Abstract
Redox imbalance in regions of the CNS controlling blood pressure is increasingly recognized as a leading factor for hypertension. Nucleus tractus solitarius (NTS) of the dorsomedial medulla is the main region receiving excitatory visceral sensory inputs that modulate autonomic efferent drive to the cardiovascular system. This study sought to determine the capacity of reduced glutathione, a major bioactive antioxidant, to modulate NTS-mediated control of cardiovascular function in unanaesthetized rats. Male Fischer 344 rats were used for microinjection experiments. Cardiovascular responses to L-glutamate were first used to verify accurate placement of injections into the dorsomedial region comprising the NTS. Next, responses to GSH or vehicle were recorded followed by responses to L-glutamate again at the same site. GSH microinjection increased mean arterial pressure (MAP) compared to vehicle and abrogated responses to subsequent injection of L-glutamate. These data indicate that GSH microinjection into the NTS affects blood pressure regulation by dorsomedial neuronal circuits and blunts L-glutamate driven excitation in this region. These findings raise the possibility that increased antioxidant actions of GSH in NTS could contribute to autonomic control dysfunctions of the cardiovascular system.
Keywords: blood pressure, glutathione, L-glutamate, nucleus tractus solitaries
Introduction
Glutathione is produced in the CNS by glial cells and to a lesser extent by neurons [26]. The mechanism of glutathione action in the CNS has mainly been considered to depend on its antioxidant properties. In its reduced form (GSH), it is readily oxidized in the CNS and thus can protect against harmful effects of free radicals [reviewed by [14]]. However, GSH peptide is also capable of modulating excitatory neurotransmission due to its ability to directly interact with and modify functional properties of glutamate receptors. Indeed, Ogita and colleagues reported that oxidized glutathione (GSSG) as well as GSH each decrease binding affinity of [3H] glutamate without affecting the number of binding sites [21]. These findings suggested that glutathione could act to reduce glutamatergic neurotransmission. However, subsequent studies revealed that more physiological millimolar concentrations of glutathione have agonist properties at N-methyl-D-aspartate (NMDA) receptors [20]. Hence, whether the dominant neuromodulatory role of GSH is inhibitory or excitatory has not been established and, indeed, may vary among neuronal cell types and across brain regions.
Other studies have raised the possibility that glutathione acts at specific receptors in the CNS. This provides a possible mechanism of action that is distinct from those related to modulation of excitatory amino acid neurotransmission. Studies indicate that glutathione binding sites reside mainly on astrocytes and suggest that glutathione acts as an indirect neuromodulator [11, 12]. However, earlier observations suggested the presence of glutathione binding sites in synaptic membranes – suggesting direct neuronal actions – and this was confirmed recently in cortical synaptic membranes [15].
Despite these findings, few studies have sought to determine the functional significance of glutathione in modulating CNS neuronal activity. Available evidence suggests that thiol-containing compounds might be important modulators in neural pathways of autonomic cardiovascular control. For example, Ohta and colleagues showed that microinjection of the thiol-containing amino acid L-cysteine into the nucleus tractus solitarius (NTS) elicited pronounced hypotensive and bradycardic responses [23] that were abolished by acute pharmacological blockade of ionotropic glutamate receptors [23], suggesting that reduced thiols in NTS facilitate glutamatergic neurotransmission. On the other hand, data in the literature shows that H2O2, an oxidant agent, also produced robust bradycardic and hypotensive responses when injected into the NTS and is abrogated by the blockade of ionotropic glutamate receptors as well [6].
Since GSH is an endogenously generated thiol-containing compound and an antioxidant, its main effect on glutamatergic pathways within the dorsomedial medulla, specially the NTS, has yet to be determined. Hence, this study sought to investigate in conscious freely moving rats the effect of GSH microinjection in NTS on ongoing arterial pressure and heart rate and on responses of these variables to acute microinjection of L-glutamate.
Materials and Methods
Animals
Male Fischer 344 rats (220 – 250 g) from the main breeding stock of the institutional animal facility (CCA-UFOP – Federal University of Ouro Preto UFOP) were used. Animals were housed in collective cages (maximal four per cage) under temperature (22±3°C) and humidity (40 to 60%) controlled conditions and with a 12:12h light:dark cycle (lights on: 07:00 h AM). After surgical procedures, animals were housed in individual cages until experimentation. Rats received standard chow (Guabi Rat Chow, Paulinia, SP, Brazil) and water ad libitum. Experiments were performed in accordance with Brazilian Society for Neuroscience guidelines and Behavior Guidelines for Animal Experimentation and were approved by the institutional animal care and use committee (CEUA) from the Federal University of Ouro Preto (CEUA-UFOP, protocol n° 2010/85).
