Abstract
Tumor markers are important tools for early diagnosis, prognosis, therapy response and endometrial cancer monitoring. A large number of molecular and pathologic markers have been described in types I and II endometrial cancers, which has served to define the main oncogenic, epidemiological, genetic, clinical and histopathological features. Ongoing attempts to stratify biological markers of endometrial cancer are presented. However, data on changes in tumor marker profiles in obesity-related endometrial cancer are scarce. Obesity is a pandemic in Western countries that has an important impact on endometrial cancers, albeit through not very well-defined mechanisms. Although endometrial cancer is more common in Caucasian women, higher mortality is found in African Americans who also show higher incidence of obesity. Here, we describe how obesity signals (estrogen, leptin, leptin induced-molecules, Notch; cytokines and growth factors) could affect endometrial cancer. Leptin signaling and its crosstalk may be associated to the more aggressive and poor prognosis type II endometrial cancer, which affects more postmenopausal and African-American women. In this regard, studies on expression of novel molecular markers (Notch, interleukin-1 and leptin crosstalk outcome) may provide essential clues for detection, prevention, treatment and prognosis.
Keywords: endometrial cancer, leptin, NILCO, obesity, tumor markers
Introduction
Endometrial cancer (EmCa) is an adenocarcinoma of the endometrium and the most common cancer of the female reproductive tract in developed countries and the seventh most common cancer in women worldwide [1].
The development of cancer is characterized by self-sufficiency in growth signals, insensitivity to growth inhibition, evasion of apoptosis, angiogenesis, invasion and metastasis. All these features are unique in EmCa and have given origin to two major divisions. Type I, associated with endometrioid histology, unopposed estrogen exposure and often preceded by precancerous disease, occurs primarily in pre- and peri-menopausal women and is most common in White population usually carrying a good prognosis. In contrast, type II EmCa, with no endometrioid histology and no association with hormonal risk, occurs most commonly in postmenopausal women and are mostly seen in African-American women (AAW), with an aggressive clinical course and generally with poorer prognosis [2].
The incidence of EmCa is higher in well-developed countries and countries with high obesity rates [2]. As of 2013, there were 49,560 new cases of EmCa reported and 8190 deaths reported in the USA. The American Cancer Society has estimated that 810,320 American women will suffer from cancer and 275,710 will die in the USA in 2014. From these, 6% will present uterine cancer. Cancer lifetime probabilities show that 2.69% (1 in 37) of American women will present with uterine cancer, which places it as the fourth most frequent cancer among women behind breast, lung and colorectal cancers. These figures have been almost steady from 1975 to date in the USA [3].
Caucasian women (CSW) are at a higher risk of developing EmCa when compared to AAW in the USA. However, AAW are more likely to develop the more aggressive form of EmCa and are more likely to die from this disease [4]. The incidence rate of EmCa in CSW is 24.8 per 100,000 women, whereas in AAW it is 20.9 per 100,000 women [5]. The reason(s) for this disparity is unknown [3].
Mainly modifiable factors impacting the rise of cancer incidence are the consumption of highly caloric diets and low physical activity. EmCa is associated with obesity, diabetes and excessive estrogen exposure. Indeed, in comparison with all obesity-related cancers, EmCa incidence and death are associated most with increasing body mass index (BMI) [5].
Several tumor markers have been described for EmCa, which are mainly related to classification, treatment outcome and epidemiology of EmCa. High levels of leptin characterize obesity. Leptin is an adipokine with proliferative, pro-angiogenic and pro-inflammatory effects on many cancer types [6]. Leptin crosstalks to oncogenic signaling molecules, i.e., Notch and IL-1 could play important roles in the incidence and progression of EmCa, particularly in obese patients. However, currently, data on the expression patterns and effects of such leptin signaling crosstalk in EmCa and its relation to obesity are scarce.
Endometrial cancer
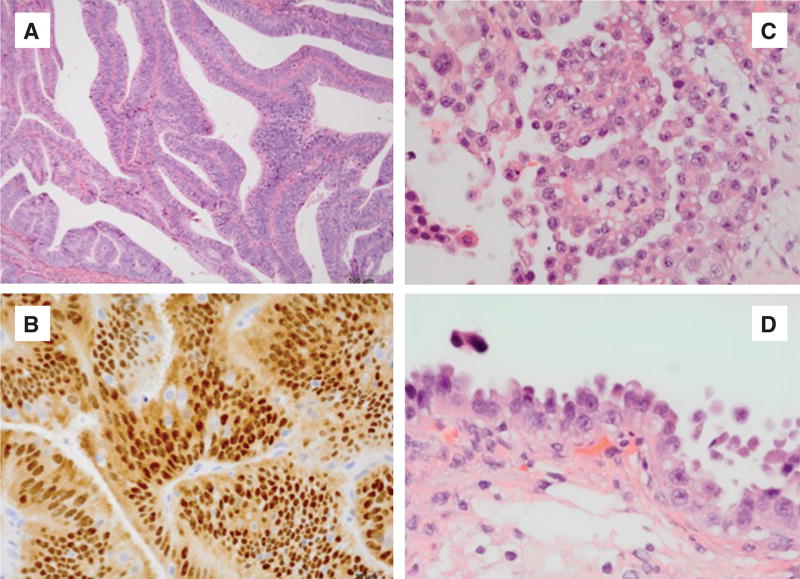
EmCa is classified into two main types: type I, which is estrogen dependent, and type II, which is usually associated with endometrial atrophy and is estrogen independent [2]. Type II is the more aggressive form with a poor prognosis. Approximately 85% of all EmCas are type I, low grade with an excellent prognosis. In contrast, type II is usually high-grade tumors with some patients exhibiting early metastasis. Type II tumors are associated with high recurrences. Approximately half of all recurrences occur in patients with type II tumors [2] (Figure 1).
Figure 1. Type I EmCa (A, B) and type II EmCa (C, D).
(A) Endometrioid carcinoma containing glandular features that resemble glands of the benign endometrium (HE, 10×). (B) Estrogen receptor positive tumor (HE, 40×). (C) Serous carcinoma of the endometrium (HE, 40×). (D) Endometrial carcinoma in situ (HE, 60×).
The common pathological type I is an endometrioid adenocarcinoma with some resemblance to endometrial morphology. Type I EmCa is more differentiated and dependent on hormonal cues and has a better prognosis (Figure 1A and B). In contrast, type II represents 10% of EmCa and has serous or clear cell appearance (Figure 1C and D). Type II EmCa is less differentiated, independent of sex hormones stimuli, more aggressive and shows poor prognosis. There are other less abundant EmCa types that include the mixed mesenchymal Mullerian malignant tumor (MMMT) or carcinosarcoma, mucinous, clear cell, squamous cell, mixed and undifferentiated. Additionally, uterine sarcoma is a more rare type and forms in the muscles or other tissues of the uterus [7].
Type I EmCa is probably derived from atypical hyperplasia, and its progression is closely related to unopposed estrogenic stimulation. Type I EmCa shows few p53 mutations but numerous mutations in other tumor suppressors and oncogenes (i.e., PTEN, PI-3K and KRAS) and CTNNB1 or β-catenin [8]. Type II develops from atrophic endometrium. Type II EmCa had extensive copy number alterations, few DNA methylation changes, low estrogen receptor (ER)/progesterone receptor levels and frequent p53 mutation. Additionally, type II serous EmCa have extensive mutations in DNA polymerase ε (POLE) exonuclease domain. However, about 10% of type I EmCa also shows high number of POLE mutations [9].
Remarkably, type II EmCa shares genomic features with basal-like breast cancer [8], which shows low levels or absence of ER, progesterone and EGFR2 (Her2) receptors. Basal-like breast carcinomas (triple negative) are very aggressive and have poor prognosis. Type II EmCa are also very aggressive, have poor prognosis and no targeted therapies.
EmCa stage is an important feature related to progression of tumor and invasion of surrounding tissues, which is currently based on the tumor-node-metastases (TNM) system 7 and FIGO (International Federation of Gynecology and Obstetrics) staging [3]. The FIGO system and the American Joint Committee on Cancer TNM staging system are basically the same. They both classify EmCa on the basis of three factors: the extent of the tumor (T), whether the cancer has spread to lymph nodes (N) and whether it has spread to distant sites (M). The American Cancer Society classifies EmCa by grades: grade 1 present ≥95% of the cancerous tissue forming glands; grade 2 tumors show 50% to 94% of tissue forming glands and grade 3 <50% of tissue forming glands. Grade 3 tumors are more aggressive and show poor prognosis [3].
Endometrial cancer and obesity
Obesity is a pandemic, particularly in developed countries. It is predicted that in 2015 about 700 million people will be obese worldwide [10]. Obesity, mainly due to unhealthy diets and lifestyles, is a proven factor contributing to higher risk and poor prognosis of cancer [11]. Currently, obesity is considered the second major risk factor for several cancer types, only surpassed by smoking. Interestingly, smoking is inversely correlated to EmCa. That could be associated to the transformation of estrogen via 2-hydroxylation, which produces 2-hydroxyestradiol and its methoxy derivative that are not proliferative factors for EmCa but anti-apoptotic and can inhibit inflammatory cytokine actions [12, 13]. However, the precise causes for the protective role of smoking on EmCa are not completely understood.
Accumulated evidence supports the notion that obesity is a risk factor for EmCa. Approximately 40% of EmCa cases are related to obesity [14]. An increase in EmCa incidence in the last 30 years is believed to be due to the increasing number of elderly people and increasing rates of obesity. Indeed, a 5 kg/m2 increment of BMI correlated to a significant increase of EmCa [RR: 1.50 (1.42–1.59)] [15].
Several studies have found significant associations between obesity and increase of EmCa mortality. In a large prospective epidemiological study including approximately half a million women followed for 16 years, it was demonstrated that obesity increased EmCa mortality [11]. Obesity was associated with earlier age at diagnosis of endometrioid-type EmCa. Similar associations were not, however, observed with non-endometrioid cancers, consistent with different pathways of tumorigenesis [11]. An association of BMI with age at diagnosis was found in 985 cases of EmCa. Age at diagnosis was inversely proportional to BMI only in patients suffering from type I EmCa (y=67.89–1.86×, R=0.049, p<0.001) but not with type II EmCa [16]. In vitro studies show the influence of adipose-derived factors on EmCa. Conditioned media from adipose-derived stem cells increased proliferation and secretion of vascular endothelial growth factor (VEGF) by Ishikawa cells (type I EmCa), which demonstrates that adipose tissue secretes factors inducing EmCa cell growth [17]. However, the specific mechanisms involved in obesity-related cancer incidence are still not completely understood [18, 19].
