | |
| +PII | 16.8 ± 2.0 | 1.0 ± 0.4 | 2.3 ± 0.6 | |
| pH 7.8, no GlcNAc-6P | +NanE | 5.4 ± 0.4 | 2.1 ± 0.3 | 4.8 ± 0.5 |
| –NanE | 3.5 ± 0.9 | 2.1 ± 0.8 | 8.0 ± 2.0 | |
| pH 6.8, 0.2 mM GlcNAc-6P | +NanE | 28.9 ± 3.5 | 1.9 ± 0.6 | 2.0 ± 0.4 |
| –NanE | 13 ± 1.3 | 1.4 ± 0.4 | 1.9 ± 0.4 | |
The kinetic parameters of NagB were measured with respect to the GlcN-6P concentration in the presence or absence of PII or U-PII with 0.4 mM GlcNAc-6P at pH 8.1 and in the presence or absence of NanE at pH 7.8 (no GlcNAc-6P) or at pH 6.8 with 0.2 mM GlcNAc-6P. “U” indicates micromoles per minute. The pH values used were those that appeared to give the largest activation of NagB.
The effect was greater at pH 7.5 than at pH 8 due to the allosteric behavior of the Ser-1 mutant NagB, resulting from an increased Hill coefficient, also noticed for the wild-type NagB (8). Accordingly, U-PII at 0.2 μM substantially decreased the NagB Khalf for GlcN-6P when the kinetics were measured at pH 7.5. The increase in NagB activity was >10-fold in the presence of U-PII at low or high concentrations of the substrate GlcN-6P. The potential activation of NagB by GlnD was tested in the presence of different effectors, and no activation or inhibition under the conditions described for the NagB assay was detected.
The pH dependency of NagB with a standard concentration of GlcNAc-6P has been published (8), and the kcat does not vary between pH 6 and pH 9 (27). However, the Hill coefficient changes substantially with an optimum at pH 7.7 (Hill coefficient of 2.6), dropping below pH 7 or above pH 8.8. All of our results obtained at different pH values (pH 8.0, 7.8, 7.5, and 6.8) are in agreement with this statement (Table 2) and with the previously published results (27). The conditions used are relevant to the physiological conditions of intact E. coli cells.
NanE-dependent activation of NagB is not dependent on GlcNAc-6P.
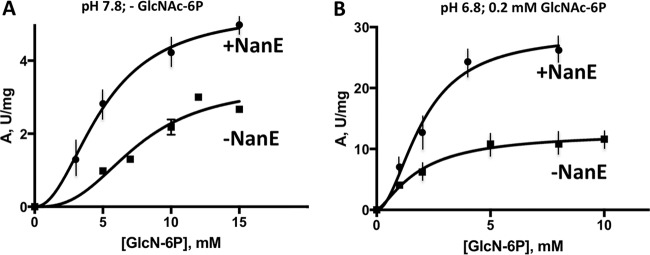
NanE activation of NagB was measured in the presence and absence of GlcNAc-6P. The activity measurements with respect to GlcN-6P concentration are shown in Fig. 4. The effect of 0.7 μM NanE at pH 7.8 is shown in Fig. 4A without the effector, GlcNAc-6P. Under these conditions, NagB should be largely in the poorly active T-state. Increased activity of >2-fold was observed, particularly at low concentrations of the substrate, GlcN-6P. The kinetics with 0.2 mM GlcNAc-6P (the partially activated state of NagB), measured at pH 6.8 and pH 7.8 in the presence or absence of 0.7 μM NanE, are presented in Fig. 4B and Table 2.
FIG 4.
Allosteric activation of NagB by NanE. (A) Steady-state kinetics of NagB were determined as a function of the GlcN-6P concentration (0 to 15 mM) in the absence or presence of 0.7 μM NanE at pH 7.8. (B) The same steady-state kinetics of NagB activity at pH 6.8 and in the presence of the allosteric effector, 0.2 mM GlcNAc-6P, in the absence or presence of 0.7 μM NanE. The resultant kinetic parameters are presented in Table 2. The assay is described in Materials and Methods.
Synergistic effects of HPr- and U-PII-dependent and of HPr- and NanE-dependent activation of NagB measured at fixed concentrations of both GlcNAc-6P and GlcN-6P.
HPr has previously been shown to activate NagB (12). The synergistic effects of HPr and U-PII at nonsaturating concentrations on NagB activity were measured with a 0.4 mM concentration of the allosteric effector, GlcNAc-6P, and a 2 mM concentration of the substrate GlcN-6P (Fig. 2B). A 0.1-ml portion of the assay mixture contained 0.2 M Tris (pH 7.5), 1 mM ATP, 1 mM dithiothreitol (DTT), 2 mM phosphate, 20 mM KCl, 10 mM MgSO4, 2 mM NADP, 1.2 U of phosphoglucose isomerase (Pgi), and 1.2 U of glucose 6-phosphate dehydrogenase (Zwf). The activation effect for U-PII (at 0.05 μM) was 2-fold, and for HPr it was 4-fold. The synergistic effect when both U-PII and HPr were added together was 10-fold when the same concentrations of these proteins were present (0.05 and 0.5 μM, respectively).
