Abstract
Background
Sample processing for real-time reverse transcriptase polymerase chain reaction (rRT-PCR) based diagnostic assays requires stabilizing sample ribonucleic acid (RNA) in lysis buffer prior to testing. The stability of viral RNA prior to processing is difficult to assure. It is unknown if clinical sample integrity is compromised by delays in processing, as may occur due to weekends and holidays. We sought to examine the integrity of respiratory specimens with variable processing times.
Study Design
Upper respiratory specimens were collected during 3 influenza seasons 2009-2012 and tested for influenza virus and internal control human RNase P (RNP) RNA by rRT-PCR. Time to processing was measured in hours from specimen collection to placement in lysis buffer.
Results
635 (11.4%) of 5583 samples were influenza positive. Mean and median times to processing were 11.5 hours and 6.0 hours respectively (min 0.1h, max 105.2h). There were no significant associations between time to processing and presence of RNP (OR=1.0, p=0.740), or detection of influenza (OR=1.0, p=0.060). Longer duration of illness was associated with a lower likelihood of influenza detection (OR=0.92, p<0.001) and with increased influenza A cycle threshold (Ct) values (p<0.001), while older age was associated with increased influenza B Ct values (p=0.001), indicating the presence of less amplifiable RNA.
Conclusion
Delays in time to processing of upper respiratory specimens up to 105 hours were not associated with decreased detection of amplifiable RNA, suggesting specimen integrity is not compromised by such delays.
Keywords: Influenza, real-time RT PCR, Processing, Swabs, Respiratory
Background
Real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) has become the gold standard for the detection of respiratory viruses. With this method of detection, specimen processing requires nucleic acid stability rather than organism viability previously needed for culture. Many studies have compared various types of specimens, swabs, and processing buffers for influenza and other respiratory virus RT-PCR assays [1–4]. Laboratory-based studies have found that influenza RNA was stable on dry swabs for days prior to processing if kept at room temperature or cooler [5, 6]. However, another study found that delayed processing of respiratory specimens resulted in lower influenza detection by molecular testing [7]. Our study evaluated the presence and amount of both influenza and human RNA in clinical samples with variable processing times using rRT-PCR.
It is common for laboratories to suspend testing over weekends and holidays due to technician staffing. Additionally, such laboratories typically batch influenza testing during studies. We sought to examine the integrity of respiratory specimens with variable processing times for the testing of influenza. A secondary objective was to evaluate the association between presence and amount of amplifiable RNA and processing time, adjusting for age and duration of illness.
Study Design
Study Population
5633 patients with acute respiratory illness presenting for medical care were enrolled from various inpatient and outpatient clinical settings in the Nashville area during three influenza seasons 2009-2012. Demographic and clinical information were collected using questionnaires and case report forms [8]. Duration of illness was defined as total days from onset of symptoms until date of specimen collection.
Clinical Samples and Laboratory Testing
Both nasal and throat swabs were collected from each study participant and placed into the same vial of viral transport medium at time of collection. Samples were kept on ice until they arrived at the laboratory where they were placed into a 4°C refrigerator until processed. Processing included placing an aliquot into lysis buffer prior to RNA extraction, and followed by testing for influenza A, influenza B, and RNAse P (RNP) RNA using real time RT-PCR [9]. Time to processing was defined as time in minutes from collection of specimen until the specimen was placed in lysis buffer. Samples were processed on weekdays only, leading to delayed processing for weekend and holiday samples.
Statistical Analysis
Descriptive analyses were conducted. Two-sample T-tests were used to compare the mean cycle threshold (Ct) values between early and late processed specimens. Multivariable logistic and linear regression models were used to evaluate the association between presence and amount of amplifiable RNA respectively with processing time adjusting for age at time of illness, and duration of illness. Variables included in both the multivariable logistic and linear regression models were time to processing, age at time of illness, and duration of symptoms prior to specimen collection. Ct values were used as a semi-quantifiable surrogate for amount of RNA present which was the outcome variable in the multivariable linear regression model. All analyses were performed using Stata 12.1.
