Abstract
Learned associations between drugs of abuse and the drug administration environment have an important role in addiction. In rodents, exposure to a drug-associated environment elicits conditioned psychomotor activation, which may be weakened following extinction (EXT) learning. Although widespread drug-induced changes in neuronal excitability have been observed, little is known about specific changes within neuronal ensembles activated during the recall of drug–environment associations. Using a cocaine-conditioned locomotion (CL) procedure, the present study assessed the excitability of neuronal ensembles in the nucleus accumbens core and shell (NAccore and NAcshell), and dorsal striatum (DS) following cocaine conditioning and EXT in Fos-GFP mice that express green fluorescent protein (GFP) in activated neurons (GFP+). During conditioning, mice received repeated cocaine injections (20 mg/kg) paired with a locomotor activity chamber (Paired) or home cage (Unpaired). Seven to 13 days later, both groups were re-exposed to the activity chamber under drug-free conditions and Paired, but not Unpaired, mice exhibited CL. In a separate group of mice, CL was extinguished by repeatedly exposing mice to the activity chamber under drug-free conditions. Following the expression and EXT of CL, GFP+ neurons in the NAccore (but not NAcshell and DS) displayed greater firing capacity compared to surrounding GFP− neurons. This difference in excitability was due to a generalized decrease in GFP− excitability following CL and a selective increase in GFP+ excitability following its EXT. These results suggest a role for both widespread and ensemble-specific changes in neuronal excitability following recall of drug–environment associations.
Introduction
Exposure to drug-associated environmental cues or contexts elicits anticipatory responses including conditioned locomotor hyperactivity in rodents (Post et al, 1981) and conditioned emotional, behavioral, and physiological responses in humans (O'Brien et al, 1998). These learned associations between drug effects and the drug administration environment have an important role in addiction and may be weakened through extinction learning (Michel et al, 2003), and methods such as cue-exposure therapy utilize such inhibitory learning to reduce the impact of drug-associated stimuli (Conklin and Tiffany, 2002). Thus, understanding the neurobiological mechanisms of how the strength of these associations are modulated is crucial to better understanding drug addiction.
We and others have reported that drug–environment associations are encoded in sparsely activated populations of neurons called neuronal ensembles (Carelli, 2002; Koya et al, 2009). Recent studies utilizing Fos-GFP mice that express the green fluorescent protein (GFP) in behaviorally activated, Fos-expressing neurons, suggest that the ensembles that encode these associations exhibit unique adaptations at glutamatergic synapses compared with their surrounding neurons (Koya et al, 2012; Whitaker et al, 2016). These data indicate that ensemble-specific modifications may be implicated in the storage of drug-associative memories.
Neurons may alter their signal processing through synaptic adjustments or intrinsic excitability modulation (Wolf, 2010), such as changes in the firing capacity of neurons and/or in ion channel function. Widespread intrinsic excitability changes in the striatum, a brain area that subserves various cocaine-induced behaviors (Everitt and Robbins, 2013), have been observed following repeated cocaine exposure (Kourrich and Thomas, 2009; Ma et al, 2013; Mu et al, 2010; Zhang et al, 1998). These studies have enhanced our understanding of the long-term effects of repeated cocaine on intrinsic excitability. However, to further understand how cocaine-environment associations are encoded in the brain, in addition to the widespread drug-induced changes, the neuronal excitability properties from neurons that are specifically activated by cocaine-associated memory recall must be characterized.
The aim of this study was to investigate neuronal excitability changes in striatal ensembles following cocaine and extinction (EXT) memory retrieval utilizing a cocaine-conditioned locomotion (CL) procedure in Fos-GFP mice. We focused our investigation on the nucleus accumbens shell (NAcshell), core (NAccore) and dorsal striatum (DS), as these three striatal areas have been shown to have related yet distinct involvement in encoding drug–environment associations (Caprioli et al, 2017; Chaudhri et al, 2010; Everitt and Robbins, 2013). We hypothesized that changes in the strength of a drug–environment association may be accompanied by alterations in neuronal excitability on striatal neuronal ensembles.
Materials and methods
For more detailed information of the procedures below, see Supplementary Methods.
Behavioral Experiments
Animals and apparatus
Male wild-type C57Bl/6 (Charles River, UK) and heterozygous Fos-GFP mice (aged 10–12 weeks on test day) were used in immunohistochemical and electrophysiology experiments, respectively. All experiments were conducted in accordance with the UK 1986 Animal Scientific Procedures Act. The behavioral experiments were performed in square clear acrylic locomotor chambers (20 × 20 × 20 cm). Ethovision software (Noldus, RRID:SCR_000441) was used for automated behavioral tracking.
Procedures
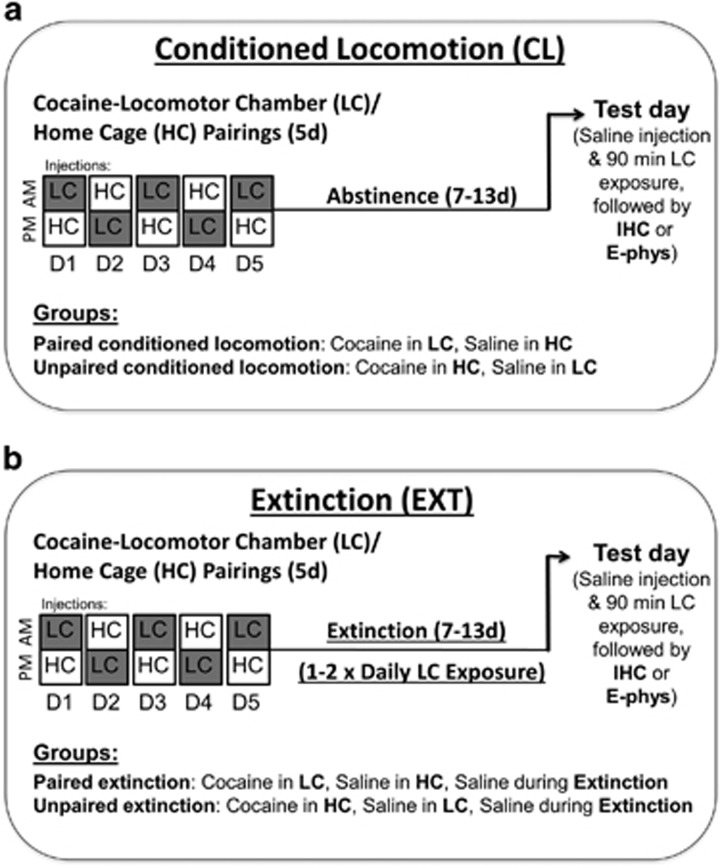
Cocaine locomotor conditioning: Mice were randomly assigned to CL groups ‘Paired CL’ or ‘Unpaired CL’ in which cocaine injections (20 mg/kg, i.p.; MacFarlan Smith, UK) were paired with a novel environment (locomotor chamber) or with the home cage, respectively (Figure 1a). Mice received two injection sessions per day; on one session, the Paired and Unpaired CL mice received a cocaine and saline injection, respectively, before being placed in the locomotor chambers for 30 min. In an alternate session, Paired and Unpaired CL mice received saline and cocaine injections, respectively, in the home cage. Conditioning proceeded for five sessions with cocaine injections counterbalanced between morning (0800–1200 h) and afternoon (1500–1800 h) sessions.