When NanE and HPr were added together in the same assay mixture, but with a 0.2 mM concentration of the allosteric effector, GlcNAc-6P, and a 3 mM concentration of the substrate, GlcN-6P, there was substantial synergism at concentrations of HPr greater than 0.8 μM (Fig. 5). Titration with nonphosphorylated HPr showed sigmoidal kinetics in the presence of 0.3 μM NanE, showing that activation by HPr exhibits strong cooperativity in the presence of NanE. Titration results with HPr in the presence of 0.05 μM U-PII are shown in Fig. 5.
FIG 5.
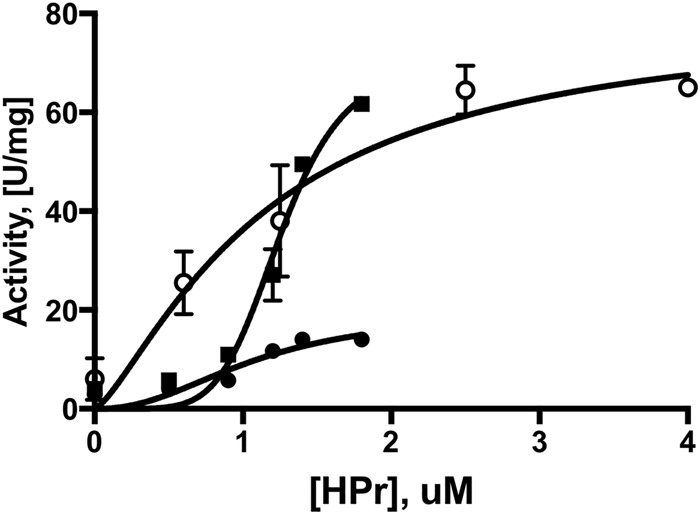
The activity of NagB was measured as a function of HPr concentration, 0 to 1.8 μM, with 3 mM GlcN-6P in the presence of 0.3 μM NanE (■) or 0.05 μM U-PII (○) and in the absence of both proteins and the presence of the effector GlcNAc-6P at 0.2 mM (●). Synergy of HPr and NanE was observed at concentrations of HPr in excess of 0.8 μM.
Modeling of HPr/U-PII and of HPr/NanE binding to NagB.
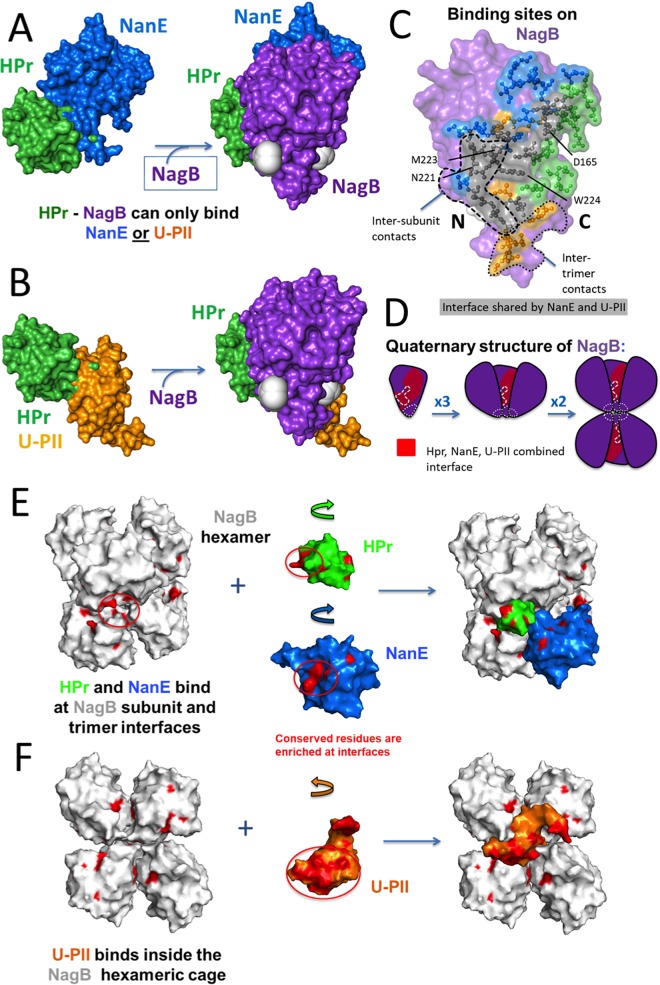
It appears that U-PII (PDB 5L9N chain A) and HPr (PDB 3CCD chain A), as well as NanE (SMR model, NanE_Ecoli:A) and HPr, exert synergistic effects on NagB (PDB 1FS5 chain A) through simultaneous binding. The structure used for NagB modeling (1FS5 chain A) was the ligand-bound, active-site R conformer, with GlcNAc-6P removed, rather than the T conformer. From HADDOCK protein-protein docking, it was possible to find energetically favorable conformations for the binary complexes of HPr/NagB, U-PII/NagB, and NanE/NagB where HPr would not sterically prevent either U-PII or NanE binding, although U-PII and NanE would sterically exclude each other. This was revealed by aligning the three separate binary complexes involving NagB and observing the positions of HPr, U-PII, and NanE. All three binary complexes had significant HADDOCK and z-scores and were in the top four clusters of docked results. All three binary complexes were either the largest or second-largest cluster in their respective docking outputs.
