Abstract
This commentary summarizes the presentations and discussions from the 2016 Gilbert W. Beebe symposium “30 years after the Chernobyl accident: Current and future studies on radiation health effects.” The symposium was hosted by the National Academies of Sciences, Engineering, and Medicine (the National Academies). The symposium focused on the health consequences of the Chernobyl accident, looking retrospectively at what has been learned and prospectively at potential future discoveries using emerging 21st Century research methodologies.
INTRODUCTION
The Chernobyl nuclear reactor accident in Ukraine, a Republic of the former Soviet Union at the time, began during the night of April 26, 1986. Two explosions, the result of a flawed reactor design coupled with a series of operator mistakes during a systems test, destroyed reactor unit 4 and led to large radioactive releases for approximately 10 days. Most of the radioactive releases from the Chernobyl accident were deposited over Ukraine, Belarus and Russia, and to some extent over other parts of Europe. The short-lived 131I (half-life 8 days) and the long-lived 137Cs (half-life 30 years), were particularly significant for the radiation doses received by the exposed populations at different time periods after the accident. Iodine-131 led to considerable thyroid exposure of local residents through inhalation and ingestion of contaminated foodstuffs, especially milk. Cesium-137 continues to give rise to external and internal radiation exposure.
This commentary summarizes the presentations and discussions from the 2016 Gilbert W. Beebe Symposium hosted by the National Academies of Sciences, Engineering, and Medicine (the National Academies) titled “30 years after the Chernobyl accident: Current and future studies on radiation health effects.” The symposium focused on the health consequences of the Chernobyl accident, looking retrospectively at what has been learned and prospectively at potential future discoveries using emerging 21st Century research methodologies.
The Gilbert W. Beebe Symposium was established by the National Academies in 2002 to honor the scientific achievements of the late Dr. Gilbert W. Beebe. The 2016 Gilbert W. Beebe Symposium topic was most appropriate for honoring Dr. Beebe, whose illustrious career in radiation research culminated with studies of the consequences of the Chernobyl accident. He was quick to recognize the potential for generating new understanding of radiation risks and launched multidisciplinary studies of workers and the general population in cooperation with investigators from Belarus and Ukraine. The collaborations he set in motion produced important work, including the identification of the epidemic of thyroid cancer in children (1, 2). Many of the studies initiated by Dr. Beebe continue today.
Symposium presenters reported both recent well-established research findings published in scientific journals as well as preliminary observations that have not yet undergone thorough formal scientific peer review. In the latter cases, the authors of this Commentary declare them as such and attribute them to a specific symposium presenter (the investigator’s name and affiliation is provided in parenthesis). Where appropriate, specific strengths and limitations of the studies are mentioned.
Here, we report the findings and preliminary observations from the studies as they were presented at the symposium or in the original literature cited at the symposium. As a consequence, in this Commentary there is variation of quantities used to describe radiation exposure and risk estimates. For example, radiation exposure is described as either absorbed organ dose expressed in gray (Gy) or effective dose expressed in sievert (Sv). Risk estimates may be presented as excess odds ratio per Gy, excess relative risk per Gy or some other form.
This article was prepared by the authors as a factual summary of what occurred at the symposium. The statements made are those of the authors or individual symposium participants and do not necessarily represent the views of all symposium participants or the National Academies.
PLENARY SESSION
Dr. Mikhail Balonov (Institute of Radiation Hygiene, St. Petersburg, Russia) provided an overview of the Chernobyl accident. He noted that reliable information about the accident and the resulting radioactive contamination was initially unavailable. The people residing in the affected areas, including the neighboring city of Pripyat, learned about the accident primarily through hearsay rather than from official government reports. This lack of communication likely contributed to the high levels of exposure of some nearby populations who did not have the information needed to take protective actions.
Dr. Vadim Chumak (National Academy of Medical Sciences of Ukraine, Kiev, Ukraine), described the exposures related to the time course of radiation releases and commented on how they determined the adjacent populations exposed from the Chernobyl accident. These populations, consisting of individuals from Belarus, Russia and Ukraine, can be broadly categorized into the following four groups:
Emergency operation workers (approximately 1,000) who responded on site within the first day of the immediate emergency.
Recovery operation workers, i.e., “liquidators” (approximately 530,000), who worked at the Chernobyl site or were involved in various cleanup activities from 1986 to 1990. Of these liquidators, 240,000 worked in 1986 and 1987, when doses were highest.
Residents of contaminated areas (approximately 115,000) who were evacuated.
Residents of contaminated areas who were not evacuated (approximately 6.4 million).
Table 1 summarizes the approximate size and average doses these groups received, as presented by Dr. Andre Bouville (National Cancer Institute, Rockville, MD), unless stated otherwise. Dr. Ilya Veyalkin, (Republican Research Centre for Radiation Medicine and Human Ecology, Gomel, Belarus), presented the size of these groups in the Belarusian State Registry (data not shown). The registry was established to monitor those exposed from Chernobyl for cancer and other disease occurrence. Tables 2 and 3 summarize some basic characteristics of major studies of recovery operation workers and of children exposed from the Chernobyl accident.
TABLE 1.
Size and Dose Estimates for the Main Population Groups Exposed from Chernobyl
| Population group | Size | Average whole-body dose (mGy) |
Average thyroid dose (mGy) |
|---|---|---|---|
| Emergency operation workers | 1,000 | Not available | Not available |
| Workers with acute radiation sickness (ARS) | 134 | 800–16,000 | |
| Recovery operation workers | 530,000 | 120 | 200a |
| Residents of contaminated areas who evacuated | 115,000 | 31 | 490 |
| Children (0–6 years old) | ~31 | 1,500 | |
| Adults | ~31 | 350 | |
| Residents of contaminated areas who were not evacuated | 6.4 million | 9 | 100 |
| Residents of contaminated areas of strict radiation control (3) | 216,000 | 61 | Not available |
From external and internal exposure.
TABLE 2.
Major Studies of Cancer in Cohorts of Chernobyl Recovery Operation Workers
| Country/Countries | ||||
|---|---|---|---|---|
|
|
||||
| Ukraine (4) | Russia, Belarus and Baltics (5, 6) |
Russia (7) | Baltic countries (Estonia, Latvia, Lithuania) (8, 9) |
|
| Cohort size | 110,645 | Total: 146,000 | 104,947 | Total: 17,040 |
| Russia: 66,000 | Estonia: 4,810 | |||
| Belarus: 65,000 | Latvia: 5,546 | |||
| Baltics: 15,000 | Lithuania: 6,684 | |||
| Average external dose (mGy) | 92 | Red bone marrow: 45 | ~100 | Estonia: 99 |
| Thyroid: 29 | Latvia: 118 | |||
| Lithuania: 109 | ||||
| Source of dose estimate | RADRUEa (10) | RADRUEa (10) | Chernobyl State Registryb | Chernobyl State Registryb |
Notes. Cohorts generally defined by region of residence, age during recovery operation and years on site. Numbers in specific analyses presented in the text may be based on subsets (e.g., those on site in 1986).
RADRUE = method using interview-based realistic analytical dose reconstruction with uncertainty estimation.
Official dose records of the Chernobyl State Registry.
TABLE 3.
Studies of Thyroid Cancer in Chernobyl-Exposed Children
| Country: cohort | |||
|---|---|---|---|
|
|
|||
| Ukraine: UkrAm (11, 12) | Belarus: BelAm (13, 14) | Ukraine: In utero (15) | |
| No. in screening cohort | 13,243 | 11,918 | 2,582 |
| No. of screening cycles | Five cycles | Three cycles | Two cycles |
| Dates of screening cycles | 1998–2000 | 1997–2000 | 2003–2006 |
| 2000–2003 | 2002–2004 | 2012–2015 | |
| 2003–2005 | 2004–2006 | ||
| 2005–2007 | |||
| 2012–2015 | |||
| Mean thyroid 131I dose (mGy) | 650 (16) | 680 (17) | 72 (18) (131 in third trimester) |
| Basis for dose estimate | Thyroid activity measurements; environmental transfer model | Thyroid activity measurements; environmental transfer model | 131I activity in mother’s thyroid; biokinetic ecological model (19) |
Note. Subjects were residents of contaminated regions, 0–18 years old at the time of the accident.
