Abstract
This study investigated the changes in the major etiologic organisms and clinical phenotypes of nontuberculous mycobacterial lung disease (NTM-LD) over a recent 15-year period in Korea. The increase of number of patients with NTM-LD was primarily due to an increase of Mycobacterium avium complex (MAC) lung disease (LD). Among MAC cases, the proportion of M. avium increased compared with M. intracellulare, whereas the incidence of M. abscessus complex and M. kansasii LD remained relatively stable. The proportion of cases of the nodular bronchiectatic form increased compared with the fibrocavitary form of NTM-LD.
Keywords: Nontuberculous Mycobacteria, Mycobacterium avium Complex, Mycobacterium abscessus, Mycobacterium kansasii, Epidemiology, Republic of Korea
Graphical Abstract
Nontuberculous mycobacteria (NTM) generally refer to mycobacteria other than Mycobacterium tuberculosis complex and M. leprae.1 The incidence and prevalence of NTM lung disease (NTM-LD) are increasing worldwide including Korea,2,3 and among the more than 150 officially recognized NTM species, the most frequent human pathogens are M. avium complex (MAC), M. abscessus complex (MABC), and M. kansasii.1 MAC, in which M. avium and M. intracellulare are the main pathogens, is the most common etiology of NTM-LD.2,3 MABC is the second most common etiology of NTM-LD in many countries and is currently divided into three species (M. abscessus, M. massiliense, and M. bolletii), with M. abscessus complex lung disease (MABC-LD) due predominantly to M. abscessus and M. massiliense.2,3 Although previous studies suggest that species differentiation for these complexes has epidemiologic and clinical implications,4,5 many studies report MAC or MABC as single entities.6 In Korea, previous studies showed that the NTM isolation rate and the number of patients with NTM-LD have increased steadily.7,8,9,10,11,12 However, the long-term trend of the changes of causative organisms of NTM-LD was not available in Korea.
NTM-LD typically comprises two major clinical phenotypes, namely, the fibrocavitary and nodular bronchiectatic forms.1 The fibrocavitary form (also known as upper lobe cavitary form) is characterized by cavitary lesions, which occur predominantly in the upper lobes, and usually develops in older males with underlying lung disease (LD) such as pulmonary tuberculosis and/or chronic obstructive pulmonary disease.1 The nodular bronchiectatic form occurs predominantly in non-smoking postmenopausal women and presents as bilateral bronchiectasis with multiple nodules and tree-in-bud opacities on high-resolution computed tomography (HRCT).1 The pathogenesis and prognosis of NTM-LD differ according to the two clinical phenotypes.1 However, there is very limited information on the long-term change in the proportions of these clinical NTM-LD phenotypes. The purpose of this study was to investigate the changes in the major etiologic organisms and clinical phenotypes of NTM-LD over a recent 15-year period in Korea.