The gray balls in Fig. 6C are labeled with “N” and “C,” indicating the N and C termini of NagB. NagB has been rotated in Fig. 6C to show the NagB interaction interface and, consequently, the N and C termini are in different orientations than in Fig. 6A.
FIG 6.
Synergistic effects of the NagB protein-protein interactions (PPIs) is possible as a result of simultaneous binding of protein partners. (A and B) Structural models of simultaneous binding are shown for NagB plus HPr plus NanE (A), as well as NagB plus HPr plus U-PII (B). The binding of HPr to NagB does not sterically obstruct the binding of either NanE (A) or U-PII (B) in the model, but the binding of NanE to NagB does sterically obstruct the binding of U-PII (and vice versa). Although the figure shows that NanE and HPr are together before binding to NagB in panel A and U-PII and HPr are together before binding to NagB in panel B, there is no evidence that this actually occurs. (C) Binding sites for HPr (green), NanE (blue), and U-PII (orange) on the surface of NagB. Note that the majority of the NanE and U-PII interfaces on NagB overlap each other (shown in gray). Residues indicated by black lines are shared among all three NagB partners. (D) Functional NagB is a hexamer in which the cavity interacts with solvent (red); the intersubunit trimer contacts, a loop spanning residues 216 to 223, are indicated by heavy dashed lines, while the intertrimeric contacts (residues 244 to 250) are indicated by light dashed lines. The orientation of the NagB monomer with respect to the trimer and hexamer is indicated. (E) Overlay of the HPr and NanE with a hexamer of NagB. HPr (green) and NanE (blue) attach at trimer and subunit interfaces, respectively, with very little steric hindrance, at highly conserved residues (red) on both themselves and NagB. Green and blue arrows indicate how HPr and NanE, respectively, rotate from position shown to position on NagB hexamer (45° clockwise from top). (F) Overlay of U-PII with a hexamer of NagB (two NagB subunits removed for clarity). U-PII binds within the hexameric cage of NagB, attaching using highly conserved residues. A loop of U-PII (top right) sterically hinders one of the six NagB subunits (not shown). The orange arrow indicates how U-PII rotates from position shown to position in NagB hexamer (45° counterclockwise from top). PDB identification codes and chains used for NagB, HPr, and U-PII were 1FS5 chain A (open R conformer), 3CCD chain A, and 5L9N chain A (uridylylated PII), respectively. For NanE, the full-length Swiss Model Repository model based on the template 3IGS chain A (79.7% sequence identity) was used. For the NagB hexamer, PDB 1CD5, chains A to F, was used and six 1FS5 A chains were aligned onto the hexamer, proving a close approximation of the open, R conformer NagB hexamer.
The structure of NagB used (PDB 1FS5 chain A) is the free active-site, GlcNAc-6P ligand-bound, R conformer of a monomer of NagB. The R form seemed more appropriate than the unbound T form since the structural modeling being performed was for NagB binding several protein partners, which were found in this study to promote activation of NagB. However, functional E. coli NagB is a hexamer (28). Therefore, we placed the model onto a NagB hexamer. HPr and NanE from our model fit onto the NagB hexamer with very little steric hindrance, on the contrary appearing to bind neatly within the NagB intertrimer and intersubunit clefts (Fig. 6E), respectively, using highly conserved residues. U-PII appears to bind within the NagB hexameric cage (Fig. 6F), in close proximity to all six NagB subunits, also using highly conserved residues. U-PII displays significantly more steric hindrance, as a loop (residues 100 to 107, containing a turn between residues 102 to 104) clashes with one of the six NagB subunits. However, it is likely that this loop is highly flexible and could be rearranged upon binding, with minimal energy penalties.
With respect to whether complete information about known interacting residues was used, the docking program used, HADDOCK, is a local refinement docking program and is optimized for finding the most biologically accurate conformation, but it requires an approximation of the interface as a guide. CPORT, a highly sensitive consensus prediction server, combining predictions from several distinct interface prediction servers, was used to generate this approximation. It is likely that CPORT captured all known interacting residues, including those in the enzyme active sites. This may also be why it captured subunit interface residues (see Discussion).
