Abstract
Background
Autism is a neurodevelopmental disorder that is first manifested during early childhood. Postmortem experiments have identified significantly elevated expression of metabotropic glutamate receptor 5 (mGluR5) in cerebellar vermis and prefrontal cortex of individuals with autism.
Methods
In the current study we employed the mGluR5 tracer [18F]-3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile ([18F]-FPEB) to quantify mGluR5 binding in vivo in adults with autism vs. healthy controls using positron emission tomography (PET).
Results
We identified significantly higher [18F]-FPEB binding potential in the postcentral gyrus and cerebellum of individuals with autism. There was a significant negative correlation between age and [18F]-FPEB binding potential in the cerebellum but not in the postcentral gyrus. In the precuneus, [18F]-FPEB binding potential correlated positively with the lethargy subscale score for the Aberrant Behavioral Checklist (ABC). In cerebellum, there were significant negative correlations between [18F]-FPEB binding potential and ABC total score, ABC hyperactivity subscale score, and the ABC inappropriate speech subscale score.
Conclusions
These novel findings demonstrate for the first time that mGluR5 binding is altered in critical brain areas of subjects with autism, suggesting abnormal glutamate signaling in these regions. Finally, the correlations between altered [18F]-FPEB binding potential in the cerebellum and precuneus suggest that some autistic symptoms may be influenced by abnormal glutamate signaling.
Keywords: PET, Autism, mGluR5, Cerebellum, Postcentral gyrus, Precuneus, [18F]-3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile
Background
Autism is a severe neurodevelopmental disorder with a rising incidence of 14.7 per 1000 (1 in 68) in the United States [1]. Autism is characterized by impairments in social communicative behavior and patterns of repetitive behavior [2]. Due to the heterogeneous nature of autism, the identity of autism-specific biomarkers remains elusive. Nevertheless, much of the focus has been placed on investigating neurotransmitter systems in the brains of subjects with autism. One of them, glutamate is the primary excitatory neurotransmitter in brain and spinal cord and is important in brain development and neuroplasticity [3]. Multiple studies have demonstrated elevated levels of glutamate in plasma of both adults and children diagnosed with autism when compared with controls [4–6]. Furthermore, magnetic resonance spectroscopy (MRS) studies have identified higher levels of glutamate in multiple brain regions of children and adolescents with autism including the anterior cingulate cortex (ACC), left striatum, left cerebellar hemisphere, and left frontal lobe [6, 7]. In contrast, reduced levels of the main inhibitory brain neurotransmitter, gamma-aminobutyric acid (GABA), have been observed in multiple brain regions of subjects with autism including the temporal lobe (auditory cortex), primary motor cortex, and postcentral gyrus (somatosensory cortex) [8–10]. These findings suggest that there is an imbalance in excitatory/inhibitory neurotransmission in the brains of subjects with autism. This imbalance could potentially explain the impairments that define the core symptoms of autism [11].
Accumulating data has implicated metabotropic glutamate receptor 5 (mGluR5) in the pathology of autism spectrum disorders [12–14]. Postmortem experiments have demonstrated increased expression of dimerized and total mGluR5 in cerebellar vermis and superior frontal cortex [Brodmann Area 9 (BA9)] in children with autism [12, 13]. In vitro binding assays employing [3H]-labeled 3-methoxy-5-pyridin-2-ylethynylpyridine (MPEPy) in prefrontal cortex tissue homogenates from subjects with fragile X syndrome (FXS) vs. controls found a marginally significant increase in mGluR5 density (p < 0.058) in subjects with FXS [14]. These findings are important in light of proposed treatments for autism [15] as well as schizophrenia and affective disorders [16] via the modulation of mGluR5 activity. If successful, mGluR5 modulation may improve symptoms of psychiatric disorders including autism in patients who have not improved via currently available treatment modalities.
The current study represents the first time that positron emission tomography (PET) imaging has been used to determine mGluR5 binding in vivo in brains of adults with autism and controls, using [18F]-3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile ([18F]-FPEB), a potent, selective, and systemically active antagonist of mGluR5 [17]. We aimed to investigate if mGluR5 expression in vivo replicated the results obtained by postmortem experiments and whether these changes (if any) positively correlated with autism symptom severity.
Methods
Patient recruitment
All study procedures were approved by the Johns Hopkins University Institutional Review Board (IRB) and all enrolled study subjects signed and dated IRB-approved consent forms. Informed consent was obtained from all individual participants included in the study. People with autism (n = 6) were recruited from a sample of children who had previously completed separate investigations at the Kennedy Krieger Institute (Baltimore, MD). Healthy control volunteers (n = 3) were recruited from the surrounding community via IRB-approved advertisements. Two historic controls, who had previously participated in a PET study conducted at Johns Hopkins University (JHU) were included. One of the historic controls could not be reached to complete new bloodwork and the Structured Clinical Interview for DSM-IV Axis I disorders – Clinician Version.