IMMEDIATE AND LATE HEALTH EFFECTS OF EMERGENCY OPERATION WORKERS
The Chernobyl accident resulted in almost one-third of the reported cases of acute radiation sickness (ARS) that have been studied worldwide to date (20). The diagnosis of ARS was initially considered for 237 emergency operation workers based on symptoms of nausea, vomiting and diarrhea. It was ultimately confirmed with detailed clinical analyses in 134 individuals. These workers received doses ranging from 0.8 to 16 Gy (see Table 1). Twenty-eight of these emergency operation workers died within the first few months after the accident, primarily due to bone marrow failure. Acute radiation sickness did not occur in anyone in the general population exposed to radiation by the Chernobyl accident.
Thirteen workers diagnosed with ARS received allogenic bone marrow transplantation to reverse the bone marrow failure and six received human fetal liver cell transplantation. (Fetal liver cells serve as a source of hematopoietic stem cells.) However, these interventions were not successful for those with ARS from Chernobyl. All but one worker who received allogenic bone marrow transplantation or human fetal liver cell transplantation died. Deaths were due to acute radiation injury effects on the skin, liver and intestines, viral/bacterial infections or, to a lesser extent, transplant complications. The worker who received an allogenic bone marrow transplant but survived had recovered his own marrow and rejected the transplant. Survivors of ARS received long-term treatment including therapy for beta burn fibrosis and skin atrophy as well as for cataracts (20).
Long-term health effects of emergency response workers who were diagnosed with ARS from Chernobyl and survived have not been studied comprehensively. This is because a systematic follow-up of this exposed population as a whole has not been established, presumably due to the difficulties of tracking the cohort, now spread across several countries. A subpopulation of 91 confirmed ARS cases from Ukraine has been followed, however, findings from this subpopulation are expressed solely as death counts without an analysis of expected deaths using a comparison group. As of 2016, there have been 52 deaths among the confirmed ARS cases from Ukraine: 18 from cancer and leukemia, 20 from cardiovascular causes and 17 from other causes (Dr. Dimitry Bazyka, National Academy of Medical Sciences of Ukraine). Lacking a comparison group, these deaths cannot necessarily be attributed to radiation exposure from the Chernobyl accident.
Key Points
The Chernobyl accident resulted in 134 confirmed cases of ARS among emergency response workers. Allogenic bone marrow transplantation and human fetal liver cell transplantation were not successful interventions for those with ARS from Chernobyl. There has been no systematic follow-up of the emergency response workers who were diagnosed with ARS from Chernobyl, presumably due to the difficulties of tracking this cohort, now spread across several countries. This represents a missed opportunity to assess long-term health effects in this population.
LATE HEALTH EFFECTS OF RECOVERY OPERATION WORKERS
Long-term health effects among the much larger population of recovery operation workers, who worked at the Chernobyl site or were involved in various cleanup activities from 1986 to 1990, have been studied in some detail. The doses for these studies were estimated partly from self-reported exposure histories. End points studied include leukemia [including chronic lymphocytic leukemia (CLL)], breast and thyroid cancers.
Zablotska and colleagues performed a nested case-control study using data from the Ukrainian cohort of recovery operation workers (see Table 2 for some details). The investigators found evidence of a significant linear dose-response relationship for all types of leukemia (160 cases) after external radiation doses of approximately 100 mGy on average (4). A surprising finding was nonsignificant dose-response associations of similar magnitude for both non-CLL and CLL (79 cases). CLL has not been clearly linked to ionizing radiation exposure previously. However, most earlier studies had few CLL cases; for example, in the Life Span Study of the Japanese atomic bomb survivors there were only 11 cases (21).
Nonsignificant increases of both types of leukemia were also reported in cohorts of recovery operation workers in Russia, Belarus and the Baltic countries along with a significant dose-dependent increase in non-Hodgkin lymphoma (NHL) (5), a neoplasm morphologically similar to CLL. A significant time-restricted increase in leukemia (excluding CLL) for the period 1986–1997 was seen in the Russian recovery operation worker cohort (22).
Kesminiene and colleagues performed a collaborative case-control study of 107 thyroid cancer cases nested in the cohorts of recovery operation workers in Russia, Belarus and the Baltic countries (see Table 2). They found a statistically significant dose-response relationship with total thyroid dose (6). The radiosensitivity of the thyroid gland during childhood had been well established prior to the Chernobyl accident, however, there was little evidence for risks after adulthood exposure (23). For example, the risk estimates from the Japanese A-bomb survivors exposed in adulthood were ten times lower than those reported by Kesminiene and colleagues (6). A case-control study of thyroid cancer in recovery operation workers in Ukraine is still in progress.
Ecological studies comparing the cancer incidence rates in the recovery operation workers with the general population have several limitations, but have nevertheless raised interesting hypotheses about possible increases in multiple myeloma and female breast cancer (D. Bazyka).
Additional studies are underway to examine cardiovascular risks after radiation exposure. There is some evidence of an increased risk of heart disease among younger recovery operation workers (less than 40 years old) who received doses greater than 150 mGy. There is also a report of an increase in cerebrovascular disease in the cohort of Russian recovery operation workers (24). Whether cardiovascular disease (CVD) can be caused by low doses of ionizing radiation is an important public health question (see Other Health Effects section, for further discussion on cardiovascular risks after radiation exposure.)
Key Points
Recovery operation workers likely experienced an increased risk of leukemia, already known to be a radiogenic cancer. Other possible health effects, which were not expected, include a possible increased risk for CLL, NHL, multiple myeloma, thyroid cancer after adulthood exposure and CVD at low radiation doses.
LATE CANCER-RELATED HEALTH EFFECTS IN THE GENERAL POPULATION
Discussions on late cancer-related health effects in the general population focused on thyroid cancer, leukemia and breast cancer.
Thyroid Cancer
Most of the evidence regarding the radiosensitivity of the thyroid gland during childhood has been derived from studies of external exposure, such as the Japanese atomic bomb survivor studies and tinea capitis cohorts (25). At the time of the Chernobyl accident cancer risks from protracted internal 131I exposure were uncertain, but it was thought that the risks would be lower than from acute external exposure. As described earlier, the most significant exposures to the general population from the Chernobyl accident were from internal 131I exposures. The thyroid was the most heavily exposed organ, with young children who evacuated receiving on average 1.5 Gy (see Table 1) from ingestion of milk and other contaminated foods.
Dr. Ausrele Kesminiene, [International Agency for Research on Cancer (IARC), Lyon, France], noted that early reports of a rise in thyroid cancer incidence after the Chernobyl accident in the highly affected areas (1) were initially received with skepticism. These studies were mostly ecological and thought to be influenced by increased surveillance and screening. Collaborations among investigators in affected countries, the NCI, IARC and other international institutes resulted in two large cohort studies of exposed children in Ukraine and Belarus, known as UkrAm and BelAm, respectively (see Table 3). The UkrAm and BelAm cohorts of approximately 25,000 exposed children have several strengths, including use of dose estimates based on direct thyroid activity measurements. Using interview data, an ecological model is then applied to account for environmental transfer. All the children in these studies were systematically screened to eliminate the impact of surveillance bias on estimates of relative risk.
The UkrAm and BelAm cohorts of exposed children provided definitive evidence that absorption of 131I increases thyroid cancer risk and that the linear dose response up to 5 Gy is similar in magnitude to the risk from external exposure, even after accounting for dose uncertainties (11–14, 23). The excess risk was evident by five years after exposure and remains elevated for more than two decades of follow-up. However, in contrast to the studies of external childhood exposure (23), a reduced risk with increasing age at exposure was not clearly apparent.
Ecological studies of thyroid cancer in the general population exposed in adulthood suggested higher incidence rates in high- compared to low-exposure regions of Ukraine (26) and Russia (7), but not in Finland (27), although doses in the Finnish study (27) were generally very low (<0.5 mSv).
Leukemia and Breast Cancer
Leukemia and breast cancer are also highly radiogenic cancers, especially if exposure occurs in childhood. A risk projection analysis for the period 1986–2065 showed that in a population of approximately 11 million residing in the most contaminated regions in Belarus, Russia and Ukraine, there could be 500 additional leukemia cases and 1,000 additional breast cancer cases from radiation exposure (28). The average whole-body dose in the period of 1986–2005 in this population was 6.1 mSv. These projections have not been verified with epidemiological studies. For reference, the projections for leukemia and breast cancer cases from other causes for the same period were 75,000 and 200,000, respectively.