DISCUSSION
The PII protein is known to be a regulator of both the activity and the synthesis of glutamine synthetase (GS; GlnA) in enteric bacteria, and of nitrogen metabolism in many other bacteria, archaea, and eukaryotes, in response to the availability of nitrogen sources (Fig. 1) (29–33). The pathways that regulate glnA gene expression and GS enzymatic activity both involve the covalent modification of proteins (Fig. 1). The regulation of GS activity involves deadenylylation for activation and adenylylation for inactivation with both reactions catalyzed by the same enzyme, adenylyltransferase/adenylylase (GlnE). The direction of GS modification is dictated by the PII protein, the state of which is also regulated by reversible covalent modification by uridylylation catalyzed by GlnD, another bifunctional enzyme regulated oppositely by αKG and glutamine. The modified form, U-PII, is essential for the deadenylylation reaction acting on GS.
We found that the modified form of PII, U-PII, activates NagB. Coordinate activation of both NagB and GS by U-PII makes teleological sense since activation of the former releases NH3, while activation of GS facilitates its incorporation into glutamine for the synthesis of numerous other nitrogenous compounds. The effects and consequences of the NagB allosteric interactions can be summarized as follows. (i) The presence of amino sugars in the medium dephosphorylates HPr and activates NagB if and only if GlcNAc-6P is present. (ii) An increase in the cytoplasmic GlcNAc-6P concentration promotes high levels of nagB expression and high NagB activity. (iii) The activation of NagB by U-PII promotes successful utilization of amino sugars, thereby increasing levels of both carbon and nitrogen in the cell (Fig. 7). (iv) GS will be converted to the unmodified active form, allowing the incorporation of the NH3 released from GlcN-6P into glutamine. (v) NanE activation of NagB only occurs when NANA is available, promoting high-level expression of the nanE gene.
FIG 7.
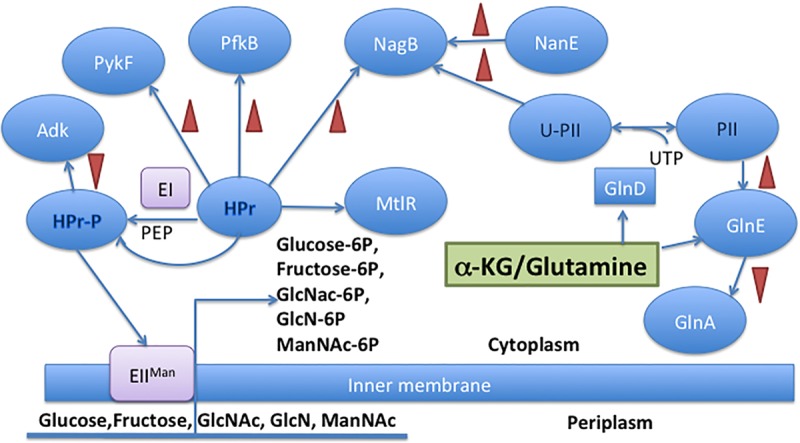
Protein-protein interaction networks for known regulatory effects of HPr, U-PII, and NanE on NagB. The left side of the diagram shows the effects of HPr on carbon and energy metabolism, including its effect on NagB (12), while the right side shows the effects of the indicated proteins on nitrogen metabolism, including NagB. See the text for protein abbreviations. Regulation of the mannitol catabolic regulator, MtlR, has been described (40). NagB synthesis is regulated by NagC.
The pathways for the utilization of different amino sugars—NANA, ManNAc-6P, and GlcNAc-6P—converge with the production of GlcN-6P, the substrate of NagB (Fig. 1). The protein-protein interactome data for HPr have led to the suggestion that NagB is a connecting point with the nitrogen regulatory module, with the U-PII protein playing a central role (Fig. 7). If GlcN or GlcNAc is transported into the cell by the PTS, HPr, present in the nonphosphorylated form, will activate NagB in response to the availability of PTS sugar substrates if and only if cytoplasmic GlcNAc-6P is present (12). However, what if NANA is utilized? During exogenous NANA utilization, the transporter is NanT, and no accumulation of GlcNAc-6P occurs; this means that the PTS protein, HPr, should be largely phosphorylated (HPr-P), and HPr-P has no effect on the activity of NagB (12). Under these conditions, nanE gene expression is induced in response to the availability of cytoplasmic NANA, so that even in the absence of GlcNAc-6P, NagB will be activated by NanE. The concentration of GlcNAc-6P can be low during NANA utilization, since NanE is the epimerase for ManNAc-6P, catalyzing a reversible reaction to GlcNAc-6P as product. nagB expression is dependent on the GlcNAc-6P concentration, and during NANA utilization, when cytoplasmic concentrations of GlcNAc-6P are low, activation of NagB by NanE is physiologically relevant.
In the situation where the nitrogen source is limiting, the utilization of low concentrations of amino sugars produces the essential level of nitrogen, and the synergistic effect of U-PII- and HPr-dependent activation of NagB (as sensors of nitrogen limitation and extracellular amino sugar availability, respectively) is also physiologically relevant.