Patient screening
All subjects were first screened using an IRB-approved telephone script to determine if they met the inclusion and exclusion criteria for the study. Additionally, all potential study subjects provided a complete medical and medication history, underwent a physical exam, vital signs, laboratory tests, and a 12-lead electrocardiogram (ECG). All study subjects (except for one historical control) underwent the Structured Clinical Interview for DSM-IV Axis I disorders – Clinician Version (SCID-CV) [18] in order to determine if they presented with psychiatric disorders. Autistic symptoms were determined by scores on the Autism Diagnostic Observation Schedule (ADOS) [19], the Autism Diagnostic Interview-Revised (ADI-R) [20], Autism Spectrum Screening Questionnaire (ASSQ) [21], the Clinical Global Impression (CGI) [22], the Aberrant Behavior Checklist (ABC) [23], the Lifetime Social Communication Questionnaire (SCQ) [24], and the Global Assessment of Functioning (GAF) [25, 26]. ADI-R and ADOS scores were obtained when the subjects with autism were children and were provided by the Kennedy Krieger Institute. SCID-5-CV, ASSQ, GCI, ABC, and GAF scores were obtained during patient screening.
Inclusion criteria for people with autism included: 1) male or female subjects, 18-35 years old; 2) previous diagnosis of autism spectrum disorder based on ADI-R and ADOS criteria; 3) weight of at least 100 lbs. (45.4 kg); 4) absence of other major serious, current medical, psychiatric, or neurologic issues other than autism and its comorbid deficits (i.e., seizure disorder, intellectual impairment). Subjects with comorbid diagnoses of attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), or anxiety disorder were not excluded as there are high rates of comorbidity of these conditions in individuals diagnosed with autism [27, 28]; 5) provision of informed consent for testing from subject or an authorized decision maker. Inclusion criteria for healthy controls included the following: 1) male and female subjects, aged 18-35 years old, in good physical health; 2) have clinical laboratory test results within the reference ranges for the population or results within acceptable deviations that are not considered by the investigators to be clinically significant; 3) absence of other serious, current comorbid psychiatric disorders as determined by the SCID-CV; and 4) provision of written informed consent and ability to comply with the study restrictions. Exclusion criteria for people with autism and healthy controls were as follows: 1) change in behavioral treatments or life change such as change in residence, work site, or community service provider (within the past month); 2) pregnant or lactating women; 3) diagnosis of Tourette syndrome, FXS, or Rett syndrome; 4) patients with significant self-injury or with severity requiring inpatient treatment; 5) concurrent other psychiatric illness including substance abuse, or severe systemic disease based on history and physical exam; 6) laboratory tests with clinically significant abnormalities; 7) prior participation in other research protocols or clinical care in the last year such as radiation exposure that would exceed the annual limits; 8) suffer from claustrophobia and would be unable to undergo magnetic resonance imaging (MRI) or PET scanning; 9) implanted or embedded metal objects, prostheses, or fragments in the head or body that would present a risk during the MRI scanning procedure; 10) clinically significant abnormal MRI; 11) positive human immunodeficiency virus (HIV) test; 12) alcohol consumption within 48 h before the PET scan; 13) currently a user of any illicit drugs or alcohol abuse, or has a positive drug screen; 14) BMI of > 40 kg/m2 15) use of prescription stimulants in the two days before the PET scan; 16) currently on medications that affect the glutamate system.
MRI
Subjects, who met the enrollment criteria following the initial screening assessments, underwent an MRI scan with a set format of structural sequences including a spoiled gradient recalled (SPGR) acquisition sequence (124 slices with image matrix 256 × 256, pixel size 0.93 × 0.93 mm, slice thickness 1.5 mm) imaging for a three-dimensional anatomical data set of the brain [29] using a 3-T Magnetom Trio scanner (Siemens Medical Solutions). The MRI scans were obtained on each subject in order to co-register PET and MRI images for analysis of PET data.
18F-FPEB preparation
18F-FPEB was prepared at high specific activity as previously described [17].
PET scan
All PET scans were performed at the JHU PET Center using a second-generation, High-Resolution Research Tomograph (HRRT; CPS Innovations, Inc.) (2 mm axial resolution). A venous catheter was placed prior to the scan for radiotracer injection. Approximately 10 min before 18F-FPEB injection, a transmission scan was acquired for attenuation correction. Subjects were administered intravenously approximately 185 megabecquerel (MBq) [5 mCi (mCi)] [18F]-FPEB dose in saline. Dynamic PET scans of the brain began immediately after [18F]-FPEB administration and images were acquired for 90 min after tracer administration.
