Abstract
Child sexual abuse (CSA) is an important public health problem with long-standing neurobiological, developmental, and psychiatric abnormalities. The present review analyzes the long-term effects of CSA from a developmental, psychiatric morbidity, neurochemical and neurobiological perspective and then tries to posit a developmental neurobiological trajectory from CSA to the genesis of psychopathology in later life. The role of various neurotransmitters such as serotonin and dopamine affected by CSA are discussed. Serotonin abnormalities have been reported in various studies among participants exposed to CSA. Structures such as the prefrontal cortex, superior temporal gyrus, corpus callosum, parietal lobes, hippocampus, and cerebellum all demonstrate volumetric and structural changes in response to the trauma of CSA. Neurocognitive studies demonstrate memory and spatial awareness as well as decrements in general cognitive performance and memory when compared to normal individuals. The hypothalamic–pituitary–adrenal axis has also been implicated in CSA, and there is an alteration in corticotropin-releasing hormone response due to the continuous cumulative trauma of CSA. This paper also reviews a section on the role of genetic and epigenetic factors in the development of psychiatric disorders as a result of exposure to episodes of CSA where studies have demonstrated changes in DNA methylation in response to CSA. This review tries to hypothesize a developmental trajectory framework which is individual for every case where exposure to CSA may lead to psychopathology and psychiatric morbidity later in life. Rapid and emerging fields such as developmental traumatology in relation to CSA are also discussed.
Keywords: Child sexual abuse, developmental psychopathology, genetics, neurobiology, neuroimaging
Child sexual abuse (CSA) is an important public health problem; however, there is a scarcity of studies about the effects of abuse on the brain and neurobiological development.[1] This article posits CSA as a risk factor for the future developmental of psychiatric disorders and tries to propose a neurobiological model that looks at the developmental trajectory in this direction and context.
CSA and other forms of maltreatment are significantly associated with a wide range of psychiatric disorders in adulthood that range from depression, posttraumatic stress disorder (PTSD), panic disorder, and substance abuse to schizophrenia and antisocial personality disorder.[2,3] Numerous studies also show that adults who report suffering maltreatment/abuse as children have more physical and psychological problems both medically explained and unexplained.[4] At any given time, 20%–40% of psychiatric patients carry with them a history and burden of CSA.[5] The pathway of genesis of a psychiatric disorder in response to CSA is complex and determined by a number of intertwining factors such as genetics, epigenetics, neurobiological changes, neurochemical and synaptic changes as well as effects of the neuroendocrine axes due to cumulative stress.[6] This paper looks at various neurobiological factors and tries to find a final common pathway that may lead to a psychiatric disorder that stems from CSA as a risk factor.
METHOD OF CONDUCTING THIS REVIEW
For identifying articles that focused on the long-term development of psychiatric disorders after exposure to CSA, the terms “child sexual abuse,” “neuroimaging and child sexual abuse,” “neurobiology and child sexual abuse,” “developmental psychopathology and child abuse,” or “stress and neurobiology” were used. For identifying articles that focused on specific terms, searches with words such as “anxiety and child abuse,” “depression and child abuse,” “child abuse and neurocognitive abnormalities,” “epigenetics and child abuse,” “child abuse genetics,” and other terms were used. These two search strategy results were combined in the following databases with the time frame being specified from 1995 to 2015. The databases used were MEDLINE, PubMed, and the Cochrane Database on Systematic Reviews. In total, 641 articles were identified which included reviews, mini-reviews, and research studies in populations exposed to CSA. The original research studies reviewed were those that focused on elucidating a neurobiology of CSA.
The papers were reviewed by all authors and for this article include review articles, commentaries, and research papers on CSA. A total of 154 papers were reviewed for this paper of which 95 references finally appear in this paper. This has been supplemented with insights from the personal clinical experience of all the authors who work regularly with patients that have been exposed to CSA.
PSYCHIATRIC DISORDERS AS A SEQUEL TO CHILD SEXUAL ABUSE
Inquiry into the sequel of CSA has underlined the high prevalence of this life event to individual and public health. Estimates of sexual abuse in retrospective population-based samples range from 8% to 43% for women and 2%–26% for men.[7] While earlier studies of childhood abuse focused particularly on sexual abuse, research has since expanded to include multiple forms of maltreatment in current studies. For the purpose of this paper, we restrict ourselves to CSA though other forms of maltreatment may overlap it.
