Abstract
Background
Methamphetamine use increases the risk of HIV-1 infection among seronegative users, and can exacerbate disease progression in HIV-positive users. The biological mechanisms underlying these associations remain unclear. In this cross-sectional pilot study, we examine the associations between recent methamphetamine use and inflammation in the rectal mucosa and peripheral blood compartments in HIV-1 seropositive and seronegative men who have sex with men (MSM).
Methods
HIV-seronegative and seropositive MSM participants were enrolled (N=24). Recent methamphetamine use was determined by urine drug screen. Cytokines were quantified using multiplex arrays from collected plasma and rectal sponge samples, and peripheral blood T cell activation was assessed by flow cytometry.
Results
Methamphetamine use was associated with consistently increased rectal inflammatory cytokines, specifically interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha, regardless of HIV-1 serostatus in this pilot study. This association was significant after adjusting for age, HIV-serostatus, and receptive anal intercourse frequency using regression analysis. Similar increases were not uniformly observed in peripheral blood.
Conclusions
Methamphetamine use is associated with increased local mucosal inflammatory cytokine production. These findings may help explain the increased HIV-1 risk seen in methamphetamine users, and contribute to increased inflammation among HIV-seropositive users.
Keywords: mucosal immunity, methamphetamine, men who have sex with men, HIV-1, gastrointestinal-associated lymphoid tissue
BACKGROUND
Methamphetamine (MA) is a widely used illicit stimulant drug popular among men who have sex with men (MSM)1. MA use increases risk of HIV-1 acquisition in seronegative users, with MA accounting for attributable risk percent for HIV-1 infection between 16.3% and 33%2,3, and 1.46 increased relative hazard of HIV-1 seroconversion4. Among HIV-1 infected MA-users, clinical indices such as plasma viral RNA and CD4 counts are adversely affected5–7. Some studies suggest increased immune activation in the blood of HIV-infected MA-users, even after controlling for antiretroviral therapy (ART) adherence8,9. The mechanism underlying these clinical observations is likely multifactorial including both changes in risk behavior and direct immune effects of the drug. The extent to which each of these factors contributes to this growing epidemic remains unanswered.
MA triggers both systemic and localized inflammation, including in the context of HIV-1 infection7–9. Studies in mice have shown that MA induces activation of microglial cells in the central nervous system, with resultant induction of inflammatory cytokine production and gene expression10. In vivo studies using simian immunodeficiency virus (SIV)-infected macaques showed that MA increases local central nervous system viral concentrations via increased inflammation11,12. In humans, stimulant-induced immune activation was also associated with decreased tryptophan9, suggesting that modulation of the indoleamine-(2,3)-dioxygenase (IDO) pathway could be a potential mechanism for stimulant-associated immune dysregulation. While MA can penetrate mucosal tissues13, it is not known whether similar inflammatory effects occur at mucosal sites. Mucosal inflammation contributes to immune activation in HIV-infected persons14, as well as increased risk of HIV-1 transmission in HIV-negative populations15,16, making this an important area of investigation.
To further explore the effect of MA on inflammation, particularly at sexually-exposed mucosal sites, we examined the effects of recent MA use on HIV-1 relevant immune parameters in both the rectal mucosal and circulating blood compartments in HIV-seronegative and HIV-seropositive MSM. Sexual behavior data were collected to control for confounding variables in our analysis. We hypothesized that recent MA use would correspond with significant increases in immune measures independent of HIV-serostatus.
METHODS
Participants and study design
All participants in this pilot study provided written informed consent under a UCLA Institutional Review Board approved protocol. HIV-seropositive and seronegative MSM over age 18 were selected from another longitudinal study (mSTUDY NIH U01DA036267, UCLA IRB #13-001749) or recruited from the UCLA Mucosal Immunology Core Registry (UCLA IRB #10-000528). Additional inclusion criteria for HIV seropositive: antiretroviral therapy (ART)-naïve or self-report non-adherence/discontinuation and screening plasma HIV RNA >1000 copies/ml and CD4+ T cells >200 cells/µl. Exclusion criteria for all participants: Positive urine or rectal nucleic acid amplification test for Chlamydia trachomatis or Neisseria gonorrhoeae. At the study visit participants provided a urine specimen for point of care drug screen toxicity test, filled out a demographic and behavioral questionnaire, then had phlebotomy and rectal sponge collected via anoscopy. All specimens were stored at −80 degrees until batch processing.