Figure 1.
Timeline for conditioned locomotion (CL) and extinction (EXT) experiments. (a) In CL (cocaine memory retrieval) experiments, two groups of mice were exposed to the locomotor chamber for once daily 30 min sessions over a 5-day period; Paired CL mice received 20 mg/kg i.p. cocaine injections prior to these sessions while Unpaired CL mice received saline. Across the five conditioning days, Unpaired CL and Paired CL mice were given cocaine and saline injections in the home cage, respectively. Home cage and locomotor chamber injections were counterbalanced across morning (0800–1200 h) and afternoon (1500–1800 h) sessions. This regime ensured that the psychostimulant effects of cocaine were paired with the locomotor chamber in the Paired CL group only. Following an abstinence period of 7–11 days (IHC; immunohistochemistry) or 7–13 days (E-phys; electrophysiology), free of experimental intervention, both Paired CL and Unpaired CL mice were given a single saline injection and placed in the locomotor chambers for 90 min, before being killed for further immunohistochemistry or electrophysiology experiments. (b) In EXT experiments (EXT memory retrieval), Paired EXT and Unpaired EXT mice underwent an identical cocaine injection procedure as during the CL experiments. One day following the final cocaine injection, Paired EXT and Unpaired EXT mice began an EXT phase consisting of 30 min, 1–2 × daily exposures to the locomotor chamber, each started immediately following a saline injection. Following 7–13 days (10–16 sessions; electrophysiology) or 7–11 days (10–14 sessions, immunohistochemistry) of EXT, mice were given a final 90 min EXT session before being killed for further experiments.
CL test: Locomotor tests were conducted 7–13 (electrophysiology) or 7–11 (immunohistochemistry) days following the final conditioning session. Mice received a single saline injection and were placed in the locomotor chamber for 90 min after which their brains were extracted for electrophysiological or immunohistochemical analyses.
EXT learning and behavioral testing: Paired and Unpaired EXT mice received cocaine locomotor conditioning as described above (similar to Paired and Unpaired CL, respectively). However, 1 day following the final conditioning session, both groups of mice underwent 1–2 × daily EXT sessions, consisting of a saline injection preceding a 30 min locomotor chamber exposure (Figure 1b). Following 7–13 (10–16 sessions; electrophysiology) or 7–11 (10–14 sessions; immunohistochemistry) days of EXT, a 90 min EXT test session was conducted.
Fos Immunohistochemistry
Ninety minutes following the final test session, wild-type mice were anesthetized and perfused with 4% paraformaldehyde before visualization of Fos using DAB-nickel immunohistochemistry.
Electrophysiology Experiments
Ninety minutes following the final test session, Fos-GFP mice were deeply anesthetized with 150 mg/kg ketamine and 20 mg/kg xylazine, and 250 μm striatal sections were dissected, and current clamp recordings from the NAcshell, NAccore, and DS were performed from GFP+ and GFP− cells.
Data Analysis
All data were analyzed using GraphPad Prism (Graphpad Software, RRID:SCR_002798) and SPSS (IBM, RRID:SCR_002865). Data points outside 2 SD from the mean were excluded from analysis. Group data are presented as mean±SEM. Post-hoc tests were conducted using Fisher’s LSD.
Behavior and immunohistochemistry
Distance travelled in the locomotor chamber and Fos+ cells/mm2 (analyzed independently for the NAcshell, NAccore, and DS) were analyzed using a two-way ANOVA including the factors Group (Paired vs Unpaired) and Extinction (EXT vs no EXT). Three mice were excluded from the immunohistochemical analysis due to poor perfusion and/or section quality.
Electrophysiology
Typically one to four GFP+ and GFP− cells each were recorded per animal. Where applicable (ie, when data from more than one GFP+ or GFP− cell were obtained), we utilized the average data from multiple cells from each cell type per animal. Spike counts were analyzed using a three-way mixed ANOVA including the factors of Group (Paired, Unpaired), GFP (GFP+, GFP−), and a repeated-measures factor of Current (30-210 pA range in 30 pA increments). Active and passive membrane properties (Tables 1, 2 and 3) were analyzed using a two-way ANOVA with Group (Paired, Unpaired) and GFP (GFP+, GFP−) as factors. Electrophysiological parameters were analyzed separately for CL and EXT experiments.
Table 1. Basic Membrane Properties from NAcshell MSNs of Paired and Unpaired Mice Following Cocaine and Extinction Memory Retrieval.
| NAc shell |
Conditioned locomotion |
Extinction |
||||||
|---|---|---|---|---|---|---|---|---|
|
Unpaired |
Paired |
Unpaired |
Paired |
|||||
| GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | |
| Resting Vm (mV) | −70.63±0.80 | −71.00±0.93 | −70.19±0.46 | −68.80±0.41 | −71.06±0.49 | −70.56±0.52 | −70.39±0.39 | −70.87±0.85 |
| Rheobase (pA) | 115.63±11.78 | 110.71±12.84 | 116.88±6.74 | 134.33±30.50 | 115.00±12.38 | 107.22±12.34 | 108.75±9.99 | 92.14±13.35 |
| Ri (MΩ) | 207.34±22.89 | 228.63±28.97 | 200.22±15.06 | 223.86±53.98 | 185.38±16.30 | 223.48±25.07 | 205.65±19.12 | 260.76±33.26 |
| AP peak (mV) | 68.21±3.88 | 70.46±2.30 | 69.53±2.01 | 66.99±2.31 | 71.99±2.99 | 68.14±4.32 | 70.39±0.96 | 72.11±3.07 |
| AP half-width (ms) | 1.40±0.06 | 1.55±0.14 | 1.41±0.08 | 1.42±0.09 | 1.32±0.03 | 1.38±0.04 | 1.34±0.04 | 1.45±0.05 |
| Threshold (mV) | −35.83±1.58 | −36.59±0.99 | −35.47±0.81 | −34.17±0.87 | −36.95±0.88 | −35.63±1.26 | −36.26±0.34 | −38.72±1.21 |
| fAHP (mV) | −6.72±0.57 | −6.81±0.89 | −8.30±0.49 | −6.86±0.24 | −7.75±0.73 | −7.60±0.56 | −6.92±0.39 | −7.16±0.88 |
| mAHP (mV) | −8.01±0.50 | −7.61±0.65 | −7.88±0.35 | −7.64±0.53 | −7.83±0.35 | −8.48±0.56 | −7.15±0.38 | −7.71±0.79 |
Data are expressed as mean±SEM. Liquid junction potential was −13.7 mV and was not adjusted for. Spike characteristics were determined from the first action potential (AP) of spike runs consisting of six to eight spikes. Input resistance was calculated from the slope of the I/V curve measured in response to 10 pA current steps ranging from −30 to 70 pA. Spike threshold was measured using the third differential method (Cotel et al, 2013) with Mini Analysis software. The AP peak was calculated as the difference between the AP peak and AP threshold. Half-width was measured as the AP width at half-maximal spike. Post-spike fAHPs and mAHPs were measured 3 and 30 ms following the AP threshold, respectively, similar to (Ishikawa et al, 2009).