Although there is some indication of an excess of leukemia among the recovery operation workers after the Chernobyl accident (see previous section), to date this has not been convincingly reported in the general population. Ecological studies have again been inconsistent or null [e.g. see refs. (29–31)], and a case-control study which showed a significant association of leukemia risk with increasing radiation dose in Ukraine (but not in Belarus or Russia) could be due to sampling-derived bias (32). In the UkrAm and BelAm cohorts of children there have only been 11 cases of leukemia, suggesting a small but nonsignificant increase in risk (33, 34).
Results from ecological studies of breast cancer in the general population have been inconsistent with some suggesting increased risks [e.g. see refs. (35, 36)] and others were null [e.g. see ref. (37)]. In the UkrAm and BelAm cohorts of children there are only 10 reported breast cancer cases to date. Dr. Lydia Zablotska (University of California, San Francisco) noted that the latency period for solid cancers, including breast cancer, is long. For example, it was not until approximately 20 years after the atomic bombings in Japan that an increase of solid cancers, other than thyroid cancer, became evident (38). Therefore, further follow-up of potential Chernobyl radiation-related breast cancers appears to be warranted. Also, the women in these studies are currently younger than 50 years old, below the age of onset for most breast cancers.
Outside the highly affected countries there have been a number of reports and ecological studies suggesting possible increased cancer risks in Europe due to fallout from Chernobyl. Since the doses were low (<2 mSv), and not possible to estimate on an individual basis, it seems unlikely that there have been detectable excesses. A risk projection study estimated that there could be 1,000 additional leukemia cases and 2,000 additional breast cancer cases related to Chernobyl fallout in the rest of Europe by the year 2065, which is a very small fraction compared to the background rates in this population of approximately 500 million people (28).
Key Points
Studies of the general population who were exposed to 131I in the months after the Chernobyl nuclear accident have demonstrated significant excess of thyroid cancers after exposure in childhood, which was substantially greater than originally expected (but compatible with external exposure risks). To date there are no measurable increases of breast cancer or leukemia in the general population exposed to radiation from Chernobyl. Longer-term passive follow-up via cancer registries of the cohorts of children with well-characterized doses could be useful to confirm that these findings for thyroid cancer, breast cancer and leukemia hold later into adulthood.
OTHER HEALTH EFFECTS
Symposium participants discussed three health effects, other than cancer, from exposures to the Chernobyl nuclear accident: cataracts, cardiovascular health effects and health effects from in utero exposure. These effects are described in the following sections.
Cataracts
Depending on the type and delivery of ionizing radiation, exposures can lead to the loss of clarity in the lens of the eye, later resulting in clouding or opacifications known as cataract, which can cause significant visual impairment. Dr. Norman Kleiman (Columbia University, New York, NY), noted that in the past, eye exposure guidelines were based on the notion that radiation-induced cataract is a “deterministic” effect with a relatively highthreshold radiation dose (of perhaps 2 Gy for acute exposures and 5–8 Gy for chronic exposures). These guidelines were based primarily on information from radiation therapy patients. However, there is more recent epidemiologic evidence to suggest that, although significant uncertainty still exists, the data are consistent with a lower (perhaps even as low as 0.5 Gy for acute and protracted exposures) or even a nonthreshold model for radiation-induced cataract (39). Most recent guidelines from international (39, 40) and national (41) radiation protection organizations recommend lower exposure limits for the lens of eye. The implications and applications of such limits are now being explored (42, 43).
Chernobyl studies have contributed to the recent change in guidelines for lens protection. Key studies include the resident school children exposed to radioactive contamination of 137Cs deposits in the Ukraine (44), initial dosimetry (including to the eye lenses) for the recovery operation workers (45), a small cohort study that included people with ARS (46) and a comprehensive study of 8,607 recovery operation workers exposed between 1986 and 1987 and examined 12–14 years postirradiation (47, 48). For the recovery operation workers, significantly increased risk was noted for several cataract types (i.e., posterior subcapsular, cortical and mixed cataracts) with suggested thresholds of less than 0.5 Gy. Experimental animal studies, as well as dilated slit lamp eye examinations of mice and voles living in the Chernobyl exclusion zone, appear to support such findings [N. Kleiman; (49)].
Cardiovascular Health Effects
Dr. Kiyo Mabuchi (NCI) noted that CVD effects of low-dose radiation continue to be evaluated in Chernobyl studies and other populations, including the Japanese atomic bomb survivors (50, 51). With respect to radiation protection standards, the high-baseline rates of CVD in many populations increases the concern regarding absolute risks of CVD.
The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (52) reviewed epidemiological data on CVD effects after the Chernobyl accident. Most notable was a significant dose-response relationship found for various CVD end points in the Russian recovery operation workers (53, 54). Since the 2008 UNSCEAR report, analyses of cerebrovascular data in the Russian recovery operation workers cohort (24), have been updated and continue to suggest a similar excess relative risk per Gy. While this population can offer a unique opportunity for continued study, there are several difficulties in studies of CVD outcomes, including the following:
The large number of potential confounding variables (e.g., smoking, dietary factors and concurrent diseases and conditions) for CVD.
Misclassification of CVD diagnosis.
Lack of understanding of the biological mechanisms related to CVD risk at low radiation doses.
The International Commission on Radiological Protection (ICRP) (39) evaluated the epidemiological data suggesting the persistence of CVD effects after radiation exposure in various settings, including Chernobyl study populations. The ICRP concluded that “a judgment can be made of a threshold acute dose of approximately 0.5 Gy for both cardiovascular disease and cerebrovascular disease” (39). In the absence of specificity in the available evidence, the threshold dose is assumed to be the same for acute, fractionated and chronic exposures.
Health Effects of In Utero Radiation Exposure
Several reports on the effects of in utero exposure of the embryo and fetus have been developed by the ICRP (55), UNSCEAR (52) and most recently by the National Council on Radiation Protection and Measurements (NCRP) (56). However, these reports address exposure to external radiation from prenatal medical procedures, and not with the radioiodines that are present in nuclear accident fallout. There have been few studies on 131I in utero exposure (57, 58). Dr. Maureen Hatch (NCI) noted that since 2002, the NCI and the Institute of Endocrinology and Metabolism in Kiev, Ukraine have been following a cohort of 2,582 mother–child pairs exposed to radiation from Chernobyl during pregnancy [(15); see Table 3].
Iodine-131 readily crosses the placenta and concentrates in the thyroid gland. By approximately 10–12 weeks, when the thyroid becomes active in the developing fetus, there is rapid uptake of 131I. By late gestation, 131I concentration in the fetal thyroid is many-fold higher than in the maternal thyroid. In the initial research in Ukraine after in utero exposure from the Chernobyl accident (i.e., thyroid screening examinations 2003–2006), nonsignificant increases were found based on seven thyroid cancers and one Hurthle cell neoplasm detected during a screening examination in adolescence (15). Preliminary data from the second cycle of screening (i.e., thyroid screening examinations 2012–2015) continue to show similar increases in thyroid cancer, which remain statistically non-significant. Preliminary data also show an increased and significant risk of thyroid nodules (excess odds ratio per Gy 1.26; P = 0.036) and especially large nodules greater than or equal to 10 mm (excess odds ratio per Gy 4.68; P < 0.001). These findings on thyroid nodules are consistent with findings from the Japanese atomic bomb survivors in utero cohort (M. Hatch, NCI).
Because thyroid function may govern growth, the most recent research on the in utero exposed Chernobyl cohort also examined radiation dose in relationship to anthropometry. Preliminary results suggest a dose-related reduction in head circumference with the greatest reductions for those exposed early in gestation and a decrement at 1 Gy of approximately 1 cm (<1 mm at mean of 62 mGy) (M. Hatch, NCI). The biological mechanism for the observed association between radiation dose and head circumference after in utero exposure to 131I exposure is not clear.
Key Points
Cataracts, cardiovascular health effects and health effects from in utero exposure are three health effects other than cancer that have been studied after radiation exposures from Chernobyl. Radiation-induced cataract studies, including continuing follow-up of the recovery operation workers, may facilitate the further refinement of the appropriate risk guidelines for accidental and/or occupational exposures to radiation. For CVD risk, additional studies could help resolve the shape of the dose-response relationship, identify specific target cells or tissues and end points, characterize the modifying effects of age and other factors and elucidate the underlying biological mechanisms. Studies of in utero exposure of the embryo and fetus after internal radiation exposure can provide useful guidance on environmental dose restrictions for pregnant civilians, radiation workers and/or patients.