Participants in this cross-sectional study were recruited into four groups: (1) HIV-positive, MA-positive, (2) HIV-positive, MA-negative, (3) HIV-negative, MA-positive, and (4) HIV-negative, MA-negative. MA users were defined as self-reported use of methamphetamine (any route of administration) in the 30 days preceding enrollment and urine drug screen positive for methamphetamine at time of study visit and sample collection, but negative for other drugs except nicotine, alcohol, or marijuana. MA non-users reported no use of drugs except nicotine, alcohol, or marijuana and negative urine drug screen.
Cytokine quantification
Detailed sample processing description is available in Supplementary Materials. In brief, rectal secretions were collected via sponge and eluted by centrifugation. Plasma was prepared by centrifugation from whole blood. Concentrations of interleukin (IL)-1β, 6, 12p70, interferon (IFN)-γ, macrophage inflammatory protein (MIP)-1α, tumor necrosis factor (TNF)-α, and regulated on activation, normal T cell expressed and secreted (RANTES) were measured using Milliplex Human Cytokine kits (Millipore) according to manufacturer’s instructions. Samples were run in duplicate and discarded if coefficient of variation (%CV) >15%. The same kits were used for both plasma and rectal samples to ensure comparability of results.
Peripheral blood T cell phenotyping
PBMC were prepared via Ficoll density centrifugation then stained with fluorochrome-conjugated antibodies and analyzed by flow cytometry. Details regarding specific antibodies and gating analysis available in Supplementary Materials. Analysis was performed using FlowJo and expressed as percentages of activated (CD38 and/or HLA-DR) CD4+ or CD8+ T cells.
Statistical Analysis
Initial analysis compared the unadjusted differences in participant characteristics and mean cytokine concentrations or activated T cell percentages between methamphetamine users and non-users within HIV-1 serogroups. Nonparametric exact Wilcoxon rank-sum (Mann–Whitney U) tests were used for all biomarkers and continuous variables and Fisher's exact tests for binary variables. We then adjusted for multiple comparisons using Benjamini-Hochberg false discovery rate (FDR)17. Correlation coefficients between plasma and rectal cytokines were computed using nonparametric Spearman rank correlation. Cytokines for further investigation in linear regression models were selected based on p-values computed among the HIV-seropositive participants (due to largest N) with cutoff for further study unadjusted p<0.05 and/or FDR <0.2. Log-transformed biomarker values were regressed on an indicator variable for methamphetamine use (versus non-use) along with potential confounding variables including age, HIV-serostatus, and receptive anal intercourse (RAI) frequency in the past 30 days. The regression coefficients were exponentiated to present adjusted ratios of cytokine medians for methamphetamine users relative to those of non-users (equivalent to the ratios of the geometric means). Statistical analyses were performed using SAS software version 9.4 for Windows (SAS Institute, Cary, NC USA) and Prism 7 (GraphPad, San Diego, CA USA).
RESULTS
The study included 24 MSM comprised of 16 HIV-seropositive and 8 HIV-seronegative participants. Participants were further classified as MA-positive or negative based on urine toxicology screen at the time of study visit, and included 12 MA-positive and 12 MA-negative participants. Table 1 provides details regarding demographics. Within the HIV-seropositive group, 13 participants were treatment naïve and three reported treatment non-adherence or discontinuation prior to enrollment. We found no significant difference in plasma HIV RNA copies (p=0.17) or CD4+ T cells (p>0.8) associated with MA use (Table 1). Self-reported frequency of receptive anal intercourse (RAI) also did not differ with MA use (p=0.23).
Table 1.