Statistical analysis
Quantitative analysis of the non-displaceable binding potential (BPND) of [18F]-FPEB was obtained to measure the density of the mGluR5 availability (Bmax) in selected brain regions. Cerebellar white matter was defined as the reference tissue for [18F]-FPEB. We determined volumes of interest (VOIs) based on co-registered MRI and PET scans for analysis with a multilinear reference tissue, two parameter model. Brain regional BPNDs were obtained. Descriptive and analytical methods for small samples were employed. To determine differences in binding potential of [18F]-FPEB between individuals with autism vs. controls, two-tailed student’s t-tests were run for each of the brain regions with significance set at p < 0.05. Two-tailed Pearson correlations were calculated to determine potential relationships between [18F]-FPEB binding potential and measures of autistic symptoms and scores on psychometric tests in subjects with autism.
Results
A total of six individuals with autism were used for data analysis. Three control individuals were included, two of whom had previously been scanned with [18F]-FPEB as part of an earlier study. The mean age of controls was 27 ± 3.61 and the mean age of subjects with autism was 20 ± 2.10 (p < 0.0067). All subjects with autism and controls were male. With regard to race, for the controls one was white, one was African American, and one was Asian American. For the subjects with autism five were white and one was Asian American. Table 1 summarizes demographic data and Table 2 summarizes mean scores for the study subjects on measures of autistic pathology.
Table 1.
Demographics of study subjects
| Control | Autism | P | |
|---|---|---|---|
| Age | 27 ± 3.61 | 20 ± 2.10 | 0.0067 |
| Sex | 3 M | 6 M | – |
| Race | 1 W, 1 A, 1AA, | 5 W, 1A | – |
A Asian, AA African American, M male, W white
Italicized value represents a statistically significant finding
Table 2.
Measures of autism symptoms of study subjects
| Measure | Range of scores | Autism cutoff score | Score |
|---|---|---|---|
| Autism Diagnostic Observation Schedule (ADOS) | |||
| Language and communication total | (0-12) | > 3 | 4.50 ± 1.87 |
| Reciprocal social interaction total | (0-18) | > 6 | 8.00 ± 3.54 |
| Language and communication and reciprocal social interaction total | (0-30) | > 10 | 12.50 ± 3.90 |
| Autism Diagnostic Interview-Revised (ADI-R) | |||
| Reciprocal social interaction total | (0-32) | > 10 | 15.83 ± 5.12 |
| Communication total | (0-26) | > 8 | 12.67 ± 4.08 |
| Restricted, Repetitive and stereotyped behavior total | (0-16) | > 3 | 5.33 ± 1.86 |
| Total | (0-70) | NA | 34.00 ± 7.96 |
| Autism Spectrum Screening Questionnaire (ASSQ) | |||
| Total | (0-54) | > 17 | 17.17 ± 7.88 |
| Aberrant Behavior Checklist (ABC) | |||
| Irritability total | (0-45) | NA | 3.50 ± 5.68 |
| Lethargy total | (0-48) | NA | 10.50 ± 8.36 |
| Stereotypy total | (0-21) | NA | 1.50 ± 1.22 |
| Hyperactivity total | (0-48) | NA | 7.83 ± 6.01 |
| Inappropriate speech total | (0-12) | NA | 1.83 ± 1.72 |
| Total | (0-174) | NA | 25.17 ± 14.27 |
| Clinical Global Impression | |||
| Severity of illness | (0-7) | NA | 3.50 ± 1.05 |
| Global Improvement | (0-7) | NA | 3.17 ± 1.33 |
| Efficacy index | (0-16) | NA | 9.33 ± 4.03 |
| Lifetime Social Communication Questionnaire (SCQ) | |||
| Total for verbal children | (0-39) | > 15 | 16.17 ± 3.92 |
| Global Assessment of Functioning (GAF) | |||
| Total | (0-90)a | NA | 69.83 ± 9.15 |
NA not applicable; aFor this scale, a higher score indicates better functioning
[18F]-FPEB binding potential was measured in 21 brain regions, all of which are known to express mGluR5 [17, 30–37] and have previously been implicated in the pathology of autism [38–46]. We identified significantly elevated [18F]-FPEB binding potential in cerebellum (p < 0.016) and postcentral gyrus (p < 0.036), indicating increased mGluR5 binding in these brain regions (Table 3, Fig. 1). Moreover, we identified trends towards significant elevation of [18F]-FPEB binding potential in the entorhinal area (p < 0.065) and the precuneus (p < 0.071) (Table 3, Fig. 1). Because age was significantly different between controls and subjects with autism, Pearson correlations were run to analyze this demographic measure for an effect on [18F]-FPEB binding potential. For cerebellum, there was a significant negative correlation between age and [18F]-FPEB binding potential (r = − 0.68, p < 0.042). However, correlations were not significant for postcentral gyrus (r = − 0.43, p < 0.25), precuneus (r = − 0.36, p < 0.34) or entorhinal area (r = − 0.38, p < 0.32).
Table 3.