In a community sample that responded to mailed questionnaires, 14.2% of men and 32.3% of women reported experiencing sexual abuse (such as unwanted kissing or touching in a sexual way by someone 5 or more years older to them) before the age of 18 years.[8] Physical abuse (such as being hit with a fist, kicked, thrown, burned, or bruised on purpose) was reported by 22.2% of men and 19.5% of women.[8] Approximately 21% of those who had been abused were abused both sexually and physically. Among all respondents, 37% reported either sexual or physical abuse in childhood.[8] Data from the National Comorbidity Survey indicated a relationship between CSA and the subsequent onset of psychiatric disorder, and findings revealed that 78% of women and 82% of men who reported CSA met diagnostic criteria for at least one lifetime psychiatric disorder versus 49% and 51%, respectively, among those who did not report CSA.[9,10]
The odds ratio (OR) for lifetime history of depression was 1.8 among both men and women who reported a history of CSA versus those who did not.[10] Overall, after controlling for other childhood adversities, CSA was found to be significantly associated with mood, substance use, and anxiety disorders in both sexes.[11] In a recent meta-analysis that looked at 37 longitudinal studies from across the world between 1980 and 2008 (20 cohort and 17 case-control studies) with over 3,160,000 participants, it was calculated that there was a statistically significant association between sexual abuse and a lifetime diagnosis of anxiety (OR: 3.09), depression (major depressive disorder [MDD]) (OR: 2.66), eating disorders (OR: 2.72), PTSD (OR: 2.34), sleep disorders, and suicide attempts. This association persisted irrespective of gender and age at which CSA occurred.[12] The same authors conducted another systematic review and meta-analysis that demonstrated an association between CSA and medical symptoms such as chronic pelvic pain, functional gastrointestinal disorders, psychogenic seizures, and nonspecific pain and somatic symptoms.[13] In both, the meta-analyses mentioned above the associations were strengthened when a history of rape was present.
Other studies have shown similar findings and reported that the association between CSA and lifetime occurrence of psychiatric disorder persists irrespective of gender, socioeconomic status, relationship to the abuser, number of abuse episodes, and type of CSA that may have occurred.[14] An important factor to note in CSA studies is that sexual abuse may be coupled with other forms of abuse including physical and verbal abuse. This may often confound the causation of psychiatric disorders and observations reported.[15]
CSA is not a single-episode event but often occurs a number of times during one's lifetime. It therefore makes sense to inquire about dose–response relationships, that is, whether greater severity (e.g., intrusiveness and higher frequency) of abuse or exposure to a greater number of different categories of maltreatment/sexual abuse is associated with a greater risk of psychiatric or other sequelae in adulthood. Authors have reported a linear dose–response relationship between abuse severity (i.e., none, mild, moderate, and severe) and lifetime MDD.[16] Among those reporting sexual abuse in childhood, rates of chronic or recurrent MDD were 83% for those who suffered “marked” abuse, 70% for those who experienced “moderate” abuse, and 55% for those who experienced “mild” abuse.[17] The greater the number of categories of maltreatment the individual reported the higher the probability of lifetime chronic or recurrent depression.[18,19] CSA resulting in intercourse was more strongly associated with each of the psychiatric conditions examined and then was any other category of abuse.[20]
The experience of CSA is also associated with increased risk of suicide attempts nonsuicidal self-injury.[21] In a study involving more than 1900 women in primary care, sexual or physical abuse in childhood was associated with a significantly higher frequency of attempted suicide in adulthood.[22] Evidence of a significant association between childhood abuse and attempted suicide has also been reported in another primary care sample.[23] In a co-twin study, the risk of suicide attempt was significantly higher among those with reported CSA history than among those without such history.[24] Studies find significantly higher frequency of suicide attempts among those who reported physical, sexual, or psychological abuse. In addition, the more categories of such experience reported the higher the likelihood of suicidal behavior.[25] Among psychiatric disorders, MDD is most frequently associated with attempted and completed suicide.[26] Patients reporting a history of childhood sexual or physical abuse were significantly more likely to have attempted suicide than those with no such history.[27] Several studies have examined relationships between self-reported abuse history and particular features of depression such as onset and course. It was found that self-reported history of childhood maltreatment was associated with earlier onset of depression, greater number of depressive episodes, and more extensive comorbidity.[28,29] In a large sample of chronically depressed patients, it was found that those who met criteria for early childhood trauma responded significantly better to psychotherapy than to medication.[30,31]