Drugs
Reduced glutathione (GSH) and L-glutamate (Sigma, St. Louis, MO, USA) were diluted in phosphate buffered saline (PBS) to final concentrations of 20 nmol and 1 nmol, respectively. GSH was neutralized with an equimolar amount of sodium bicarbonate prior to dilution.
Animal instrumentation
Stainless guide cannulae directed to the NTS were implanted in rats anesthetized with a ketamine (80 mg/kg, i.p.)/xylazine (7 mg/kg, i.p.) cocktail (Cristalia Produtos Químicos e Farmacêuticos, Itapira, SP). Rats were placed in a Stoelting stereotaxic instrument and a stainless steel cannula (14.0 mm×0.6 mm o.d.) was implanted so that its tip was located 14.5 mm caudal and 7.5 mm ventral to bregma and 0.5 mm lateral to midline. Two jeweler’s screws were implanted in the skull, and the cannula was anchored to the screws with acrylic cement. Immediately following surgery, rats received an intramuscular injection with 30,000 IU of penicillin (Fort Dodge Saúde Animal Ltda., Campinas, SP) and were housed singly with ad libitum access to chow and water.
Three days after stereotaxic surgery, also under ketamine/xylazine anesthesia, a polyethylene cannula (PE-10 connected to PE-50, Clay Adams, Parsippany, NJ, USA) filled with heparinized saline (125 IU/ml), was inserted into the abdominal aorta through the right femoral artery of each rat to measure pulsatile arterial pressure (PAP), mean arterial pressure (MAP) and heart rate (HR) according to procedures indicated below.
Experimental protocols
Experiments were performed in unanesthetized freely moving rats, approximately 48 h after femoral cannulation surgery. To insure minimal impact of acute stress on experimental outcomes, after connecting arterial cannulae to recording instruments rats were allowed to stabilize in their home cages for at least 1 h before commencing experiments.
Unilateral injections into the NTS were made with a 5 μl Hamilton syringe connected by polyethylene tubing (PE-10) to an injector cannula made of 32 gauge stainless steel tubing. The injector, when fully inserted, protruded ~1.5 mm beyond the tip of the guide cannula to target NTS. Each injection volume was 100 nL and was delivered over a period of 5 to 10 s.
Effects of reduced glutathione microinjections in NTS of conscious rats
Effects of GSH (20 nmol/100 nL) in NTS (lateral aspect of the commissural NTS) were tested 40 min after the injection site was functionally identified with L-glutamate (L-glu, 1 nmol/100 nL), which typically produced a robust decrease in MAP and HR [5, 6]. From a group of 9 rats with positive functional identification of the NTS, 4 rats were used to teste the effects of GSH on cardiovascular responses to L-glu into the same site. In this group, vehicle (100 nL) was injected 20 minutes after the first L-glu injection and 20 minutes before the GSH injection. Cardiovascular responses to L-glutamate at the same site were retested 60 min after GSH microinjection. In an additional control group (3 rats), an injection of vehicle (100 nL) replaced the GSH injection.
Histology
At the end of each experiment, injection sites were marked with 2% Evan’s Blue solution (100 nL). Immediately after the injection, animals were deeply anesthetized with sodium thiopental (70 mg/kg of body weight, i.v.) and perfused through the heart with 0.9% NaCl (30 ml) followed by 10% buffered formalin (100 ml). Brains were removed and kept in 10% buffered formalin solution for at least 24 hours. Coronal slices were cut to a thickness of 50 μm on a freezing microtome, mounted on glass slides, stained with Neutral Red and viewed under a light microscope to determine the location and distribution of dye at the injection site. When the distribution of dye encompassed the NTS, injections were accepted and the results from those experiments were included in the data analysis.
Data analysis
Cardiovascular responses to L-glutamate and GSH microinjections were quantified by measuring the maximum change in MAP and HR taking as baseline 20 s of the records just before each NTS microinjection. Results are reported as mean ± standard error of the mean (SEM).