Consecutive patients who were diagnosed with NTM-LD caused by MAC, MABC, or M. kansasii between January 2001 and December 2015 were identified from the NTM Registry of Samsung Medical Center (a 1,979-bed referral hospital in Seoul, Korea). All patients met the diagnostic criteria for NTM-LD,1 and this study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2017-06-001). During the study period, NTM species were identified by polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis of the rpoB gene or reverse-blot hybridization of rpoB.4,5 Radiologic phenotypes were classified by chest X-ray and HRCT. The fibrocavitary and nodular bronchiectatic forms were defined by the presence of cavitary opacities in the upper lobes and presence of bronchiectasis and multiple nodules on chest HRCT, respectively. Unclassifiable disease, such as cases with solitary pulmonary nodules, lacked clear characteristics of either form.13,14
The incidence of NTM-LD was calculated as the number of NTM-LD patients per annum divided by the total number of patients, including inpatients and outpatients, who visited our hospital per year, represented as cases per 100,000 inpatient and outpatient encounters. The annual change in the observed proportion of NTM-LD was calculated using Poisson regression models. All data sets were analyzed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
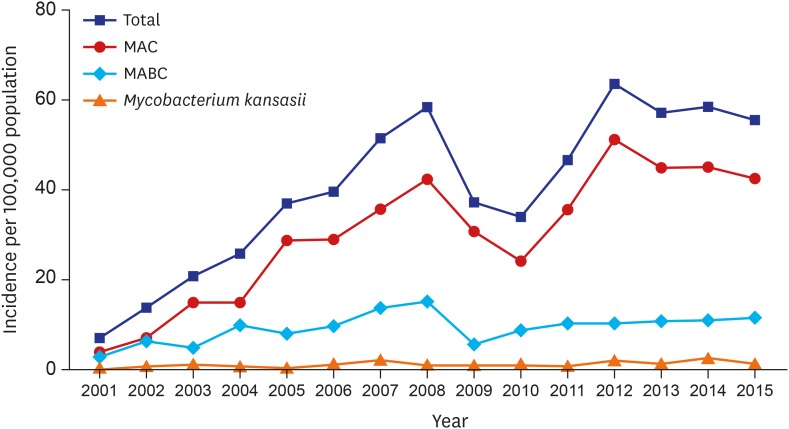
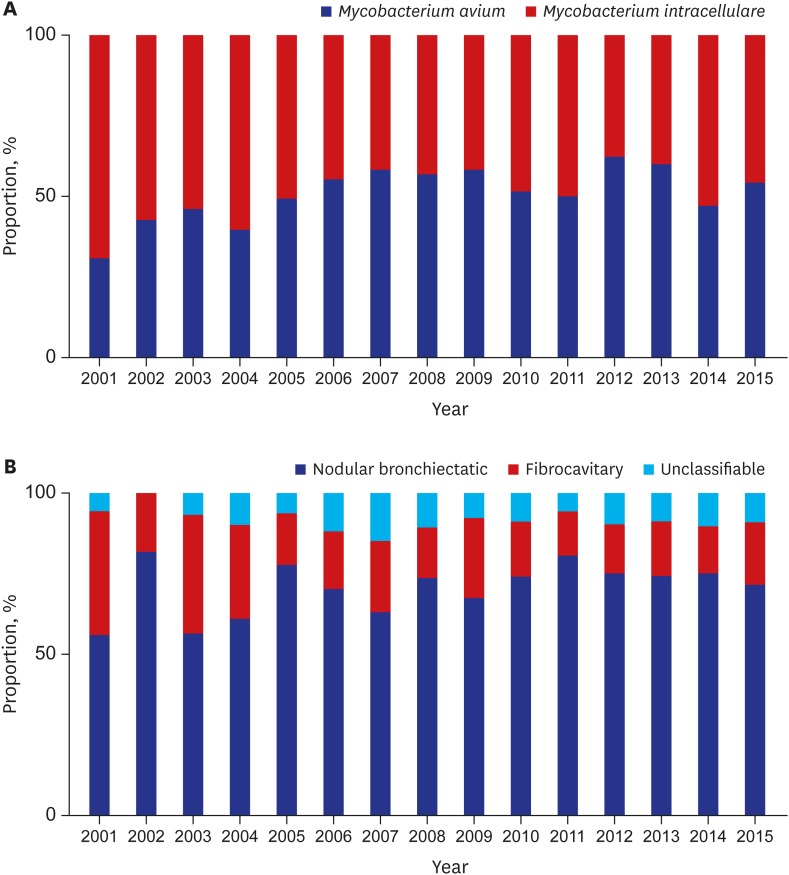
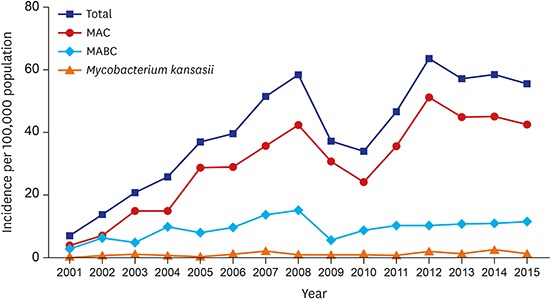
A total of 2,329 patients were diagnosed with NTM-LD during the study. The median age of patients diagnosed with NTM-LD was 60.0 years (interquartile range, 51.5–68.0 years), and 59% were women. The annual incidence of NTM-LD increased significantly from 7.0/100,000 in 2001 to 55.6/100,000 in 2015 (Fig. 1). The most common etiologic organism was MAC (n = 1,746; 75%), followed by MABC (n = 519; 22%) and M. kansasii (n = 64; 3%). The increasing trend for M. avium complex lung disease (MAC-LD) incidence was similar to that for overall NTM-LD, whereas the incidence of NTM-LD caused by MABC and M. kansasii was relatively stable over time (Fig. 1). Among cases of MAC-LD, the proportion of M. avium infections increased from 30% in 2001 to 54% in 2015 (P trend < 0.001, Fig. 2A). For MABC, M. abscessus and M. massiliense were differentiated in our institution beginning in March 2010, and the proportions of M. abscessus and M. massiliense among cases of MABC were 54% and 46%, respectively.
Fig. 1.
Annual incidence of nontuberculous mycobacterial lung disease per 100,000 inpatient and outpatient encounters.
MAC = Mycobacterium avium complex, MABC = Mycobacterium abscessus complex.
Fig. 2.
Changes in the proportion. (A) Mycobacterium avium and Mycobacterium intracellulare among patients with Mycobacterium avium complex lung disease. (B) Clinical phenotypes of nontuberculous mycobacterial lung disease.
Regarding clinical phenotypes of NTM-LD, the proportion of cases of the nodular bronchiectatic form increased significantly from 56% in 2001 to 72% in 2015 (P trend < 0.001), while the proportion of cases of the fibrocavitary form decreased from 39% in 2001 to 20% in 2015 (P trend < 0.001, Fig. 2B).
This study evaluated recent changes in the epidemiology of NTM-LD in Korea. We identified an increasing incidence of NTM-LD, largely due to an increase in MAC-LD as the incidence of LD from other etiologic organisms, including MABC and M. kansasii, remained relatively stable. We also identified an increasing proportion of M. avium compared with M. intracellulare among MAC cases, and a significant increase in the proportion of the nodular bronchiectatic form.