Potential players involved in the relationships between EmCa incidence and progression, and obesity are the elevated levels of estrogens (unopposed estrogen stimulus), insulin, insulin growth factor-1 (IGF-1), adipokines (leptin, resistin) and cytokines [20–22].
Obesity is characterized by altered profiles of several cytokines and, therefore, is considered a mild inflammatory condition [21, 22]. Adipose tissue secretes several cytokines including leptin, VEGF, interleukin-1 (IL-1), interleukin-6 (IL-6), hepatocyte growth factor (HGF) and tumor necrosis factor-alpha (TNF-α). Several of these cytokines can induce tumor angiogenesis contributing to the growth of solid tumors [6, 23–27]. Abnormal patterns of these factors are associated to obesity-induced changes in tumor and stroma cells [28, 29]. High levels of colony stimulator factor-1 (CSF-1) and its receptor, CSFR, have long been associated with poor-prognosis uterine cancer, among others [30].
Recruitment of inflammatory cells significantly contributes to adipose neovascularization and breast cancer inflammation and angiogenesis [31]. In addition, inflammatory cells and cytokines found in tumors are more likely to contribute to tumor growth, progression, and immunosuppression than they are to mount an effective host antitumor response [32]. It has been proposed earlier that chronic inflammation is a principal mechanism inducing EmCa, through the induction of mitosis, mutations and defective DNA repair [33, 34]. Higher levels of VEGF were detected in type I EmCa and increased CSF-1 and TNF-α in tumor with deep myometrium invasion. Many cytokines induce NFkB signaling pathway, which is involved in cancer cell survival. Also, inflammatory cytokines and cells induce estrogen production [33].
High levels of estrogen are produced by adipose tissue via overexpression of aromatase (that converts androgens into estradiol and estrone) after the sharp decline of ovarian estrogen production in postmenopausal women. Obesity induces high levels of estrogen, which in turn increase the growth of endocrine responsive of cancer, in particular, type I EmCa. In addition, skeletal and vascular tissues and the brain are important sites of postmenopausal estrogen production. Furthermore, obesity leads to the development of metabolic syndrome, which is generally characterized by insulin resistance [16], which is a risk factor for EmCa [35].
Insulin is produced mainly by the pancreatic β cells and is a proliferation factor for EmCa, which expresses insulin receptor (IR). Activation of IR induces direct and indirect effects that contribute to the development of EmCa [36]. Insulin/IR directly promotes EmCa cell proliferation and survival via PI-3K/Akt and Ras/MAPK signaling pathways. Additionally, IGF-1 mainly synthesized in the liver is also a proliferation factor for endometrial cells. Insulin crosstalk to IGF-1 contributes to the progression of EmCa [37]. IGF-1 is mainly regulated by the actions of the growth hormone (GH). Insulin can increase IGF-1 through the upregulation of GH receptors (GHR) and crosstalk to GH/GHR signaling [38]. Furthermore, hyperinsulinemia reduces the levels of IGF binding proteins that increase IGF availability [39]. Insulin induces changes of sex hormone levels that further increase the levels of estrogen in obese individuals. ERs can also crosstalk to IGF-1 signals inducing proliferation and survival changes in EmCa cells [40].
Metformin, a common drug used in diabetes type II patients, was earlier shown to inhibit aromatase expression in adipocytes [41]. In addition to normalizing insulin levels, metformin has been shown to downregulate IGF-1R and upregulate IGF binding protein-1 in EmCa cell lines, and inhibit IGF-1 pathway in type I EmCa [42]. A recent report from a 10-year retrospective cohort study of diabetic patients suffering from type II EmCa (non-endometrioid EmCa) and treated with metformin showed an increase of overall survival compared with a similar cohort of non-diabetic and EmCa patients not treated with metformin. The association was significant (hazard ratio=0.54, 95% CI: 0.30–0.97, p<0.04) after adjusting several variables (age, clinical state, grade and treatment). However, no association between the use of metformin and overall survival in diabetics with endometrioid histology (type I EmCa) was observed [43]. Another recent report shows that non-diabetic EmCa patients that did not use metformin had worst free (95% CI: 1.1–2.9, p=0.02) and overall survivals (95% CI: 1.3–4.2, p=0.005) compared to EmCa diabetic and obese patients treated with metformin after adjusting for age, stage, grade, histology and adjuvant treatment [44].
Leptin and endometrial cancer
Leptin is a small non-glycosylated protein (16 kDa; 167-amino acid non-glycosylated) secreted by adipose tissue that is coded by the obese (ob) gene. It is the most studied adipokine since this protein was first cloned in 1994 [45]. Leptin is a pleotropic cytokine that regulates energy intake and energy expenditure. The role of leptin involves the regulation of glucose homeostasis, growth response, reproduction and immune response [6]. Leptin is secreted by adipocytes as well as cancer cells, and its circulating levels are proportional to total body fat. Obese individuals exhibit high circulating levels of leptin in the body due to what is known as leptin resistance [6].
Leptin exists as a unique protein with pro-inflammatory functions that belongs to the family of helical cytokines. Leptin is structurally similar to IL-6, IL-12, IL-15, prolactin, GH, granulocyte CSF (G-CSF) and oncostatin M. The N-terminal region (94 amino acids) of leptin is essential for both the biological and the receptor binding activities [46].
The ob gene is preserved in mammals providing a high sequence identity for leptin. A nonsense mutation in codon 105 (ob/ob) causes the lack of protein synthesis resulting in morbid obesity, hyperphagia, hypothermia, insulin resistance and infertility [45]. Leptin functions as a long-term signal from adipose tissue that regulates appetite and energy balance. Exogenous administration of leptin on mutant obese mice recovered the normal lean phenotype in C576J mice, which early demonstrated leptin’s role in energy balance [47].
Leptin receptor [OB-R, the product of the diabetic (db) gene], in contrast to leptin, shows at least six alternative spliced isoforms: a long isoform (OB-RL, OB-Rb or LEPR) with full intracellular signaling capabilities and shorter isoforms with less biological activity (OB-Rs or OB-Ra) [48] and a soluble leptin isoform (OB-Re or sOB-R) [49]. The large extracellular domain of OB-R (816 amino acids) is common to all OB-R forms, and the variable length cytoplasmatic tail (300 amino acid residues) distinguishes the several isoforms [50]. OB-R has a helical structure that is similar to those of gp130, the common signal-transducing receptor component for the IL-6 family of cytokines, G-CSF (granulocyte colony stimulating factor) and LIF (leukemia inhibitory factor) receptor. OB-R is related to class I cytokine receptors, which includes the receptors of IL-1, IL-2, IL-6 and GH. This super-family of receptors lacks auto-phosphorylation capabilities and needs auxiliary kinases for activation [51].
Leptin binding to OB-R seems to be very specific and triggers several canonical (i.e., JAK2/STAT3; MAPK; PI-3K/AKT1) and non-canonical signaling pathways (p38MAK; JNK and AMPK). OB-RL and membrane-bound shorter isoforms of OB-R have a cytoplasmatic motif (Box 1) required for JAK (Janus kinases)-related activation of PI-3K (phosphatidylinositol-3-kinase) and MAPK (mitogen-activated protein kinase) pathways. A second docking site (cytoplasmatic tail motif; Box 2) is essential for the activation of the JAK-STAT (signal transducers and activators of transcription) pathway. Induced mutations of the OB-Rb intracellular domain showed that Tyr 113s controls STAT3 [52]. In addition, the SH2 domains of SOCS (suppressor of cytokine signaling) bind the phosphorylated tyrosine residues on JAK2 regulating OB-Rb [53]. SOCS-3 plays an important role as a negative regulator of leptin signaling. It seems that SOCS-3 is activated by a feedback induced by leptin. The over-expression of SOCS-3 inhibits leptin-induced tyrosine phosphorylation of JAK2 and ERK activation by binding to phosphorylated Tyr 985 of OB-Rb [54].
Although the primary source of leptin is adipose tissue, several other tissues have been found to synthesize leptin, however, in lower quantities (i.e., stomach, skeletal muscle, brain, placenta and endometrium at the time of embryo implantation) [6, 55]. Leptin levels are higher in women compared to males (pre-menopausal females>post-menopausal females>males) even after correction by body weight [56]. These gender differences in leptin levels could be related to subcutaneous synthesis and estrogen and androgen regulations [57]. Leptin/OB-R seems to be involved in early embryo implantation [55, 58]. An active crosstalk between leptin secreted by embryos and OB-R expressed by decidual tissue has been described. Moreover, it seems that leptins/OB-R function as essential upstream events in the embryo implantation process [58].
Remarkably, leptin exhibits low or undetectable expression levels in normal endometrial cells, but it is synthesized by uterine cancer cells [59]. Additionally, several other cancer types express leptin including breast, ovarian, prostate, melanoma, esophagus, thyroid, brain, lung and colon [6]. Leptin expressed by adipose or EmCa can promote in a paracrine or autocrine manner the proliferation of cancer cells. However, the correlation between leptin and OB-R expressions in the EmCa clinicopathology is still unclear [60].
We earlier reported that EmCa cell lines expressed higher levels of OB-R (full-length OB-Rb and short isoforms) in contrast to benign primary non-malignant endometrial cells [61]. Leptin and OB-R are found in EmCa tissue. Positive staining for STAT3, HIF-1, leptin and OB-R was detected in 75%, 79%, 60% and 31% of endometroid adenocarcinomas (type I), respectively [59]. Leptin and OB-R overexpression correlated to ER expression, tumor invasion, metastasis and poor prognosis (3-year survival rate) [60].
Leptin signaling crosstalk in endometrial cancer
Elevated lifetime estrogen exposure is a major risk factor for EmCa. This is also true for other hormone-dependent cancers (i.e., breast and ovarian) [62]. ER signaling regulates an elevated number of genes affecting cancer proliferation and vascular function. Antiestrogens could also stimulate the synthesis and release of leptin in the adipocytes [63].
A complex crosstalk between leptin and pro-angiogenic, inflammatory and mitogenic factors occur in breast cancer, which could also be present in EmCa. Leptin actions would provide a link between pro-inflammatory and proangiogenic actions of IL-1, VEGF and macrophages in cancer progression [6]. However, the individual contributions of these factors to obesity-related cancers, including EmCa, are not well understood. We earlier reported that leptin signaling was associated to several pro-angiogenic factors in EmCa cell lines. In malignant endometrial epithelial cells (An3Ca, SK-UT2 and Ishikawa) leptin regulated in a dose-dependent manner VEGF, IL-1β, LIF and their respective receptors, VEGFR2, IL-1R tI and LIFR. However, IL-1β was only increased by leptin in benign primary endometrial cells [61]. Additional factors involved in leptin crosstalk are TNFα, IL-6 and resistin [6].