Overall, the evidence is substantial that CSA is a risk factor for a wide range of adult psychiatric disorders such as depression, schizophrenia, anxiety disorders, and personality disorders. It is also worthwhile mentioning that when examining epidemiological data like those in the studies one must take into strict consideration the definition of CSA used and this is the most common variable that can confound and distort findings and prevalence across studies worldwide.[31]
NEUROBIOLOGICAL EFFECTS OF CHILD SEXUAL ABUSE ON LONG-TERM BRAIN DEVELOPMENT
General considerations
Current neuroscience research is focused on strategies to prevent or reverse the detrimental effects of early life stress on the central nervous system.[32] The identification of the neurobiological substrates of early adverse experience is of paramount importance for the development of novel treatments for children, adolescents, and adults.[33] Tremendous inroads have been made into understanding the neurobiological basis of mood and anxiety disorders and the influence of life events on risk and resilience.[34] Persistent sensitization of neural circuits as a consequence of early life stress, which are integrally involved in the regulation of stress and emotion, may represent the underlying biological substrate of an increased vulnerability to subsequent stress as well as to the development of depression and anxiety.[35] There is a strong heritable component to psychiatric illnesses that, when coupled with environmental influences, results in increased vulnerability.[36] However, most psychiatric disorders, including mood and anxiety disorders, are polygenetic in nature rather than determined by traditional Mendelian genetics.[37] Intensive research efforts have been expended to better characterize the genetic underpinnings of mental illness. Recent technological advances, including the completion of the human genome inventory, chromosome mapping, high-throughput DNA sequencing, and others, offer the promise of someday identifying the genetic basis of mental illnesses.[38]
Serotonin system
Numerous studies have confirmed the link between reduced serotonergic function and serious suicidal acts.[39] Studies have localized the changes to the ventral prefrontal cortex and suggested how genetics, childhood rearing, alcoholism, substance abuse, gender, age, and cholesterol intake can modulate suicide rates through effects on the serotonin system.[40] The serotonin system is a stress response system that activates both anxiogenic and anxiolytic pathways and is involved in complex neuronal communication across diverse areas of the brain.[41] Disruptions in the serotonin system may play a pivotal role in the development of clinical and subthreshold PTSD symptoms in children who have experienced CSA but may also predispose them to develop major depression and aggressiveness in later life.[42]
Corticotropin-releasing factor and limbic–hypothalamic–pituitary–adrenal axis
Early-life adversity, such as physical or sexual abuse during childhood, results in long-lasting changes in the corticotropin-releasing factor (CRF)-mediated stress response and a greatly increased risk of depression in genetically predisposed persons.[43] Neural circuits containing CRF have been identified as an important mediator of the stress response. Identification and cloning of CRF receptors and characterization of their role in the stress response have enabled a better understanding of maladaptive responses to early-life adversity.[44] In addition, studies of the CRF system have suggested molecular targets for new drug development, biological risk factors, and predictors of treatment response.[45]
Inconsistencies exist in literature examining hypothalamic–pituitary–adrenal (HPA) axis activity in children and adults who have experienced childhood abuse. Hence, the extent and manner to which childhood abuse may disrupt HPA axis development is largely unknown.[46] In a study to address these inconsistencies, the developmental course of nonstress cortisol in a long-term longitudinal study was assessed at six time points from childhood to adolescence and into young adulthood to determine whether childhood abuse results in disrupted cortisol activity.[47] It was observed that the CSA group showed attenuation in cortisol activity starting in adolescence with significantly lower levels of cortisol by early adulthood. The findings demonstrate how the experience of childhood abuse might disrupt the neurobiology of stress, providing some support for the attenuation hypothesis that victims of abuse may experience cortisol hyposecretion subsequent to a period of heightened secretion.[47] Early stress is associated with long-term alterations in brain circuits and systems that mediate the stress response. A possible long-term effect of trauma is to prime the limbic–hypothalamic–pituitary–adrenal axis such that corticotropin-releasing hormone (CRH) is set a lower 24 h levels. This serves to cause a hyperresponse when a new stressor is experienced. Early stressors have lasting effects on the HPA axis and norepinephrine systems.[48] Other brain systems that are involved include benzodiazepine, opiate, dopaminergic, and various neuropeptide systems.[49] These neurochemical systems modulate function in brain regions including the hippocampus, amygdala, and prefrontal cortex.[50] Long-term alterations in these brain regions are hypothesized to play a role in the maintenance of PTSD, depression, and other psychiatric symptoms after CSA.[51] Variations of the CRH receptor 1 (CRHR1) gene appear to moderate the development of depression after childhood trauma.[52] Studies have examined sex differences in the effects of the CRHR1 gene on the relationship between childhood trauma and adult depression. Gender differences in environmental exposures could thus be reflected into a product of sex-specific CRHR1 and child abuse interactions.[53] A number of preclinical studies suggest that early life stress induces long-lived hyper (re) activity of CRF systems as well as alterations in other neurotransmitter systems, resulting in increased stress responsiveness.[54] Many of the findings from these preclinical studies are comparable to findings in adult patients with mood and anxiety disorders.[55]