Heart rate variability (HRV) was evaluated before, immediately after GSH microinjection and 20 minutes after GSH microinjection. Consecutive systolic intervals (SI) of 880 to 1050 heart beats were analyzed by the Heart Rate Variability module of the LabChart 8.1.5 for Windows software (ADInstruments Pty Ltd, Australia). Spectra was divided in low frequency (LF) and high frequency (HF) bands and each was set to range from 0.2 to 0.75 Hz and 0.75 to 2.50 Hz respectively. Ratio between LF and HF was used to estimate sympathovagal balance modulation. Power density was measured as μs2 and band’s power density were calculated as percent of total power density in the spectrum.
Analysis of variance (ANOVA) for repeated measures was used for group comparisons followed by post-hoc pair-wise comparisons with Bonferroni’s post-test. All data were statistically analyzed using Prism software version 6.00 (GraphPad Software, San Diego California USA). Differences were considered significant when the probability of a Type I error was less than 5% (p<0.05). Statistic power (β) was calculated for pairwise comparisons using the calculator tool available in the RSS Research website and considered valid when higher than 0.80.
Results
Microinjections made in 9 rats were considered to specifically target NTS. Histology showed that dye microinjected (100 nL) into NTS encompassed most of the intermediate subnucleus within the sub-postremal region, with some diffusion to surrounding areas as shown in figure 1 (panel c). Baseline mean arterial pressure (MAP) and heart rate (HR) in these experiments averaged 116 ± 9 mmHg and 346 ± 39 bpm, respectively.
Figure 1.

MAP and HR effect of GSH microinjection into NTS of conscious freely moving rats. (a) Traces of pulsatile arterial pressure (PAP), mean arterial pressure (MAP) and heart rate (HR) showing responses reduced glutathione (GSH, 20 nmol/100 nL). Dashed lines delineate microinjection time. (b) Summary data showing that GSH (20 nmol/100 nL) microinjection in NTS significantly increased MAP when compared to vehicle microinjection. (c) Schematic drawing of the microinjection sites (top) and photomicrograph of a neutral red stained coronal section (50 μm) through the dorsal medulla oblongata including the NTS (lateral aspect of the commissural NTS), indicated by the dashed lines (botton). Black circles indicate the center of GSH microinjection and white circles indicate the center of vehicle microinjections. Triangle indicates site of injection and arrow indicates the central canal. *different from vehicle; unpaired t-test; p<0.05.
Microinjections of GSH into the NTS significantly increased MAP (19.4 ± 1.4 mmHg, n=9) compared to vehicle (2.5 ± 0.8 mmHg, n=4) (unpaired t-test; p<0.05) and reduced HR (GSH, −36.8 ± 4.2 bpm, n=9; vehicle, −5.9 ± 2.5 bpm, n=4; unpaired t-test; p<0.05) as shown in figure 1 (panels a and b). Pressor responses to GSH lasted ~5 minutes. Changes in MAP and HR elicited by microinjections of vehicle (100 nL) into the NTS followed the same protocol described for GSH microinjections.
Figure 2 shows that the initial microinjection of L-glutamate into NTS produced depressor (−50 ± 14 mmHg; n=4) and bradycardic (−237 ± 16 bpm; n=4) responses. Prior microinjection of GSH into the same site effectively abolished (p<0.05; β = 0.929) responses to subsequent injection of L-glutamate (1 ± 2 mmHg and −7 ± 4 bpm; n=4). Both L-glutamate-evoked responses were compared to responses elicited by vehicle microinjections 20 minutes before the injection of GSH (2 ± 1 mmHg and6 ± 3 bpm, n=4). The MAP (−49 ± 12 mmHg) and HR (−250 ± 25 bpm) responses to L-glutamate before (n=3) and after (MAP, −48 ± 11 mmHg; HR, −242 ± 15 bpm, n=3; β = 0.95) vehicle injections were not different.
Figure 2.

Changes in MAP and HR elicited by L-glutamate microinjections in NTS before and after GSH or vehicle in conscious freely moving rats. (a) Reduced glutathione (GSH, 20 nmol/100nL) microinjection into NTS abrogated depressor and bradycardic responses to subsequent injection (60 min later) of L-glutamate (L-glu, 1 nmol/100nL). Vehicle (100 nL) microinjection had no effect on either response to a second L-glu microinjection. (b–e) Summary data. *different from vehicle and L-glu after GSH; ANOVA followed by Bonferroni’s post test; p<0.05; statistic power 0.929 for the comparison between L-glu responses before and after GSH.