Additionally, vitamin D3 and microRNA signaling regulate the effects of leptin signaling on EmCa growth. Molecular analyses showed that leptin increased human telomerase reverse transcriptase (hTERT) mRNA expression and cell growth through ERα activation. Real-time polymerase chain reaction analyses revealed an inverse correlation between hTERT mRNA and miR-498 in response to vitamin D3 (1,25(OH)2D3) in estrogen-sensitive cancers, including EmCa. The studies suggest that miR-498-mediated hTERT downregulation is a key event mediating the anti-leptin activity of 1,25(OH)2D3 in estrogen-sensitive EmCa [64].
Notch-system signaling
Notch signaling is a complex transduction process initiated by the binding of a membrane-bound ligand (in adjacent cells) to a membrane-bound receptor in the target cells. Notch affects processes such as proliferation, apoptosis, cell survival, epithelial-mesenchymal transition, differentiation and angiogenesis. Currently, four Notch receptors have been identified in mammals (Notch 1–4). Each receptor consists of an extracellular domain, which is involved in ligand binding and a cytoplasmic domain involved in signal transduction. Five ligands for Notch have been identified: Jagged (JAG1 and JAG2) and Delta-like (DLL1, DLL3 and DLL4). Once the ligand binds to its receptor, the Notch receptor is proteolytically cleaved at the extracellular domain by an α-secretase (ADAM10), which is subsequently followed by cleavage of the receptor’s intracellular domain by γ-secretase, resulting in the formation of the intracellular domain of Notch (NICD or Notch-IC). The Notch canonical pathway is characterized by the translocation of the cleaved NICD to the nucleus to initiate transcription of target genes. Inside the nucleus, NICD interacts with a transcription factor CSL (RBPjk), converting CSL from a transcriptional repressor to a transcriptional activator [65]. Simultaneously, coactivators (CoA) are recruited and form a transcription-activating complex with CSL to modulate the expression of the genes HES and HERP (hairy/enhancer of split and hairy/enhancer of split-related protein), respectively [66, 67]. Polyubiquitination and degradation of NICD can occur in a proteasome-dependent manner [65, 66]. However, Notch signaling can be regulated by canonical (CSL, ADAM) and non-canonical pathways (Wnt/β-catenin) [68].
An uncommon characteristic of the Notch signaling pathway is the lack of an amplification step during the canonic signal transduction process. Amplification usually involves phosphorylation of multiple core proteins within the pathway to augment the signaling process. Additionally, Notch signaling exhibits a 1:1 ratio of signaling input and output in each reaction, wherein the Notch receptor is consumed yielding only one NICD. Therefore, to generate an appropriate cellular response, signal strength is an important factor [69]. Hence, the Notch signaling pathway can be extremely sensitive to deviations in gene expression [70].
Aberrant activation of Notch signaling can lead to various pathological conditions such as cancer [71]. Therefore, Notch is a hallmark for many cancers [65]. In tumorigenesis, irregular Notch activation can be initiated through the abnormal expression of Notch ligands, receptors and target genes, all of which have been reported in many solid tumors, including breast, prostate and pancreatic tumors [70, 71]. The Notch signaling pathway exhibits oncogenic properties in some tumors and suppressive properties in others, which suggests a dual role in carcinogenesis [71]. In salivary gland carcinomas, the Notch pathway acts as an oncogene via the translocation of a Notch transcriptional co-activator (Mastermind-like gene). In contrast, Notch functions as a tumor suppressor in skin carcinomas through Wnt and Hedgehog signaling pathways [72]. Hence, Notch signaling is cell and context dependent [65].
Expression of Notch in endometrial cancer
Notch signaling has been studied in many gynecologic cancers, including ovarian cancer and cervical cancer [73]. A role for Notch signaling molecules has been suggested in normal and malignant endometria. The function of Notch signaling in EmCa is poorly understood. However, there are only few reports of Notch signaling in EmCa. Moreover, the role of Notch signaling in EmCa is controversial [70, 74, 75].
Notch1 expression was significantly higher in the proliferative phase of the endometrium when compared to the other phases, which may suggest that Notch signaling is necessary for cell proliferation [74]. Additionally, the expression of Notch signaling molecules was higher in EmCa when compared to normal endometrium [75]. Notably, Notch1 expression was increased in tumors with invasive properties. Consequently, Notch1 expression was positively correlated with high FIGO staging and lower survival rates for patients. Moreover, high expression levels of JAG1/Notch1 was associated with a poor prognosis [75]. In contrast, it has been suggested that Notch signaling could function as a tumor suppressor in EmCa due to the down-regulated expression of certain Notch ligands and receptors [70]. Notch receptors (Notch 1–4), ligands (JAG1, JAG2 and DLL1) and target (HES1) had an overall decreased expression of mRNA in EmCa when compared to the normal endometrium [70]. Therefore, more studies must be conducted to identify the specific role Notch plays in EmCa.
Notch crosstalk to many signaling pathways in endometrial cancer
An active crosstalk occurs between Notch, Wnt and Hedgehog signaling pathways. Remarkably, Notch, Wnt and Hedgehog are involved in embryonic development. Abnormal activation of these pathways is linked to many cancers. Indeed, the proliferation of EmCa cells involves the aberrant activation of the Hedgehog signaling pathway [76, 77]. However, there are few reports of Wnt signaling in EmCa. An essential player of Wnt signaling, β-catenin, was abnormally expressed in type I EmCa. Deregulation of the Wnt/β-catenin signaling pathway by inactivating β-catenin mutations was found in approximately 10%–45% of EmCa [77]. In contrast, no significant difference in Wnt1, FZD1 and Wnt5 expression between EmCa and normal endometrium was found [78]. Additionally, Wnt and Hedgehog expressions were lower in EmCa compared to normal endometrium, which may suggest that Notch acts a tumor suppressor in these pathways [70, 79].
Notch, IL-1 and leptin crosstalk in endometrial cancer
Leptin is an important pro-inflammatory, pro-angiogenic and mitogenic factor [5, 80]. Studies have shown that high leptin levels are linked to poor cancer prognosis [5, 80]. Leptin produced by cancer cells act in an autocrine and paracrine manner to promote tumor cell proliferation, migration and invasion, pro-inflammation and angiogenesis [5, 81, 82]. Obesity and leptin significantly alter the profiles of numerous proteins linked to cellular processes in cancerous tissues such as Notch and IL-1 [6, 65, 83]. OB-R short isoforms are higher expressed than OB-R long isoform in EmCa [81]. High levels of leptin and OB-R are associated with metastasis and decreased survival rates in breast cancer patients [80]. However, it is unknown whether this association is found in EmCa.
Leptin induces IL-1 system in endometrial and breast cancer cells [61, 82, 84, 85]. The IL-1 system is composed of ligands (IL-1α and IL-1β), receptors (IL-1R tI and IL-1R tII) and an antagonist (IL-1Ra). IL-1β is the more abundant ligand that preferably binds IL-1R tI in normal and cancer cells. The IL-1 system is involved in various roles in both physiological and pathological states [84]. In cancer cells, IL-1 promotes angiogenesis, tumor growth and metastasis [85]. IL-1 is known to be up-regulated in many tumor types. Indeed, the presence of IL-1 in some human cancers is associated with aggressive tumor biology [86]. IL-1 has been shown to up-regulate leptin levels in some cancer cells. Overexpression of IL-1 is seen in breast cancer and linked to proliferation of breast cancer cells [84]. However, we previously showed that leptin up-regulates the IL-1 system in endometrial cells [61]. Moreover, we have shown that IL-1R tI levels were increased in EmCa cells in the presence of leptin, which was related to the activation of JAK2/STAT3, MAPK/ERK1/2 and mTOR pathways [61].
An active crosstalk exists between Notch, IL-1 and leptin in breast cancer termed NILCO [83, 84, 87, 88]. Notch, IL-1, and leptin crosstalk outcome (NILCO) is involved in the induction of breast cancer cell proliferation and migration. In these cells, leptin up-regulates Notch ligands, receptors and target genes. Additionally, leptin up-regulates IL-1 [85, 89]. Remarkably, the blockade of IL-1R tI abrogated leptin up-regulation of Notch [84]. Interestingly, IL-1/IL-1R tI signaling has been shown to mediate leptin up-regulation of VEGF/VEGFR-2 in breast cancer [89]. Leptin can directly induce VEGF/VEGFR-2 up-regulation and indirectly up-regulates VEGF/VEGFR-2 through IL-1 and Notch [83, 84, 88–90]. However, this crosstalk has not been investigated in EmCa.
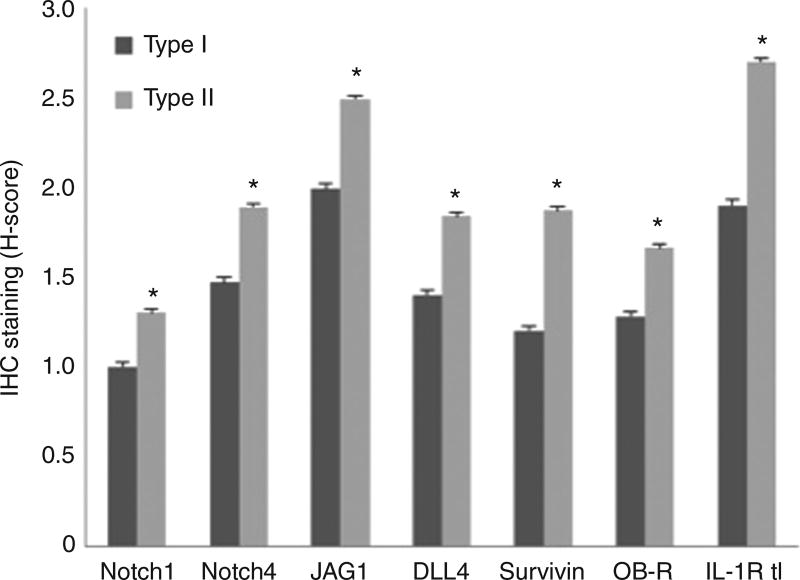
Currently, our lab is investigating NILCO in EmCa. We have shown that NILCO is expressed significantly higher in type II EmCa, the more aggressive non-hormonal form of EmCa [91]. Paraffin sections were analyzed using malignant and surrounding benign tissue biopsies from obese AAW (n=21). The patient samples were classified by a histopathologist as either type I or type II EmCa. Patients had well-annotated clinicopathological data (including race, age, parity, body weight and pathology). Institutional Review Board approval was obtained from Morehouse School of Medicine and Grady Memorial Hospital, Atlanta, GA. Expression levels of NILCO components (Notch 1–4, JAG1 and DLL4) and targets (survivin, Hey2, IL-1R tI and OB-R) were determined via immunohistochemistry (IHC) [91]. Staining intensity were assigned using semi-quantitative H-score [Σpi (i+1), where “i” is the intensity with a value of 0, 1, 2 or 3 (negative, weak, moderate or strong, respectively) and “pi” is the percentage of stained cells for each intensity] calculated by two independent observers in three different fields (100 cells/each) [87]. Preliminary immunohistochemistry results showed that Notch1 and 4 (receptors), JAG1 and DLL4 (ligands), survivin, OB-R and IL-1R tI were expressed higher in type II EmCa (Figure 2).