Thus, we propose that NanE activates NagB when cytoplasmic or exogenously derived NANA is available and metabolized. HPr activates NagB only when the PTS is used for amino sugar uptake, and U-PII activates NagB primarily under nitrogen-limiting conditions. Thus, NanE transmits a signal indicating the presence of cytoplasmic NANA, HPr signals the availability of an extracellular amino sugar substrate, and U-PII signals nitrogen deficiency since GlnD, which uridylylates PII, senses the ratio of cytoplasmic αKG to glutamine. These signal transducing systems (Fig. 7) allow the bacteria to respond to at least three different signals, all converging to regulate the activity of glucosamine-6-phosphate deaminase in response to nitrogen and carbon source availability.
We have further shown that HPr and U-PII, as well as HPr and NanE, act synergistically under appropriate conditions, enhancing the activating effect of either one. In contrast, U-PII and NanE had no measurable synergistic effect, suggesting that they bind to the same site or overlapping sites on NagB or the NagB-HPr complex. One noteworthy aspect of the NagB interface (adjacent/overlapping binding sites for U-PII, NanE, and HPr) is that it is highly disordered. There is almost no secondary structure at all, although there are loops. Also noteworthy is that this disordered area is large, approximately one-fourth of the entire surface area of NagB. This suggests that the NagB interface is highly flexible. Analysis of the models suggests that HPr, U-PII, and NanE bind very close to each other on NagB. It is possible that the flexibility of the NagB interface allows for the simultaneous binding of HPr/U-PII, as well as HPr/NanE. However, an entire alpha helix of NanE overlaps with the core of the U-PII binding site, making simultaneous binding of these two NagB activators unlikely, no matter what extent of flexibility is allowed. Moreover, the modeling of the HPr/U-PII/NagB and HPr/NanE/NagB complexes suggested which residues are involved in the simultaneous interactions of the two proteins (HPr/U-PII or HPr/NanE) with NagB (data not shown). They explain why the possibility of simultaneous U-PII/NanE binding could be excluded.
MATERIALS AND METHODS
GlcNAc-6P, GlcN-6P, NADP, UTP, l-glutamine, α-ketoglutarate, DTT, and other chemicals were purchased from Sigma-Aldrich, USA.
Protein-protein interaction analysis for the NagB interactome.
The scores recorded in Table 1 represent the log likelihood scoring (LLS), which was computed by integrating the HyperGeometric Spectral Counts score (HGSCore [34]) and the Comparative Proteomic Analysis Software Suite (ComPASS) S-score (35) into a single combined score to define high-quality associations. The procedures for the LLS calculation and precise recombination and perfect in-frame fusion of the SPA-tag to the natural C terminus of the target proteins are described elsewhere (13).
Cloning nagB into pMST3.
The nagB gene, encoding the GlcN-6P deaminase, NagB, was PCR amplified from the E. coli BW25113 chromosome using the oligonucleotides nagB-Bam-F (ATAGGATCCAGACTGATCCCCCTGACTACCGCTGAAC) and nagB-Sal-R (CTCGTCGACTTACAGACCTTTGATATTTTCTGCTTC). The product was gel purified, digested with BamHI and SalI, and then cloned into the pSMT3 vector digested with the same restriction endonucleases. Individual clones were confirmed by colony PCR and subsequently by DNA sequencing. The resultant recombinant plasmid, pMST3-nagB, carried the nagB structural gene (without the first codon) fused to the 3′ end of the SUMO gene (without its stop codon) encoding the SMT3-His tag. Expression of “SUMO:nagB” was under the control of the T7 promoter. The SMT3 tag, present in the fusion protein, was removed using the Ulp1 Sumo protease. The resultant NagB enzyme (Ser1-NagB) has a serine residue instead of the N-terminal methionine residue (Met1-NagB).
Protein purification.
Recombinant proteins NagB, NanE, PII (GlnB), GlnD, Zwf, Pgi, Tsf, and BglA, all containing an N-terminal His6 tag, were overexpressed in E. coli and purified using Ni2+-chelating chromatography. E. coli OE strains for NanE, PII, GlnD, Zwf, Pgi, Tsf, and BglA, all from the ASKA collection (36), were used for protein purification. Strains were grown in Luria-Bertani medium (50 ml), induced by the addition of 0.6 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and harvested after 4 h of shaking. Rapid purification of recombinant proteins on Ni-nitrilotriacetic acid-agarose minicolumns was performed as described previously (37). PII protein was refolded as described previously (12).
NagB activity measurements.