Recent studies have reported an association between exposure to childhood abuse or neglect and alterations in brain structure or function.[56] Preclinical studies on the effects of exposure to early life stress can demonstrate causality and can enrich our understanding of the consequences of early abuse which are predominantly mediated through the induction of stress responses.[57]
Neuroimaging findings related to child sexual abuse exposure
The period from birth to adulthood is one of intense brain development, wiring and rewiring. Increased volume of the brain occurs after birth that completes by the age of 5.[58] Neuronal development is lifelong but is more intense during the first 7 years of life with repeated neurogenesis, synaptogenesis, synaptic pruning, and changes in synaptic and neuronal density occurring. This is coupled with increase in neuronal size and synaptic size as well as enhanced myelination.[59] Gray matter and white matter changes that reflect this neurodevelopment has been noted on imaging across the first three decades of life while it is most prevalent in childhood.[60] Changes in the volumes of the hippocampus, amygdala, medial prefrontal cortex, and corpus callosum are other markers of early neurodevelopment.[61] The elevated and increased response of catecholamines and CRF as discussed above can result in mechanisms that cause accelerated neuronal loss, decreased neuronal size, reduced number of synapses, abnormalities in developmentally appropriate pruning, and inhibition of neurogenesis at vital periods along with inadequate production and expression of brain-derived neurotrophic factor.[62,63,64,65,66] Studies that have looked at the role of gonadal hormones on brain development hypothesize that male brains are more prone to the adverse effects of CSA or any other trauma and thereby show greater reactions to life adversities.[67]
An interesting finding noted in neuroimaging studies related to CSA is that hippocampal abnormalities are more common in adults than in children.[68] One of the theories in this regard that early trauma or CSA has a gradual effect on the hippocampus, and the effects may not be visible till adolescence or adulthood.[69] Furthermore, noted is the fact that early disclosure of the abuse by children and therapeutic intervention may in turn increase hippocampal plasticity and neurogenesis. Thus, intervention in childhood when exposed to CSA may mask the effects of trauma on the hippocampus and prevent further neuronal damage. This is also an area that warrants further investigation.[70]
The prefrontal cortex is an important brain area which is constantly developing throughout childhood and adolescence. It is an area of the brain that serves the function of planning, thinking, attention, concentration, working memory, and executive function.[71] It normally exhibits an inhibitory control over the amygdala and the limbic system and has a neurotransmission system that is primarily dopaminergic in nature. The stress-based release of catecholamines may lead to a switching off of this control over the limbic system and may exacerbate emotional and PTSD-related symptoms in response to any trauma/CSA.[72] Enhanced dopamine due to stress within the prefrontal cortex may cause hypervigilance and paranoia in developing children with decline in attention and academic performance as well as subtle neurocognitive abnormalities.[73]
Children who have experienced CSA and resulting PTSD have shown smaller superior temporal gyrus volumes on neuroimaging studies.[74] A greater left-to-right asymmetry has been reported and this has been linked to abnormalities such as slower emotional, auditory, and facial stimuli processing.[75] Another structure brought up in some neuroimaging and CSA studies is the cerebellar vermis. This is a structure that is intricately linked to multisensory integration and limbic activation.[76] Studies have found that cerebellar volumes positively correlated with age of onset of the trauma and negatively correlated with the duration of the trauma in maltreated children and adolescents with the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition PTSD.[77] Significant reduction in corpus callosum volumes has been noted in response to CSA. Studies show that this reduction is greater in the medial portion of the corpus callosum leading to poor communication between the two hemispheres and its neurocognitive effects.[78]
Neurocognitive findings in child sexual abuse