PSYCHOSOCIAL EFFECTS AND COMMUNITY RESILIENCE
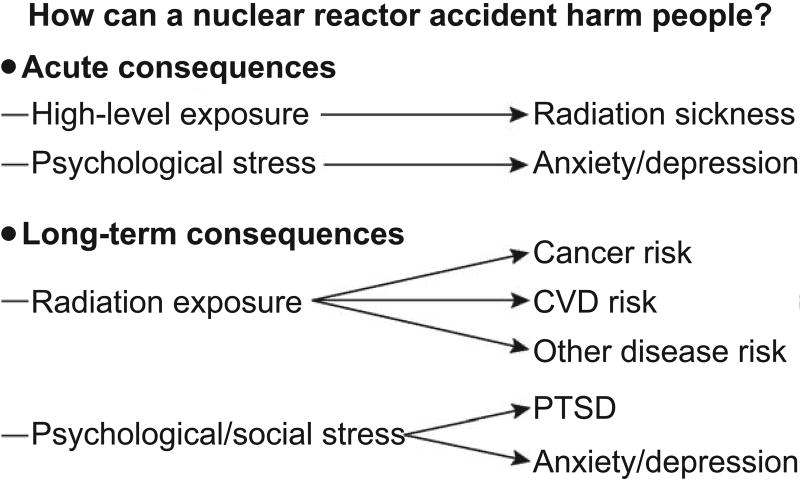
Radiation exposure has been termed “the most dreaded” of all risks (59). Emergencies that involve exposure to radiation can amplify the effect of any incident on mental health due to uncertainty about the exposure and its risks, the extent and nature of health effects and the time frame for development of adverse effects (see Fig. 1). These adverse effects may follow immediately after exposure (if doses received are high, e.g., ARS) or emerge after several years (if doses are lower, e.g., cancer). Psychological stress on the other hand can manifest both immediately after exposure (or perceived exposure) and remain for many years. The most salient mental health consequences after radiation exposure are post-traumatic stress disorder (PTSD), depression, panic disorder, substance use problems and generalized anxiety disorder (60, 61).
FIG. 1.
Time frame of adverse effects of a nuclear reactor accident. PTSD = post-traumatic stress disorder; CVD = cardiovascular disease.
Dr. Evelyn Bromet (Stony Brook University School of Medicine, Stony Brook, NY) noted that the first attempt to quantify mental health issues in a population exposed to radiation was made after the Three Mile Island (TMI) accident in the United States in 1979 (62, 63). The mental health of previously exposed populations including the atomic bomb survivors has not been systematically studied. There were only three reports published indicating increased mental retardation in the in utero cohort (64), no schizophrenia in the in utero cohort (65) and an increase in generalized anxiety disorder in adults closest versus furthest from the blast of the atomic bombings (66).
The nuclear plant accidents that followed TMI, that is Chernobyl in 1986 and Fukushima in 2011, highlighted widespread mental stress in the general population and the workers exposed to radiation. For Chernobyl, in addition to PTSD, depression and increased risk of poor mental health in the population evacuated, an increased number of suicides was reported among the recovery operation workers of the Estonian cohort (67) (see Table 2). The Chernobyl Forum reported PTSD and poor subjective health in mothers of young children and emergency recovery workers, but higher resilience in young children (68). However, no intervention programs were established for those affected nor was mental health screening incorporated into the various studies of health effects after Chernobyl. Mental health problems and impaired social well-being were also major issues after the Fukushima accident (69, 70).
Dr. Bromet made several suggestions related to research on accidents that involve radiation, including the following:
Involve study participants in all phases of research (that is in planning, content, execution, interpretation and presentation of results).
Assess physical and mental health effects equally.
Identify an appropriate comparison group.
Plan feedback methods for participants.
Dr. Dean Kilpatrick (Medical University of South Carolina, Charleston, SC) and Dr. Benjamin Springgate (Louisiana State University, Baton Rouge, LA) discussed parallels in mental health effects of disasters that involve radiation with those that do not. They defined “disasters” as large-scale incidents that effect many people at the same time, involve threats of potential physical harm or mortality, widespread destruction of property and infrastructure with concomitant disruption of access to basic resources (e.g., food, water, shelter, utilities, social services, medical aid). They noted that community resilience is key to adapting to or recovering from both natural and man-made disasters and that it requires individual, organizational and community engagement. Elements of community resilience include information and communication, economic development, social support and community wellness, including access to health and social services (71). Plans to build community resilience after a radiological incident are in the preliminary stages.
Key Points
Psychosocial effects after a nuclear reactor accident are a great burden on the population at large. These effects need to be better investigated by incorporating mental health screening into the various studies of health effects after an accident.
ADVANCES IN RADIATION RESEARCH
The Chernobyl accident did much more than improve our understanding of radiation health effects. It contributed greatly to advances in radiation research tools and to the creation of an infrastructure for further epidemiological and radiobiological studies. The advances in radiation research discussed at the symposium were on biodosimetry, medical countermeasures, tissue banking and genetic markers. These presentations focused on efforts from individual institutions and did not provide an overview of the current state of advances in radiation research.
Biodosimetry
Biodosimetry is the measurement of physiological or biological response to ionizing radiation to determine the exposure dose to an individual. Dr. Lynne K. Wathen [Biomedical Advanced Research and Development Authority (BARDA), Washington, DC] noted that in all potential radiation incidents, the population is likely to encounter a number of complex radiation exposure scenarios, including different dose ranges and dose rates. Therefore, triage and definitive radiation biodosimetry will require multiple tests to measure absorbed dose.
There are five quantitative biodosimetry assays currently under development by the U.S. Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response (ASPR)-BARDA Biodosimetry Program. These assays fall under the following two types of testing:
Point-of-care testing to determine whether an individual has absorbed a minimum threshold radiation dose and needs further medical care.
High-throughput laboratory-based testing to estimate the actual absorbed dose that a person has received.
Point-of-care testing typically involves minimal complexity of operation and results are available quickly, within 15–30 min. High-throughput laboratory-based testing typically requires trained personnel to run the tests and results are available within 24 h. Both types of testing are intended to evaluate up to one-million individuals within seven days after an event, and their results are intended to be used in conjunction with signs, symptoms and hematology tests, to give health care providers additional information to treat those exposed.
Dr. Wathen provided some details about the five assays currently under development. Two of these assays are intended for point-of-care testing and three are intended for high-throughput laboratory-based testing.
Point-of-care assays currently under development are a multiplex lateral-flow immunoassay test and a multi-array cartridge-based electrochemiluminescent test. Both assays use capillary (fingerstick) blood samples to detect protein biomarkers that increase after gamma or X-ray exposure. Two of the three high-throughput assays under development use changes in gene expression patterns to determine the extent of radiation damage from whole blood drawn into specialized collection and stabilization blood tubes. The third high-throughput assay advancing toward a product measures chromosomal damage and micronucleus generation to predict absorbed dose (L. Wathen, BARDA).
Medical Countermeasures
Medical countermeasures are intended to treat the exposed population after a large-scale radiological incident. To date, two radiation medical countermeasures have been approved by the Food and Drug Administration (FDA) to treat ARS leading to bone marrow failure after total-body irradiation (72, 73). Dr. David Cassatt [National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, MD] noted two pieces of data that were instrumental in obtaining FDA approval for these medical countermeasures:
Data on the natural history of radiation injury after the atomic bombings in Japan and the Chernobyl accident, in which the LD50 (lethal dose of radiation resulting in 50% mortality in humans) was established at 4.1 Gy and 8.9 Gy, respectively (74).
Data on the human neutrophil response to radiation exposure derived from the Chernobyl emergency and recovery operation workers (75).
Regarding the first piece of data, the disparity between the two doses is due to the medical management received by individuals exposed at Chernobyl compared to lack of medical management of ARS victims after the atomic bombings. For the second piece of data, investigators were able to reproduce comparable mortality curves as well as demonstrate similar patterns of radiation-induced neutropenia in primates (76). This led to the establishment of primates as valid animal models for radiation medical countermeasure testing. In these models, both FDA-approved medical countermeasures improved 60-day post-irradiation survival and improved neutrophil recovery in the primates irradiated with an LD50 (76, 77).