MAC was the most common etiologic organism identified in our study, and the increased incidence of MAC-LD was the reason for the increased incidence of NTM-LD in Korea, consistent with the reported experience of other countries.6 Although M. avium and M. intracellulare are infrequently differentiated in clinical practice and in many previous epidemiologic studies, these organisms have a different epidemiology and pathogenesis. Regarding MAC-LD in East Asia, M. avium is the most common causative species in Japan, whereas M. intracellulare is the predominant organism in mainland China.15,16 Another study showed that household tap water was the main source of M. avium pulmonary infection, whereas M. intracellulare pulmonary infections were acquired from environmental sources like soil.17 These findings suggest that natural environmental factors, including soil, humidity, and temperature; artificial environmental factors, such as modernized water supply systems; and human behaviors, such as showering, may be responsible for the change in epidemiology of NTM-LD in certain geographical regions.15
Within MABC, M. abscessus is the most common pathogen (45%–65%) followed by M. massiliense (20%–55%), but their relative proportions vary according to geographic location.6 Although species differentiation was only available for the latter part of the present study, our results are consistent with previous reports showing that the proportion of M. abscessus and M. massiliense are similar to each other in Korea.5,14
The proportion of the nodular bronchiectatic form of NTM-LD has increased significantly in recent years. Patients with nodular bronchiectatic NTM-LD are typically postmenopausal women with a unique body morphotype, consisting of a slender body habitus, altered immunophenotype, and mucociliary dysfunction18 that increases their susceptibility to environmental NTM exposure. Indeed, the prevalence of bronchiectasis increases substantially with age in these patients, and they tend to have a high risk of acquiring NTM-LD.19 However, it is unclear whether NTM-LD is a consequence or the cause of bronchiectasis. There are increasing evidence that support both contentions.20
There were several limitations of the present study. First, the data were collected from a single referral center with specialized NTM clinics, and the number of patients transiently decreased in 2009–2010 and 2013–2015 in our hospital due to shortage of medical staff. Therefore, the data may not be representative of Korea due to referral bias. Second, environmental factors for each patient were not reviewed as part of this retrospective study.
In summary, the increase of number of patients with NTM-LD was primarily due to an increase of MAC-LD. The proportion of M. avium infections among MAC and the proportion of cases of the nodular bronchiectatic form of NTM-LD have significantly increased over time.
Footnotes
Funding: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1A2A1A01003959) and by a grant from the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C2778). The study sponsors had no role in the design of the study, collection and analysis of the data, or preparation of the manuscript.
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Ko RE, Moon SM, Koh WJ. Data curation: Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, Huh HJ, Ki CS, Lee NY. Formal analysis: Ko RE, Moon SM, Ahn S. Investigation: Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, Huh HJ, Ki CS, Lee NY. Writing - original draft: Ko RE, Moon SM. Writing - review & editing: Koh WJ.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci. 2016;31(5):649–659. doi: 10.3346/jkms.2016.31.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis (Seoul) 2016;79(2):74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142(6):1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 5.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus . Am J Respir Crit Care Med. 2011;183(3):405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 6.Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010;14(8):1069–1071. [PubMed] [Google Scholar]
- 8.Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis (Seoul) 2012;72(5):409–415. doi: 10.4046/trd.2012.72.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis (Seoul) 2013;75(5):199–204. doi: 10.4046/trd.2013.75.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Lee Y, Lee S, Kim YA, Sun YK. Recent trends in clinically significant nontuberculous Mycobacteria isolates at a Korean general hospital. Ann Lab Med. 2014;34(1):56–59. doi: 10.3343/alm.2014.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim N, Yi J, Chang CL. Recovery rates of non-tuberculous mycobacteria from clinical specimens are increasing in Korean tertiary-care hospitals. J Korean Med Sci. 2017;32(8):1263–1267. doi: 10.3346/jkms.2017.32.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon HJ, Choi HY, Ki M. Nontuberculosis mycobacterial infections at a specialized tuberculosis treatment centre in the Republic of Korea. BMC Infect Dis. 2017;17(1):432. doi: 10.1186/s12879-017-2532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017;50(3):1602503. doi: 10.1183/13993003.02503-2016. [DOI] [PubMed] [Google Scholar]
- 14.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64(3):309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 15.Namkoong H, Kurashima A, Morimoto K, Hoshino Y, Hasegawa N, Ato M, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan (1) Emerg Infect Dis. 2016;22(6):1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. 2016;73(6):558–567. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Wallace RJ, Jr, Iakhiaeva E, Williams MD, Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. J Clin Microbiol. 2013;51(6):1747–1752. doi: 10.1128/JCM.00186-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187(2):197–205. doi: 10.1164/rccm.201206-1035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksamit TR, O'Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels ML, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest. 2017;151(5):982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith DE, Aksamit TR. Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med. 2012;33(2):283–295. doi: 10.1016/j.ccm.2012.02.002. [DOI] [PubMed] [Google Scholar]