Table 2. Basic Membrane Properties from NAcCore MSNs of Paired and Unpaired Mice Following Cocaine and Extinction Memory Retrieval.
| NAc core |
Conditioned locomotion |
Extinction |
||||||
|---|---|---|---|---|---|---|---|---|
|
Unpaired |
Paired |
Unpaired |
Paired |
|||||
| GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | |
| Resting Vm (mV) | −70.16±0.48 | −71.33±0.67 | −71.93±0.57 | −70.38±0.75 | −71.37±0.60 | −72.06 ±0.24 | −71.81±0.47 | −71.25±0.69 |
| Rheobase (pA) | 169.06±11.81 | 180.71±19.34 | 248.24±# 14.59 | 143.88±* 16.75 | 189.44±14.91 | 211.50±11.21 | 197.30±16.27 | 135.30±* 12.68 |
| Ri (MΩ) | 123.66±8.21 | 104.24±9.75 | 83.12±# 4.89 | 133.15±* 15.50 | 112.84±14.88 | 84.21±7.51 | 96.21±6.08 | 145.49±* 13.23 |
| AP peak (mV) | 72.06±2.72 | 78.70±3.51 | 73.46±2.16 | 78.08±1.31 | 71.45±3.11 | 76.61±2.73 | 75.37±2.62 | 71.55±1.79 |
| AP half-width (ms) | 1.38±0.06 | 1.25±0.05 | 1.27±0.07 | 1.35±0.07 | 1.36±0.08 | 1.26±0.06 | 1.35±0.04 | 1.39±0.05 |
| Threshold (mV) | −34.50±1.04 | −34.53±1.32 | −32.08±0.87 | −35.61±0.98 | −32.91±0.96 | −34.78±0.79 | −34.54±1.35 | −33.24±1.06 |
| fAHP (mV) | −6.20±0.32 | −7.29±0.69 | −8.83±# 0.71 | −6.70±* 0.35 | −7.32±0.74 | −8.70±0.53 | −7.11±0.68 | −8.22±0.49 |
| mAHP (mV) | −7.95±0.20 | −9.31±0.45 | −10.37±# 0.22 | −8.63±* 0.30 | −8.88±0.53 | −9.78±0.36 | −8.39±0.36 | −8.96±0.55 |
Data are expressed as mean±SEM. Asterisks indicates a significant interaction effect (*p<0.05, #indicates differences between Paired GFP− vs Unpaired GFP−). Spike kinetics were calculated as detailed in Table 1.
Table 3. Basic Membrane Properties from DS MSNs of Paired and Unpaired Mice Following Cocaine and Extinction Memory Retrieval.
| DS |
Conditioned locomotion |
Extinction |
||||||
|---|---|---|---|---|---|---|---|---|
|
Unpaired |
Paired |
Unpaired |
Paired |
|||||
| GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | GFP− | GFP+ | |
| Resting Vm (mV) | −71.00±0.66 | −71.57±0.53 | −72.41±0.64 | −72.46±0.72 | −71.87±0.74 | −72.58±1.04 | −71.10±0.37 | −72.00±0.33 |
| Rheobase (pA) | 237.86±41.13 | 205.00±24.84 | 233.33±40.55 | 171.90±30.22 | 236.25±17.62 | 176.95±22.25 | 248.33±20.73 | 210.00±23.53 |
| Ri (MΩ) | 101.95±13.06 | 116.69±18.90 | 102.62±13.06 | 132.73±23.39 | 73.44±5.18 | 100.01±17.97 | 89.77±10.02 | 94.24±9.11 |
| AP peak (mV) | 76.86±4.82 | 85.04±2.82 | 84.50±6.41 | 91.91±1.63 | 86.74±5.01 | 83.99±4.00 | 80.03±3.21 | 85.89±2.79 |
| AP half-width (ms) | 1.24±0.07 | 1.18±0.11 | 1.24±0.08 | 1.24±0.10 | 1.16±0.05 | 1.26±0.09 | 1.22±0.05 | 1.17±0.05 |
| Threshold (mV) | −35.93±2.54 | −37.94±0.82 | −37.88±2.29 | −42.18±0.91 | −35.94±2.17 | −38.71±0.96 | −35.94±1.15 | −39.29±1.10 |
| fAHP (mV) | −8.39±1.09 | −9.00±1.09 | −7.85±1.14 | −9.47±0.92 | −10.21±1.38 | −9.20±1.71 | −8.59±1.07 | −9.27±1.00 |
| mAHP (mV) | −8.86±0.42 | −9.63±0.38 | −9.48±0.92 | −10.79±0.42 | −10.47±0.69 | −9.65±0.48 | −9.27±0.44 | −9.67±0.37 |
Data are expressed as mean±SEM. Spike kinetics were calculated as detailed in Table 1.
Results
Locomotor Activity and Striatal Fos Expression Following Cocaine and EXT Memory Retrieval
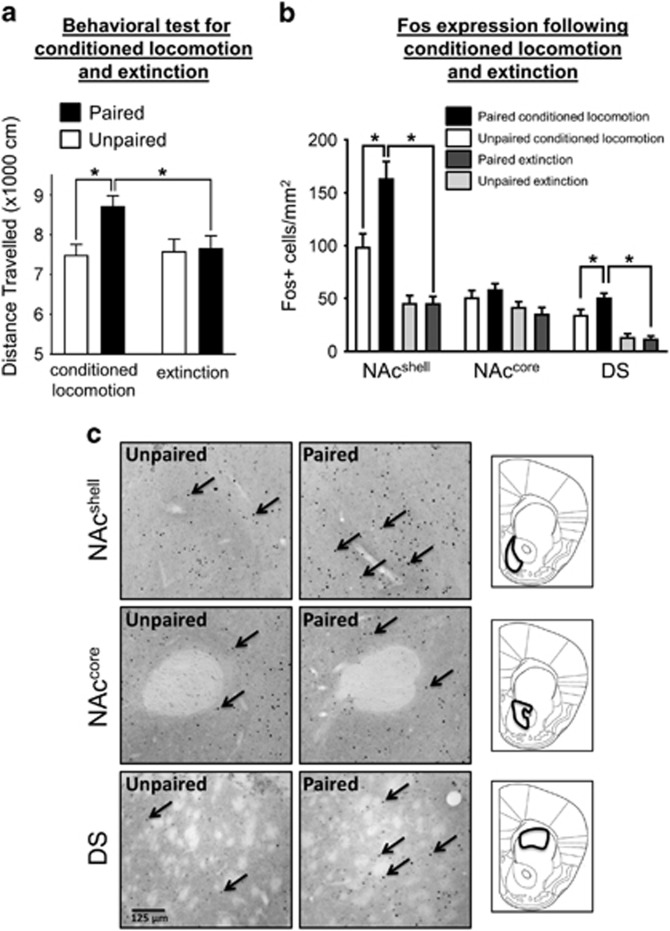
We trained four groups of mice to assess the expression (CL: Paired CL, Unpaired CL) (Figure 1a) and EXT (EXT: Paired EXT, Unpaired EXT) (Figure 1b) of conditioned locomotor activity. A two-way ANOVA on the locomotor activity (indicated by distance travelled) during the test session revealed a significant interaction of Group × EXT (F1,40=4.17, p<0.05) and a significant main effect of Group (F1,40=5.41, p<0.05) (Figure 2a and Supplementary Figure 1A). Post-hoc tests indicated that Paired CL mice displayed significantly higher locomotor activity compared to Unpaired CL mice (p<0.01). In addition, Paired EXT mice displayed significantly lower locomotor activity compared to Paired CL mice (p<0.05), at levels similar to Unpaired EXT mice. These data indicate that Paired CL and Paired EXT mice retrieved a cocaine and EXT memory, respectively.