Dr. Cassatt noted that future research will focus on addressing the use of the two FDA-approved medical countermeasures in special populations such as pediatric and geriatric populations, pregnant women and people with preexisting medical conditions. Additional future research focus is the development of medical countermeasures against other ARS system failures (e.g., gastrointestinal, lung, cardiovascular, renal and neuronal).
Tissue Banking
The Chernobyl Tissue Bank was established in 1998 and contains tissue samples from patients with thyroid carcinoma or cellular follicular adenoma who were children or young adults residing in highly contaminated areas in Ukraine and Russia at the time of the Chernobyl accident. Dr. Geraldine Thomas (Imperial College London, London, UK) noted that the Chernobyl Tissue Bank is supported by the governments of Ukraine and Russia, with financial support from the NCI, the European Commission, the Sasakawa Foundation of Japan and the World Health Organization (WHO). Imperial College London is the coordinating center for the bank.
Dr. Thomas noted that the Chernobyl Tissue Bank is the first tissue bank of its type; it provides multi-format and pathologically assured, biological samples to international research groups and an infrastructure to track and collate research results from each individual sample. The aim of the Chernobyl Tissue Bank is to establish a data repository for studies taking an “integrated biology” approach to understanding the mechanisms that underpin development of thyroid cancer. The Chernobyl Tissue Bank currently archives information and samples from more than 4,500 thyroid cancer cases. It has supported over 30 research projects by administering tissue samples and linked data.
Genetic Markers
Dr. Yuri Nikiforov (University of Pittsburgh Medical Center, Pittsburgh, PA) discussed the evolution of understanding the genetics of post-Chernobyl radiation-induced thyroid cancers. He noted that early studies were driven by specific hypotheses and followed single gene approaches to identify somatic alterations of genes encoding proteins involved in the mitogen-activated protein kinase (MAPK) signaling pathway, including point mutations of the BRAF and RAS genes (78–80) and gene fusions involving RET (81–83) and NTRK1 tyrosine kinases (84). More recent studies are hypothesis-neutral and have used whole genome sequencing approaches. These studies have demonstrated a relationship between gene fusions and 131I exposure in post-Chernobyl thyroid cancer and have provided further evidence for the link between chromosomal rearrangements and radiation exposure.
A prototypic example of such chromosomal rearrangements is the RET gene fusion known as RET/PTC (85), seen in 30–80% of post-Chernobyl cancers. More recently, another rearrangement, ETV6/NTRK3, has been found to be common and detectable in approximately 15% of radiation-related thyroid cancers. Dr. Nikiforov said that these rearrangements appear to be associated with 131I dose received during the accident and can be induced in cultured human thyroid cells exposed to various types of ionizing radiation.
Genes participating in RET/PTC rearrangements are located close to each other in the nuclei of normal thyroid cells, providing a structural basis for misjoining free DNA ends located in proximity to each other. Dr. Nikiforov noted that it remains unknown if such oncogenic chromosomal rearrangements are formed as a direct result of DNA double-strand breaks induced by exposure to radiation or indirectly and via what mechanism of DNA repair.
Dr. Stephen Chanock (NCI) described two NCI-Ukraine collaborations that pioneer whole-genome-sequencing in radiation-exposed populations. The first is a study that aims to characterize the genomes of radiation-related thyroid cancers. The goal is to compare tissue samples from 450 radiation-related thyroid cancers that were collected as part of the UkrAm cohort (see Table 3) to 50 sporadic thyroid cancers as well as to normal adjacent tissue or peripheral blood. All tissues used in this study are archived at the Chernobyl Tissue Bank. The study is modeled on The Cancer Genome Atlas (TCGA) and will include copy number variation, methylation, microRNA, large RNA and whole genome/exome sequencing.
Preliminary analysis of the pilot study of 50 tumor tissues showed a relatively “quiet” mutational signature of the radiation-induced thyroid cancer genomes; there were 20 to 40 times fewer mutations compared to melanoma or lung adenocarcinoma, cancers that are linked to ultraviolet-(UV) light exposure and cigarette smoke, respectively. The mutations that were observed to occur in radiation-related thyroid cancers were discrete events as opposed to a clustering within a certain region of the DNA, a phenomenon known as “kategis,” which is common in brain and bone tumors. Point mutations on the BRAF gene were observed among older individuals exposed to lower radiation doses (<100 mGy) (S. Chanock, NCI).
Dr. Chanock noted that this quiet mutational signature makes it more difficult to understand, in terms of the genome, what drives these radiation-related thyroid cancers. Interestingly, the mutational signature observed [denoted as signature 1 in Sanger Institute’s Catalogue of Somatic Mutations in Cancer (COSMIC); see: http://cancer.sanger.ac.uk/cosmic/signatures] correlates positively with age at time of cancer diagnosis. When the analysis is complete using all 450 available radiation-induced thyroid cancer tissues, Dr. Chanock and colleagues hope to draw more robust conclusions on the mutational signature of radiation-induced thyroid cancers, if any exist, and investigate the relationship with radiation dose.
Dr. Chanock also discussed the application of whole genome sequencing to investigate heritable effects (referred to as “genetic effects” in the 1950s) of radiation exposure. To date, information on the genetic effects of radiation comes almost entirely from animal studies. The Trios Study, another NCI-Ukraine collaborative study, searches for mutational patterns in the exposed parents (father and/or mother) that may have been transmitted to the offspring. It aims to recruit 450 trios of mother, father and child born more than one year after the Chernobyl accident, from the cohorts of Ukrainian recovery operation workers and evacuees. A pilot study of 50 trios is close to completion and preliminary results are expected by 2018 (S. Chanock). The analysis plan includes whole genome sequencing, SNPmicroarray, methyl-microarray and RNA sequencing to assess rates of minisatellite mutations, de novo mutations, recombination rates and methylation patterns (S. Chanock).
Malformations after exposure from Chernobyl such as rates of neural tube defects, conjoined twins and teratomas were not discussed at the symposium.
Key Points
The application of cutting-edge genetics in the Chernobyl populations promises to provide novel insights into radiation carcinogenesis in the next decade. The Chernobyl Tissue Bank has facilitated these unique studies and serves as a model for other scientists interested in establishing similar resources.
RESEARCH ON THE AFTERMATH OF CHERNOBYL
Dr. Jonathan Samet (University of Southern California, Los Angeles, CA), gave a presentation titled “Research on the aftermath of Chernobyl: Have lessons been learned?” Here, he used the holistic WHO definition of “health,” which embraces not only disease but also well-being. Based on this definition, “radiation health effects” extend beyond the physical health effects of radiation exposure, such as cancer, to include psychosocial effects after the acute and chronic stress of potential exposure to radiation and loss of normalcy due to displacement, loss of employment, diminished economic opportunities and stigmatization. Figure 1 summarizes how a nuclear reactor accident can have acute and long-term consequences and captures what has followed the Chernobyl accident over the last 30 years.
Of the four major nuclear reactor accidents to date [Windscale (UK, 1957); Three Mile Island (U.S., 1979); Chernobyl (Ukraine, 1986); and Fukushima (Japan, 2011], Chernobyl is by far the most studied. The examples of surveillance, screening and systematic evaluation of cancer incidence in the general population in the areas around Chernobyl have provided background information for the Japanese government and researchers in their response to the 2011 Fukushima Daiichi nuclear power plant accident. However, one critical issue is the extent to which the findings from Chernobyl can be extended to Fukushima or other accidents and radiological incidents that may occur in the future. There were unique aspects of the societal and historical context of the Chernobyl accident that may limit extending the findings on psychosocial consequences to Japan post-Fukushima or elsewhere. Nonetheless, there is a large volume of literature on studies of those exposed to Chernobyl and even more literature on disasters in general. These studies provide valuable background information and foundation for predicting, monitoring and, to some extent, managing the psychosocial consequences of future accidents and incidents.
Dr. Samet addressed the rationale for doing research after a nuclear reactor accident. He recognized that even by 1986, when the Chernobyl accident occurred, much was known about cancer risks associated with radiation exposure, due in particular to the studies of the Japanese atomic bomb survivors. Reasons were given to justify further research:
Advance understanding of what is already known, e.g., refining cancer risk estimates;
Address identified uncertainties, e.g., completing hazard identification for additional outcomes; and
Monitor for unanticipated consequences, which may be unexpected or inconsistent with strongly held evidence-based priors.