Demographics and clinical characteristics in HIV-positive and HIV-negative participants
| HIV | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| POSITIVE | NEGATIVE | |||||
|
|
||||||
| Methamphetamine Use | + (N=9) |
− (N=7) |
p-valuea | + (N=3) |
− (N=5) |
p-value |
|
|
||||||
| Demographics and Clinical | ||||||
| Age, mean (SD) | 38.56 (7.40) | 37.57 (6.90) | 0.78 | 31.33 (9.45) | 26.80 (2.39) | 0.77 |
| Ethnicity, N (%) | ||||||
| Hispanic/Latino | 2 (22) | 2 (29) | >0.8 | 2 (67) | 2 (40) | 0.57 |
| Black (Non-Hispanic) | 3 (33) | 4 (57) | 1 (33) | 3 (60) | ||
| White (Non-Hispanic) | 1 (11) | 0 | 0 | 0 | ||
| Unknown | 3 (33) | 1 (14) | 0 | 0 | ||
| Plasma HIV RNA (log copies/ml), mean (SD) | 4.60 (0.68) | 4.26 (0.56) | 0.17 | NA | NA | |
| CD4+ T cells (103 cells/ml), mean (SD) | 352 (239.50) | 348 (99.50) | >0.8 | NA | NA | |
| Sexual Behavior | ||||||
| RAI frequency (# of times in past 30 days), mean (SD) | 1.38 (1.69) | 0.83 (2.04) | 0.23 | 0 (0) | 1.33 (2.31) | >0.8 |
P-values computed using nonparametric Wilcoxon rank-sum tests (exact) for continuous variables and Fisher’s exact tests for binary variables.
SD, standard deviation; NA, not applicable; RAI, receptive anal intercourse
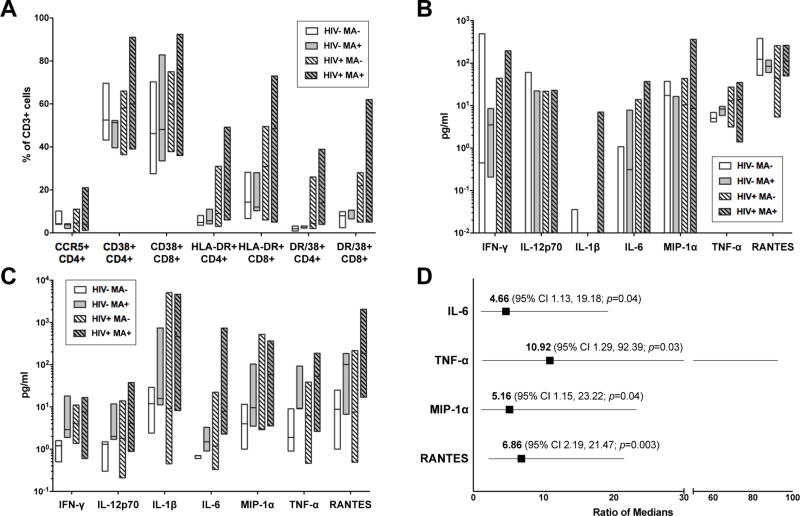
To assess differences in immune parameters related to MA use we independently compared MA-users to non-users by HIV-serostatus. We examined both plasma inflammatory cytokines and peripheral blood T cell activation, which have been separately associated with HIV-1 progression18,19. We saw no significant differences in percentages of activated T cells using CD38 and HLA-DR as markers (Figure 1A). We also found no significant differences in plasma inflammatory cytokine production in either HIV-seropositive or HIV-seronegative groups (Figure 1B).
Figure 1. Effects of methamphetamine (MA) on immune activation and cytokine production in HIV+ and HIV− MSM.
(A) Peripheral blood T cell activation plotted by HIV-serostatus and MA use. Bars outline minimum to maximum values with line indicating median. T cell activation was assessed by flow cytometry using cell surface markers CD38, HLA-DR, and CCR5 on either CD4+ or CD8+ T cells. Data expressed as percent of total CD3+ T cells for indicated subsets. (B) Plasma and (C) rectal cytokine quantification by HIV-serostatus and MA use. Bars outline minimum to maximum values with line indicating median. Values at or below the lower limit of assay detection were plotted at the lower limit (0.01 pg/ml). (D) Regression analysis of log-transformed cytokines from MA-using and non-MA using participants. Models for each cytokine were adjusted for age (continuous), HIV-1 serostatus (positive vs negative), and receptive anal intercourse (RAI) frequency in the past 30 days (# of encounters, continuous). Square indicates ratio of median cytokine concentrations of MA-users versus non-MA users adjusted for covariates with lines indicating 95% confidence interval (CI). p-value calculated using Wald Chi-Square test for linear regression model coefficients.