18F-FPEB binding potential throughout the brain as revealed by positron emission tomography (PET)
| Region | Control | Autism | t-value | P |
|---|---|---|---|---|
| Amygdala | 3.93 ± 0.64 | 3.69 ± 0.57 | 0.58 | 0.58 |
| Caudate nucleus | 4.28 ± 0.47 | 4.56 ± 0.62 | 0.67 | 0.52 |
| Cerebellum | 1.07 ± 0.10 | 1.25 ± 0.068 | 3.17 | 0.016 |
| Cingulate | 4.33 ± 0.43 | 4.42 ± 0.23 | 0.42 | 0.68 |
| Entorhinal area | 1.78 ± 0.71 | 2.75 ± 0.59 | 2.19 | 0.065 |
| Frontal lobe | 4.11 ± 0.39 | 4.28 ± 0.22 | 0.87 | 0.41 |
| Fusiform gyrus | 3.86 ± 0.49 | 3.69 ± 0.52 | 0.47 | 0.65 |
| Globus pallidus | 1.12 ± 0.14 | 1.28 ± 0.15 | 1.58 | 0.16 |
| Hippocampus | 3.74 ± 0.67 | 3.66 ± 0.52 | 0.21 | 0.84 |
| Insula | 4.58 ± 0.62 | 4.49 ± 0.51 | 0.22 | 0.83 |
| Occipital lobe | 3.22 ± 0.39 | 3.35 ± 0.15 | 0.79 | 0.46 |
| Paracentral gyrus | 2.89 ± 0.44 | 3.36 ± 0.32 | 1.83 | 0.11 |
| Parahippocampus | 3.25 ± 0.09 | 3.55 ± 0.40 | 1.22 | 0.26 |
| Parietal lobe | 3.93 ± 0.36 | 4.10 ± 0.14 | 1.08 | 0.32 |
| Precentral gyrus | 3.55 ± 0.38 | 3.86 ± 0.12 | 1.89 | 0.10 |
| Precuneus | 3.87 ± 0.24 | 4.19 ± 0.20 | 2.13 | 0.071 |
| Postcentral gyrus | 3.44 ± 0.22 | 3.82 ± 0.20 | 2.60 | 0.036 |
| Putamen | 3.90 ± 0.54 | 4.18 ± 0.33 | 0.91 | 0.39 |
| Temporal lobe | 4.23 ± 0.57 | 4.23 ± 0.30 | 0.02 | 1.00 |
| Thalamus | 2.43 ± 0.28 | 2.50 ± 0.29 | 0.37 | 0.72 |
| Ventral striatum | 4.75 ± 0.66 | 5.10 ± 0.50 | 0.89 | 0.40 |
Italicized values represent significant or near-significant findings
Fig. 1.
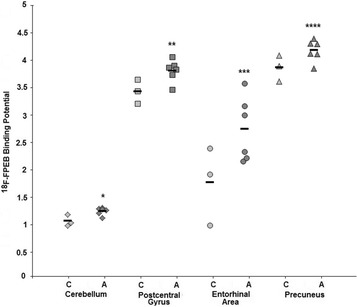
[18F]-FPEB binding potential in selected brain regions. Scatter plot showing [18F]-FPEB binding potential in cerebellum, postcentral gyrus, entorhinal area, and precuneus of subjects with autism vs. controls. A, autism; C, control; *, p < 0.016; **, p < 0.036; ***, p < 0.065; ****, p < 0.071
In cerebellum, there were significant negative correlations between [18F]-FPEB binding potential and ABC total score (r = − 0.904, p < 0.013), ABC hyperactivity subscale score (r = − 0.861, p < 0.028), and the ABC inappropriate speech subscale score (r = − 0.928, p < 0.008) (Fig. 2). In precuneus, there was a significant positive correlation between [18F]-FPEB binding potential and ABC lethargy subscale score (r = 0.843, p < 0.035) (Fig. 2).
Fig. 2.
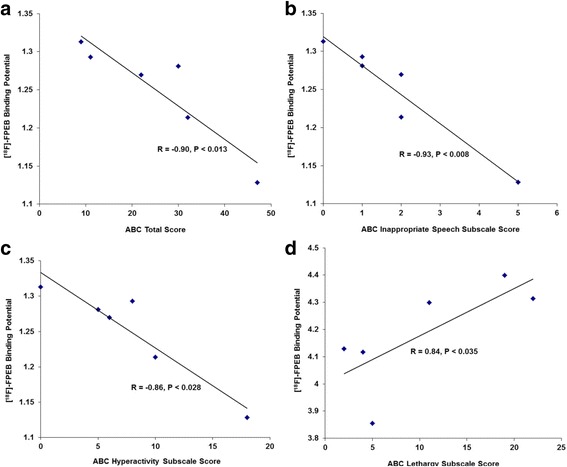
Correlations between [18F]-FPEB binding potential and scores on psychometric tests. Scatter plots showing correlations between [18F]-FPEB binding potential and ABC total score (a), ABC inappropriate speech subscale score (b), ABC hyperactivity subscale score (c), and ABC lethargy subscale score (d) in subjects with autism. a, b, and c [18F]-FPEB binding potentials are from cerebellum; d [18F]-FPEB binding potential is from precuneus
Discussion
We observed significantly elevated [18F]-FPEB binding potential in two brain regions of males with autism: the cerebellum (in its entirety) and the postcentral gyrus. We also observed trends for significantly elevated [18F]-FPEB binding potential in the precuneus and the entorhinal area. In subjects with autism, we identified significant, negative correlations between [18F]-FPEB binding and ABC total score, ABC hyperactivity subscale score, and ABC inappropriate speech subscale score in cerebellum. We also identified one significant positive correlation between [18F]-FPEB binding and ABC lethargy subscale score in precuneus.