The activity of the purified recombinant NagB protein was routinely assayed in a cuvette at 37°C using a standard enzymatic coupling assay involving phosphoglucose isomerase (Pgi) and glucose 6-phosphate dehydrogenase (Zwf) by measuring the increase in absorbance at 340 nm resulting from the reduction of NADP as described previously (26). NagB kinetics as a function of the GlcN-6P concentration were measured using 0 to 0.4 mM GlcNAc-6P in a 0.1-ml assay mixture in the presence of 0.2 M Tris (pH 6.5 to 8.1), 5 mM phosphate, 10 mM MgSO4, 3 mM NADP, 50 mM KCl, 1.2 U of Zwf, 1.2 U of Pgi, and 10 to 50 nM NagB.
Effect of different protein-protein interactions on NagB activity.
We examined the effects of His-tagged, purified, recombinant proteins, NanE, PII (GlnB), GlnD, Zwf, Tsf, and BglA on NagB activity. GlnD proved to have no effect on NagB activity under the conditions used in the assay mixture. The activity of NagB was measured after purification with a His tag, followed by proteolytic removal of the His tag resulting in the Ser-1 NagB derivative. Met-1 in NagB has been shown to play a role in activation by GlcNAc-6P (10), possibly explaining the higher Ka of 2.1 mM (12) in the activation of NagB by GlcNAc-6P reported here compared to that reported previously (9). E. coli NagB activity was measured by monitoring the increase in absorbance at 340 nm resulting from the reduction of NADP in a coupled assay involving Pgi and Zwf. This assay is based on the conversion of GlcN-6P to fructose 6-phosphate by NagB, followed by isomerization to glucose 6-phosphate by Pgi and further oxidation of glucose 6-phosphate to gluconate 6-phosphate by Zwf. We showed that the E. coli Zwf (NADPH producing), under the conditions used, had no effect on NagB activity (data not shown). The Zwf with a different cofactor specificity (NADH-producing) from Leuconostoc mesenteroides was used as the coupling enzyme (38) to test the effect of E. coli Zwf, and the NagB kinetics were the same regardless of which coupling enzyme was used.
GlnD-dependent uridylylation of the PII protein.
GlnD was assayed in a 1-ml assay mixture containing the purified refolded PII recombinant protein at a concentration of 10 μM. The reaction for the covalent modification of PII included 0.2 M Tris (pH 7.5), 1 mM ATP, 3 mM UTP, 1 mM DTT, 200 nM GlnD, 50 mM KCl, and 0.5 mM α-ketoglutarate. The reaction mixture was incubated at 30°C for 20 min and for 4 h at 25°C. The level of PII uridylylation was measured by native gel electrophoresis (data not shown). The fully posttranslationally modified U-PII was not purified from the PII uridylylation reaction mixture, but no effect of this mixture on NagB activity was noticed. In this control, the PII uridylylation mixture without the PII protein was added to the NagB assay mixture. It should be noted that the former mixture was added to the latter mixture with a 10-fold dilution (data not shown). Loss of uridylylation occurred during incubation of the U-PII protein for 3 days at 4°C in the elution buffer.
Structural modeling of the U-PII/HPr and NanE/HPr protein interactions with NagB.
HPr, NanE, and U-PII were each docked to NagB individually using the HADDOCK webserver with CPORT-predicted interface residues as active and passive restraints. All structures from clusters with negative z-scores (below average energy scores among clusters of the top 200 structures) were considered in the modeling. Docked complexes for HPr/NagB, NanE/NagB, and U-PII/NagB were aligned by NagB in Pymol. The four, one, and three clusters for HPr/NagB, NanE/NagB, and U-PII/NagB, respectively, were selected for further analysis, since these allowed unobstructed orientations for HPr and NanE, as well as HPr and U-PII, in their bound states with NagB. The HPr/NagB and NanE/NagB clusters were both the largest (greatest number of docking models) clusters from their respective docking runs, while the U-PII/NagB cluster was the second largest. The PDB identification codes and chains used for NagB, HPr, and U-PII were IFS5 chain A (1FS5 chain A), 3CCD chain A (3CCD chain A), and 5L9N chain A (5L9N chain A), respectively. 1FS5 chain A is a structure of the open, “R” conformation of NagB, and 5L9N chain A is a structure of uridylylated PII. For NanE, the full-length Swiss Model Repository model based on the template 3IGS chain A (79.7% sequence identity) was used. For fitting of the model into the NagB hexamer, PDB 1CD5 chains A to F were used. 1CD5 is the NagB hexamer in the closed T form. For studying the fitting of the model within the hexamer, six copies of the open R conformer, 1FS5 chain A, were aligned onto each of the six subunits of 1CD5. For conservation analyses ConSurf-DB (39) was used, and only the most highly conserved (level 9/9) residues are highlighted in Fig. 6.
ACKNOWLEDGMENTS
We thank Jimmy Do for help with plasmid purification and Zhongge Zhang for constructing the nagB overexpression strain.
This study was supported by NIH grant GM109895.
I.A.R. completed the experiments, M.H.S. supervised the study, I.A.R. and M.H.S. analyzed the data and wrote the manuscript, P.U. and N.G. performed modeling for the protein-protein interaction, A.E. conducted interactome studies, and M.B. conducted the interactome studies reported in Table 1.