Dr. Samet made a distinction between “research” for the purpose of generating new knowledge versus “surveillance” for the purpose of tracking populations to identify events that may need follow-up and intervention. Both research and surveillance potentially have roles after a radiological incident, as documented by the Chernobyl accident; the epidemic of childhood thyroid cancer was identified through clinical encounters with the background of known 131I exposures while more formal research studies were undertaken with dose reconstruction so that quantitative risks could be estimated. Over time, the research results have served some of the original purposes, including refining estimates of the risks to health posed by radiation exposure and building biospecimen banks that have become increasingly useful to methods as they emerge. By contrast, surveillance has been limited and fragmented, in part because of major geopolitical changes in the nations affected by the disaster in the ensuing three decades. Specifically, the breakup of the Soviet Union in 1991 brought many fundamental changes, including economic collapse and reformation of the health system in some of the countries that emerged.
Research results presented at the symposium captured the most critical findings from the epidemiological studies initiated after the accident: the unexpectedly high rate of thyroid cancer in children; the suggested increased risk of CLL in recovery operation workers; increased risk of the non-stochastic outcome, cataract, at lower than expected doses, based on prior studies; and widespread and lasting psychosocial effects. Although psychosocial effects have previously been cited as one of the most significant adverse consequences of the Chernobyl nuclear accident, the relevant research has been less systematic and poorly funded in comparison to the various cohort studies directed at physical health effects of radiation exposure. The work of Dr. Bromet (Stony Brook University School of Medicine) and colleagues, in particular, underscores the psychosocial effects and the need for immediate and sustained tracking and interventions to mitigate them. Dr. Bromet offered specific recommendations on methodologies for addressing psychological consequences (see previous section).
The presentation by Dr. Samet ended with a list of lessons learned (Table 4). In addition to this, he cited the work of Dr. Andre Bouville and colleagues in proposing guidelines for exposure assessment after a nuclear accident (86). The proposed guidelines were, to the extent possible:
Compile a list of those exposed to the accident;
Collect individual-based radiation measurements;
Collect information that can be used for radiation dose estimation (e.g., location at the time of exposure and relevant dietary information);
Collect information on the spatial and temporal variations of the radiation field (e.g., exposure rates of airborne radionuclides at all locations); and
Validate the dose estimates and estimate associated uncertainties.
TABLE 4.
Lessons Learneda from 30 Years of Research on Chernobyl
| Analytical methods |
|---|
| Propagation of uncertainty |
| Addressing measurement error |
| Validation studies |
| Models for measurement error correction |
| New approaches for dose-response modeling |
| Cancer risks |
| Strong evidence on risks for some cancers (strong priors established; for example, chronic lymphocytic leukemia) |
| Risk estimates can be refined |
| Surprises happen |
| Some new questions will inevitably arise. What populations are appropriate to answer these new questions? |
| When should studies be extended to “get the full picture”? |
| Other health risks |
| Acute radiation sickness: Can data capture and follow-up be done more effectively? |
| Cardiovascular disease: A very important contributor to disease burden but many questions remain |
| In utero exposures: complex territory and of great potential importance; what next? |
| Cataract: dose-response and risks at lower end of dose range |
| Psychological consequences |
| The “orphan” of adverse effects |
| Community-based methods can be used to assess this effect |
| Approach with multi-level models that include individuals and communities |
| Tie research needs to intervention and evaluation |
These “lessons learned” were presented at the symposium by Dr. Jonathan Samet, University of Southern California.
The availability of these guidelines offers a needed starting point for a rapid response in the event of another accident involving a nuclear power plant.
Looking to the future, Dr. Samet’s presentation highlighted the opportunities of 21st Century science presented at the symposium: biomarkers of dose and application of genomics and other “omics” to biobank specimens. This type of 21st Century science has already been applied to provide insights in radiation-induced thyroid carcinogenesis. Such research is best performed by multidisciplinary teams that include epidemiologists, laboratory scientists and exposure assessors, along with analysts who can integrate the resulting data. At closing, Dr. Samet raised the question of whether organized, formal prospective planning is needed to provide guidelines, templates and decision points in the event of future disasters. Such prospective planning might have been of benefit in responding to the Fukushima power plant accident.
Acknowledgments
The symposium was sponsored by the Environmental Protection Agency, the National Cancer Institute and the National Institute of Allergy and Infectious Diseases. It was organized by a planning committee of five experts who are also authors of this article (J. Samet, A. Berrington de González, L. Dauer, F. Mettler and M. Satyamitra). We thank Drs. Michael Balonov (Institute of Radiation Hygiene, St. Petersburg, Russia), Andre Bouville and Stephen Chanock (both of the NCI) and Yuri Nikiforov (University of Pittsburgh Medical Center) for reviewing sections of the workshop report for accuracy.
References
- 1.Likhtarev IA, Sobolev BG, Kairo IA, Tronko ND, Bogdanova TI, Oleinic VA, et al. Thyroid cancer in the Ukraine. Nature. 1995;375:365. doi: 10.1038/375365a0. [DOI] [PubMed] [Google Scholar]
- 2.Astakhova LN, Anspaugh LR, Beebe GW, Bouville A, Drozdovitch VV, Garber V, et al. Chernobyl-related thyroid cancer in children of Belarus: A case-control study. Radiat Res. 1998;150:349–56. [PubMed] [Google Scholar]
- 3.Balonov M, Crick M, Louvat D. In: STUK, editor. Update of Impacts of the Chernobyl accident: Assessments of the Chernobyl Forum (2003–2005) and UNSCEAR (2005–2008); Proceedings of the Third European IRPA Congress; 2010 Jun 14–18; Helsinki, Finland. Helsinki. 2011. pp. 1519–32. Radiation and Nuclear Safety Authority. [Google Scholar]
- 4.Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect. 2013;121:59–65. doi: 10.1289/ehp.1204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170:721–35. doi: 10.1667/RR1231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitise J, Stengrevics A, et al. Risk of thyroid cancer among Chernobyl liquidators. Radiat Res. 2012;178:425–36. doi: 10.1667/RR2975.1. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov VK, Kashcheev VV, Chekin SY, Maksioutov MA, Tumanov KA, Vlasov OK, et al. Radiation-epidemiological studies of thyroid cancer incidence in Russia after the Chernobyl accident (estimation of radiation risks, 1991–2008 follow-up period) Radiat Prot Dosimetry. 2012;151:489–99. doi: 10.1093/rpd/ncs019. [DOI] [PubMed] [Google Scholar]