Increased mGluR5 expression has previously been observed in cerebellar vermis and BA9 of children with autism [12, 13]. These findings suggest pathologic activation of mGluR5 which may be prenatal or early postnatal. The observed PET data of elevated [18F]-FPEB binding potential in young adults with autism supports this hypothesis. In contrast, a recent study found reduced mGluR5 immunoreactivity in the dorsolateral prefrontal cortex (DLPFC) of subjects with autism [47]. However, this finding may be due to the mixing of adult and child subjects. Additionally, the authors used immunohistochemistry and not western blotting or imaging, which may account for possible differences between their results and ours. As shown in the comparison between age of study subjects and [18F]-FPEB binding potential, there was a significant negative correlation between age and [18F]-FPEB binding potential in the cerebellum, while there was no effects in the postcentral gyrus, precuneus, or the entorhinal area. These results are likely impacted by low sample size. Thus, repetition of these experiments involving a larger sample is needed to determine the effect of age on [18F]-FPEB binding potential. The negative correlations between [18F]-FPEB binding potential and ABC total score as well as hyperactivity and inappropriate speech subscale scores in the cerebellum provide some evidence that increased mGluR5 binding in autism is not associated with greater symptom severity. The one positive correlation between [18F]-FPEB binding and the ABC-lethargy subscore in precuneus is intriguing given that the precuneus is a principal component of the default mode network (DMN). The DMN is active when the individual is engaged in internally focused tasks including social cognition, a major impairment in individuals with autism [48]. As reviewed by Padmanabhan et al. [48], there are structural and functional abnormalities in the DMN of individuals with autism. Excitatory/inhibitory imbalance as indicated by changes in levels of glutamate and/or GABA could potentially impact functioning of the DMN and autistic symptomatology including lethargy as measured by the ABC. Further studies are needed however, to confirm these findings.
The four brain regions that showed significant increased or trends for increased [18F]-FPEB binding potential are involved in important cognitive domains that are impaired in autism including motor control, facial recognition, and memory [49–55]. The cerebellum plays a crucial role in learning and control of action through sensorimotor adaptation of signals in motor, premotor, and prefrontal cortex [51, 54]. The postcentral gyrus contains the primary somatosonsory cortex and is thereby crucial to motor control and learning and facial recognition [50]. The precuneus is located in the medial parietal cortex and maintains functional connection with the prefrontal cortex [56]. PET studies have shown precuneus activation during episodic memory tasks [52, 53]. The entorhinal cortex is the main region of interaction between the hippocampus and neocortical regions due to its reciprocal connections between these two areas and is involved with memory [55].
Studies have previously demonstrated functional and morphological changes in all four of these brain regions in people with autism [38–40, 42]. A number of motor learning studies have suggested that people with autism show a bias towards reliance on proprioceptive (as opposed to visual) feedback [57–59], with evidence that abnormalities in the sensorimotor regions of the cerebellum that may contribute to this bias. Our findings provide additional support for abnormalities in these somatosensorimotor-cerebellar circuits, crucial to development of skilled actions necessary in social and communicative behavior. The postcentral gyrus of subjects with autism has been shown to display reduced cortical thickness and reduced gray matter concentration when compared with controls [42]. Cheng et al. [40], found that gray matter volume was reduced in postcentral gyrus while gray matter volume is increased in cerebellum in subjects with autism vs. controls using voxel-based morphometric analysis. An analysis of whole-brain voxel-based unbiased resting state functional connectivity found reduced connectivity in multiple brain regions including left and right precuneus and left and right postcentral gyrus of subjects with autism [39]. Moreover, functional connectivity changes in these two regions were significantly associated with ADOS severity scores. Morphological changes in the cerebellum of subjects with autism have been identified including: 1) altered Purkinje cell density; 2) abnormalities in deep cerebellar nuclei; and 3) changes in total cerebellar volume [51]. These changes may contribute to motor and cognitive deficits associated with autism. Small tightly packed neurons have been consistently found in the entorhinal cortex of subjects with autism [38]. These anatomical changes, coupled with potential changes in glutamate signaling, likely contribute to impaired cognitive domains in autism.
