Abstract
Hypercholesterolemia is one of primary risk factors of cardiovascular disease, together with metabolic syndrome, hypertension and diabetes. Although progress has been made, the search for novel methods of preventing and treating dyslipidemia is ongoing and current therapies for cardiovascular disease induce various side effects. β-glucans are linear unbranched polysaccharides found in various natural sources, such as mushrooms. Due to their structure they are able to interact with innate immunity receptors, however they also act as dietary fibers in the digestive tract. As there are two forms of β-glucans, insoluble and soluble forms, they are able to interact with lipids and biliary salts in the bowel and consequently reduce cholesterol levels. Therefore, they may be developed as a suitable therapeutic option to treat patients with dyslipidemia, as they are natural molecules that do not induce any significant side effects. The current review discusses the evidence supporting the effects of β-glucans on cholesterol levels.
Keywords: cholesterol, β-glucans, diet, fiber, disease, diabetes, obesity
Introduction
According to statistics published by the World Health Organization, cardiovascular disease (CVD) is a leading cause of mortality and morbidity worldwide (1). Between 1980 and 2014, the prevalence of obesity worldwide doubled. Currently, nearly 40% of adults aged ≥18 years old, (~2 billion people) are overweight and ~13% (~600 million) are obese (2). Furthermore, 41 million children aged <5 years old are overweight or obese (3). According to statistics compiled by the Center for Disease Control and Prevention, ~610,000 people succumb to heart disease annually in the US alone, representing 1 out of every 4 mortalities (4). Various environmental and behavioral factors may increase the risk of CVD, however, a prevailing body of evidence suggests that contemporary lifestyles, comprising sedentary lifestyles, low energy expenditure together with overnutrition and the inadequate composition of nutrition are primary reasons behind the increase in rates of obesity (5–8). Obese individuals exhibit excessive adipose tissue and dyslipidemia and it has been suggested that these conditions may cause many chronic diseases, including metabolic syndrome, hypertension, hypercholesterolemia and diabetes, all of which are major risk factors for CVD (5–8). Excessive adiposity is accompanied by the accumulation of activated macrophages, which release proinflammatory cytokines, including tumor necrosis factor α, interleukin-6 (IL-6) and IL-1 (9,10). These pathological conditions increase endogenous formation of blood cholesterol and its subsequent deposition in blood vessel walls (11,12). Identifying ways of reducing elevated low density lipoprotein cholesterol (LDL-C) levels is therefore a key public health challenge and introducing novel dietary supplements may be an important method of achieving this (13).
A number of studies aiming to develop novel methods of regulating cholesterol levels are currently in progress. It has been proposed that products of natural origin, including glucomannans, plant sterols, berberine or red yeast rice, which are known as nutraceuticals, can be used either alone (14) or in combination with commonly used statins, such as 3-hydroxy-3-methylglutaryl coenzyme A inhibitors. Although statins represent an important method of regulating cholesterol levels, they may induce significant side effects on skeletal muscle, including myalgia and rhabdomyolysis (15,16), as well as on the homeostasis of bowel microbiota, including dysbiosis with intestinal dysfunction (17,18).
Prebiotics, such as dietary fiber, have been evaluated due to their ability to modulate intestinal microbiota. They may be able to improve the selection of protective bacteria such as Lactobacillus and Enterococcus and therefore help to prevent cancer (19–21). Studies have suggested that the risk of colorectal cancer is increased by consumption of processed and unprocessed meat, but is decreased suppressed by consumption of fiber. Food composition may affect cancer risk due to its effects on the metabolism of colon microbes. The gut microbiota ferment complex dietary residues that are resistant to digestion by enteric enzymes. This process provides energy for the microbiota but culminates in the release of short-chain fatty acids (22).