Since the majority of HIV-1 transmission occurs at mucosal sites, we quantified local rectal inflammatory cytokine production using rectal sponge samples. We found that MA use was associated with consistently increased mucosal inflammatory cytokine production in both HIV-seropositive and HIV-seronegative MSM on initial analysis, but did not meet statistical significance in this pilot study (Figure 1C). We next adjusted for potential confounders which could affect rectal inflammation, specifically age, HIV-serostatus, and frequency of RAI, using linear regression on selected cytokines. In this analysis, recent MA use was a strong predictor of increased rectal cytokine production (Figure 1D). We also considered the potential effects of direct MA contact with the mucosa (“booty bumping”), but were unable to include this in the analysis as none of the participants reported rectal MA administration. Finally, we examined the correlation between plasma and rectal cytokines using Spearman rank correlation and no significant correlation was seen for any cytokine (data not shown).
DISCUSSION
This small pilot study provides compelling evidence that acute MA use may have direct, mucosal immune effects in both HIV-1 seropositive and seronegative MSM. We compared both peripheral and rectal mucosal cytokine production, and while no significant changes in plasma cytokines or T cell activation were noted we did find increases in rectal inflammatory cytokines IL-6 and TNF-α associated with MA use (Figure 1C). This linkage was significant after adjusting for age, HIV-serostatus, and RAI frequency (Figure 1D). Moreover, we found no correlation between plasma and rectal cytokines, highlighting the importance of examining mucosal measures to assess local inflammation.
Local changes in cytokine and chemokine concentrations can impact HIV-1 transmission. The chemoattractant chemokines MIP-1α, MIP-1β, and RANTES recruit CCR5+ CD4+ T lymphocytes, a primary target cell for HIV-1 infection, thereby increasing the opportunity for infection and localized spread20. Localized inflammation, including production of inflammatory cytokines, further influences HIV-1 transmission and replication21. IL-6, for example, may further enhance HIV-1 transmission through promotion of Th17 cells, an early target for HIV-1 infection22,23. Our findings of increased mucosal inflammatory cytokines may therefore provide suggestion of a biological mechanism underlying the clinical association between MA use and increased HIV-1 risk that warrants further study.
MA is known to activate inflammatory responses, though its effect in the gastrointestinal mucosa had not been previously examined. Our study showed that following recent, confirmed MA use, evidence of inflammatory cytokine production was detected in the rectal mucosa but not in plasma. The reasons for this compartmentalized response remain unclear. One possibility includes the increased availability of MA-susceptible inflammatory cells, such as intestinal epithelial cells or resident lymphoid cells, in the rectal mucosa than in blood. Similarly, the distribution of MA in the distal gastrointestinal mucosa is not clear, but based on one study using radiolabeled MA it does appear to have remarkably high concentrations at mucosal sites including stomach and lung13.
Contrary to other studies5,7, we found no significant difference in plasma HIV RNA concentrations between MA users and non-users. This small study was possibly underpowered to detect such differences, and the cutoff of >1000 copies/ml for inclusion in our study may have further reduced our ability to detect differences between groups. We did not observe significant differences in plasma cytokines related to MA, perhaps related to our small pilot design, but did find increased rectal IL-6 and MIP-1α which have been associated with MA-use in prior studies of peripheral blood7. We also found no difference in T cell activation in our study (Figure 1A), somewhat discordant with Massanella et al8, although our study did not examine T cell proliferative capacity. Surprisingly, no significant difference in self-reported RAI associated with MA use were observed in either serogroup. However, this lack of difference may further suggest there is a unique association between MA and rectal mucosal cytokines independent of other inflammatory stimuli, such as RAI.