Strengths of the current study include: 1) high resolution tomograph (the HRRT PET scanner is the highest resolution dedicated brain PET scanner available) with kinetic modeling; 2) the use of state of the art technology and one of the most optimal PET radiotracers for mGluR5 human brain imaging; and 3) carefully screened and characterized subjects. Limitations of the current study include: 1) small sample size; 2) differences in racial/ethnic diversity; 3) the age differences between subjects with autism vs. controls; and 4) the lack of inclusion of both sexes. Of the limitations, small sample size is important and as we have mentioned previously, further studies using a larger sample size are needed to confirm our results. The significant difference in age between subjects with autism vs. controls was an important limitation, which moreover had an effect on [18F]-FPEB binding potential in the cerebellum, but not the postcentral gyrus, precuneus, or entorhinal area. This may be an artifact that is tied to small sample size. In our previous studies of mGluR5 expression in children and adults with autism vs. controls, analysis of confounds did not find an effect of age on our results for cerebellar vermis [13]. The differences in racial/ethnic diversity between groups are a limitation that is also partially tied to small sample sizes. It is thus, difficult to determine whether these differences have an impact on [18F]-FPEB binding potential. Finally, while autism is more prevalent in males than in females [60], further studies should include females as well to ensure that the observed differences are not sex-specific.
Conclusions
The current study represents the first measurement of mGluR5 concentration in vivo in young adults with autism. Our findings partially validated our hypotheses in that some brain regions displayed significant elevations in elevated [18F]-FPEB binding potential and there was one significant positive correlation between [18F]-FPEB binding potential and a symptom of autism. Based on this pilot study, further experiments measuring mGluR5 binding in individuals with autism are warranted. mGluR5 may prove an important target of therapeutic intervention in autism spectrum disorders.
Acknowledgments
This work is supported by the Winston and Maxine Wallin Neuroscience Discovery Fund. S.H. Fatemi is also supported by the Bernstein Endowed Chair in Adult Psychiatry. Special thanks to Ayon Nandi, Andrew Crabb, Arman Rahmim, Lorena Gapasin of the JHU Section of High Resolution Brain Imaging and Wong lab.
Funding
This work is supported by the Winston and Maxine Wallin Neuroscience Discovery Fund. S.H. Fatemi is also supported by the Bernstein Endowed Chair in Adult Psychiatry.
Availability of data and materials
The raw MRI and PET images generated and analyzed during the course of the study are not publicly available due to data storage on local (Johns Hopkins University) share drives but are available, upon a reasonable request, from the corresponding author.
Abbreviations
- [18F]-FPEB
[18F]-3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile
- ABC
Aberrant Behavior Checklist
- ACC
Anterior cingulate cortex
- ADHD
Attention deficit hyperactivity disorder
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS
Autism Diagnostic Observation Schedule
- ASSQ
Autism Spectrum Screening Questionnaire
- BA9
Brodmann area 9
- Bmax
The ratio of the available receptor density
- BMI
Body mass index
- BPND
Non-displaceable binding potential
- CGI
Clinical Global Impression
- DLPFC
Dorsolateral prefrontal cortex
- DSM
Diagnostic and Statistical Manual
- ECG
Electrocardiogram
- FXS
Fragile X syndrome
- GABA
Gamma-aminobutyric acid
- GAF
Global Assessment of Functioning
- HIV
Human immunodeficiency virus
- HRRT
High-Resolution Research Tomograph
- IRB
Institutional review board
- JHU
Johns Hopkins University
- KD
Radioligand equilibrium dissociation constant
- MBq
Megabecquerel
- mCi
Millicurie
- mGluR5
Metabotropic glutamate receptor 5
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- OCD
Obsessive compulsive disorder
- PET
Positron Emission Tomography
- SCID-CV
Structured Clinical Interview for DSM-IV Axis I disorders – Clinician Version
- SCQ
Lifetime Social Communication Questionnaire
- SPGR
Spoiled gradient recalled
Authors’ contributions
SHF conceived of the study, participated in its design, supervised conduct of all experiments, and contributed to the drafting of the manuscript. DFW coordinated the study at Johns Hopkins University and contributed to study design. JRB contributed to study design, screened study subjects, and conducted the PET and MRI scans. AM was involved in patient recruitment and study design. HK analyzed PET data. TDF coordinated between Johns Hopkins University and the University of Minnesota and contributed to the drafting of the manuscript. GMR and SJ advised on issues related to autism. JVP provided insight into interpretation of PET results and contributed to the drafting of the manuscript. SL performed statistical analysis of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All study procedures involving human subjects were approved by the Johns Hopkins University Institutional Review Board (IRB) and all enrolled study subjects signed and dated IRB-approved consent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. Hossein Fatemi, Phone: 612-626-3633, Email: fatem002@umn.edu.
Dean F. Wong, Email: dfwong@jhmi.edu
James R. Brašić, Email: brasic@jhmi.edu
Hiroto Kuwabara, Email: hkuwaba1@jhmi.edu.
Anil Mathur, Email: amathur4@jhmi.edu.