Figure 2. H-scores from immunohistochemistry staining of NILCO components and targets in type I and type II EmCa from AAW.
H-scores were significantly higher in type II versus type I EmCa for Notch1 (p=0.0044), Notch4 (p=0.0081), JAG1 (p=0.0116), DLL4 (p=0.0042), survivin (p=0.0144), OB-R (p=0.0008) and IL-1R tI (p=0.0028).
These results were assessed in preliminary studies using tissue microarray from type II EmCa from Chinese patients (data not shown). Preliminary data suggested that the more aggressive and non-hormonal form of EmCa (type II) could be dependent on Notch signaling. The results also might suggest that an active crosstalk between obesity signals (leptin) and Notch occurs in EmCa. Therefore, NILCO expression in EmCa may serve as a new tumor marker. Moreover, novel therapy strategies targeting Notch and leptin signaling could be a new way to treat type II EmCa. Identification of the role of NILCO is a current research challenge that could provide information to better understand the mechanisms involved in obesity-induced effects on EmCa.
Endometrial cancer markers
Tumor markers are molecules found in malignant cells or body fluids. These molecules are produced by the cancer or host normal cells in response to the presence of cancer. Tumor markers can be used to differentiate malignant from normal tissue. These molecules include proteins and genes (i.e., cell receptors, growth and angiogenic factors, cytokines, extracellular and cell adhesion molecules, serum proteins and tumor suppressor genes among others). Since the description of first tumor marker by Henry Bence-Jone in 1846, several tumor markers have been reported. Tumor markers are useful to identify the risk, screen for early cancer, establish diagnosis, monitor disease progression and response to therapy, detect recurrence and estimate cancer prognosis [92].
Mismatch repair genes (MMR) and microsatellite instability (MSI)
DNA repair and the mismatch repair system (MMR) play crucial roles in promoting genetic stability. Microsatellites are simple repetitive DNA sequences in the genome susceptible to replication errors. Microsatellite instability (MSI) is due to inactivation of intranuclear proteins, which comprises the MMR, resulting in accumulation of structural mutations during DNA replication [93]. MSI is a molecular phenotype found in approximately 20% of sporadic endometrioid EmCas (type I EmCa) of all grades. MHL1 inactivation (MutL homolog 1, a component of MMR) is the most common altered mechanism of DNA mismatch repair in the endometrium, which is accomplished by hypermethylation of CpG islands in gene promoters (epigenetic silencing). MSI may specifically target for inactivation of those genes which contain susceptible repeat elements, such as transforming growth factor beta receptor II (TGF-RII), BAX, insulin growth factor receptor (IGFIIR) and hMSH3 resulting in secondary tumor subclones with altered capacity to invade and metastasize. However, MSI is rare (<5%) in type II EmCa, where the primary genetic defect is in the p53 gene [94].
Tumor suppressor genes
Tumor suppressor genes code for proteins that inhibit tumor growth. When mutated, they become inactive and tumor growth is allowed. Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene encoding a phospholipid phosphatase, which acts to maintain G1 arrest and enable apoptosis, antagonizing the P13K/AKT pathway. Inactivation of PTEN tumor suppressor gene is the most common genetic defect in type I EmCa. PTEN mutations are found in endometrial hyperplasia suggesting that it is an early event in carcinogenesis and is involved in the early phases of endometrial tumorigenesis [95, 96]. Discrete premalignant phase of EmCa precedes the inactivation of PTEN in up to 83% of endometrial abnormalities. PTEN inactivation may be caused by mutations, deletions or promoter hypermethylation, and decreased PTEN activity causes increased cell proliferation, cell survival and angiogenesis [92].
The serine/threonine kinase Akt (protein kinase B), a major downstream effector of PI-3K and PTEN, is very frequent activated. This occurs in human cancers not only by inactivation of PTEN but also through activation of Ras (a small GTPase family of kinases), PI3K and growth factor receptors [95, 97]. The deficiency of Akt1 has been shown to reduce the development of tumors in endometrium induced by PTEN inactivation in mouse models [98]. Thus, inhibition or ablation of Akt activity could be an approach of prognostic value [99].
The p53 gene regulates cell cycle, apoptosis and differentiation [95]. It can initiate cell cycle arrest as a response to DNA damage, by increasing the cyclin-dependent kinase inhibitor p21. p53 mutations have been found in 10%–20% of endometrial carcinomas [97, 98]. However, p53 protein overexpression is more frequent in serous papillary (type II) than endometrioid tumors (type I) [99] and has been associated with higher FIGO stage. Furthermore, overexpression of p53 (detected by IHC) is associated in several retrospective studies with an unfavorable prognosis of EmCa [100, 101].
Additionally, the inactivation of the p21 gene, a downstream effector in the p53 pathway of cell growth control, may potentially lead to EmCa progression. Low expression of p21 protein has been associated with significantly decreased survival of EmCa patients, including those without p53 alterations [102].
Cell cycle regulation, proliferation and apoptosis
Ki67 (proliferation marker) is increased in serous papillary and high-grade tumors and at the invasive front of EmCas [103, 104]. Ki67 expression has been correlated with clinical stage and histological grade in a series of primary untreated EmCa patients [105], which could be a strong prognostic indicator of EmCa recurrence. Moreover, Ki67 detection is related to the efficacy of endocrine treatments [106]. Additionally, a few other studies estimating cell proliferation rate by determining cells in S phase have associated cell proliferation with significant prognostic outcome in EmCa [106].
Mutations in Bax, an apoptosis inducer gene, induces a loss of Bax protein expression in endometrial carcinomas [107, 108]. In contrast, Bcl-2, an inhibitor of apoptosis, is highly expressed in endometrial hyperplasia but shows decreased expression in EmCa [109].
Cables is a cell cycle regulatory protein and tumor suppressor that is up-regulated by progesterone and down-regulated by estrogen actions in the endometrium. Cables expression is lost in type II EmCa and more that 80% of type I EmCa. Nuclear immunostaining for Cables is lost in a high percentage of cases of endometrial hyperplasia and EmCa, which are likely the product of unopposed estrogen. Thus, loss or suppression of Cables may be an early step in the development of endometrial cancer [110].
Steroid receptors
The presence of hormone receptors has been relevant for the phenotype classification and targeted treatment in EmCa [100]. Activated ER can suppress the expression of Bax by upregulating a group of microRNAs including hsa-let-7 family members and hsa-miR-27a. Therefore, ER promotes the increase of Bcl2/Bax ratio as well as enhanced survival and proliferation of endometrial cells. ER-regulated hsa-let-7 microRNAs can be detected in most hyperplastic endometria, suggesting their potential utility as indicators of estrogen over-exposure [107].
Protein kinase C-α (PKC-α) is aberrantly expressed in endometrioid tumors and is an important mediator of EmCa cell survival, proliferation, and invasion. PKC-α signaling, via PI-3K/Akt, may be a critical element of the hyperestrogenic environment and activation of ER. This signaling crosstalk is thought to trigger the development of estrogen-dependent endometrial hyperplasia and malignancy [92].
Progesterone receptor (PR) expression has been correlated to EmCa grade, histology, adnexal spread and recurrence [111]. However, EmCa recurrence occurs more in PR-negative tumors [112]. In recent years, the expression of PR-A and PR-B isoforms in endometrial adenocarcinoma has been significantly associated with increasing tumor differentiation [101].
Markers of invasion and metastasis
Genetic alterations in cellular adhesion molecules, such as catenins and cadherins, are important for tumor stroma and tumor vascular interactions. It was found that several features of type I EmCa occur significantly more often in tumors expressing nuclear β-catenin. These results suggest that abnormal Wnt/beta-catenin signaling pathway could be a molecular feature of a subset of type I EmCa [113]. Mutations in the β-catenin gene have been associated with a low metastatic potential [114].
CD146 (cell surface glycoprotein MUC 18) is a cell adhesion molecule found overexpressed in various cancers including breast and ovarian cancers. CD146 promotes tumor growth, angiogenesis and metastasis, and its levels were higher in EmCa and positively correlated with histological grade and myometrial invasion [114, 115].
DNA ploidy
Aneuploidy tumors are present in 20%–30% of endometrial carcinomas associated with a high grade, non-endometrioid subtype, deep myometrial invasion and high FIGO stage. DNA ploidy was the strongest independent predictor of poor outcome in series of EmCa and was correlated to recurrence and survival patterns [116–118].
Serum tumor markers
Elevated pre-diagnostic concentrations of tumor necrosis factor-alpha (TNF-α) and its soluble receptors TNFR1 and TNFR2 were related to a higher risk and advanced EmCa stage [119, 120]. However, lower levels of TNF-α were found in endometrial hyperplasia compared to normal controls. Estrogen-stimulated TNF-α expression from EmCa cells induced the stromal expression of HGF that could be targeted with NK4 (HGF-antagonist/angiogenesis inhibitor) [120].
Serum human epididymis protein 4 levels correlated with an aggressive tumor phenotype and may constitute an independent prognostic factor for poorly differentiated EmCa [121].
YKL-40 (human cartilage glycoprotein-39) was elevated in 76% of EmCa patients, and its pre-operative serum level may predict worse clinical outcome [100]. YKL-40 correlated to VEGF overexpression and co-activation of syndecan-1 (S1), integrin αvβ3 and focal adhesion and MAP kinases [122].
Higher levels of M-SCF (stem cell factor) were detected in 25%–73% of EmCa cases and were predictive of aggressive clinical course [111, 114]. Elevated levels of serum sFas (Fas ligand, a pro-apoptotic molecule) were found in endometrioid adenocarcinoma (p<0.0001). Additionally, human serum amyloid A protein was overexpressed and actively secreted by grade-3 endometrioid adenocarcinoma and serous papillary carcinoma [112].
Elevated serum CA 125 (cancer antigen 125 or MUC16) levels have been detected in 11%–43% of EmCa and related to disease stage, myometrial invasion, peritoneal cytology and lymph node metastasis. CA 125 serum cutoff of 20 U/mL had a sensitivity of 69.0%, specificity of 74.1%, positive predictive value of 58.8% and negative predictive value of 81.6% for assessment of myometrial infiltration. Serum CA 125 level usually parallels the clinical course of the disease [123]. Other tumor serum markers, CA 15.3 and CA 72.4 were found in 47% of EmCa patients with occult stage III compared to 18% of those with stages I and II [123].