Frequency-domain variability of the heart rate analysis showed an increase in HF band power immediately after (40.4±4.4%; ANOVA followed by Bonferroni’s post test; p<0.05; n=9) compared with before (27.8±2.3%) GSH microinjection into the NTS (figure 3). No differences were found between 20 minutes after GSH and before or 20 minutes after and immediately after (figure 3). LF band and LF/HR ratio were not different among the periods evaluated (figure 3).
Figure 3.
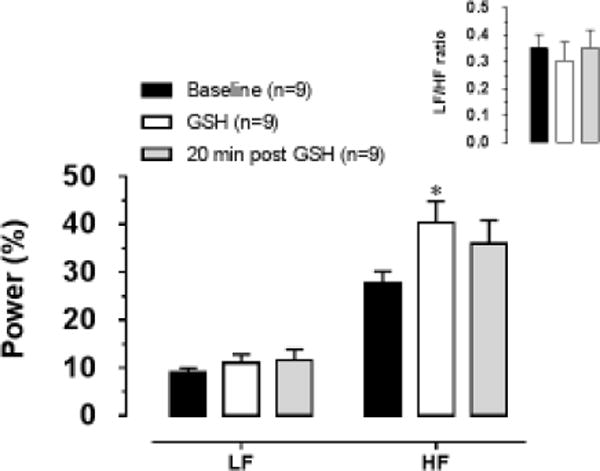
Percent contributions of the LF and HF bands to the total power density (μs2) measured in the spectra acquired before (Baseline), immediately after (GSH) and 20 minutes after (20 min post GSH) GSH (20 nmol/100nL) microinjection into the NTS. The inset graph represents the LF/HF ratio. LF and HF bands ranged from 0.2 to 0.75 Hz and 0.75 to 2.50 Hz respectively. * different of Baseline; Two Way ANOVA followed by Bonferroni’s post test; p<0.05.
Discussion
Results from this study showed that GSH microinjection into NTS, at the concentration used in these experiments (20 nmol/100 nL), elicited mild pressor response and blunted depressor and bradycardic responses to NTS microinjection of L-glutamate in conscious freely moving rats, thereby suggesting that GSH has an inhibitory effect on glutamatergic neurotransmission in NTS.
The concept that brain GSH plays a physiological role in regulating cardiovascular function is consistent with literature evidence that thiol containing compounds as cysteine, cysteine sulfinic acid [29] and S-nitrosocysteine [18, 23] elicit robust cardiovascular responses when injected into the NTS. The cardiovascular actions of the L-cysteine into the NTS seem to be largely diminished by the L-glutamate receptors antagonist kynurenic acid [23] suggesting that cardiovascular responses evoked by L-cysteine may depend on glutamatergic pathways in the NTS. On the other hand, overall brain and plasma GSH seem to be reduced in renovascular [7] and Dahl salt [4] hypertension animal models. These findings suggest that keeping CNS and peripheral glutathione contents within normal range is likely an important factor for mechanisms controlling cardiovascular function. Consistent with this, chronic administration (in the tap water) of the glutathione synthase inhibitor buthionine-sulfoximine has been reported to increase blood pressure in rats [9]. Taken together, these findings raise the possibility that GSH may contribute to cardiovascular pathologies in different ways depending on what mechanisms are affected and how they are affect by changes in GSH levels. Here we examine the cardiovascular effects of acute changes in GSH levels exclusively within the dorsomedial medulla and how it affected local glutamatergic transmission.