Timothy D. Folsom, Email: folso013@umn.edu
Suma Jacob, Email: sjacob@umn.edu.
George M. Realmuto, Email: realm001@umn.edu
José V. Pardo, Email: jvpardo@umn.edu
Susanne Lee, Email: leexx310@umn.edu.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbid Mortal Wkly Rep Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5®) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Spencer AE, Uchida M, Kenworthy T, Keary CJ, Biederman J. Glutamatergic dysregulation in pediatric psychiatric disorders: a systematic review of the magnetic resonance spectroscopy literature. J Clin Psychiatry. 2014;75(11):1226–1241. doi: 10.4088/JCP.13r08767. [DOI] [PubMed] [Google Scholar]
- 4.El-Ansary A, Al-Ayadhi L. Gabaergic/glutamatergic imbalance relative to excessive neuroinflammation in autism. J Neuroinflamm. 2014;11:189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30(8):1472–1477. doi: 10.1016/j.pnpbp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Hassan TH, Abdelrahman HM, Abdel Fattah NR, El-Masry NM, Hashim HM, El-Gerby KM, et al. Blood and brain glutamate levels in children with autistic disorder. Res Autism Spect Disord. 2013;7:541–548. doi: 10.1016/j.rasd.2012.12.005. [DOI] [Google Scholar]
- 7.Joshi G, Biederman J, Wozniak J, Goldin RL, Crowley D, Furtak S, et al. Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: a pilot study at 4T. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):379–384. doi: 10.1007/s00406-012-0369-9. [DOI] [PubMed] [Google Scholar]
- 8.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision regional cortical variation. NeuroImage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism. 2017;10(4):608–619. doi: 10.1002/aur.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. NeuroImage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzunova G, Pallanti S, Hollander E. Excitatory/inhibitory balance in autism spectrum disorders: implications for interventions and therapeutics. World J Biol Psychiatry. 2016;17(3):174–186. doi: 10.3109/15622975.2015.1085597. [DOI] [PubMed] [Google Scholar]
- 12.Fatemi SH, Folsom TD. Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of subjects with autism: a postmortem brain study. Mol Autism. 2011;2:6. doi: 10.1186/2040-2392-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec. 2011;294(10):1635–1645. doi: 10.1002/ar.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohith TG, Osterweil EK, Fujita M, Jenko KJ, Bear MF, Innis RB. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol Autism. 2013;4(1):15. doi: 10.1186/2040-2392-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gürkan CK, Hagerman RJ. Targeted treatments in autism and fragile X syndrome. Res Autism Spectr Disord. 2012;6(4):1311–1320. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 17.Wong DF, Waterhouse R, Kuwabara H, Kim J, Brašić JR, Chamroonrat W, et al. 18F-FPEB, a PET radiopharmaceutical for quantifying metabotropic glutamate 5 receptors: a first-in-human study of radiochemical safety, biokinetics, and radiation dosimetry. J Nucl Med. 2013;54(3):388–396. doi: 10.2967/jnumed.112.107995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 19.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 21.Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29(2):129–141. doi: 10.1023/A:1023040610384. [DOI] [PubMed] [Google Scholar]
- 22.Busner J, Targum SD. The clinical global impression scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 23.Aman MG, Singh NN. Aberrant behavior checklist (ABC) – community supplementary manual. East Aurora, New York: Slosson Educational Publishing, Inc; 1994. [Google Scholar]
- 24.Rutter M, Bailey A, Berument SK, Lord C, Pickles A. Lifetime Social Communication Questionnaire (SCQ): Western Psychological Services; 2003. https://www.wpspublish.com. Accessed 1 Mar 2017
- 25.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, DSM-III-R. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 26.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the global assessment of functioning. Br J Psychiatry. 1995;166(5):654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 27.Davis NO, Kollins SH. Treatment of co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics. 2012;9(3):518–530. doi: 10.1007/s13311-012-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Steensel FJ, Bogels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev. 2011;14(3):302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brašić JR, Zhou Y, Musachio JL, Hilton J, Fan H, Crabb A, et al. Single photon emission computed tomography experience with (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine in the living human brain of smokers and nonsmokers. Synapse. 2009;63(4):339–358. doi: 10.1002/syn.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballester-Rosado CJ, Sun H, Huang JY, Lu HC. mGluR5 exerts cell-autonomous influences on the functional and anatomical development of layer IV cortical neurons in the mouse primary somatosensory cortex. J Neurosci. 2016;36(34):8802–8814. doi: 10.1523/JNEUROSCI.1224-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownell A-L, Jokivarsi K, Isacson O. Modulation of metabotropic glutamate subtype 5 receptor (mGluR5) in dyskinesia. J Nucl Med. 2012;53(Suppl. 1):468. [Google Scholar]