Low levels of serum taurine (an organic acid) are found in EmCa patients [124]. Apolipoprotein A, pre-albumin and transferrin levels were found higher in early and late-stage EmCa (71/88% sensitivity and 82/86% specificity) [125].
Despite the abundant literature on EmCa markers, there are scarce data on relationships between these molecular markers and obesity cues.
Treatment of endometrial cancers
High risk of EmCa recurrence correlates to deeply tumor invasion, type II and advanced age. For advanced stage EmCa, hysterectomy is the first therapy choice. Chemotherapy reduces the mortality due to recurrent EmCa by a quarter and also reduces the risk of developing the first recurrence outside the pelvis. Radiotherapy is commonly used in conjunction with chemotherapy and surgery to treat EmCa [3].
Initial management of early EmCa is surgical staging with total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic and para-aortic lymph node dissection and pelvic washings. Minimally invasive approach, such as laparoscopy or robotic assistance, is preferred in certain situations over laparotomy due to similar outcomes and decreased postoperative adverse events. Vaginal hysterectomy may be appropriate for patients with increased risk of morbidity, however does not allow lymphadenectomy [126].
Lymphadenectomy is useful in triaging need for adjuvant therapy but can be eliminated for patients identified as low risk by the Mayo criteria, with grade 1–2 of type I endometrioid tumors, <50% myometrial invasion and tumor of 2 cm or less. Sentinel node dissection may further clarify patients who need nodal dissection while minimizing morbidity. Vaginal brachytherapy is the adjuvant therapy of choice for patients with early stage EmCa. For type II EmCa with high risk of recurrence, adjuvant therapy in addition to brachytherapy is often considered, and there is a Gynecologic Oncology Group study ongoing; however, no prospective data are currently available [126]. For advanced or recurrent EmCa, aggressive surgical cytoreduction including exenteration have been shown to improve progression-free and overall survival. Adjuvant therapy is given as combination of chemotherapy with paclitaxel and carboplatin, along with radiation. Protocols often studied employ the sandwich technique with three chemotherapy cycles, radiation and then additional three chemotherapy cycles. Patients who are not candidates for surgery may be treated with primary radiation therapy with adjuvant chemotherapy [127].
Conservative management with hormonal agents has been studied in women who are poor surgical candidates or who desire fertility-sparing treatment. Most often studied are medroxyprogesterone acetate and megestrol acetate. Other regimen studies include diverse progestins, oral contraceptives, tamoxifen and intrauterine device containing levonorgestrel. Failure or recurrence may occur. If medical therapy fails or childbearing has been completed, definitive surgical therapy may be recommended [127].
Oncogenes and targeted treatment of EmCa
Oncogene overactivation stimulates cell division. However, few oncogenes have been found over-activated in EmCa. Among them, mutations of K-Ras (a proto-oncogene involved in growth control and differentiation) could be related to the progression of several cancers. K-Ras mutations occur in 10%–30% of EmCa, predominantly in type I as well as endometrial hyperplasia. These data suggest that K-Ras mutation is an early event in the development of type I EmCa [128].
Her2 overexpression was found in approximately 20% endometrioid and serous carcinomas. Her2 was associated with EmCa aggressive phenotype and poor survival. However, trastuzumab therapy (anti-Her2 antibody) as a single agent did not demonstrate activity against EmCa with Her2 overexpression or gene amplification [129].
Somatic mutations in the fibroblast growth factor receptor 2 (FGFR) were found in 12% of the EmCa. Altered ligand specificity and constitutively activated FGFR2 mutations have oncogenic roles in EmCa cell lines [130]. Dovitinib, a FGFR2 inhibitor induced complete EmCa regressions in a long-term in vivo study in FGFR2 wild-type EmCa xenograft models. mTOR pathway in concert with oncogenic FGFR2 may drive EmCa growth [131]. Indeed, Ridaforolimus, a selective inhibitor of mTOR, reduced EmCa growth in vivo [132]. Additionally, amplifications of PI-K3CA, a catalytic subunit of PI-3K, correlated to PI-3K activation and PTEN mutations in EmCa. This suggests that these molecules are potential targets for therapy [133].
Increased levels of VEGF and angiogenic markers are associated with poor outcome and high grade in type I EmCa [134, 135]. Detection of VEGF and its receptor type 1, VEGFR-1, could be useful markers for predicting 5-year disease-free survival in endometrioid EmCa [136]. Avastin, a humanized antibody against VEGF-A, retarded tumor growth in athymic mice. Interestingly, c-Jun oncogene was detected in bevacizumab-treated EmCa that suggests that c-Jun-mediated pathway(s) contributes to bevacizumab resistance [137].
Cancer stem cells
Accumulating evidence has revealed that there are rare populations of cells that display adult stem cell properties of self-renewal and differentiation in both epithelium and stroma of the human endometrium. These cells are probably responsible for the regenerative capacity of the endometrium. Epithelial stem cells might be located in the basal layer of the endometrium [138]. Stem cells can be classified as embryonic stem cells [139], germ stem cells [140], fetal stem cells [141], cancer stem cells (CSCS) and EmCa side population cells (ECSP) as well as somatic or adult stem cells [142, 143].
Embryonic stem cells are pluripotent cells derived from the inner cell mass of the blastocyst. Germ stem cells are defined as pluripotent stem cells having derived from germ cells. Fetal stem cells are responsible for the initial development of all tissues before birth; they can be isolated not only from fetal blood and hemopoetic organs but also from fetal organs, amniotic fluid and placental membranes. Adult stem cells are found in several tissues, and it has also been suggested that stem-like cells exist in cancerous tissues [143].
The human endometrium contains rare epithelial and stromal cells able to produce colony-forming units: endometrial epithelial stem cell-like (also referred to as side population) and endometrial mesenchymal stem cell-like (MSC). MSC are found in perivascular and basalis and functionalis endometrial layers [144]. MSC express CD146+ (MCAM), PDGF-Rβ+ (CD140B), ITGB1 (9CD29), CD44, NT5E (CD730), THY1 (CD90) and ENG (CD105) but not endothelial or hematopoietic markers [145]. Similar populations of cells, called EmCa stem cells (CSCS), have been described.
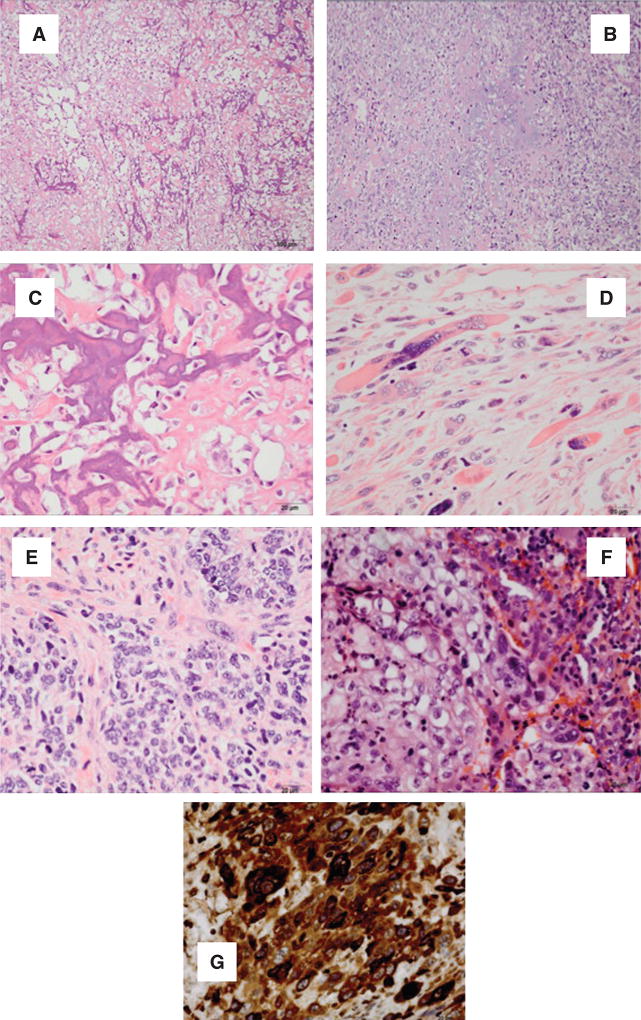
CSCS have unlimited proliferative potential, show reduced level of differentiation markers, resistance to conventional chemotherapeutic agents and high DNA repair capacity [138]. ECSP isolated and characterized from EmCa cell line express CD133, CD44, CD146, PDGFRβ and aldehyde dehydrogenase-1 markers. These cells may be identified as label-retaining cells using their property to retain DNA synthesis label bromo-deoxyuridine (BrdU) [146]. ICD133 (+) EmCa cells and/or ECSP cells can initiate tumor formation and recapitulate the phenotype of the original tumor (Figure 3) [145].
Figure 3. EmCa derived from EmCa stem cells (ECSC). (A, B) Osteosarcomas; (C, D) chondrosarcomas; (E) rhabdosarcoma; (F) epithelial sarcomatous and (G) choriocarcinoma differentiation.
(A) Sarcomatous stromal cells produce osteoid. Tumoral osteoid represented by amorphous fibrillary eosinophilic deposits. Early osteoid deposition forms a lace-like pattern around tumor cells, and advanced osteoid shows evidence of mineralization (darker pink-purple color) (HE, 10×). (B) High magnification shows highly malignant cells with high nuclear-to-cytoplasmic ratio, anaplastic hyperchromatic nuclei or clearing of the chromation and conspicuous, cherry-red nucleoli (HE, 40×). (C) Sarcomatous stromal cells produce chondroid matrix (HE, 10×). (D) Malignant nuclear anaplastic features (HE, 40×). (E) Anaplastic rhabdomyosarcoma showing large strap cells with abundant cytoplasm and striations. The cells are mononuclear or multinucleated. Numerous mitoses are identified (HE, 40×). (F) Epithelial and sarcomatous component blend in this tumor. The malignant epithelial component is composed of cells with high nuclear-to-cytoplasm ratios that show numerous mitoses. Spindle cells with large anaplastic nuclei and prominent nucleoli representing the sarcomatous component are immediately adjacent to the epithelial component (HE, 40×). (G) Malignant polygonal/round cells with single nucleus are reminiscent of the cytotrophoblast. Few multinucleated cells reminiscent of the syncytiotrophoblast are also present. Hemorrhagic background and necrosis areas are present in this tumor (HE, 40×).