Although GSH actions may have important implication for improving disease treatment, the functional relevance of GSH changes to neural control of blood pressure is not well understood. The brain concentration of GSH averages between 2–3 mM, with the concentration in astrocytes (~4 mM) being higher than in neurons (2.5 mM) [reviewed in [26]]. Largely on this basis, it has been proposed that fluctuations of GSH levels more profoundly impact the function of glia compared to neurons. However, this may not be the case. Indeed, some studies have reported that glutathione can influence neuronal behavior both by modulating glutamate receptor function [20, 21] and by acting at specific glutathione receptors expressed by neurons [15, 22]. L-glutamate is an important mediator of neuronal activation by inputs arising from peripheral cardiovascular sensors and is a key excitatory transmitter within CNS circuitry that mediates cardiorespiratory reflexes [3, 8, 13, 16, 17, 19, 25, 28, 30, 31]. Studies have revealed that both reduced (GSH) and oxidized (GSSG) glutathione are capable of displacing L-glutamate from its receptor [20]. Janáky and colleagues further demonstrated that GSH binds via its gamma-glutamyl moiety to ionotropic glutamate receptors and, at micromolar concentrations, displaces excitatory agonists, acting to halt their physiological actions (citation of the Janaky paper). The aforementioned findings clearly point toward glutathione having a net inhibitory effect on neurotransmission mediated by L-glutamate. However, other studies point toward an alternate action. At millimolar concentrations, GSH increase current through NMDA receptor channels by acting through its free cysteinyl thiol group to modulate redox sites on the NMDA receptor [14]. Thus, given evidence for millimolar concentrations of GSH in the CNS, facilitation of glutamatergic neurotransmission also appears possible [14, 20, 27].
The present study indicates that the dominant effect of GSH on local glutamatergic transmission within the dorsomedial medulla comprising the NTS in conscious rats was to abrogate excitatory actions of L-glutamate. It was evident from our observation that cardiovascular responses evoked by L-glutamate in NTS were nearly abolished by prior microinjection of GSH into the same site. Based on that, the GHS actions in the NTS seem to be correlated to its antioxidant property. In a previous study by our group, we showed that H2O2 microinjections into the NTS elicited robust bradycardic and hypotensive responses which were abrogated by previous kynurenic acid microinjection [6]. Since GSH and H2O2 are in the opposite sides of the redox balance, we speculate that both may act through redox modulatory sites on glutamate receptors to increase or diminish local neuronal activity. The existence of redox modulatory site have already been proposed on both NMDA and non NMDA glutamate receptors [1, 2, 27] as well as functional data have provide further evidences of the importance of such redox modulatory sites in regulate neuronal excitability by L-glutamate [6, 18, 23, 24]. In addition, GSH microinjection by itself acutely increased blood pressure, even though GSH was microinjected only unilaterally. This finding indicates that an acute increase in GSH availability within NTS interferes with blood pressure regulation at rest possibly by interfering with glutamatergic neurotransmission. A caveat to this interpretation is that GSH could have concentration-dependent effects that could participate in observed responses. The present study, for example, cannot rule out the possibility that GSH either reduced NTS neuronal excitability or perhaps enhanced local inhibitory neurotransmission, either of which could have contributed to its ability to increase resting MAP and to blunt responses to subsequent injection of L-glutamate. Another mechanism que could explain the lack of glutamate responses after GSH is the possible selective depletion of glutamate from neurons/astrocytes produced by the high tissue concentrations of GSSG (oxidized glutathione) or GSH. If that was the case in our study, GSH microinjections would cause a massive increase in extracellular glutamate which would naturally binds to its post-synaptic receptors at last-order neurons and produce huge depression in blood pressure levels. That would be like the injection of L-glutamate into the same site. However, data do not support this hypothesis because GSH microinjection into the NTS produced the opposite response, a mild increase in blood pressure. Thereby, we do not believe this could be a major mechanism by which GSH is acting within dorsomedial medulla.
To assess how GSH-mediated blockade of L-glutamate receptors could impact autonomic control of the heart, we evaluated heart rate variability. Spectral analysis of the systolic interval revealed that LF band power density was not affected and that HF band power density was increased by GSH microinjection into NTS. LF/HF ratio was not affect by GSH suggesting that autonomic balance was kept within normal range despite of increased HF band power density just after GSH microinjection. It has been proposed in the literature that HF band mostly reflect respiratory sinus arrhythmia, which can be linked to parasympathetic cardiovagal outflow [10]. This mater have been reviewed recently in the literature and raised evidences led to two important conclusions: 1) LF power seems to reflect baroreflex function and not cardiac sympathetic tone and 2) LF/HF ratio may reflect modulation of cardiac autonomic outflows by the baroreflex and not the cardiac autonomic outflows directly [10]. Since LF band was similar before and after the unilateral GSH microinjection, we suggest that baroreflex may not be significantly affect by the GSH microinjection in this study. Furthermore, increased modulation of parasympathetic activity to the heart, as indexed by HF band power density, may result from functional compensation of the circuit unreached by the GSH in response to the mild increase in blood pressure, thereby explaining the apparent contradiction.