- 32.Guimaraes IM, Carvalho TG, Ferguson SS, Pereira GS, Ribeiro FM. The metabotropic glutamate receptor 5 role on motor behavior involves specific neural substrates. Mol Brain. 2015;8:24. doi: 10.1186/s13041-015-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holst SC, Sousek A, Hefti K, Saberi-Moghadam S, Buck A, Ametamey SM, et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprevation. elife. 2017;6:e28751. doi: 10.7554/eLife.28751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kågedal M, Cselényi Z, Nyberg S, Jönsson S, Raboisson P, Stenkrona P, et al. Non-linear mixed effects modeling of positron emission tomography data for simultaneous estimation of radioligand kinetics and occupancy in healthy volunteers. NeuroImage. 2012;61(4):849–856. doi: 10.1016/j.neuroimage.2012.02.085. [DOI] [PubMed] [Google Scholar]
- 35.Ohnuma T, Tessler S, Arai H, Faull RL, McKenna PJ, Emson PC. Gene expression of metabotropic glutamate receptor 5 and excitatory amino acid receptor 2 in the schizophrenic hippocampus. Brain Res Mol Brain Res. 2000;85(1-2):24–31. doi: 10.1016/S0169-328X(00)00222-9. [DOI] [PubMed] [Google Scholar]
- 36.Romano C, van den Pol AN, O’Malley KL. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA splice variants, and regional distribution. J Comp Neurol. 1996;367(3):403–412. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Simon L, Toth J, Molnar L, Agoston DV. MRI analysis of mGluR5 and mGluR1 antagonists, MTEP and R214127 in the central forebrain of awake, conscious rats. Neurosci Lett. 2011;505(2):155–159. doi: 10.1016/j.neulet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Bauman ML, Kemper TL. The neuropathology of autism spectrum disorders: what have we learned? Novartis Found Symp. 2003;251:112. [PubMed] [Google Scholar]
- 39.Cheng W, Rolls ET, Gu H, Zhang J, Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain. 2015;138(Pt. 5):1382–1393. doi: 10.1093/brain/awv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y, Chou KH, Fan YT, Lin CP. ANS: aberrant neurodevelopment of the social cognition network in adolescents with autism spectrum disorders. PLoS One. 2011;6(4):e18905. doi: 10.1371/journal.pone.0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyle-Thomas KA, Kushki A, Duerden EG, Taylor MJ, Lerch JP, Soorya LV, et al. The effect of diagnosis, age, and symptom severity on cortical surface area in the cingulate cortex and insula in autism spectrum disorders. J Child Neurol. 2013;28(6):732–739. doi: 10.1177/0883073812451496. [DOI] [PubMed] [Google Scholar]
- 42.Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual or other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31(4):556–566. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Dev Cogn Neurosci. 2011;1(3):260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagani M, Manouilenko I, Stone-Elander S, Odh R, Salmaso D, Hatherly R, et al. Brief report: alterations in cerebral blood flow as assessed by PET/CT in adults with autism spectrum disorder with normal IQ. J Autism Dev Disord. 2012;42(2):313–318. doi: 10.1007/s10803-011-1240-y. [DOI] [PubMed] [Google Scholar]
- 45.Van Rooji D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, et al. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry. 2018; in press [DOI] [PMC free article] [PubMed]
- 46.Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, et al. Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 2017;134:537–566. doi: 10.1007/s00401-017-1736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chana G, Laskaris L, Pantelis C, Gillett P, Testa R, Zantomio D, et al. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain Behav Immun. 2015;49:197–205. doi: 10.1016/j.bbi.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Padmanabhan A, Lynch CJ, Schaer M, Menon V. The default mode network in autism. Biol Psych Cogn Neurosci Neuroimaging. 2017;2(6):476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16(23):7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borich MR, Brodie SM, Gray WA, Inota S, Boyd LA. Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation. Neuropsychologica. 2015;79(Pt. B):246–255. doi: 10.1016/j.neuropsychologia.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, et al. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. Brain. 1999;122(Pt. 2):255–263. doi: 10.1093/brain/122.2.255. [DOI] [PubMed] [Google Scholar]
- 53.Platel H, Baron JC, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks. NeuroImage. 2003;20(1):244–256. doi: 10.1016/S1053-8119(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 54.Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takehara-Nishiuchi K. Entorhinal cortex and consolidated memory. Neurosci Res. 2014;84:27–33. doi: 10.1016/j.neures.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Cavanna AE, Trimble MR. The precuneus: a role of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt. 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 57.Haswell C, Izawa J, Dowell L, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neurosci. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izawa J, Pekny SE, Marko MK, Haswell C, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Res. 2012;5(2):124–136. doi: 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH. Behavioral and neural basis of anomalous motor learning in children with autism. Brain. 2015;138(Pt. 3):784–797. doi: 10.1093/brain/awu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw MRI and PET images generated and analyzed during the course of the study are not publicly available due to data storage on local (Johns Hopkins University) share drives but are available, upon a reasonable request, from the corresponding author.