Figure 2.
Behavioral response and neuronal activation following conditioned locomotion (CL) and extinction (EXT). (a) Distance travelled by Paired and Unpaired mice during the first 30 min of the CL and EXT test sessions in the immunohistochemistry study (n=10–12/group). Paired CL group mice show increased locomotion compared to Unpaired CL mice following cocaine memory retrieval. Following EXT memory retrieval, no increase in locomotion was observed (Paired EXT group). (b) Quantification of Fos+ cells/mm2 in the nucleus accumbens shell (NAcshell), nucleus accumbens core (NAccore), and dorsal striatum (DS) following cocaine and EXT memory retrieval (n=10–12/group). (c) Representative images of Fos immunostaining in the NAcshell, NAccore, and DS. Arrows indicate Fos+ neurons. Scale bar, 125 μm. Right: identification of sampling area for the NAcshell, NAccore, and DS in both immunohistochemistry and electrophysiology experiments; coronal slice represents bregma 1.34 mm. All data are expressed as mean±SEM; *p<0.05.
We next examined neuronal ensemble activation in the NAcshell, NAccore, and DS by quantifying the number of Fos+ cells/mm2.
Nucleus accumbens shell
In the NAcshell, a two-way ANOVA revealed a significant Group × EXT interaction (F1,41=8.31, p<0.01) and significant main effects of Group (F1,41=8.20, p<0.01) and EXT (F1,41=58.24, p<0.001) (Figures 2b and c). Post-hoc tests indicated that Fos expression in Paired CL mice was significantly higher compared with Unpaired CL mice (p<0.001). Paired EXT mice displayed significantly lower Fos compared with Paired CL mice (p<0.001), at levels similar to Unpaired EXT mice.
Nucleus accumbens core
In the NAccore, a two-way ANOVA revealed no significant interaction between Group × EXT (F1,40=1.54, p=0.22), but a significant main effect of EXT (F1,40=8.03, p<0.01) (Figures 2b and c).
Dorsal striatum
In the DS, there was a significant Group × EXT interaction (F1,39=6.09, p<0.05) and main effect of Group (F1,39=4.43, p<0.05) and EXT (F1,39=69.66, p<0.001) (Figures 2b and c). Post-hoc tests revealed a significant difference between Paired CL and Unpaired CL mice (p<0.01). Paired EXT mice displayed significantly lower Fos compared with Paired CL mice (p<0.001), at levels similar to Unpaired EXT mice.
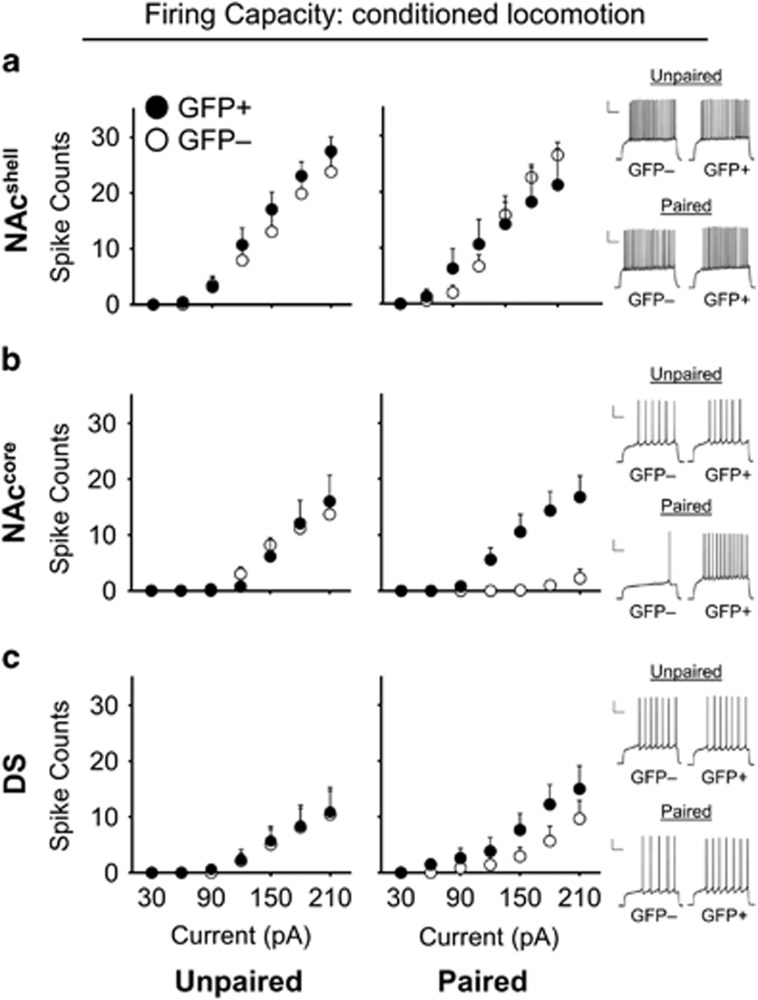
Striatal MSN Excitability Following Cocaine Memory Retrieval
We next examined the excitability of ‘activated,’ GFP-expressing (GFP+) and ‘non-activated,’ non-expressing (GFP−) medium spiny neurons (MSNs) in the Fos-GFP mouse following cocaine memory retrieval. To that end, we examined the number of action potentials (firing capacity) across a range of positive current injection steps (30–210 pA), as a broad measure of excitability. We then examined active and passive membrane properties to investigate underlying adaptations to MSNs which may modulate firing capacity changes (indicated in Figure 5 and Tables 1, 2 and 3).
Nucleus accumbens shell
There was a significant interaction of Group × GFP × Current (F6,144=2.38, p<0.05; Figure 3a), but no significant interactions for Group × Current (F6,144=0.27, p=0.95) and GFP × Current (F6,144=0.68, p=0.67; Figure 3a). In addition, a two-way mixed ANOVA separately conducted on Paired and Unpaired mice did not reveal a significant GFP × current interaction in either group (Paired: F6,66=2.03, p=0.07, Unpaired: F6,78=0.83, p=0.57). Furthermore, we found no significant interaction effects in any of the passive or active membrane properties measured.
Figure 3.
Excitability of GFP+ and GFP− striatal MSNs following cocaine memory retrieval. (a) In the nucleus accumbens shell (NAcshell), the spike counts of Paired CL mice were not significantly different between GFP+ neurons (n=5) and GFP− neurons (n=8); this was similar to the Unpaired CL group (GFP+ n=7; GFP− n=8). Right: example traces of Paired CL and Unpaired CL GFP+ and GFP− neurons at 150 pA stimulation from the NAcshell following cocaine memory retrieval. (b) In the nucleus accumbens core (NAccore), GFP+ neurons were significantly more excitable than GFP− neurons in Paired CL mice (GFP+ n=9; GFP− n=9) but not Unpaired CL mice (GFP+ n=7; GFP− n=7). Right: example traces of Paired CL and Unpaired CL GFP+ and GFP− neurons at 150 pA stimulation from the NAccore following cocaine memory retrieval. (c) Excitability of GFP+ and GFP− MSNs in the dorsal striatum (DS) (GFP+ Paired CL n=7, Unpaired CL n=8), (GFP− Paired CL n=6, Unpaired CL n=8). Right: example traces of Paired CL and Unpaired CL GFP+ and GFP− neurons at 180 pA stimulation from the DS following cocaine memory retrieval. All data are expressed as mean±SEM; n=number of animals. Scale bars, 20 mV, 200 ms.