Recent findings from cognitive neuroscience and cognitive psychology may help explain why recovered memories of trauma are sometimes illusory. In particular, the notion of defective source monitoring has been used to explain a wide range of recently established memory distortions and illusions.[79] Conversely, the results of a number of studies may potentially be relevant to forgetting and recovery of accurate memories, including studies demonstrating reduced hippocampal volume in survivors of sexual abuse, and recovery from functional and organic retrograde amnesia.[80] Other recent findings of interest include the possibility that state-dependent memory could be induced by stress-related hormones, new pharmacological models of dissociative states, and evidence for “repression” in patients with right parietal brain damage.[81] Scientists investigated the relationship between childhood trauma exposure and cognitive function in 47 healthy adults, who were identified as part of a larger study from the general population in which the Cambridge Neuropsychological Test Automated Battery and the Wide Range Achievement Test-3 were used to examine cognitive function and individual achievement. The results suggest that physical neglect and emotional abuse might be associated with memory deficits in adulthood, which in turn might pose a risk factor for the development of psychopathology.[82] Variations in social cognition serve as one possible mechanism by which these environmental experiences influence aggressive behavior during adolescence. Children who have been maltreated tend to display negatively biased social-cognitive processing styles, which may in turn increase their likelihood of reacting aggressively in ambiguous social situations.[83] It has been noted that alterations in memory in the form of dissociative amnesia are an important part of exposure to traumatic stressors, such as childhood abuse. Extreme stress has long-term effects on memory. These findings may provide a model for understanding the mechanisms involved in dissociative amnesia as well as a rationale for phenomena such as delayed recall of childhood abuse.[84] Studying the effects of CSA on intelligence, various forms of memory, cognitive skills, and cognitive processing is an interesting line of research to follow.
Genetics and epigenetics in relation to child sexual abuse
To date, preclinical studies have guided clinical investigations and will continue to provide important insight into studies on molecular mechanisms and gene–environment interactions. Neglecting psychosocial factors in the causes of aggressive behavior would also be naïve.[85] There is some evidence of an association between childhood abuse and neglect and adult antisocial personality disorder, but this relationship might be merely an artifact of personality dysfunction positing an interaction between inherited susceptibility and environmental factors such as childhood trauma.[86] The role of trauma in the development of personality disorder and especially for borderline personality disorder (BPD) remains unclear. Although recent studies suggest that BPD is not a trauma-spectrum disorder and that it is biologically distinct from PTSD, high rates of childhood abuse and neglect do exist for individuals with personality dysfunction.[87] Gene–environment interactions involving childhood maltreatment are demonstrated in recent studies on antisocial behaviors and aggression in animals.[88] Some of the strongest evidence of the role of social cognition as a mechanism in this association comes from intervention studies which reduced aggressive behavior by targeting negatively biased social-cognitive processing styles.[89]
In humans with a history of CSA, epigenetic studies have evoked the greatest interest.[90] Epigenesis is defined as the production of stable changes in DNA expression that do not result from changes in the DNA sequence.[90] DNA methylation is mechanism of epigenesis that has been studied in most cases with relationships between DNA methylation and gene activity being well established.[91] It has been hypothesized that CSA would cause epigenetic changes by inducing a sustained and substantial stress response resulting in epigenetic reprogramming. This would in turn predispose an individual to increased likelihood of psychopathology. CSA would be also a predictor of methylation and thus some of long-term psychopathology resulting as a sequel to CSA may be attributed to epigenetics.[92] It is also worthwhile mentioning that CSA may not induce epigenetic changes in the same manner in every individual. A number of factors in the environment including the total psychiatric genetic load of the person who has experienced CSA such as the presence of psychiatric disorders in the family shall determine the extent to which epigenetic factors will function as causative mechanisms.[93]
LOOKING AT AN INTEGRATIVE TRAJECTORIAL FRAMEWORK
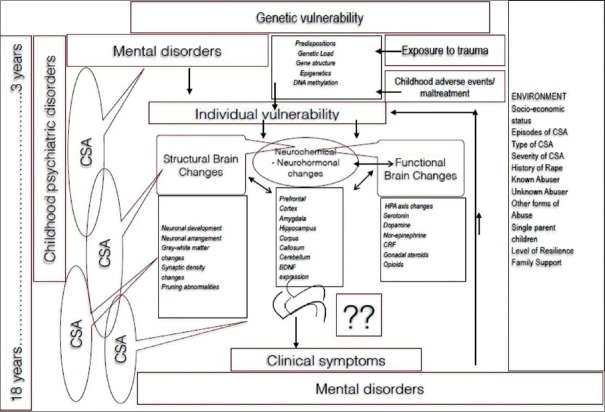
This section aims to propose a unified model of the neurobiological trajectory of adult psychopathology that arises from exposure to CSA. It is important at the outset to understand that this trajectory is highly individual and varies from case to case. There is no general sequel to CSA which is uniform, and the pathway chosen is a conglomeration of genetic, biological, and psychosocial factors. On the one hand, the alterations are caused by exposure to early stress, and on the other hand, they also may serve as a means for the child to cope and come out unscathed from the trauma of CSA. It is sometimes that these developmental changes become nonadaptive, useless, and damaging from an allostatic load framework and may serve as the path toward psychiatric illness.[94] CSA has structural, functional, and neuroendocrine effects on the developing brain. One has to find room to integrate the complex molecular biological, genetic, cognitive, psychological, and neurobiological changes that occur to synthesize how CSA may ultimately contribute to psychiatric illness [Figure 1].