In this study, we first demonstrated that the endogenous tripeptide GSH seem to abrogate local glutamatergic transmission within the dorsomedial medulla in conscious rats. Such strong inhibition is commonly reported for glutamate receptors antagonists and may uncover important role for GSH in the reflex control of blood pressure. From our findings, we can now draw a possible regulatory function for GSH in vivo as a powerful endogenous regulator of the glutamatergic transmission in cardioregulatory neural circuits. Different of cysteine, GSH does not act activating glutamate receptors [23] and may have a complete distinct mechanism of action from other thiol-containing compounds which have been demonstrated produce cardiovascular responses when injected into the NTS[18, 23]. Consequently, results presented here provide a new and potential important line of investigation that may uncover potential role for GHS as a neuromodulator involved with neural cardiovascular control.
Highlights.
Unilateral microinjection of GSH into the NTS produced pressor response in conscious rats.
GSH into the NTS increased LF band of the spectral analysis but produced no change in LF/HF ratio.
GSH blunts the cardiovascular responses to exogenous L-glutamate into the NTS.
L-glutamate cardiovascular responses were still blocked even after blood pressure reached its peak to GSH.
Acknowledgments
We thank Dr. Marcelo Eustáquio Silva for complementary animal supply.
Funding: This work was supported by grants from the Minas Gerais Research Foundation (FAPEMIG) and the National Council for Scientific and Technological Development (CNPq). GM Toney was supported, in part, by NIH grants HL102310 and HL088052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abele R, Lampinen M, Keinanen K, Madden DR. Disulfide bonding and cysteine accessibility in the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit GluRD. Implications for redox modulation of glutamate receptors. J Biol Chem. 1998;273:25132–25138. doi: 10.1074/jbc.273.39.25132. [DOI] [PubMed] [Google Scholar]
- 2.Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–846. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- 3.Arnolda L, Minson J, Kapoor V, Pilowsky P, Llewellyn-Smith I, Chalmers J. Amino acid neurotransmitters in hypertension. Kidney Int Suppl. 1992;37:S2–S7. [PubMed] [Google Scholar]
- 4.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Canesin RO, Bonagamba LG, Machado BH. Bradycardic and hypotensive responses to microinjection of L-glutamate into the lateral aspect of the commissural NTS are blocked by an NMDA receptor antagonist. Brain Res. 2000;852:68–75. doi: 10.1016/s0006-8993(99)02196-4. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso LM, Colombari DS, Menani JV, Toney GM, Chianca DA, Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius, American journal of physiology. Regulatory, integrative and comparative physiology. 2009;297:R462–469. doi: 10.1152/ajpregu.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ersahin M, Sehirli O, Toklu HZ, Suleymanoglu S, Emekli-Alturfan E, Yarat A, Tatlidede E, Yegen BC, Sener G. Melatonin improves cardiovascular function and ameliorates renal, cardiac and cerebral damage in rats with renovascular hypertension. J Pineal Res. 2009;47:97–106. doi: 10.1111/j.1600-079X.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 8.Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1675–R1682. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ganafa AA, Walton M, Eatman D, Abukhalaf IK, Bayorh MA. Amlodipine attenuates oxidative stress-induced hypertension. Am J Hypertens. 2004;17:743–748. doi: 10.1016/j.amjhyper.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes, Experimental physiology. 2011;96:1255–1261. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo N, McIntosh C, Shaw C. Glutathione: new candidate neuropeptide in the central nervous system. Neuroscience. 1992;51:835–842. doi: 10.1016/0306-4522(92)90524-6. [DOI] [PubMed] [Google Scholar]