Results
There were 5583 of 5633 (99.1%) enrollees who had amplifiable human RNA indicating the majority of samples were adequate in regards to sample collection and successful nucleic acid extraction. All 5633 samples were included in further analysis to evaluate for an association between the presence of amplifiable RNA and processing time using the multiple logistic regression model. The 50 samples that failed to amplify internal control RNA were excluded in the multiple linear regression model. Mean and median sample time to processing was 11.5 hours and 6.0 hours, respectively, with a range of 0.1 to 105.2 hours. Study demographics and clinical information are shown in Table 1. Specimens with negative RNP test results were not significantly different from the specimens that amplified internal control RNA when comparing mean (12.5h vs 11.5h; p=0.267) or median (6h vs 6.3h; p=0.444) time to processing, mean (32.4y vs 32.6y; p=0.518) or median (36.5y vs 29.2y; p=0.448) age of patient, and mean (3.7d vs 4.1d; p=0.151) or median (3d vs 3d) duration of illness respectively (data not shown). Of the 5583 internal control positive samples, 635 (11.4%) were influenza positive; 469 (8.4%) influenza A, 163 (2.9%) influenza B, and 3 (0.1%) influenza A and B co-infections.
Table 1.
Demographic and Clinical Information.
| Specimens (5633) | RNP+ | 5583 (99.1) |
| Influenza + (RNP+) | 635 (11.4) | |
| Influenza A+ (RNP+) | 469 (8.4) | |
| Influenza B+ (RNP+) | 163 (2.9) | |
| Influenza A and B+ (RNP+) | 3 (0.3) | |
|
| ||
| Season | 2009-2010 | 3688 (65.5) |
| 2010-2011 | 1624 (29.1) | |
| 2011-2012 | 321 (5.7) | |
|
| ||
| Location | Inpatient | 2321 (41.2) |
| Outpatient | 1725 (30.6) | |
| ED | 1587 (28.2) | |
|
| ||
| Sex | Male | 3043 (54.0) |
| Female | 2590 (46.0) | |
|
| ||
| Age | Range (Years) | 0.01-102.4 |
| Mean ± SD (Years) | 32.6 ± 28.0 | |
| <18yr | 2289 (40.6) | |
| ≥18yr | 3344 (59.4) | |
|
| ||
| Race | White | 3224 (57.2) |
| Black | 1488 (26.4) | |
| Hispanic | 810 (14.4) | |
| Asian | 49 (0.9) | |
| Other | 62 (1.1) | |
|
| ||
| Days III | Range (Days) | 0-30 |
| Mean ± SD (Days) | 3.7 ± 4.1 | |
|
| ||
| Time to Processing | Range (Hrs) | 0.1-105.2 |
| Mean ± (Hrs) | 11.5 ± 11.7 | |
| Median (IQR: 25%, 75%) (Hrs) | 6.0 (4.2, 20.5) | |
ED, Emergency Department; Hrs, hours; SD, Standard Deviation; IQR, Interquartile Range
Mean Ct values were similar for influenza A, influenza B, and human RNP assays and there were no statistical significant difference between samples processed ≤ 24 hours and >24 hours (Table 2).
Table 2.
Ct of influenza A, influenza B, and human RNAse P RNA for samples processed ≤ 24 hours and >24 hours.
| Processed <24Hr (n=4733) | Processed>24Hr (n=900) | ||||
|---|---|---|---|---|---|
|
|
|||||
| Total (%) | Mean Ct | Total (%) | Mean Ct | P Value | |
| Influenza A | 393 (83) | 29.81 | 242 (17) | 29.42 | 0.564 |
| Influenza B | 141 (84) | 26.68 | 23 (16) | 27.72 | 0.339 |
| RNP | 4691 (84) | 29.65 | 892 (16) | 29.83 | 0.112 |
Mean cycle threshold (Ct) values determined using real time RT-PCR. P-values are comparing Ct values.
Specimens with increased time to processing trended towards negative influenza test results though this was not statistically significant. There was no association between increased time to processing and the presence of either influenza (p=0.062; OR=1.0) or human (p=0.740; OR=1.0) RNA using multivariable logistic regression models (Figure 1 A–B). Older age (p<0.001; OR=0.99) and longer duration of illness (p<0.001; OR=0.92) were both associated with a lower likelihood of influenza detection (Figure 1C and 1E).