Figure 1.
Mechanisms by which child sexual abuse causes mental illnes
Some authors have coined the term “developmental traumatology” to enclose developmental psychology, developmental neuroscience, Early Childhood Cognitive Development, and how trauma affects each one of them.[95] The long-term sequel of CSA is multisystemic. Genetic load inducing potent epigenetic changes tied to biological cascades leading to alterations in early neurodevelopment and unstable cellular microenvironments all play a role in the genesis of the psychiatric disorders that ensues in the long term. It is not only the hardware of the brain that changes but also the malfunction in software as well that plays a role in the process. Thus, there are multiple factors that play a role in the transition from CSA to the development of adult psychiatric disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Priebe G, Svedin CG. Child sexual abuse is largely hidden from the adult society. An epidemiological study of adolescents’ disclosures. Child Abuse Negl. 2008;32:1095–108. doi: 10.1016/j.chiabu.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Chen LP, Murad MH, Paras ML, Colbenson KM, Sattler AL, Goranson EN, et al. Sexual abuse and lifetime diagnosis of psychiatric disorders: Systematic review and meta-analysis. Mayo Clin Proc. 2010;85:618–29. doi: 10.4065/mcp.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CF. Child sexual abuse. Lancet. 2004;364:462–70. doi: 10.1016/S0140-6736(04)16771-8. [DOI] [PubMed] [Google Scholar]
- 4.Kellogg N. American Academy of Pediatrics Committee on Child Abuse and Neglect. The evaluation of sexual abuse in children. Pediatrics. 2005;116:506–12. doi: 10.1542/peds.2005-1336. [DOI] [PubMed] [Google Scholar]
- 5.Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. J Psychol. 2001;135:17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- 6.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Polusny MA, Follette VM. Long-term correlates of child sexual abuse: Theory and review of the empirical literature. Appl Prev Psychol. 1995;4:143–66. [Google Scholar]
- 8.Molnar BE, Berkman LF, Buka SL. Psychopathology, childhood sexual abuse and other childhood adversities: Relative links to subsequent suicidal behaviour in the US. Psychol Med. 2001;31:965–77. doi: 10.1017/s0033291701004329. [DOI] [PubMed] [Google Scholar]
- 9.Maniglio R. The impact of child sexual abuse on health: A systematic review of reviews. Clin Psychol Rev. 2009;29:647–57. doi: 10.1016/j.cpr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Jumper SA. A meta-analysis of the relationship of child sexual abuse to adult psychological adjustment. Child Abuse Negl. 1995;19:715–28. doi: 10.1016/0145-2134(95)00029-8. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 12.Stoltenborgh M, van Ijzendoorn MH, Euser EM, Bakermans-Kranenburg MJ. A global perspective on child sexual abuse: Meta-analysis of prevalence around the world. Child Maltreat. 2011;16:79–101. doi: 10.1177/1077559511403920. [DOI] [PubMed] [Google Scholar]
- 13.Pereda N, Guilera G, Forns M, Gómez-Benito J. The prevalence of child sexual abuse in community and student samples: A meta-analysis. Clin Psychol Rev. 2009;29:328–38. doi: 10.1016/j.cpr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Davis MK, Gidycz CA. Child sexual abuse prevention programs: A meta-analysis. J Clin Child Psychol. 2000;29:257–65. doi: 10.1207/S15374424jccp2902_11. [DOI] [PubMed] [Google Scholar]
- 15.Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, et al. Sexual abuse and lifetime diagnosis of somatic disorders: A systematic review and meta-analysis. JAMA. 2009;302:550–61. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- 16.Spataro J, Mullen PE, Burgess PM, Wells DL, Moss SA. Impact of child sexual abuse on mental health: Prospective study in males and females. Br J Psychiatry. 2004;184:416–21. doi: 10.1192/bjp.184.5.416. [DOI] [PubMed] [Google Scholar]
- 17.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Maniglio R. Child sexual abuse in the etiology of depression: A systematic review of reviews. Depress Anxiety. 2010;27:631–42. doi: 10.1002/da.20687. [DOI] [PubMed] [Google Scholar]
- 19.Hill J, Pickles A, Burnside E, Byatt M, Rollinson L, Davis R, et al. Child sexual abuse, poor parental care and adult depression: Evidence for different mechanisms. Br J Psychiatry. 2001;179:104–9. doi: 10.1192/bjp.179.2.104. [DOI] [PubMed] [Google Scholar]