Musashi-1, an evolutionary conserved marker for neural stem cells, was co-expressed with Notch1 in a subpopulation of endometrial cells. Additionally, Musashi-1 and telomerase expressing cells were found significantly increased in proliferative endometrium, endometriosis and EmCa [147]. Also, Notch1 pathway was increased in EmCa and endometriosis suggesting the concept of a stem cell origin of EmCa and endometriosis [147].
ECSP possess the following characteristics: (i) reduced expression levels of differentiation markers, (ii) long-term repopulating properties, (iii) self-renewal capacity, (iv) enhanced migration and podia formation, (v) enhanced tumorigenicity and (vi) bi-potential development (tumor cells and stroma-like cells), suggesting that they have cancer stem-like cell features. Recently, sodium butyrate, a histone deacetylase inhibitor, was shown to inhibit the self-renewal capacity of ECSP by inducing a DNA damage response [148].
Race and endometrial carcinomas
The lifetime risk of being diagnosed with EmCa is as follows: CSW>AAW>Hispanics>Asian Pacific islanders>Native American women. However, there is a large disparity in the death rate. AAW have a 12% decrease in incidence rate and an 86% increase in death rate in comparison with CSW [148].
The 5-year survival rate of AAW is lower than in CSW for every stage of disease at the time of diagnosis. AAW have a higher grade and more aggressive tumor types (serous, clear cell type and carcinosarcomas) [149]. However, divergent data on race factor impact on survival have been shown [149, 150]. CSW were more likely to have PTEN mutation, present in type I EmCa histology and associated with better prognosis [151]. In contrast, AAW suffering from EmCa have more p53 mutations and Her2 expression in cancer tissue, and show poor treatment response, which are associated with type II Emca [4, 152, 153]. Additionally, incidence of obesity is significantly higher in AAW than in CSW. Obesity contributes to EmCa morbidity, progression, recurrence and mortality [14]. These data suggest that incidence of types I and II EmCa, obesity and socio-economic factors may impact on the AAW survival differences [4, 154, 155].
Expert opinion
In spite of the important role suggested for tumor markers and CSCS in EmCa pathology, recurrence and response to treatments, there are not comprehensive data available on how obesity and race could impact on these factors. Moreover, whether health disparity in AAW could be influenced by obesity-induced changes in CSCS and EmCa markers is unknown. There is a need to better understand the mechanisms involved in obesity-related EmCa. NILCO markers may provide additional information for obesity-related type II EmCa. These important questions on EmCa biology, detection, potential prevention, treatment and recurrence warrant further investigations.
Outlook
Determination of NILCO and other obesity-related molecules together with traditional tumor markers may be used as additional tools to predict the impact of obesity on EmCa prevention, treatment and recurrence.
Highlights
EmCa is a multifactorial disease classified in two major types: type I and type II.
Type I EmCa is responsive to steroid hormonal medium, more differentiated and shows better prognosis.
Type II EmCa is unresponsive to steroid hormones, less differentiated and shows poor prognosis.
CSW show higher incidence of EmCa, but AAW show higher grade and poor prognosis.
Obesity correlates to higher incidence and poor prognosis of EmCa.
Race, obesity and socioeconomic factors could be related to higher incidence of type II EmCa in AAW. Frequent overexpression of p53 has been reported in type II EmCa from AAW. More studies are needed to better understand how race and obesity could impact on EmCa health disparity.
Several tumor and serum markers are being used to detect and predict treatment response in EmCa. However, no data are available on relationships between EmCa markers, CSCS profiles and obesity cues.
NILCO may serve as novel biomarker for obesity-related type II EmCa.
Acknowledgments
This work was partially supported by the National Institutes of Health (NIH) and National Cancer Institute (NCI) grant U54 CA118638 and Department of Defense (DOD), U.S. Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs (CDMRP) Idea Award Number W81XWH-13-1-0382 to R.R.G.P.; the National Center for Advancing Translational Sciences of the NIH Award 5TL1TR000456-07 and 5T32HL103104-04 (MPI) to D.D-B; and facilities and support services at Morehouse School of Medicine (NIH RR03034 and 1C06 RR18386) and NIH/NCRR grant 1G12RR026250-03. We would like to thank Dr. Uma Krishnamurti, Allegheny General Hospital and Canonsburg General Hospital Pittsburgh, PA, for providing the glass slides showing MMMT with osteosarcoma and chondrosarcoma differentiation, and Dr. Florencia Elena Castaneda Valladares, Universidad Autonoma del Estado de Mexico, Obstetrics and Gynecology PY3, Hospital Regional ISSEMYM Tlalnepantla, for her contribution to Endometrial cancer markers section.
Footnotes
The authors declare that they have no conflict of interests.
Contributor Information
Danielle Daley-Brown, Department of Microbiology, Biochemistry and Immunology, Morehouse School of Medicine, 720 Westview Dr. SW., Atlanta, GA 30310, USA.
Gabriela M. Oprea-Ilies, Department of Pathology, Emory University, 201 Dowman Drive, Atlanta, GA 30322, USA
Regina Lee, Department of Gynecology and Obstetrics, Morehouse School of Medicine, 720 Westview Dr. SW., Atlanta, GA 30310, USA.
Roland Pattillo, Department of Gynecology and Obstetrics, Morehouse School of Medicine, 720 Westview Dr. SW., Atlanta, GA 30310, USA.
Ruben R. Gonzalez-Perez, Department of Microbiology, Biochemistry and Immunology, Morehouse School of Medicine, 720 Westview Dr. SW., Atlanta, GA 30310, USA, Phone: +1-404-752-1581, Fax: +1-4040-752-1179, rgonzalez@msm.edu
References
- 1.NIH/NCI website. 2014 http://www.cancer.gov.
- 2.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society, Statistics, 2014. 2014 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures.
- 4.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol. 2013;130:652–9. doi: 10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205:518–25. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825:207–22. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database Syst Rev. 2011;(10) doi: 10.1002/14651858.CD003175.pub2. PMC4164379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein YR, Weigelt B, Levine DA, Schoolmeester JK, Dao LN, Balzer BL, Liles G, Karlan B, Köbel M, Lee CH, Soslow RA. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) 2014 http://www.who.int/cancer/prevention/en.
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–9. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 13.Terry PD, Rohan TE, Franceschi S, Weiderpass E. Cigarette smoking and the risk of endometrial cancer. Lancet Oncol. 2002;3:470–80. doi: 10.1016/s1470-2045(02)00816-1. [DOI] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. World Cancer Report 2014. World Health Organization; 2014. Chapter 5.12. [Google Scholar]
- 15.World Cancer Research Fund International. http://www.wcrf.org/int/cancer-facts-figures/link-between-lifestyle-cancer-risk/cancers-linked-greater-body-fatness.
- 16.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Levitt J, Girda E, Goldfinger M, Goldberg GL, Einstein MH. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–6. doi: 10.1097/AOG.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 17.Bellone S, Watts K, Cane S, Palmieri M, Cannon MJ, Burnett A, Roman JJ, Pecorelli S, Santin AD. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol. 2005;98:92–8. doi: 10.1016/j.ygyno.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;1807:664–78. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103–13. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hlavna M, Kohut L, Lipkova J, Bienrtova-Vasku J, Dostalova Z, Chovanec J, Vasku A. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58:124–8. doi: 10.4149/neo_2011_02_124. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 23.Coxon A, Bolon B, Estrada J, Kaufman S, Scully S, Rattan A, Duryea D, Hu YL, Rex K, Pacheco E, Van G, Zack D, Feige U. Inhibition of interleukin-1 but not tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis. Arthritis Rheum. 2002;46:2604–12. doi: 10.1002/art.10546. [DOI] [PubMed] [Google Scholar]
- 24.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–3. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 25.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–41. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–66. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 28.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–9. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 29.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831–52. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 30.Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995;27:79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- 31.Coussens LM, Werb Z. Inflammatory cells and cancer. Think different. J Exp Med. 2001;193:f23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 33.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840–7. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 34.Smith HO, Stephens ND, Qualls CR, Fligelman T, Wang T, Lin CY, Burton E, Griffith JK, Pollard JW. The clinical significance of inflammatory cytokines in primary cell culture in endometrial carcinoma. Mol Oncol. 2013;7:41–54. doi: 10.1016/j.molonc.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005;12:210–5. doi: 10.1097/00042192-200512020-00016. [DOI] [PubMed] [Google Scholar]
- 36.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–7. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Wang T, Ning G, Bloomgarden Z. Diabetes and cancer relationships. J Diabetes. 2013;5:378–90. doi: 10.1111/1753-0407.12057. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Messina JL. Crosstalk between growth hormone and insulin signaling. Vitam Horm. 2009;80:125–53. doi: 10.1016/S0083-6729(08)00606-7. [DOI] [PubMed] [Google Scholar]
- 39.Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–41. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 40.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 41.Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res Treat. 2010;123:591–6. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- 42.Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS One. 2013;8:e61537. doi: 10.1371/journal.pone.0061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, Conroy E, Goldberg GL, Einstein MH. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132:236–40. doi: 10.1016/j.ygyno.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C, Havrilesky LJ, Secord AA, Moore DT, Gehrig PA, Bae-Jump V. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014;132:438–42. doi: 10.1016/j.ygyno.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa K, Numata Y, Katsuura G, Sakaguchi I, Morita A, Kikuoka S, Matumoto Y, Tsuji T, Tamaki M, Sasakura K, Teraoka H, Hosoda K, Ogawa Y, Nakao K. Structure-function studies of human leptin. J Biol Chem. 1998;273:35245–9. doi: 10.1074/jbc.273.52.35245. [DOI] [PubMed] [Google Scholar]