A limitation to our study is the small sample size. Despite screening many individuals, only the 24 included met the stringent inclusion/exclusion criteria for this pilot study. However, by using such strict parameters including exclusion of antiretroviral therapy and using urine drug screen results to document recent MA use, we can more precisely examine links between acute drug effects and immune parameters. Indeed, even with this limited sample size, we found significant differences in rectal cytokines in our regression analysis (Figure 1D). Certainly, these novel pilot study data warrant further investigation in larger studies.
It should be noted that urine drug screens only detect MA use within the past three days. It is possible that some MA-negative participants had recently used MA, despite negative self-report, but were not detected due to the short detection window. Since the rectal mucosa is known to rapidly repair we expect that changes in inflammation may be short lived and thus wanted to limit our study only to recent MA use. Any misclassification could potentially diminish the observed differences in cytokines between groups. Additionally, MA use was not quantified in this study, limiting the potential to make any inference regarding the amount of MA use and immune effects. These data are the first to report inflammatory consequences in the rectal mucosa linked to acute MA use, and are suggestive of a direct activating effect of MA on mucosal immune activation in both HIV-1 seropositive and seronegative MSM. Further efforts toward reducing MA use among MSM may have beneficial impact in reducing inflammation, and thereby potentially lowering HIV-1 acquisition risk and improving clinical outcomes.
Supplementary Material
Acknowledgments
We are most grateful for all the participants in this study for their generous participation. We thank Zi Liu for technical assistance and Terry Saunders, PA-C for clinical assistance. The authors also thank Dr. Grace Aldrovandi for insightful discussions.
Source of Funding:
This work was supported by National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) and National Institute of Mental Health (NIMH) [U01 DA036267 and P30 MH058107 to S.S. and P.M.G]. J.A.F. was supported in part by National Institutes of Health (UCLA CTSI) [KL2 TR001882]. The UCLA AIDS Institute and Center for AIDS Research (CFAR) provided supplementary funding and the CFAR Mucosal Immunology Core and Flow Cytometry Core provided technical assistance [NIH/NIAID P30 AI028697].
Footnotes
Conflicts of Interest
There are no declared conflicts of interest.
References
- 1.Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annual review of public health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 3.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr. 2009;51(3):349–355. doi: 10.1097/QAI.0b013e3181a24b20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 6.Shoptaw S, Stall R, Bordon J, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23(8):576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Wang M, Liang B, et al. In vivo effects of methamphetamine on HIV-1 replication: A population-based study. Drug and alcohol dependence. 2016;159:246–254. doi: 10.1016/j.drugalcdep.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Massanella M, Gianella S, Schrier R, et al. Methamphetamine Use in HIV-infected Individuals Affects T-cell Function and Viral Outcome during Suppressive Antiretroviral Therapy. Sci Rep. 2015;5:13179. doi: 10.1038/srep13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrico AW, Johnson MO, Morin SF, et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain, behavior, and immunity. 2008;22(8):1257–1262. doi: 10.1016/j.bbi.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva AP, Martins T, Baptista S, Goncalves J, Agasse F, Malva JO. Brain injury associated with widely abused amphetamines: neuroinflammation, neurogenesis and blood-brain barrier. Current drug abuse reviews. 2010;3(4):239–254. doi: 10.2174/1874473711003040239. [DOI] [PubMed] [Google Scholar]
- 11.Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. The American journal of pathology. 2010;177(1):355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najera JA, Bustamante EA, Bortell N, et al. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC immunology. 2016;17(1):7. doi: 10.1186/s12865-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Fowler JS, Wang GJ, et al. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One. 2010;5(12):e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 15.Masson L, Passmore JA, Liebenberg LJ, et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clinical Infectious Diseases. 2015 doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Introini A, Bostrom S, Bradley F, et al. Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants. PLoS Pathog. 2017;13(5):e1006402. doi: 10.1371/journal.ppat.1006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 18.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 21.McGowan I, Elliott J, Fuerst M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37(2):1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 22.Stieh DJ, Matias E, Xu H, et al. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe. 2016;19(4):529–540. doi: 10.1016/j.chom.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez Y, Tuen M, Shen G, et al. Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J Virol. 2013;87(19):10843–10854. doi: 10.1128/JVI.01838-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.