- 8.Rahu K, Auvinen A, Hakulinen T, Tekkel M, Inskip PD, Bromet EJ, et al. Chernobyl cleanup workers from Estonia: follow-up for cancer incidence and mortality. J Radiol Prot. 2013;33:395–411. doi: 10.1088/0952-4746/33/2/395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahu K, Hakulinen T, Smailyte G, Stengrevics A, Auvinen A, Inskip PD, et al. Site-specific cancer risk in the Baltic cohort of Chernobyl cleanup workers, 1986–2007. Eur J Cancer. 2013;49:2926–33. doi: 10.1016/j.ejca.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kryuchkov V, Chumak V, Maceika E, Anspaugh LR, Cardis E, Bakhanova E, et al. RADRUE method for reconstruction of external photon doses for Chernobyl liquidators in epidemiological studies. Health Phys. 2009;97:275–98. doi: 10.1097/HP.0b013e3181ac9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner AV, Tronko MD, Hatch M, Bogdanova TI, Oliynik VA, Lubin JH, et al. I-131 dose response for incident thy:roid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119:933–9. doi: 10.1289/ehp.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little MP, Kukush AG, Masiuk SV, Shklyar S, Carroll RJ, Lubin JH, et al. Impact of uncertainties in exposure assessment on estimates of thyroid cancer risk among Ukrainian children and adolescents exposed from the Chernobyl accident. PLOS One. 2014;9:e85723. doi: 10.1371/journal.pone.0085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zablotska LB, Ron E, Rozhko AV, Hatch M, Polyanskaya ON, Brenner AV, et al. Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br J Cancer. 2011;104:181–7. doi: 10.1038/sj.bjc.6605967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little MP, Kwon D, Zablotska LB, Brenner AV, Cahoon EK, Rozhko AV, et al. Impact of uncertainties in exposure assessment on thyroid cancer risk among persons in Belarus exposed as children or adolescents due to the Chernobyl accident. PLOS One. 2015;10:e0139826. doi: 10.1371/journal.pone.0139826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatch M, Brenner A, Bogdanova T, Derevyanko A, Kuptsova N, Likhtarev I, et al. A screening study of the thyroid cancer and other thyroid diseases among individuals exposed in utero to iodine-131 from Chernobyl fallout. J Clin Endocrinol Metab. 2009;94:899–906. doi: 10.1210/jc.2008-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Likhtarov I, Kovgan L, Masiuk S, Talerko M, Chepurny M, Ivanova O, et al. Thyroid cancer study among Ukrainian children exposed to radiation after the Chornobyl accident: improved estimates of the thyroid doses to the cohort members. Health Phys. 2014;106:370–96. doi: 10.1097/HP.0b013e31829f3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drozdovitch V, Minenko V, Golovanov I, Khrutchinsky A, Kukhta T, Kutsen S, et al. Thyroid dose estimates for a cohort of Belarusian children exposed to (131)I from the Chernobyl accident: assessment of uncertainties. Radiat Res. 2015;184:203–18. doi: 10.1667/rr13791.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Likhtarov I, Kovgan L, Chepurny M, Ivanova O, Boyko Z, Ratia G, et al. Estimation of the thyroid doses for Ukrainian children exposed in utero after the Chernobyl accident. Health Phys. 2011;100:583–93. doi: 10.1097/HP.0b013e3181ff391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doses to the embryo and fetus from intakes of radionuclides by the mother. ICRP Publication 88. Ann ICRP. 2001;31 doi: 10.1016/S0146-6453(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 20.Mettler FA, Jr, Gus’kova AK, Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007;93:462–9. doi: 10.1097/01.HP.0000278843.27969.74. [DOI] [PubMed] [Google Scholar]
- 21.Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res. 2013;179:361–82. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov VK, Tsyb AF, Khait SE, Kashcheev VV, Chekin SY, Maksioutov MA, et al. Leukemia incidence in the Russian cohort of Chernobyl emergency workers. Radiat Environ Biophys. 2012;51:143–9. doi: 10.1007/s00411-011-0400-y. [DOI] [PubMed] [Google Scholar]
- 23.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–77. [PubMed] [Google Scholar]
- 24.Kashcheev VV, Checkin SY, Maksioutov MA, Tumanov KA, Menyaylo AN, Kochergina EV, et al. Radiation-epidemiology study of cerebrovascular diseases in the cohort of Russian recovery operations workers of the Chernobyl accident. Health Phys. 2016;111:192–7. doi: 10.1097/HP.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 25.Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res. 2016;185:473–84. doi: 10.1667/RR14213.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuzik M, Prysyazhnyuk A, Shibata Y, Romanenko A, Fedorenko Z, Gulak L, et al. Thyroid cancer incidence in Ukraine: trends with reference to the Chernobyl accident. Radiat Environ Biophys. 2011;50:47–55. doi: 10.1007/s00411-010-0340-y. [DOI] [PubMed] [Google Scholar]
- 27.Auvinen A, Seppa K, Pasanen K, Kurttio P, Patama T, Pukkala E, et al. Chernobyl fallout and cancer incidence in Finland. Int J Cancer. 2014;134:2253–63. doi: 10.1002/ijc.28554. [DOI] [PubMed] [Google Scholar]
- 28.Cardis E, Krewski D, Boniol M, Drozdovitch V, Darby SC, Gilbert ES, et al. Estimates of the cancer burden in Europe from radioactive fallout from the Chernobyl accident. Int J Cancer. 2006;119:1224–35. doi: 10.1002/ijc.22037. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov EP, Tolochko GV, Shuvaeva LP, Ivanov VE, Iaroshevich RF, Becker S, et al. Infant leukemia in Belarus after the Chernobyl accident. Radiat Environ Biophys. 1998;37:53–5. doi: 10.1007/s004110050092. [DOI] [PubMed] [Google Scholar]
- 30.Noshchenko AG, Moysich KB, Bondar A, Zamostyan PV, Drosdova VD, Michalek AM. Patterns of acute leukemia occurrence among children in the chernnobyl region. Int J Epidemiol. 2001;30:125–9. doi: 10.1093/ije/30.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Parkin DM, Clayton D, Black RJ, Masuyer E, Friedl HP, Ivanov E, et al. Childhood leukaemia in Europe after Chernobyl: 5 year follow-up. Br J Cancer. 1996;73:1006–12. doi: 10.1038/bjc.1996.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis S, Day RW, Kopecky KJ, Mahoney MC, McCarthy PL, Michalek AM, et al. Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case-control study. Int J Epidemiol. 2006;35:386–96. doi: 10.1093/ije/dyi220. [DOI] [PubMed] [Google Scholar]
- 33.Hatch M, Ostroumova E, Brenner A, Federenko Z, Gorokh Y, Zvinchuk O, et al. Non-thyroid cancer in Northern Ukraine in the post-Chernobyl period: short report. Cancer Epidemiol. 2015;39:279–83. doi: 10.1016/j.canep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostroumova E, Hatch M, Brenner A, Nadyrov E, Veyalkin I, Polyanskaya O, et al. Non-thyroid cancer incidence in Belarusian residents exposed to Chernobyl fallout in childhood and adolescence: standardized incidence ratio analysis, 1997–2011. Environ Res. 2016;147:44–9. doi: 10.1016/j.envres.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prysyazhnyuk AY, Grishtshenko VG, Fedorenko ZP, Gulak LO, Fuzik MM. Review of epidemiological finding in study of medical consequences of the Chernobyl accident in Ukrainian population. In: Imanaka T, editor. Recent research activities about the Chernobyl NPP accident in Belarus, Ukraine and Russia [KURRI-KR-79] Kyoto: Research Reactor Institute, Kyoto University; 2002. pp. 188–202. [Google Scholar]
- 36.Pukkala E, Kesminiene A, Poliakov S, Ryzhov A, Drozdovitch V, Kovgan L, et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer. 2006;119:651–8. doi: 10.1002/ijc.21885. [DOI] [PubMed] [Google Scholar]
- 37.Dardynskaia I, Imrey PB, Okeanov A, Hryhorczuk D. Breast cancer trends in two oblasts of Belarus and the Chernobyl accident. Int J Occup Environ Health. 2006;12:415–22. doi: 10.1179/oeh.2006.12.4.415. [DOI] [PubMed] [Google Scholar]
- 38.Wanebo CK, Johnson KG, Sato K, Thorslund TW. Breast cancer after exposure to the atomic bombings of Hiroshima and Nagasaki. N Engl J Med. 1968;279:667–1. doi: 10.1056/NEJM196809262791301. [DOI] [PubMed] [Google Scholar]
- 39.Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, et al. ICRP statement on tissue reactions/early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. ICRP Publication 118. Ann ICRP. 2012;41 doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 40.International Atomic Energy Agency. Radiation protection and safety of radiation sources: International basic safety standards. Vienna, Austria: IAEA; 2011. pp. 11–39241. Report: General safety requirements, part 3 (interim edition) [Google Scholar]
- 41.National Council on Radiation Protection and Measurements. Guidance on radiation dose limits for the lens of the eye. NCRP Commentary No. 26. Bethesda: NCRP; 2016. [Google Scholar]
- 42.International Atomic Energy Agency. Report: IAEA TECDOC 1731. Vienna: IAEA; 2013. Implications for occupational radiation protection of the new dose limit for the lens of the eye: Interim guidance for use and comment. [Google Scholar]