Nucleus accumbens core
In Paired CL mice, the firing capacity of GFP+ neurons was significantly increased compared to GFP− neurons (Group × GFP × Current F6,174=5.76, p<0.001; Group × Current F6,174=3.30, p<0.01; and GFP × Current F6,174=4.33, p<0.001) (Figure 3b). Comparison of the firing capacity of GFP+ and GFP− neurons across groups suggested that although Paired CL group GFP+ neurons were not significantly more excitable than Unpaired CL GFP+ neurons (Group (GFP+ neurons only) × Current F6,84=0.53, p=0.78), the excitability of Paired CL GFP− neurons was significantly decreased compared to Unpaired CL GFP− neurons (Group (GFP− neurons only) × Current F6,90=18.50, p<0.001). This suggests that in Paired CL mice following cocaine memory retrieval, GFP+ neurons of the NAccore were relatively more excitable than surrounding GFP− neurons due to a generalized decrease in the excitability of GFP− neurons.
We next examined changes in active and passive membrane properties, which may indicate the potential mechanism by which the excitability of GFP− neurons was decreased. A two-way ANOVA indicated a significant interaction of Group × GFP for the rheobase (the minimum current required to elicit an action potential (AP)) (F1,29=13.02, p<0.01), input resistance (indicator of the density of open ion channels) (F1,29=10.65, p<0.01) (Figure 5a and Table 2) and the fast and medium afterhyperpolarization (fAHP and mAHP; components of the afterhyperpolarization potential that dampens firing) (fAHP: F1,29=8.54, p<0.01; mAHP: F1,27=26.83, p<0.001) (Figure 5b). Post-hoc tests demonstrated that the input resistance of Paired CL GFP+ neurons was significantly increased compared with Paired CL GFP− neurons (p<0.01), whereas the rheobase, fAHP, and mAHP were all significantly decreased (p<0.001, p<0.05, and p<0.001, respectively). Furthermore, the input resistance of Paired CL GFP− compared with Unpaired CL GFP− was significantly decreased (p<0.05), whereas the rheobase, fAHP, and mAHP were significantly increased (p<0.01, p<0.01, and p<0.001, respectively). We also observed a significant increase in the mAHP of Unpaired CL GFP+ neurons compared with Unpaired CL GFP− neurons (p<0.05).
Dorsal striatum
We observed no selective firing capacity alterations between GFP+ and GFP− neurons (Group × GFP × Current F6,150=0.36, p=0.91; Group × Current F6,150=0.12, p=0.99; and GFP × Current F6,150=0.43, p=0.85) (Figure 3c). In addition, there were no significant interaction effects for any of the aforementioned passive or active membrane properties.
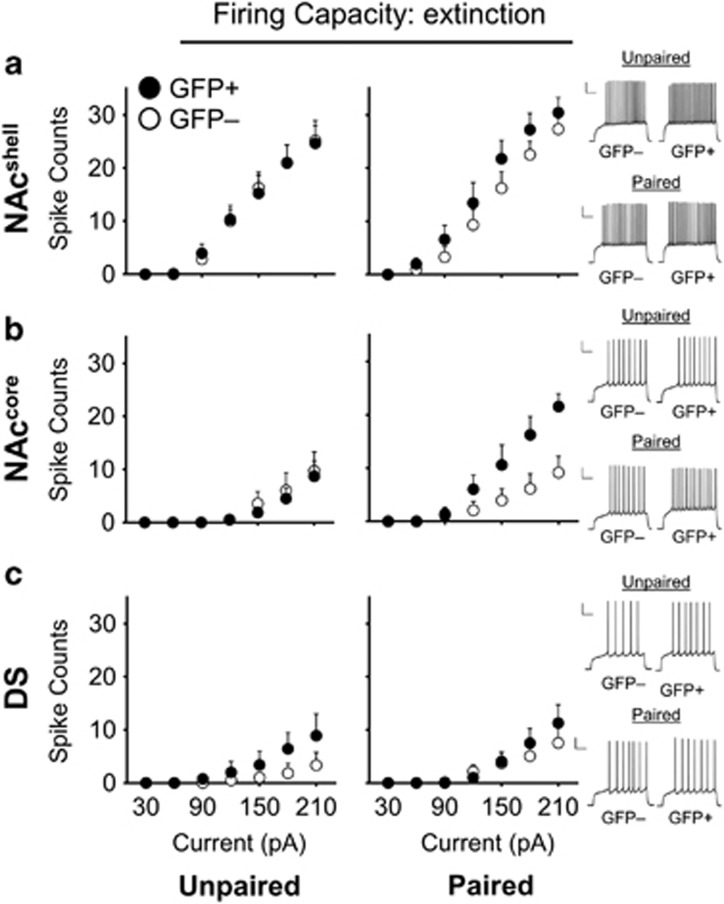
Striatal MSN Excitability Following EXT Memory Retrieval
We next examined the excitability properties of striatal MSNs following the final EXT test (ie, EXT memory retrieval) in Paired and Unpaired EXT mice.
Nucleus accumbens shell
We observed no selective firing capacity alterations between GFP+ and GFP− neurons (Group × GFP × Current F6,204=0.36, p=0.84; Group × Current F6,204=0.79, p=0.58; and GFP × Current F6,204=0.32, p=0.92) (Figure 4a). Furthermore, we found no significant interaction effects for any of the passive or active membrane properties that were measured.
Figure 4.
Excitability of GFP+ and GFP− striatal medium spiny neurons (MSNs) following extinction (EXT) memory retrieval. (a) The spike counts of nucleus accumbens shell (NAcshell) MSNs were not significantly different between Paired EXT GFP+ neurons (n=8) and Paired EXT GFP− neurons (n=10); this is similar to the Unpaired EXT group (GFP+ n=10, GFP− n=10). Right: example traces of Paired EXT and Unpaired EXT GFP+ and GFP− neurons at 150 pA stimulation from the NAcshell following EXT memory retrieval. (b) In the nucleus accumbens core (NAccore), Paired EXT GFP+ neurons (n=10) were more excitable than Paired EXT GFP− neurons (n=10). In contrast, Unpaired EXT GFP+ neurons (n=10) were not more excitable than Unpaired EXT GFP− neurons (n=9). Right: example traces of Paired EXT and Unpaired EXT GFP+ and GFP− neurons at 150 pA stimulation from the NAccore following EXT memory retrieval. (c) GFP+ and GFP− neurons in Paired EXT mice (GFP+ n=12, GFP− n=12) and Unpaired EXT mice (GFP+ n=6; GFP− n=9) following EXT memory retrieval. Right: example traces of Paired EXT and Unpaired EXT GFP+ and GFP− neurons at 180 pA stimulation from the dorsal striatum (DS) following EXT memory retrieval. All data are expressed as Mean±SEM; n= number of animals. Scale bars, 20 mV and 200 ms.