Figure 1.
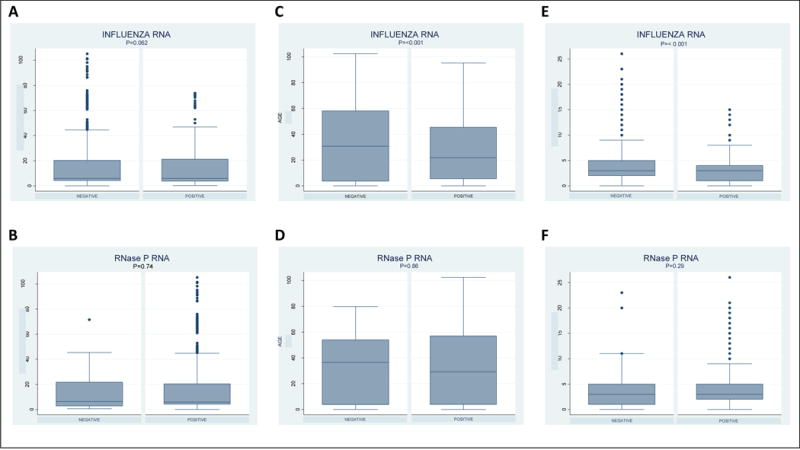
Boxplot of min, max, median and interquartile range of (A) time to processing (hours), (C) age (years), and (E) duration of illness (days) for influenza negative and positive specimens. Boxplot of min, max, median and interquartile range of (B) time to processing (hours), (D) age (years), and (F) duration of illness (days) for human RNase P (RNP) negative and positive specimens. Influenza RNA includes both influenza A and B.
The amount of amplifiable influenza A (p=0.651; 95% CI=−0.03, 0.05), influenza B (p=0.133; 95% CI=−0.02, 0.15), and human RNP (p=0.797; 95% CI=−0.01, 0.01) RNA was not impacted by increased processing time (Figure 2A). Older age was associated with less amplifiable influenza B (p=0.001; 95% CI=0.02, 0.09) and human RNP (p<0.001; 95% CI=0.02, 0.03) RNA (Figure 2B). There was less amplifiable influenza A RNA with older age though it was not statistically significant (p=0.187; 95% CI=−0.01, 0.04). Prolonged duration of illness was associated with less amplifiable influenza A (p<0.001; 95% CI=0.23, 0.65) and human RNP (p<0.001; 95% CI=0.06, 0.11) RNA (Figure 2C). There was less amplifiable influenza B RNA with prolonged duration of illness though it was not statistically significant (p=0.606; 95% CI=−0.34, 0.58).
Figure 2.

(A) Impact of time to processing (hours), (B) age (years), and (C) duration of illness (days) on the amount of influenza A, influenza B, and human RNAse P (RNP) RNA.
Discussion
In this study, increased time to processing up to 4 days, when samples were maintained at 4°C, did not affect the presence and amount of influenza and human RNA. There was a non-significant trend towards less amplifiable influenza B RNA with increased time to processing. It is unclear if this trend is insignificant due to low number of influenza B positive samples in this study or if there is truly no clinical significance.
Older patients were more likely to have negative influenza test results than younger patients. Interestingly, older patients had less amplifiable influenza B and RNP RNA with a non-significant trend towards less amplifiable influenza A RNA. The cause(s) of this is unknown, though higher annual influenza vaccine rates could explain the decrease in infection rate and possibly decreased shedding. Decreased human and viral shedding with increased age could also be explained by drier mucous membranes secondary to age and supplemental oxygen usage.
Patients presenting further into the course of illness were more likely to have a negative influenza test result. These patients also had less amplifiable influenza A RNA and, though not significant, a trend towards less amplifiable influenza B RNA highlighting early viral RNA shedding early in the course of illness.