- 47.Haalas JL. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. J Biol Chem. 1997;272:16216–23. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 49.Lewandowski K, Horn R, O’Callaghan CJ, Dunlop D, Medley GF, O’Hare P, Brabant G. Free leptin, bound leptin, and soluble leptin receptor in normal and diabetic pregnancies. J Clin Endocrinol Metab. 1999;84:300–6. doi: 10.1210/jcem.84.1.5401. [DOI] [PubMed] [Google Scholar]
- 50.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 51.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–92. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase dependent proliferation of C1H10T1/2 cells. J Biol Chem. 1997;272:12897–900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 54.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, Simon C. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J Clin Endocrinol Metab. 2000;85:4883–8. doi: 10.1210/jcem.85.12.7060. [DOI] [PubMed] [Google Scholar]
- 56.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–7. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 57.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–41. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Ramos MP, Rueda BR, Leavis P, Gonzalez RR. Leptin serves as an upstream activator of an obligatory signaling cascade in the embryo-implantation process. Endocrinology. 2005;146:694–701. doi: 10.1210/en.2004-1186. [DOI] [PubMed] [Google Scholar]
- 59.Wincewicz A, Koda M, Sulkowska M, Kanczuga-Koda L, Sulkowski S. Comparison of STAT3 with HIF-1alpha, Ob and ObR expressions in human endometrioid adenocarcinomas. Tissue Cell. 2008;40:405–10. doi: 10.1016/j.tice.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu L, Li C, Ai H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark. 2014;14:353–9. doi: 10.3233/CBM-140415. [DOI] [PubMed] [Google Scholar]
- 61.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch M, Rueda BR, Gonzalez RR. Leptin regulation of pro-angiogenic molecules in benign and cancer endometrial cells. Int J Cancer. 2008;123:2782–90. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 63.Welboren WJ, Slunnenberg HC, Sweep FC, Span PN. Identifying estrogen receptor target genes. Mol Oncol. 2007;1:138–43. doi: 10.1016/j.molonc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasiappan R, Sun Y, Lungchukiet P, Quarni W, Zhang X, Bai W. Vitamin D suppresses leptin stimulation of cancer growth through microRNA. Cancer Res. 2014;74:6194–204. doi: 10.1158/0008-5472.CAN-14-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors and cancer therapy. Cancer Res. 2007;67:1879–82. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 67.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–55. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 68.Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22:257–65. doi: 10.1016/j.tcb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 70.Jonusiene V, Sasnauskiene A, Lachej N, Kanopiene D, Dabkeviciene D, Sasnauskiene S, Kazbariene B, Didziapetriene J. Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol. 2013;30:438. doi: 10.1007/s12032-012-0438-y. [DOI] [PubMed] [Google Scholar]
- 71.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 72.Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003;3:203–5. doi: 10.1016/s1535-6108(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 73.Jayshree RS, Sreenivas A, Tessy M, Krishna S. Cell intrinsic and extrinsic factors in cervical carcinogenesis. Ind J Med Res. 2009;130:286–95. [PubMed] [Google Scholar]
- 74.Cobellis L, Caprio F, Trabucco E, Mastrogiacomo A, Coppola G, Manente L, Colacurci N, De Falco M, De Luca A. The pattern of expression of Notch protein members in normal and pathological endometrium. J Anat. 2008;213:464–72. doi: 10.1111/j.1469-7580.2008.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitsuhashi Y, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology. 2012;60:826–37. doi: 10.1111/j.1365-2559.2011.04158.x. [DOI] [PubMed] [Google Scholar]
- 76.Feng YZ, Shiozawa T, Miyamoto T, Kashima H, Kurai M, Suzuki A, Ying-Song J, Konishi I. Overexpression of hedgehog signaling molecules and its involvement in the proliferation of endometrial carcinoma cells. Clin Cancer Res. 2007;13:1389–98. doi: 10.1158/1078-0432.CCR-06-1407. [DOI] [PubMed] [Google Scholar]
- 77.Dellinger TH, Planutis K, Tewari KS, Holcombe RF. Role of canonical Wnt signaling in endometrial carcinogenesis. Exp Rev Anticancer Ther. 2012;12:51–62. doi: 10.1586/era.11.194. [DOI] [PubMed] [Google Scholar]
- 78.Menezes MD, Oshima CT, Filho LB, Gomes TS, Barrezueta LF, Stavale JN, Goncalves WJ. Canonical and noncanonical Wnt pathways: a comparison between endometrial cancer type I and atrophic endometrium in Brazil. Sao Paulo Med J. 2011;129:320–4. doi: 10.1590/S1516-31802011000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 80.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, Benedetto C, Mussa A. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–6. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 81.Yuan SS, Tsai KB, Chung YF, Chan TF, Yeh YT, Tsai LY, Su JH. Aberrant expression and possible involvement of the leptin receptor in endometrial cancer. Gynecol Oncol. 2004;92:769–75. doi: 10.1016/j.ygyno.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 82.Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol. 2002;188:219–26. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 83.Battle M, Gillespie C, Quarshie A, Lanier V, Harmon T, Wilson K, Torroella-Kouri M, Gonzalez-Perez RR. Obesity induced a leptin-Notch signaling axis in breast cancer. Int J Cancer. 2014;134:1605–16. doi: 10.1002/ijc.28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS ONE. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez RR, Leary K, Petrozza JC, Leavis PC. Leptin regulation of the interleukin-1 system in human endometrial cells. Mol Hum Reprod. 2003;9:151–8. doi: 10.1093/molehr/gag022. [DOI] [PubMed] [Google Scholar]
- 86.Faggioni R. IL-1 beta mediates leptin induction during inflammation. Am J Physiology. 1998;274:R204–8. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 87.Colbert LS, Wilson K, Kim S, Liu Y, Oprea-Ilies G, Gillespie C, Dickson T, Newman G, Gonzalez-Perez RR. Differential expression of NILCO reveals pathogenesis of human breast cancer. BMC Cancer. 2014;14:249. doi: 10.1186/1471-2407-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gillespie C, Quarshie A, Penichet M, Gonzalez-Perez RR. Potential role of leptin signaling in DMBA-induced mammary tumors by non-responsive C57BL/6J mice fed a high-fat diet. J Carcinog Mutagen. 2012;3:132. [Google Scholar]
- 89.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer. 2011;104:128–37. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 91.Daley-Brown D, Lee R, Oprea-Ilies G, Screws E, Matthews R, Pattillo R, Gonzalez-Perez RR. Role of obesity in Leptin-Notch crosstalk (NILCO) in endometrial cancer from African American and Chinese women [abstract] Cancer Res. 2014;74:5044. [Google Scholar]
- 92.Diamandis EP. Tumor markers: past, present, and future. In: Diamandis EP, Fritsche H Jr, Lilja H, Chan D, Schwartz M, editors. Tumor markers: physiology, pathobiology, technology, and clinical applications. Washington, D.C.: AACC Press; 2002. pp. 3–8. [Google Scholar]
- 93.Volgestein B, Weinberg RA. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 94.Samarnthai N, Hall K, Yeh I-T. Molecular profiling of endometrial malignancies. Obst Gynecol Intern. 2010;162363 doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, McAvoy E, Robinson RG, Duggan ME, Lindsley CW, Zhao Z, Huber HE, Jones RE. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4:271–9. [PubMed] [Google Scholar]
- 96.Kapucuoglu N, Aktepe F, Kaya H, Bircan S, Karahan N, Çiriş M. Immunohistochemical expression of PTEN in normal, hyperplastic and malignant endometrium and its correlation with hormone receptors, bcl-2, bax, and apoptotic index. Pathol Res Pract. 2007;203:153–62. doi: 10.1016/j.prp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–4. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Chen M-L, Xu P-Z, Peng X, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–74. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wullschleger S, Sakamoto K, Johnstone L, Duce S, Fleming S, Alessi DR. How moderate changes in Akt T-loop phosphorylation impact on tumorigenesis and insulin resistance. Dis Model Mech. 2011;1:95–103. doi: 10.1242/dmm.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–4. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 101.Leslie KK, Stein MP, Kumar NS, Dai D, Stephens J, Angela Wandinger-Ness A, Glueck DH. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol Oncol. 2005;96:32–41. doi: 10.1016/j.ygyno.2004.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bukholm IK, Nesland JM, Kåresen R, Jacobsen U, Børresen AL. Relationship between abnormal p53 protein and failure to express p21 protein in human breast carcinomas. J Pathol. 1997;181:140–5. doi: 10.1002/(SICI)1096-9896(199702)181:2<140::AID-PATH745>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 103.Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J, Wik E, Greulich H, Bø TH, Jonassen I, Thomas RK, Zander T, Garraway LA, Oyan AM, Sellers WR, Kalland KH, Meyerson M, Akslen LA, Beroukhim R. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106:4834–9. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol. 1999;17:1382–90. doi: 10.1200/JCO.1999.17.5.1382. [DOI] [PubMed] [Google Scholar]
- 105.Zhu C, Luo J, Shi H, Xie X, Ding Z. Expression of tubulin, p53, ki67, receptors for estrogen, and progesterone in endometrial cancer. Eur J Gynaecol Oncol. 2009;30:514–7. [PubMed] [Google Scholar]
- 106.Dowsett M. Pathology challenges for biology-driven trials: the Ki67 experience. J Eur Cancer. 2013;49:S3. [Google Scholar]
- 107.Zhang R, He Y, Zhang X, Xing B, Sheng Y, Lu H, Wei Z. Estrogen receptor-regulated microRNAs contribute to the BCL2/BAX imbalance in endometrial adenocarcinoma and precancerous lesions. Cancer Lett. 2012;314:155–65. doi: 10.1016/j.canlet.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 108.Marone M, Ferrandina G, Macchia G, Mozzetti S, de Pasqua A, Benedetti-Panici P, Mancuso S, Scambia G. Bcl-2, Bax, Bcl-xL and Bcl-xS. Expression in neoplastic and normal endometrium. Oncology. 2000;58:161–8. doi: 10.1159/000012094. [DOI] [PubMed] [Google Scholar]
- 109.Sakuragi N, Salah-eldin A, Watari H, Itoh T, Inoue S, Moriuchi T. Fujimoto S. Bax, Bcl-2, and p53 expression in endometrial cancer. Gynecol Oncol. 2002;86:288–96. doi: 10.1006/gyno.2002.6742. [DOI] [PubMed] [Google Scholar]
- 110.Zukerberg LR, DeBernardo RL, Kirley SD, D’Apuzzo M, Lynch MP, Littell RD, Duska LR, Boring L, Rueda BR. Loss of cables, a cyclin-dependent kinase regulatory protein, is associated with the development of endometrial hyperplasia and endometrial cancer. Cancer Res. 2004;64:202–8. doi: 10.1158/0008-5472.can-03-2833. [DOI] [PubMed] [Google Scholar]