Nucleus accumbens core
There was a significant increase in the firing capacity of Paired EXT GFP+ neurons compared with Paired EXT GFP− neurons (Group × GFP × Current F6,168=4.22, p<0.001; Group × Current F6,168=2.52, p<0.05; and GFP × Current F6,168=6.11, p<0.001) (Figure 4b). The firing capacity of Paired EXT GFP+ neurons was significantly increased compared with the Unpaired EXT GFP+ baseline (Group (GFP+ neurons only) × Current F6,108=6.99, p<0.001), but there were no significant differences in firing capacity between Paired EXT and Unpaired EXT GFP− neurons baseline (Group (GFP− neurons only) × Current F6,102=0.25, p=0.12).
We next examined the membrane properties (Figure 5c and d) and a two-way ANOVA indicated a significant effect in the rheobase (Group × GFP F1,35=9.12, p<0.01) and input resistance (Group × GFP F1,35=11.29, p<0.01). Post-hoc tests comparing Paired EXT GFP+ and Paired EXT GFP− neurons indicated a decrease in the rheobase (p<0.01) and an increase in the input resistance (p<0.01) of Paired EXT GFP+ neurons (Figure 5c and Table 2). This difference appeared to be due to changes in Paired EXT GFP+ neurons since no differences were observed in these properties from GFP− neurons of Paired EXT and Unpaired EXT mice.
Figure 5.
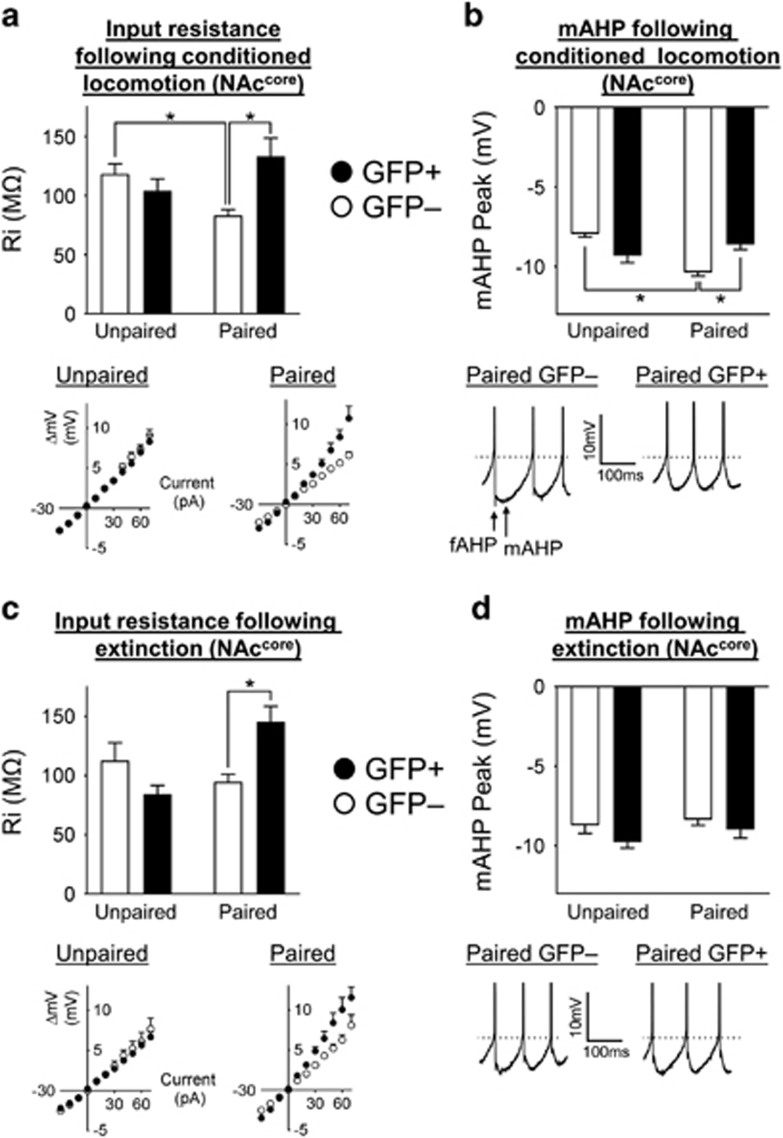
Modulation of input resistance and AHP underlies excitability changes following cocaine and extinction (EXT) memory retrieval in the nucleus accumbens core (NAccore). (a) The input resistance of GFP+ neurons in Paired conditioned locomotion (CL) mice was increased compared with Paired CL GFP− neurons. Also, the Paired CL GFP– neurons exhibited decreased input resistance compared to the Unpaired CL GFP– neurons.. In Unpaired CL mice, the input resistance of GFP+ and GFP− neurons was similar. Below: I/V curves of Paired CL and Unpaired CL GFP+ and GFP− neurons from which input resistance was calculated. In the Paired CL group, there was a significant shift in the I/V curve of GFP+ compared with GFP− neurons; in contrast, I/V curves of Unpaired CL GFP+ and GFP− neurons were similar. (b) Following cocaine memory retrieval, the mAHP of Paired CL GFP− neurons was significantly increased compared to Paired CL GFP+ and Unpaired CL GFP− neurons. Below: example traces of Paired CL GFP+ and GFP− neurons following cocaine memory retrieval, identifying the position of fast afterhyperpolarization (fAHP) and medium afterhyperpolarization (mAHP) peaks. Dashed line indicates the threshold of the first spike labeled with group means. The fAHP and mAHP of Paired CL GFP− neurons is increased following cocaine memory retrieval. Scale bar, 10 mV and 100 ms. (c) Following EXT memory retrieval, the input resistance of Paired EXT GFP+ neurons was increased compared with Paired EXT GFP− neurons. In the Unpaired EXT mice, the input resistance of GFP+ and GFP− neurons was similar. Below: I/V curves of Paired EXT and Unpaired EXT GFP+ and GFP− neurons from which input resistance was calculated. In the Paired EXT group, the I/V curve of GFP+ neurons was significantly shifted compared with GFP− neurons; in contrast, the I/V curves of Unpaired EXT GFP+ and GFP− neurons were similar. (d) The mAHP of GFP+ and GFP− neurons was not significantly different in either Paired EXT or Unpaired EXT mice. Below: example traces of Paired EXT GFP+ and GFP− neurons. The fAHP and mAHP of Paired EXT GFP+ and Paired EXT GFP− neurons were similar following EXT memory retrieval. Dashed line indicates the threshold of the first spike labeled with group means. Scale bar, 10 mV and 100 ms. All data are expressed as mean±SEM; *p<0.05.
Dorsal striatum
We observed no selective firing capacity alterations between GFP+ and GFP− neurons (Group × GFP × Current F6,210=0.13, p=0.99; Group × Current F6,210=0.83, p=0.55; and GFP × Current F6,210=1.61, p=0.15) (Figure 4c). In addition, there were no significant interaction effects for the passive or active membrane properties that were measured.