Older patients and those with a prolonged course of illness were less likely to have influenza RNA detected, pointing out the limits of these assays for this population. When testing these patients, providers should interpret negative results with caution. Additionally, this highlights the need for appropriate sample collection. Weather obtaining specimens from various sites to enhance the yield of detection in this susceptible patient population is unknown. Older adults may present with late complications of influenza at a time when virus is no longer detectable.
Additionally, there was more amplifiable human RNP RNA early in the course of influenza illness, suggesting increased epithelial shedding with active viral infection. Other possibilities include decreased epithelial shedding late in infection due to an unhealthy epithelium. Interventions routinely used during influenza infections such as supplemental oxygen, steroid nasal sprays, alpha agonist nasal sprays, and other over the counter remedies can alter the character of the nasal and oropharyngeal epithelium, affecting available amplifiable RNA.
There are limitations to this study, as it was a retrospective analysis using samples from an influenza surveillance study [8]. Mean and median time to processing was 11.5 hours and 6.0 hours respectively ranging from 0.1 to 105.2 hours with a majority (84%) of samples processed within 24 hours. Though there was not a significant impact on the presence or amount of amplifiable influenza RNA, our study only included samples with processing time up to 4 days. It remains unclear if samples processed after 4 days would be affected by such a delay, and if so, at what time would sample integrity be affected. Caselton et al. showed similar results when processing was performed up to 5 days; however, they noted a decreased rate of influenza detection when samples were processed 6 days or later [7]. In our study, there were only 163 influenza B positive samples, limiting inferences from these analyses. This study only assessed patients with influenza infection; thus, it remains unclear if detection of nucleic acid from other respiratory viruses would be impacted by similar processing delays. In addition, it is unclear whether these results apply to other populations such as infants, children, and adults in other clinical settings, including patients with respiratory symptoms up to 30 days.
Delaying diagnostic assays in patient care settings is not advocated, especially when treatment could be altered; however, many research laboratories are not routinely staffed during weekends and holidays. These data give reassurance that influenza RNA is stable on refrigerated clinical swabs up to 4 days.
Acknowledgments
Funding: Funding for this study was through multiple sources: including the CDC (1U181P000184-01: Marie Griffin MD, site PI), RTI/CDC (contract 200-2008-24624 to RTI International; Marie Griffin MD, site PI) and the NIA (Talbot, PI 1R01AG043419). The study was also supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health. The funders did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript.
Footnotes
Competing Interests: JVW serves on the Scientific Advisory Board of Quidel and on an Independent Data Monitoring Committee for GlaxoSmithKline. HKBT serves as a consultant for VaxInnate, MedImmune, and Novartis.
Ethical Approval: Not Required
Presented: Data from this study was presented at ID Week 2013, San Francisco, CA, Oct 2-6 2013 (abstract 277).
References
- 1.Abu-Diab A, et al. Comparison between pernasal flocked swabs and nasopharyngeal aspirates for detection of common respiratory viruses in samples from children. J Clin Microbiol. 2008;46(7):2414–7. doi: 10.1128/JCM.00369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irving SA, et al. Comparison of nasal and nasopharyngeal swabs for influenza detection in adults. Clin Med Res. 2012;10(4):215–8. doi: 10.3121/cmr.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spackman E, et al. Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus. BMC Vet Res. 2013;9:35. doi: 10.1186/1746-6148-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernes SS, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011;30(2):159–65. doi: 10.1007/s10096-010-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore C, et al. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real-time NASBA. J Virol Methods. 2008;153(2):84–9. doi: 10.1016/j.jviromet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Druce J, et al. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J Clin Microbiol. 2012;50(3):1064–5. doi: 10.1128/JCM.06551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caselton D, et al. Does the length of specimen storage affect influenza testing results by real-time reverse transcription-polymerase chain reaction? an analysis of influenza surveillance specimens, 2008 to 2010. Euro Surveill. 2014;19(36) doi: 10.2807/1560-7917.es2014.19.36.20893. [DOI] [PubMed] [Google Scholar]
- 8.Talbot HK, et al. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis. 2011;203(4):500–8. doi: 10.1093/infdis/jiq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstrom S. Personal Communication. Centers for Disease Control and Prevention (CDC); Atlanta, GA, USA: [Google Scholar]