- 43.Cantone MC, Ginjaume M, Miljanic S, Martin CJ, Akahane K, Mpete L, et al. IRPA guideline protocol for eye protection and eye dose monitoring of workers. St. Denis, France: IRPA; 2016. (International Radiation Protection Association) [Google Scholar]
- 44.Day R, Gorin MB, Eller AW. Prevalence of lens changes in Ukrainian children residing around Chernobyl. Health Phys. 1995;68:632–42. doi: 10.1097/00004032-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Osanov DP. The evaluation of the irradiation levels of the skin, eye lenses and gonads of the Chernobyl liquidators. Radiat Prot Dosimetry. 1997;74:235–38. [Google Scholar]
- 46.Nadejina NM, Galstian IA, Savisky AA, Kashirina OG, Filin SV, Rtischeva JN, et al. Late effects of acute radiation sickness. Med Radiol Radiat Safety [Russian] 2003;48:17–27. [Google Scholar]
- 47.Worgul BV, Kundiyev YI, Sergiyenko NM, Chumak VV, Vitte PM, Medvedovsky C, et al. Cataracts among Chernobyl clean-up workers: Implications regarding permissible eye exposures. Radiat Res. 2007;167:233–43. doi: 10.1667/rr0298.1. [DOI] [PubMed] [Google Scholar]
- 48.Chumak VV, Worgul BV, Kundiyev YI, Segiyenko NM, Vitte PM, Medvedovsky C, et al. Dosimetry for a study of low-dose radiation cataracts among Chernobyl clean-up workers. Radiat Res. 2007;167:606–14. doi: 10.1667/RR0302.1. [DOI] [PubMed] [Google Scholar]
- 49.Lehmann P, Boratynski Z, Mappes T, Mousseau TA, Moller AP. Fitness costs of increased cataract frequency and cumulative radiation dose in natural mammalian populations from Chernobyl. Sci Rep. 2016;6:19974. doi: 10.1038/srep19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 52.Effects of ionizing radiation, UNSCEAR 2006: Report to the General Assembly, with Scientific Annexes - Volume 1. Annex B - Epidemiological evaluation of cardiovascular disease and other non-cancer diseases following radiation exposure. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2008. [Google Scholar]
- 53.Ivanov VK, Maksioutov MA, Chekin SY, Kruglova ZG, Petrov AV, Tsyb AF. Radiation-epidemiological analysis of incidence of non-cancer diseases among the Chernobyl liquidators. Health Phys. 2000;78:495–501. doi: 10.1097/00004032-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 55.Streffer C, Shore R, Konermann G, Meadows A, Devi PU, Withers JP, et al. Biological effects after prenatal irradiation (embryo and fetus) Ann ICRP. 2003;33:5–206. [PubMed] [Google Scholar]
- 56.Preconception and prenatal radiation exposure: Health effects and protective guidance. Report No. 174. Bethesda: National Council on Radiation Protection and Measurements; 2013. [Google Scholar]
- 57.Sutow WW, Conard RA, Griffith KM. Growth status of children exposed to fallout radiation on Marshall Islands. Pediatrics. 1965;36:721–31. [PubMed] [Google Scholar]
- 58.Lloyd RD, Tripp DA, Kerber RA. Limits of fetal thyroid risk from radioiodine exposure. Health Phys. 1996;70:559–62. doi: 10.1097/00004032-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Slovic P. Perception of risk. Science. 1987;236:280–5. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- 60.Goldmann E, Galea S. Mental health consequences of disasters. Annu Rev Public Health. 2014;35:169–83. doi: 10.1146/annurev-publhealth-032013-182435. [DOI] [PubMed] [Google Scholar]
- 61.Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002;65:207–39. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- 62.Bromet EJ, Parkinson DK, Schulberg HC, Dunn LO, Gondek PC. Mental health of residents near the Three Mile Island reactor: a comparative study of selected groups. J Prev Psychiatry. 1982;1:225–76. [Google Scholar]
- 63.Bromet EJ, Havenaar JM. Psychological and perceived health effects of the Chornobyl disaster: A 20-year review. Health Phys. 2007;93:516–21. doi: 10.1097/01.HP.0000279635.14108.02. [DOI] [PubMed] [Google Scholar]
- 64.Otake M, Schull WJ. Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int J Radiat Biol. 1998;74:159–71. doi: 10.1080/095530098141555. [DOI] [PubMed] [Google Scholar]
- 65.Imamura Y, Nakane Y, Ohta Y, Kondo H. Lifetime prevalence of schizophrenia among individuals prenatally exposed to atomic bomb radiation in Nagasaki City. Acta Psychiatr Scand. 1999;100:344–9. doi: 10.1111/j.1600-0447.1999.tb10877.x. [DOI] [PubMed] [Google Scholar]
- 66.Yamada M, Izumi S. Psychiatric sequelae in atomic bomb survivors in Hiroshima and Nagasaki two decades after the explosions. Soc Psychiatry Psychiatr Epidemiol. 2002;37:409–15. doi: 10.1007/s00127-002-0572-5. [DOI] [PubMed] [Google Scholar]
- 67.Rahu K, Rahu M, Tekkel M, Bromet E. Suicide risk among Chernobyl cleanup workers in Estonia still increased: an updated cohort study. Ann Epidemiol. 2006;16:917–9. doi: 10.1016/j.annepidem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Bennett B, Repacholi M, Carr Z, editors. Health effects of the Chernobyl accident and special health care programmes: Report of the UN Chernobyl Forum Expert Group “Health”. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 69.Sources, Effects and Risks of Ionizing Radiation, UNSCEAR 2013: Report to the General Assembly, with Scientific Annexes – Volume 1. Scientific Annex A – Levels and effects of radiation exposure due to the nuclear accident after the 2011 great east-Japan earthquake and tsunami. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2014. [Google Scholar]
- 70.IAEA annual report 2015. Report No. GC(60)/9. Vienna: International Atomic Energy Agency; 2016. [Google Scholar]
- 71.Norris FH, Stevens SP, Pfefferbaum B, Wyche KF, Pfefferbaum RL. Community resilience as a metaphor, theory, set of capacities, and strategy for disaster readiness. Am J Community Psychol. 2008;41:127–50. doi: 10.1007/s10464-007-9156-6. [DOI] [PubMed] [Google Scholar]
- 72.FDA approves radiation medical countermeasure [Internet] Silver Spring: Food and Drug Administration; 2015. ( http://bit.ly/1Tpsbov) [Google Scholar]
- 73.Highlights of prescribing information: Reference ID: 3846752. Thousand Oaks, CA: Amgen Inc; 2015. ( http://bit.ly/22Aj474) [Google Scholar]
- 74.Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–75. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Baranov AE, Guskova AK, Nadejina NM, Nugis V. Chernobyl experience: biological indicators of exposure to ionizing radiation. Stem Cells. 1995;13:69–77. [PubMed] [Google Scholar]
- 76.Farese AM, Cohen MV, Katz BP, Smith CP, JacksonW III, Cohen DM, et al. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–82. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura ET, Nikiforova MN, Zhu ZW, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC–RAS–BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 79.Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, et al. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989;49:159–64. [PubMed] [Google Scholar]
- 80.Suarez HG, Du Villard JA, Caillou B, Schlumberger M, Tubiana M, Parmentier C, et al. Detection of activated ras oncogenes in human thyroid carcinomas. Oncogene. 1988;2:403–6. [PubMed] [Google Scholar]
- 81.Ito T, Seyama T, Iwamoto KS, Mizuno T, Tronko ND, Komissarenko IV, et al. Letters to the Editor: Activated RET oncogene in thyroid cancers of children from areas contaminated by Chernobyl accident. Lancet. 1994;344:259. doi: 10.1016/s0140-6736(94)93024-4. [DOI] [PubMed] [Google Scholar]
- 82.Fugazzola L, Pilotti S, Pinchera A, Vorontsova TV, Mondellini P, Bongarzone I, et al. Oncogenic rearrangements of the RET protooncogene in papillary thyroid carcinomas from children exposed to the Chernobyl nuclear accident. Cancer Res. 1995;55:5617–20. [PubMed] [Google Scholar]
- 83.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–4. [PubMed] [Google Scholar]
- 84.Rabes HM, Demidchik EP, Sidorow JD, Lengfelder E, Beimfohr C, Hoelzel D, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–103. [PubMed] [Google Scholar]
- 85.Leeman-Neill RJ, Brenner AV, Little MP, Bogdanova TI, Hatch M, Zurnadzy LY, et al. RET/PTC and PAX8/PPARgamma chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119:1792–9. doi: 10.1002/cncr.27893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouville A, Linet MS, Hatch M, Mabuchi K, Simon SL. Guidelines for exposure assessment in health risk studies following a nuclear reactor accident. Environ Health Perspect. 2014;122:1–5. doi: 10.1289/ehp.1307120. [DOI] [PMC free article] [PubMed] [Google Scholar]