- 20.Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, et al. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28:430–8. doi: 10.1016/j.amepre.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Klonsky ED, Moyer A. Childhood sexual abuse and non-suicidal self-injury: Meta-analysis. Br J Psychiatry. 2008;192:166–70. doi: 10.1192/bjp.bp.106.030650. [DOI] [PubMed] [Google Scholar]
- 22.Maniglio R. The role of child sexual abuse in the etiology of suicide and non-suicidal self-injury. Acta Psychiatr Scand. 2011;124:30–41. doi: 10.1111/j.1600-0447.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 23.Boudewyn AC, Liem JH. Childhood sexual abuse as a precursor to depression and self-destructive behavior in adulthood. J Trauma Stress. 1995;8:445–59. doi: 10.1007/BF02102969. [DOI] [PubMed] [Google Scholar]
- 24.Briere J, Elliott DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27:1205–22. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Bergen HA, Martin G, Richardson AS, Allison S, Roeger L. Sexual abuse and suicidal behavior: A model constructed from a large community sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:1301–9. doi: 10.1097/01.chi.0000084831.67701.d6. [DOI] [PubMed] [Google Scholar]
- 26.Martin G, Bergen HA, Richardson AS, Roeger L, Allison S. Sexual abuse and suicidality: Gender differences in a large community sample of adolescents. Child Abuse Negl. 2004;28:491–503. doi: 10.1016/j.chiabu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Klonsky ED, Muehlenkamp JJ. Self-injury: A research review for the practitioner. J Clin Psychol. 2007;63:1045–56. doi: 10.1002/jclp.20412. [DOI] [PubMed] [Google Scholar]
- 28.Lipschitz DS, Winegar RK, Nicolaou AL, Hartnick E, Wolfson M, Southwick SM. Perceived abuse and neglect as risk factors for suicidal behavior in adolescent inpatients. J Nerv Ment Dis. 1999;187:32–9. doi: 10.1097/00005053-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Bangs ME, Tauscher-Wisniewski S, Polzer J, Zhang S, Acharya N, Desaiah D, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47:209–18. doi: 10.1097/chi.0b013e31815d88b2. [DOI] [PubMed] [Google Scholar]
- 30.Hetzel-Riggin MD, Brausch AM, Montgomery BS. A meta-analytic investigation of therapy modality outcomes for sexually abused children and adolescents: An exploratory study. Child Abuse Negl. 2007;31:125–41. doi: 10.1016/j.chiabu.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–27. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 33.De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10:150–72. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- 34.Glaser D. Child abuse and neglect and the brain – A review. J Child Psychol Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- 35.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat Rev Genet. 2012;13:537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 39.van Praag HM, Kahn RS, Asnis GM, Wetzler S, Brown SL, Bleich A, et al. Denosologization of biological psychiatry or the specificity of 5-HT disturbances in psychiatric disorders. J Affect Disord. 1987;13:1–8. doi: 10.1016/0165-0327(87)90067-x. [DOI] [PubMed] [Google Scholar]
- 40.Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs. 2013;27:703–16. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 41.Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–9. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14:121–9. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 44.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–62. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–78. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 48.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–90. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 50.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 51.de Kloet ER, Joëls M, Holsboer F. Nat Rev Neurosci. 2005. Stress and the brain: From adaptation to disease; pp. 63–75. [DOI] [PubMed] [Google Scholar]
- 52.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grabe HJ, Schwahn C, Appel K, Mahler J, Schulz A, Spitzer C, et al. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1483–93. doi: 10.1002/ajmg.b.31131. [DOI] [PubMed] [Google Scholar]
- 54.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hart H, Rubia K. Neuroimaging of child abuse: A critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–58. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 59.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 61.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–66. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–25. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 65.Masten AS. Resilience in developing systems: Progress and promise as the fourth wave rises. Dev Psychopathol. 2007;19:921–30. doi: 10.1017/S0954579407000442. [DOI] [PubMed] [Google Scholar]