Discussion
We examined the size and excitability of NAcshell, NAccore, and DS neuronal ensembles following cocaine and EXT memory retrieval using a cocaine CL procedure. In the NAccore we observed a relative increase in GFP+ neuron excitability following cocaine memory retrieval, which was attributable to a general decrease in the excitability of surrounding GFP− neurons. In contrast, following EXT memory retrieval, the excitability of GFP+ neurons was increased compared to GFP− neurons without a general decrease in GFP− neuron excitability. In the NAcshell and DS, we observed no changes in ensemble excitability following cocaine and EXT memory retrieval, despite the fact that conditioning and EXT processes regulated the size of the neuronal ensemble. These adaptations were likely to be related to drug–environment exposure, as other factors such as stress were controlled for in the Unpaired group that underwent similar levels of repeated handling, injections, and activity chamber and cocaine exposure. Collectively, these data provide novel insight into how distinct adaptations may serve to increase the sensitivity of neurons activated by exposure to a drug-associated environment and following EXT.
Implications of Changes in NAccore Ensemble Excitability Following Cocaine Memory Retrieval
We found adaptations in the rheobase, input resistance, I/V curve, fAHP, and mAHP of NAccore GFP− MSNs in mice that underwent cocaine conditioning in a novel context outside of its home cage (ie, Paired mice). Many of these factors have been previously shown to be regulated in NAc MSN’s following repeated cocaine administration (Ma et al, 2013; Mu et al, 2010; Zhang et al, 1998). The input resistance and I/V curve dynamics are primarily regulated by K+ currents, including the inward-rectifying K+ (Kir) and A-type potassium currents (Nisenbaum and Wilson, 1995), although Na+ and Ca2+ currents also modulate near-threshold voltage responses (Bean, 2007; Nisenbaum and Wilson, 1995). The regulation of the firing threshold, however, is dominated by voltage sensitive Na+ currents (Cantrell and Catterall, 2001; Zhang et al, 1998), whereas the AHP (both fAHP and mAHP) is regulated by a class of voltage-dependent calcium-activated K+ currents (Ishikawa et al, 2009; Vilchis et al, 2000). These membrane currents are carried by a complex and diverse host of ion channels (Bean, 2007), regulated by striatal monoamine neurotransmitters such as dopamine and thus are a potential target for learning-induced plasticity (Cantrell and Catterall, 2001; Nicola et al, 2000).
We observed a generalized decrease in the excitability of NAccore GFP− neurons, which resulted in a relative increase in the excitability of the activated GFP+ ensemble. The NAccore is necessary for the expression of CL following exposure to psychostimulant-associated environments (Sellings and Clarke, 2006) and for encoding cues that indicate the availability of cocaine (Suto et al, 2013). The alteration in global excitability we observed following the expression of CL may function to enhance the signal-to-noise ratio of glutamatergic input by depressing the activity of neurons encoding stimuli unrelated to the drug-associated environment. This increased signal-to-noise ratio may increase the information transfer from the activated ensemble to downstream targets, such as the ventral tegmental area, substantia nigra, and ventral pallidum (Heimer et al, 1991). This in turn, may facilitate attentional bias and increased salience of drug-associated stimuli (O'Donnell, 2003; Wanat et al, 2009), a phenomena often observed in drug addicts (Robinson and Berridge, 2008).
It remains to be determined here whether the excitability changes that we observed occurred before or immediately following the behavioral test. Psychostimulant injections in a novel environment outside of the animal’s home cage (Paired mice) produces more robust behavioral sensitization, Fos expression, and glutamatergic transmission than injections in the home cage (Unpaired mice) (Badiani et al, 1998; Hope et al, 2006; Hotsenpiller et al, 2001; Mattson et al, 2007). This suggests that our observed changes may have been due to baseline differences in excitability between Paired and Unpaired mice that occurred prior to test day. Alternatively, exposure to the cocaine-associated environment may have acutely altered the excitability of NAccore neurons through release of dopamine (Di Ciano et al, 1998); but see (Brown and Fibiger, 1992), which modulates MSN excitability (Nicola et al, 2000; O'Donnell, 2003).
An interesting point to raise here is that the GFP+ neurons exhibited relatively increased excitability in the NAccore, despite the lack of Fos expression increases in this area. However, this lack of increase does not necessarily imply the lack of neuronal ensemble recruitment following exposure to the cocaine-paired context, as distinct stimuli may recruit different neuronal ensembles without concomitant increases in the number of activated neurons. For example, Suto et al (2016) recently demonstrated that cues predictive of reward availability and omission both elicit activation of a similar number of Fos-expressing neurons in the infralimbic cortex, despite these two populations of cue-activated neurons mediating opposing behavioural responses.
Implications for the Increased Excitability of GFP+ NAccore Neurons Following EXT Memory Retrieval
Following EXT, we observed a relative increase in the excitability of Paired EXT GFP+ neurons in the NAccore, which was determined by an increase in GFP+ neuronal excitability, whereas GFP− neurons were comparable with baseline controls. Hence, the generalized adaptations observed following cocaine conditioning were no longer observed following EXT learning. Previous studies have demonstrated that EXT of cocaine self-administration normalized drug-induced plasticity seen during withdrawal (Self et al, 2004), suggesting that EXT learning alone is enough to cause marked adaptations in the NAc following cocaine conditioning.
In this study, we did not examine whether the ensemble activated following CL includes the same neurons that were activated following EXT. Memories of cocaine–environment associations are robust and long lasting (Hope et al, 2006; Robinson and Berridge, 2008). Although EXT learning might suppress these drug–environment associations, exposure to certain stimuli (eg, drugs) can re-activate this memory and thus reinstate drug conditioned behaviors that may contribute to relapse (Crombag et al, 2008; Mueller and Stewart, 2000). Interestingly, persistent increases in neuronal excitability have been observed following successful EXT learning (Brons and Woody, 1980). This increase may contribute to the ‘memory savings effect,’ which facilitates re-acquisition of previously learned tasks (Ebbinghaus, 1913; Zhang and Linden, 2003). It is possible that the same NAccore neurons were activated both following cocaine and EXT memory retrieval, with relatively higher levels of excitability persisting after EXT. This persistently enhanced excitability may ‘save’ the cocaine associative memories. Such savings may explain the enduring, robust nature of drug memories and why addicts relapse even while undergoing cue exposure therapy that involves EXT learning (Conklin and Tiffany, 2002). However, one possible explanation for this persistent enhancement of excitability may be due to how we defined successful EXT learning by measuring the inhibition of conditioned general locomotor activity. As cocaine produces changes on many behavioral dimensions (eg, velocity of each movement bout, head movements) (Robinson and Berridge, 2008), it is possible that we may not have observed a full EXT of conditioned responses if other parameters were measured. Thus, we may be observing an enhanced excitability of the activated ensemble due to an incomplete, partial weakening of the CS–US association and it remains to be seen whether a more robust weakening would have resulted in the loss of the enhanced excitability. In future studies, we may examine multiple behavioral parameters when studying the EXT of CL, in order to better assess EXT learning effects.