- 111.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar NS, Richer J, Owen G, Litman E, Horwitz KB, Leslie KK. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: implications for unopposed estrogen action. Cancer Res. 1998;58:1860–5. [PubMed] [Google Scholar]
- 113.Scholten AN, Creutzberg CL, van den Broek LJ, Noordijk EM, Smit VT. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol. 2003;201:460–5. doi: 10.1002/path.1402. [DOI] [PubMed] [Google Scholar]
- 114.Saegusa M, Hashimura M, Yoshida T, Okayasu I. β-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84:209–17. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeng Q, Li W, Lu D, Wu Z, Duan H, Luo Y, Feng J, Yang D, Fu L, Yan X. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc Natl Acad Sci USA. 2012;109:1127–32. doi: 10.1073/pnas.1111053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aldovini D, Demichelis F, Doglioni C, Di V, Galligioni E, Brugnara S, Zeni B, Griso C, Pegoraro C, Zannoni M, Gariboldi M, Balladore E, Mezzanzanica D, Canevari S, Barbareschi M. M-CAM expression as marker of poor prognosis in epithelial ovarian cancer. Int J Cancer. 2006;119:1920–6. doi: 10.1002/ijc.22082. [DOI] [PubMed] [Google Scholar]
- 117.Susini T, Amunni G, Molino C, Carriero C, Rapi S, Branconi F, Marchionni M, Taddei G, Scarselli G. Ten-year results of a prospective study on the prognostic role of ploidy in endometrial carcinoma: DNA aneuploidy identifies high-risk cases among the so-called ‘low-risk’ patients with well and moderately differentiated tumors. Cancer. 2007;109:882–90. doi: 10.1002/cncr.22465. [DOI] [PubMed] [Google Scholar]
- 118.Pradhan M, Abeler VM, Danielsen HE, Sandstad B, Tropé CG, Kristensen GB, Risberg BA. Prognostic importance of DNA ploidy and DNA index in stage I and II endometrioid adenocarcinoma of the endometrium. Ann Oncol. 2012;23:1178–84. doi: 10.1093/annonc/mdr368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dossus L, Becker S, Rinaldi S, Lukanova A, Tjønneland A, Olsen A, Overvad K, Chabbert-Buffet N, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Chang-Claude J, Pischon T, Boeing H, Trichopoulou A, Benetou V, Valanou E, Palli D, Sieri S, Tumino R, Sacerdote C, Galasso R, Redondo ML, Bonet CB, Molina-Montes E, Altzibar JM, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Onland-Moret NC, Lundin E, Idahl A, Khaw KT, Wareham N, Allen N, Romieu I, Fedirko V, Hainaut P, Romaguera D, Norat T, Riboli E, Kaaks R. Tumor necrosis factor (TNF)-α, soluble TNF receptors and endometrial cancer risk: the EPIC study. Int J Cancer. 2011;129:2032–7. doi: 10.1002/ijc.25840. [DOI] [PubMed] [Google Scholar]
- 120.Choi DS, Kim H-J, Yoon J-H, Yoo S-C, Jo H, Lee SY, Min CK, Ryu H-S. Endometrial cancer invasion depends on cancer-derived tumor necrosis factor-α and stromal derived hepatocyte growth factor. Int J Cancer. 2009;124:2528–38. doi: 10.1002/ijc.24238. [DOI] [PubMed] [Google Scholar]
- 121.Bie Y, Zhang Z. Diagnostic value of serum HE4 in endometrial cancer: a meta-analysis. World J Surg Oncol. 2014;12:169. doi: 10.1186/1477-7819-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rong Shao, Kendra Hamel, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–68. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scambia C, Gadducci A, Panici PB, Foti E, Ferdeghini M, Ferrandina G, Amoroso M, Castellani C, Facchini V, Mancuso S. Combined use of CA 125 and CA 15-3 in patients with endometrial carcinoma. Gynecol Oncol. 1994;54:292–7. doi: 10.1006/gyno.1994.1213. [DOI] [PubMed] [Google Scholar]
- 124.Agouza EL, Nashar EL. Serum taurine as a marker of endometrial cancer. Open Wom Health J. 2011;5:1–6. [Google Scholar]
- 125.Farias-Eisner G, Su F, Robbins T, Kotlerman J, Reddy S, Farias-Eisner R. Validation of serum biomarkers for detection of early- and late-stage endometrial cancer. Am J Obstet Gynecol. 2010;202:73.e1–5. doi: 10.1016/j.ajog.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 126.SGO Clinical Practice Endometrial Cancer Working Group; Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, Brewer M, Boruta D, Villella J, Herzog T, Abu Shahin F Society of Gynecologic Oncology Clinical Practice Committee. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol. 2014;134:385–92. doi: 10.1016/j.ygyno.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 127.SGO Clinical Practice Endometrial Cancer Working Group; Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, Brewer M, Boruta D, Villella J, Herzog T, Abu Shahin F Society of Gynecologic Oncology Clinical Practice Committee. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014;134:393–402. doi: 10.1016/j.ygyno.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 128.Ito K, Watanabe K, Nasim S, Sasano H, Sato S, Yajima A, Silverberg SG, Garrett CT. K-ras point mutations in endometrial carcinoma: effect on outcome is dependent on age of patient. Gynecol Oncol. 1996;63:238–46. doi: 10.1006/gyno.1996.0313. [DOI] [PubMed] [Google Scholar]
- 129.Fleming GF, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, Berek JS, Chapman JA, DiSilvestro PA, Horowitz IR, Fiorica JV. Phase II Trial of Trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dutt A, Salvesen HB, Chen T-H, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong K-K, Sellers WR, Meyerson M, Greulich H. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Nat Acad Sci USA. 2008;105:8713–7. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konecny GE, Kolarova T, O’Brien NA, Winterhoff B, Yang G, Qi J, Qi Z, Venkatesan N, Ayala R, Luo T, Finn RS, Kristof J, Galderisi C, Porta DG, Anderson L, Shi MM, Yovine A, Slamon DJ. Activity of the fibroblast growth factor receptor inhibitors dovitinib (TKI258) and NVP-BGJ398 in human endometrial cancer cells. Mol Cancer Ther. 2013;12:632–42. doi: 10.1158/1535-7163.MCT-12-0999. [DOI] [PubMed] [Google Scholar]
- 132.Gozgit JM, Squillace RM, Wongchenko MJ, Miller D, Wardwell S, Mohemmad Q, Narasimhan NI, Wang F, Clackson T, Rivera VM. Combined targeting of FGFR2 and mTOR by ponatinib and ridaforolimus results in synergistic antitumor activity in FGFR2 mutant endometrial cancer models. Cancer Chemoth Pharmacol. 2013;71:1315–23. doi: 10.1007/s00280-013-2131-z. [DOI] [PubMed] [Google Scholar]
- 133.Konopka B, Janiec-Jankowska A, Kwiatkowska E, Najmoła U, Bidziński M, Olszewski W, Goluda C. PIK3CA mutations and amplification in endometrioid endometrial carcinomas: relation to other genetic defects and clinicopathologic status of the tumors. Hum Pathol. 2011;42:1710–9. doi: 10.1016/j.humpath.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 134.Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, Nugent E, Han LY, Landen CN, Jr, Spannuth WA, Lu C, Coleman RL, Gershenson DM, Sood AK. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 135.Sanseverino F, Santopietro R, Torricelli M, D’Andrilli G, Russo G, Cevenini G, Bovicelli A, Leoncini L, Scambia G, Petraglia F, Claudio PP, Giordano A. pRb2/p130 and VEGF expression in endometrial carcinoma in relation to angiogenesis and histopathologic tumor grade. Cancer Biol Ther. 2006;5:84–8. doi: 10.4161/cbt.5.1.2345. [DOI] [PubMed] [Google Scholar]
- 136.Dobrzycka B, Terlikowski SJ, Kwiatkowski M, Garbowicz M, Kinalski M, Chyczewski L. Prognostic significance of VEGF and its receptors in endometrioid endometrial cancer. Ginekol Pol. 2010;81:422–5. [PubMed] [Google Scholar]
- 137.Davies S, Dai D, Pickett G, Thiel K, Korovkina V, Leslie K. Effects of bevacizumab in mouse model of endometrial cancer: defining the molecular basis for resistance. Oncol Rep. 2011;25:855–62. doi: 10.3892/or.2011.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verdi J, Tan A, Shoae-Hassani A, Seifalian AM. Endometrial stem cells in regenerative, medicine. J Biol Eng. 2014;8:20. doi: 10.1186/1754-1611-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thomson JA, Itskovic Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 140.Hubner K, Fuhrman G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Schöler HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–6. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 141.Guillot PV, O’Donohue K, Kurota H, Fisk NM. Fetal stem cells: betwixt and between. Semin Reprod Med. 2006;24:340–7. doi: 10.1055/s-2006-952149. [DOI] [PubMed] [Google Scholar]
- 142.Lobo NA, Shimono K, Quan D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 143.Kiyoko K. Stem cell in human normal endometriosis and endometrial cancer: characteristics of side population cells. Kaohsiung J Med Sci. 2012;28:63–71. doi: 10.1016/j.kjms.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 144.Kyo S, Maida Y, Inoue M. Stem cells in endometrium and endometrial cancer: accumulating evidence and unresolved questions. Cancer Lett. 2011;308:123–33. doi: 10.1016/j.canlet.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 145.Lopez J, Valdez-Morales FJ, Benitez-Bribiesca L, Cerbon L, Garcia-Carranca A. Normal and cancer stem cells of the human female reproductive system. Rep Biol Endocrinol. 2013;11:53. doi: 10.1186/1477-7827-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szotek PP, Chang HL, Brennand K, Fujino A, Pieretti-Vanmarcke R, Lo Celso C, Dombkowski D, Preffer F, Cohen KS, Teixeira J, Donahoe PK. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc Natl Acad Sci USA. 2008;105:12469–73. doi: 10.1073/pnas.0805012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gotte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schüring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial cancer. J Pathol. 2008;215:317–29. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 148.Kato K. Endometrial cancer stem cells: a new target for cancer therapy. Anticancer Res. 2012;32:2283–93. [PubMed] [Google Scholar]
- 149.Howlader N, Noone AM, Krapch M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; www.seer.cancer.gov/csr/1975-2010. [Google Scholar]
- 150.Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;165:262–70. doi: 10.1093/aje/kwk010. [DOI] [PubMed] [Google Scholar]
- 151.Smotkin D, Nevadunsky NS, Harris K, Einstein MH, Yu Y, Goldberg GL. Histopathologic differences account for racial disparity in uterine cancer survival. Gynecol Oncol. 2012;127:616–9. doi: 10.1016/j.ygyno.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, Berchuck A. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res. 2000;6:2999–3005. [PubMed] [Google Scholar]
- 153.Clifford SL, Kaminetsky CP, Cirisano FD, Dodge R, Soper JT, Clarke-Pearson DL, Berchuck A. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I endometrial cancers. Am J Obstet Gynecol. 1997;176:S229–32. doi: 10.1016/s0002-9378(97)70380-6. [DOI] [PubMed] [Google Scholar]
- 154.Santin AD, Bellone S, Siegel ER, Palmieri M, Thomas M, Cannon MJ, Kay HH, Roman JJ, Burnett A, Pecorelli S. Racial differences in the overexpression of epidermal growth factor type II receptor (Her2/neu): a major prognostic indicator in uterine serous papillary cancer. Am J Obstet Gynecol. 2005;192:813–8. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 155.Fraley J, Risinger JI, Rose GS, Maxwell GL. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110:234–43. doi: 10.1002/cncr.22797. [DOI] [PubMed] [Google Scholar]