REFERENCES
- 1.Vimr ER, Troy FA. 1985. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol 164:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrido D, Ruiz-Moyano S, Mills DA. 2012. Release and utilization of N-acetyl-d-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 18:430–435. doi: 10.1016/j.anaerobe.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plumbridge J, Vimr E. 1999. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J Bacteriol 181:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barabote RD, Saier MH Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol Mol Biol Rev 69:608–634. doi: 10.1128/MMBR.69.4.608-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher J, Ake FM, Derkaoui M, Zebre AC, Cao TN, Bouraoui H, Kentache T, Mokhtari A, Milohanic E, Joyet P. 2014. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol Mol Biol Rev 78:231–256. doi: 10.1128/MMBR.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalivoda KA, Steenbergen SM, Vimr ER. 2013. Control of the Escherichia coli sialoregulon by transcriptional repressor NanR. J Bacteriol 195:4689–4701. doi: 10.1128/JB.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calcagno M, Campos PJ, Mulliert G, Suastegui J. 1984. Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from Escherichia coli. Biochim Biophys Acta 787:165–173. doi: 10.1016/0167-4838(84)90076-1. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Anorve LI, Bustos-Jaimes I, Calcagno ML, Plumbridge J. 2009. Allosteric regulation of glucosamine-6-phosphate deaminase (NagB) and growth of Escherichia coli on glucosamine. J Bacteriol 191:6401–6407. doi: 10.1128/JB.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara-Gonzalez S, Dixon HB, Mendoza-Hernandez G, Altamirano MM, Calcagno ML. 2000. On the role of the N-terminal group in the allosteric function of glucosamine-6-phosphate deaminase from Escherichia coli. J Mol Biol 301:219–227. doi: 10.1006/jmbi.2000.3937. [DOI] [PubMed] [Google Scholar]
- 11.Plumbridge JA. 1991. Repression and induction of the nag regulon of Escherichia coli K-12: the roles of nagC and nagA in maintenance of the uninduced state. Mol Microbiol 5:2053–2062. doi: 10.1111/j.1365-2958.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodionova IA, Zhang Z, Mehla J, Goodacre N, Babu M, Emili A, Uetz P, Saier MH Jr. 2017. The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli. J Biol Chem 292:14250–14257. doi: 10.1074/jbc.M117.795294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu M, Bundalovic-Torma C, Calmettes C, Phanse S, Zhang Q, Jiang Y, Minic Z, Kim S, Mehla J, Gagarinova A, Rodionova I, Kumar A, Guo H, Kagan O, Pogoutse O, Aoki H, Deineko V, Caufield JH, Holtzapple E, Zhang Z, Vastermark A, Pandya Y, Lai CC, El Bakkouri M, Hooda Y, Shah M, Burnside D, Hooshyar M, Vlasblom J, Rajagopala SV, Golshani A, Wuchty S, Greenblatt JF, Saier M, Uetz P, Moraes TF, Parkinson J, Emili A. 2017. Global landscape of cell envelope protein complexes in Escherichia coli. Nat Biotechnol doi: 10.1038/nbt.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forchhammer K, Luddecke J. 2016. Sensory properties of the PII signaling protein family. FEBS J 283:425–437. doi: 10.1111/febs.13584. [DOI] [PubMed] [Google Scholar]
- 15.Merrick M. 2014. Posttranslational modification of PII signal transduction proteins. Front Microbiol 5:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Heeswijk WC, Molenaar D, Hoving S, Westerhoff HV. 2009. The pivotal regulator GlnB of Escherichia coli is engaged in subtle and context-dependent control. FEBS J 276:3324–3340. doi: 10.1111/j.1742-4658.2009.07058.x. [DOI] [PubMed] [Google Scholar]
- 17.Ninfa AJ, Jiang P. 2005. PII signal transduction proteins: sensors of alpha-ketoglutarate that regulate nitrogen metabolism. Curr Opin Microbiol 8:168–173. doi: 10.1016/j.mib.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Commichau FM, Forchhammer K, Stulke J. 2006. Regulatory links between carbon and nitrogen metabolism. Curr Opin Microbiol 9:167–172. doi: 10.1016/j.mib.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Choi SK, Saier MH Jr. 2005. Regulation of sigL expression by the catabolite control protein CcpA involves a roadblock mechanism in Bacillus subtilis: potential connection between carbon and nitrogen metabolism. J Bacteriol 187:6856–6861. doi: 10.1128/JB.187.19.6856-6861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan JA, Schneider BL, Paulsen IT, Yamada M, Kredich NM, Saier MH Jr. 2002. Regulation of carbon utilization by sulfur availability in Escherichia coli and Salmonella typhimurium. Microbiology 148:123–131. doi: 10.1099/00221287-148-1-123. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH Jr. 2005. Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol 187:980–990. doi: 10.1128/JB.187.3.980-990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saier MH., Jr 1993. Regulatory interactions involving the proteins of the phosphotransferase system in enteric bacteria. J Cell Biochem 51:62–68. doi: 10.1002/jcb.240510112. [DOI] [PubMed] [Google Scholar]