Alternatively, it is possible that following EXT, we were recording from a distinct, neuronal ensemble that participates in suppression of the conditioned response. In support, recent studies have demonstrated that the suppressive effects of EXT training and omission cue exposure on food-seeking behaviors are relieved by pharmacogenetic lesioning of medial prefrontal cortex ensembles activated during EXT training and omission cue exposure, respectively (Suto et al, 2016; Warren et al, 2016). As such, it is possible that the relatively higher excitability we observed in GFP+ neurons may represent a functional adaptation in a newly recruited ‘EXT’ ensemble whose recruitment is not associated with a net increase in the number of activated neurons following EXT. This newly recruited ensemble may, in turn, inhibit the retrieval of the cocaine-environment association (Quirk and Mueller, 2008).
Lack of Changes in the NAcshell and DS Following Cocaine and EXT Memory Retrieval
We observed no selective changes in the excitability of NAcshell ensembles following cocaine and EXT memory retrieval, despite an increase and decrease in the size of the activated ensemble, respectively. A similar phenomenon has been observed previously (Jakkamsetti et al, 2013; Ziminski et al, 2017) in which exposure to novel or sucrose-conditioned stimuli increased the number of activated neurons in the hippocampus and orbitofrontal cortex, respectively, in the absence of changes to the excitability of these activated neurons. Collectively, these findings add to a body of evidence, indicating that intrinsic excitability alterations on neuronal ensembles and cue-evoked ensemble recruitment can be independently regulated.
However, in that same study we observed selective increases in NAcshell ensemble excitability following exposure to sucrose-associated cues (Ziminski et al, 2017), which were attenuated following EXT. These differences in ensemble excitability adaptations may be due to changes in conditioning parameters; drugs of abuse produce significantly more robust and longer lasting conditioned behaviors, and neuroadaptations than food rewards (Lu et al, 2003; Zombeck et al, 2008), whereas conditioning to discrete cues and contextual stimuli is subserved by different anatomical substrates (Chaudhri et al, 2010). Thus, it is likely to be that striatal brain areas respond with a diverse set of adaptations following distinct types of learning.
We did not observe any changes in ensemble-selective excitability in the DS. This area consists of two related yet distinct subdivisions, the dorsomedial striatum and the dorsolateral striatum, which have different roles in cocaine-related behaviors (Murray et al, 2012). Thus, by including both areas in our analysis, subtle ensemble excitability changes may not have been detected due to subregion-selective changes in ensemble or background neuronal excitability. In future studies these two subregions may be analyzed separately to better elucidate possible ensemble-specific adaptations following cocaine-conditioning. In addition, striatal MSNs can be further distinguished based on their dopamine 1 and 2 receptor expression, and these two neuronal subpopulations, which project to different brain areas, have distinct roles in cocaine-associated behaviors (Smith et al, 2013). In future studies, it would be crucial to identify whether GFP+ and GFP− cells are D1R- or D2R-expressing neurons using single-cell PCR, in order to determine whether associative learning induces pathway-specific neuronal ensemble changes in excitability.
Concluding Remarks
These data provide novel insight into the regulation of striatal ensemble size and excitability in encoding cocaine-associative memories. Examining the complex interaction of these factors which underlie memory encoding will be key to further understanding the contribution of drug-associated environments in addiction-related behaviors. Although the behavioral procedure used here is highly useful for studying cocaine-environment associations, it may not model certain features of drug addiction (eg drug-seeking). In future investigations, we may perform similar electrophysiological studies by using procedures that better model drug relapse, such as the contextual renewal of drug-seeking (Crombag et al, 2008).
Funding and disclosure
The University of Sussex Strategic Development Funds and The Sussex Neuroscience 4-year PhD programme supported the research described herein. The authors declare no conflict of interest.
Acknowledgments
We thank Dr Alex Hoffman at NIDA IRP (Baltimore, USA) for critically reading this manuscript. We also thank Stephanie Fisher for excellent technical support in managing the transgenic mouse colony.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE (1998). Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci 18: 10579–10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP (2007). The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465. [DOI] [PubMed] [Google Scholar]
- Brons JF, Woody CD (1980). Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. J Neurophysiol 44: 605–615. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC (1992). Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience 48: 621–629. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA (2001). Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci 2: 397–407. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT et al (2017). Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci 37: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM (2002). The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev 1: 281–296. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97: 155–167. [DOI] [PubMed] [Google Scholar]
- Cotel F, Exley R, Cragg SJ, Perrier JF (2013). Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci USA 110: 4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y (2008). Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363: 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG (1998). The relation between dopamine oxidation currents in the nucleus accumbens and conditioned increases in motor activity in rats following repeated administration of d-amphetamine or cocaine. Eur J Neurosci 10: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H (1913)Memory. A Contribution to Experimental Psychology Teachers College. Columbia University: New York. [Google Scholar]
- Everitt BJ, Robbins TW (2013). From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37(9 Pt A): 1946–1954. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991). Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41: 89–125. [DOI] [PubMed] [Google Scholar]
- Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ (2006). Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci 24: 867–875. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME (2001). Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci 14: 1843–1855. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM et al (2009). Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci 29: 5820–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F et al (2013). Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron 80: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ (2009). Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci 29: 12275–12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR et al (2012). Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci 15: 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE et al (2009). Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci 12: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT (2003). Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem 85: 1604–1613. [DOI] [PubMed] [Google Scholar]
- Ma YY, Henley SM, Toll J, Jentsch JD, Evans CJ, Levine MS et al (2013). Drug-primed reinstatement of cocaine seeking in mice: increased excitability of medium-sized spiny neurons in the nucleus accumbens. ASN Neuro 5: 257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Crombag HS, Mitchell T, Simmons DE, Kreuter JD, Morales M et al (2007). Repeated amphetamine administration outside the home cage enhances drug-induced Fos expression in rat nucleus accumbens. Behav Brain Res 185: 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A, Tambour S, Tirelli E (2003). The magnitude and the extinction duration of the cocaine-induced conditioned locomotion-activated response are related to the number of cocaine injections paired with the testing context in C57BL/6 J mice. Behav Brain Res 145: 113–123. [DOI] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA et al (2010). Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30: 3689–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J (2000). Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115: 39–47. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ (2012). Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology 37: 2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC (2000). Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ (1995). Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15: 4449–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ (1998). Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12: 15–22. [DOI] [PubMed] [Google Scholar]
- O'Donnell P (2003). Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17: 429–435. [DOI] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR (1981). Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci 28: 755–760. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363: 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS (2004). Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem 11: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB (2006). 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse 59: 374–377. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW (2013). Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr Opin Neurobiol23: 546–552.. [DOI] [PMC free article] [PubMed]
- Suto N, Elmer GI, Wang B, You ZB, Wise RA (2013). Bidirectional modulation of cocaine expectancy by phasic glutamate fluctuations in the nucleus accumbens. J Neurosci 33: 9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T et al (2016). Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. elife 5: e21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis C, Bargas J, Ayala GX, Galvan E, Galarraga E (2000). Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience 95: 745–752. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PE (2009). Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev 2: 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ et al (2016). Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci 36: 6691–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A et al (2016). Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biol Psychiatry 80: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2010). The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ (2003). The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ (1998). Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci 18: 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziminski JJ, Hessler S, Margetts-Smith G, Sieburg MC, Crombag HS, Koya E (2017). Changes in appetitive associative strength modulates nucleus accumbens, but not orbitofrontal cortex neuronal ensemble excitability. J Neurosci 37: 3160–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS (2008). Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav 93: 637–650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.