- 12.Guo N, Shaw C. Characterization and localization of glutathione binding sites on cultured astrocytes. Brain Res Mol Brain Res. 1992;15:207–215. doi: 10.1016/0169-328x(92)90110-w. [DOI] [PubMed] [Google Scholar]
- 13.Guyenet PG, Haselton JR, Sun MK. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res. 1989;81:105–116. doi: 10.1016/s0079-6123(08)62002-6. [DOI] [PubMed] [Google Scholar]
- 14.Janaky R, Ogita K, Pasqualotto BA, Bains JS, Oja SS, Yoneda Y, Shaw CA. Glutathione and signal transduction in the mammalian CNS. J Neurochem. 1999;73:889–902. doi: 10.1046/j.1471-4159.1999.0730889.x. [DOI] [PubMed] [Google Scholar]
- 15.Janaky R, Shaw CA, Varga V, Hermann A, Dohovics R, Saransaari P, Oja SS. Specific glutathione binding sites in pig cerebral cortical synaptic membranes. Neuroscience. 2000;95:617–624. doi: 10.1016/s0306-4522(99)00442-x. [DOI] [PubMed] [Google Scholar]
- 16.Le GE, Merahi N, Laguzzi R. Cardiovascular changes induced by the local application of glutamate-related drugs in the rat nucleus tractus solitarii. Brain Res. 1989;503:322–325. doi: 10.1016/0006-8993(89)91683-1. [DOI] [PubMed] [Google Scholar]
- 17.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 18.Machado BH, Bonagamba LG. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii of conscious rats decreases arterial pressure but L-glutamate does not. Eur J Pharmacol. 1992;221:179–182. doi: 10.1016/0014-2999(92)90791-2. [DOI] [PubMed] [Google Scholar]
- 19.Machado BH, Bonagamba LG, Castania JA, Menani JV. Changes in vascular resistance during carotid occlusion in normal and baroreceptor-denervated rats. Hypertension. 1992;19:II149–II153. doi: 10.1161/01.hyp.19.2_suppl.ii149. [DOI] [PubMed] [Google Scholar]
- 20.Ogita K, Enomoto R, Nakahara F, Ishitsubo N, Yoneda Y. A possible role of glutathione as an endogenous agonist at the N-methyl-D-aspartate recognition domain in rat brain. J Neurochem. 1995;64:1088–1096. doi: 10.1046/j.1471-4159.1995.64031088.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogita K, Kitago T, Nakamuta H, Fukuda Y, Koida M, Ogawa Y, Yoneda Y. Glutathione-induced inhibition of Na+-independent and -dependent bindings of L-[3H]glutamate in rat brain. Life Sci. 1986;39:2411–2418. doi: 10.1016/0024-3205(86)90482-0. [DOI] [PubMed] [Google Scholar]
- 22.Ogita K, Yoneda Y. Possible presence of [3H]glutathione (GSH) binding sites in synaptic membranes from rat brain. Neurosci Res. 1987;4:486–496. doi: 10.1016/0168-0102(87)90037-x. [DOI] [PubMed] [Google Scholar]
- 23.Ohta H, Bates JN, Lewis SJ, Talman WT. Actions of S-nitrosocysteine in the nucleus tractus solitarii are unrelated to release of nitric oxide. Brain Research. 1997;746:98–104. doi: 10.1016/s0006-8993(96)01188-2. [DOI] [PubMed] [Google Scholar]
- 24.Ohta H, Talman WT. Both NMDA and non-NMDA receptors in the NTS participate in the baroreceptor reflex in rats. Am J Physiol. 1994;267:R1065–R1070. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- 25.Pawloski-Dahm C, Gordon FJ. Evidence for a kynurenate-insensitive glutamate receptor in nucleus tractus solitarii. Am J Physiol. 1992;262:H1611–H1615. doi: 10.1152/ajpheart.1992.262.5.H1611. [DOI] [PubMed] [Google Scholar]
- 26.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 27.Sucher NJ, Lipton SA. Redox modulatory site of the NMDA receptor-channel complex: regulation by oxidized glutathione. J Neurosci Res. 1991;30:582–591. doi: 10.1002/jnr.490300316. [DOI] [PubMed] [Google Scholar]
- 28.Sved AF, Gordon FJ. Amino acids as central neurotransmitters in the barorecepor reflex pathway. News in Physiological Sciences. 1994;9:243–246. [Google Scholar]
- 29.Takemoto Y. Cardiovascular actions of L-cysteine and L-cysteine sulfinic acid in the nucleus tractus solitarius of the rat. Amino acids. 2014;46:1707–1713. doi: 10.1007/s00726-014-1733-z. [DOI] [PubMed] [Google Scholar]
- 30.Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc. 1984;43:39–44. [PubMed] [Google Scholar]
- 31.Urbanski RW, Sapru HN. Putative neurotransmitters involved in medullary cardiovascular regulation. J Auton Nerv Syst. 1988;25:181–193. doi: 10.1016/0165-1838(88)90023-9. [DOI] [PubMed] [Google Scholar]