- 23.Ninfa AJ, Jiang P, Atkinson MR, Peliska JA. 2000. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr Top Cell Regul 36:31–75. doi: 10.1016/S0070-2137(01)80002-9. [DOI] [PubMed] [Google Scholar]
- 24.Pfluger-Grau K, Gorke B. 2010. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol 18:205–214. doi: 10.1016/j.tim.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 25.van Heeswijk WC, Westerhoff HV, Boogerd FC. 2013. Nitrogen assimilation in Escherichia coli: putting molecular data into a systems perspective. Microbiol Mol Biol Rev 77:628–695. doi: 10.1128/MMBR.00025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, Rodionov DA, Li X, Laikova ON, Gelfand MS, Zagnitko OP, Romine MF, Obraztsova AY, Nealson KH, Osterman AL. 2006. Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J Biol Chem 281:29872–29885. doi: 10.1074/jbc.M605052200. [DOI] [PubMed] [Google Scholar]
- 27.Montero-Moran GM, Lara-Gonzalez S, Alvarez-Anorve LI, Plumbridge JA, Calcagno ML. 2001. On the multiple functional roles of the active site histidine in catalysis and allosteric regulation of Escherichia coli glucosamine 6-phosphate deaminase. Biochemistry 40:10187–10196. doi: 10.1021/bi0105835. [DOI] [PubMed] [Google Scholar]
- 28.Oliva G, Fontes MR, Garratt RC, Altamirano MM, Calcagno ML, Horjales E. 1995. Structure and catalytic mechanism of glucosamine 6-phosphate deaminase from Escherichia coli at 2.1 Å resolution. Structure 3:1323–1332. doi: 10.1016/S0969-2126(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 29.Lillo C. 2008. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem J 415:11–19. doi: 10.1042/BJ20081115. [DOI] [PubMed] [Google Scholar]
- 30.Muro-Pastor MI, Reyes JC, Florencio FJ. 2005. Ammonium assimilation in cyanobacteria. Photosynth Res 83:135–150. doi: 10.1007/s11120-004-2082-7. [DOI] [PubMed] [Google Scholar]
- 31.Francis SH, Engleman EG. 1978. Cascade control of E. coli glutamine synthetase. I. Studies on the uridylyl transferase and uridylyl removing enzyme(s) from Escherichia coli. Arch Biochem Biophys 191:590–601. [DOI] [PubMed] [Google Scholar]
- 32.Son HS, Rhee SG. 1987. Cascade control of Escherichia coli glutamine synthetase: purification and properties of PII protein and nucleotide sequence of its structural gene. J Biol Chem 262:8690–8695. [PubMed] [Google Scholar]
- 33.Arcondeguy T, Jack R, Merrick M. 2001. P(II) signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol Mol Biol Rev 65:80–105. doi: 10.1128/MMBR.65.1.80-105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, Beekman C, Wong C, Rhee DY, Cenaj O, McKillip E, Shah S, Stapleton M, Wan KH, Yu C, Parsa B, Carlson JW, Chen X, Kapadia B, VijayRaghavan K, Gygi SP, Celniker SE, Obar RA, Artavanis-Tsakonas S. 2011. A protein complex network of Drosophila melanogaster. Cell 147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sowa ME, Bennett EJ, Gygi SP, Harper JW. 2009. Defining the human deubiquitinating enzyme interaction landscape. Cell 138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 37.Rodionova IA, Li X, Thiel V, Stolyar S, Stanton K, Fredrickson JK, Bryant DA, Osterman AL, Best AA, Rodionov DA. 2013. Comparative genomics and functional analysis of rhamnose catabolic pathways and regulons in bacteria. Front Microbiol 4:407. doi: 10.3389/fmicb.2013.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olavarria K, De Ingeniis J, Zielinski DC, Fuentealba M, Munoz R, McCloskey D, Feist AM, Cabrera R. 2014. Metabolic impact of an NADH-producing glucose-6-phosphate dehydrogenase in Escherichia coli. Microbiology 160:2780–2793. doi: 10.1099/mic.0.082180-0. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg O, Erez E, Nimrod G, Ben-Tal N. 2009. The ConSurf-DB: precalculated evolutionary conservation profiles of protein structures. Nucleic Acids Res 37:D323–D327. doi: 10.1093/nar/gkn822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choe M, Park YH, Lee CR, Kim YR, Seok YJ. 2017. The general PTS component HPr determines the preference for glucose over mannitol. Sci Rep 7:43431. doi: 10.1038/srep43431. [DOI] [PMC free article] [PubMed] [Google Scholar]