- 66.Van den Bergh BR. Developmental programming of early brain and behaviour development and mental health: A conceptual framework. Dev Med Child Neurol. 2011;53(Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- 67.Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–8. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- 68.Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, et al. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J Trauma Stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- 69.Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: A pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–16. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 71.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 72.McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: A review of neurobiological and genetic research. J R Soc Med. 2012;105:151–6. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30:7466–72. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomoda A, Sheu YS, Rabi K, Suzuki H, Navalta CP, Polcari A, et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54(Suppl 1):S280–6. doi: 10.1016/j.neuroimage.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55:759–65. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 76.Schiffer B, Peschel T, Paul T, Gizewski E, Forsting M, Leygraf N, et al. Structural brain abnormalities in the frontostriatal system and cerebellum in pedophilia. J Psychiatr Res. 2007;41:753–62. doi: 10.1016/j.jpsychires.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Minzenberg MJ, Poole JH, Vinogradov S. A neurocognitive model of borderline personality disorder: Effects of childhood sexual abuse and relationship to adult social attachment disturbance. Dev Psychopathol. 2008;20:341–68. doi: 10.1017/S0954579408000163. [DOI] [PubMed] [Google Scholar]
- 80.Alaggia R. Many ways of telling: Expanding conceptualizations of child sexual abuse disclosure. Child Abuse Negl. 2004;28:1213–27. doi: 10.1016/j.chiabu.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Jackowski AP, de Araújo CM, de Lacerda AL, Mari Jde J, Kaufman J. Neurostructural imaging findings in children with post-traumatic stress disorder: Brief review. Psychiatry Clin Neurosci. 2009;63:1–8. doi: 10.1111/j.1440-1819.2008.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46:500–6. doi: 10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalenberg CJ, Palesh OG. Relationship between child abuse history, trauma, and dissociation in Russian college students. Child Abuse Negl. 2004;28:461–74. doi: 10.1016/j.chiabu.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Afifi TO, Boman J, Fleisher W, Sareen J. The relationship between child abuse, parental divorce, and lifetime mental disorders and suicidality in a nationally representative adult sample. Child Abuse Negl. 2009;33:139–47. doi: 10.1016/j.chiabu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Widom CS, Czaja SJ, Paris J. A prospective investigation of borderline personality disorder in abused and neglected children followed up into adulthood. J Pers Disord. 2009;23:433–46. doi: 10.1521/pedi.2009.23.5.433. [DOI] [PubMed] [Google Scholar]
- 88.Jouriles EN, McDonald R, Slep AM, Heyman RE, Garrido E. Child abuse in the context of domestic violence: Prevalence, explanations, and practice implications. Violence Vict. 2008;23:221–35. doi: 10.1891/0886-6708.23.2.221. [DOI] [PubMed] [Google Scholar]
- 89.Deblinger E, Mannarino AP, Cohen JA, Runyon MK, Steer RA. Trauma-focused cognitive behavioral therapy for children: Impact of the trauma narrative and treatment length. Depress Anxiety. 2011;28:67–75. doi: 10.1002/da.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 91.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev Psychopathol. 2012;24:143–55. doi: 10.1017/S0954579411000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Dev Psychopathol. 2011;23:1107–24. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Bellis MD, Spratt EG, Hooper SR. Neurodevelopmental biology associated with childhood sexual abuse. J Child Sex Abus. 2011;20:548–87. doi: 10.1080/10538712.2011.607753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gralton E, Muchatuta A, Morey-Canellas J, Lopez C. Developmental traumatology: Its relevance to forensic adolescent settings. Br J Forensic Pract. 2008;10:33–8. [Google Scholar]