However, it has also been demonstrated that they help to regulate hyperlipidemia (23,24). There are two types of dietary fiber: Soluble fiber, including pectin, fructo-oligosaccharides and oat β-glucan; and insoluble fiber, including cellulose found in whole wheat bread and brown rice and certain types of β-glucans, found in yeast and mushrooms. These fibers, via their digestion or fermentation by intestinal microbiota may affect cholesterol metabolism by acting on bile acids and affecting lipid absorption (25,26). They may also influence the local microbial environment through bacterial selection by butyrate production. Therefore, they may affect the colonic mucosa as well as mucosal and systemic immunity, including mucosal repair in chronic inflammation and the reduction of proinflammatory cytokines (28–32).
Among different types of fiber, β-glucans have attracted particular interest due to their well-documented activities as modulators of immunity (33,34). The immunomodulatory activities involve anti-infectious immunity, anti-cancer immunity and all aspects of cellular and humoral defense reactions. In addition, β-glucans are nutraceuticals able to decrease cholesterol levels (35,36). Therefore, β-glucans may be the best type of soluble fiber for dietary supplementation and to improve general health. The aim of the current review is to assess the most up-to-date information regarding various aspects of dietary fibers and β-glucans in order to evaluate their potential in regulating cholesterol levels.
2. β-glucans
Natural products that may prevent or treat various diseases have been identified, including β-glucans. Chemically, β-glucans are heterogeneous non-starch polysaccharides, which form the structural compounds of the cell wall of certain microorganisms, including yeast and algae, and certain protists, including mushrooms and grains, such as oats and wheat. β-glucans may be insoluble or soluble. Insoluble β-glucans fibers consist of β-(1,3/1,4)-D-linked glucose units, whereas soluble viscous β-glucans fibers consist of β-(1,3)/1,6)-D-linked glucose (37). A schematic representation of the basic molecular structure of β-glucan is presented in Fig. 1.
Figure 1.
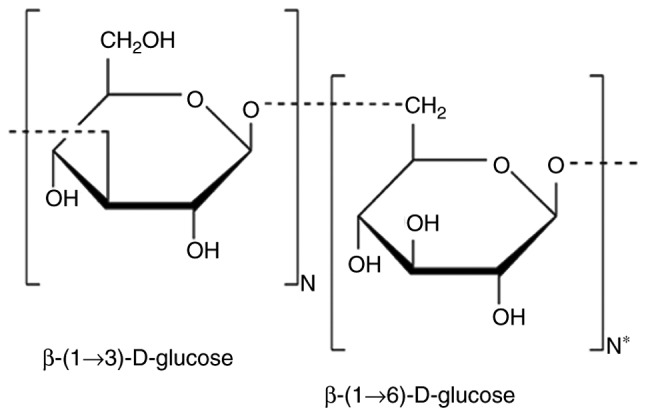
Schematic representation of the basic molecular structure of glucan molecule. N and N* represent the number of specific repetitions of each structure.
It has been demonstrated that β-glucans reduce levels of a non-high-density lipoprotein-cholesterol (non-HDL-C) fraction that contains LDL-C without affecting HDL-C or triglyceride levels (35,38–40). β-glucans have been used to reduce blood cholesterol levels since the 1960s (41). β-glucans are also used to enhance the efficiency of the immune system (42,43).
3. β-glucans and immunity
Research conducted over the previous 40 years has established the biological effects of β-glucan. Various studies have indicated that β-glucans represent important substances to be included, among other substances, within the biological response modifiers, which are substances that can modulate immune activity (44), as they exhibit strong immunomodulatory properties (45). These immunomodulatory properties primarily depend on the source they have been extracted from; studies conducted in animals and humans and confirmed that the oral administration of yeast β-glucans had a greater effect on the immune system than grain-derived β-glucans (45–48). β-glucan is active in all species from earthworms to humans, and is able to stimulate humoral and cellular immunity (45), metabolically regulate diabetes (49), stimulate wound healing (50), reduce psychophysical stress (51), attenuate chronic fatigue syndrome (52) and inhibit the development of cancer (53,54). The effects of β-glucan on the immune system are summarized in Fig. 2A. Clinical trials into the effects of β-glucan on the immune system have now been conducted (55,56) and it has been demonstrated that β-glucans not only affect the immune system but may also reduce cholesterol levels. Different types of β-glucans, not only β-glucans found in oats, are able to reduce cholesterol levels.
Figure 2.
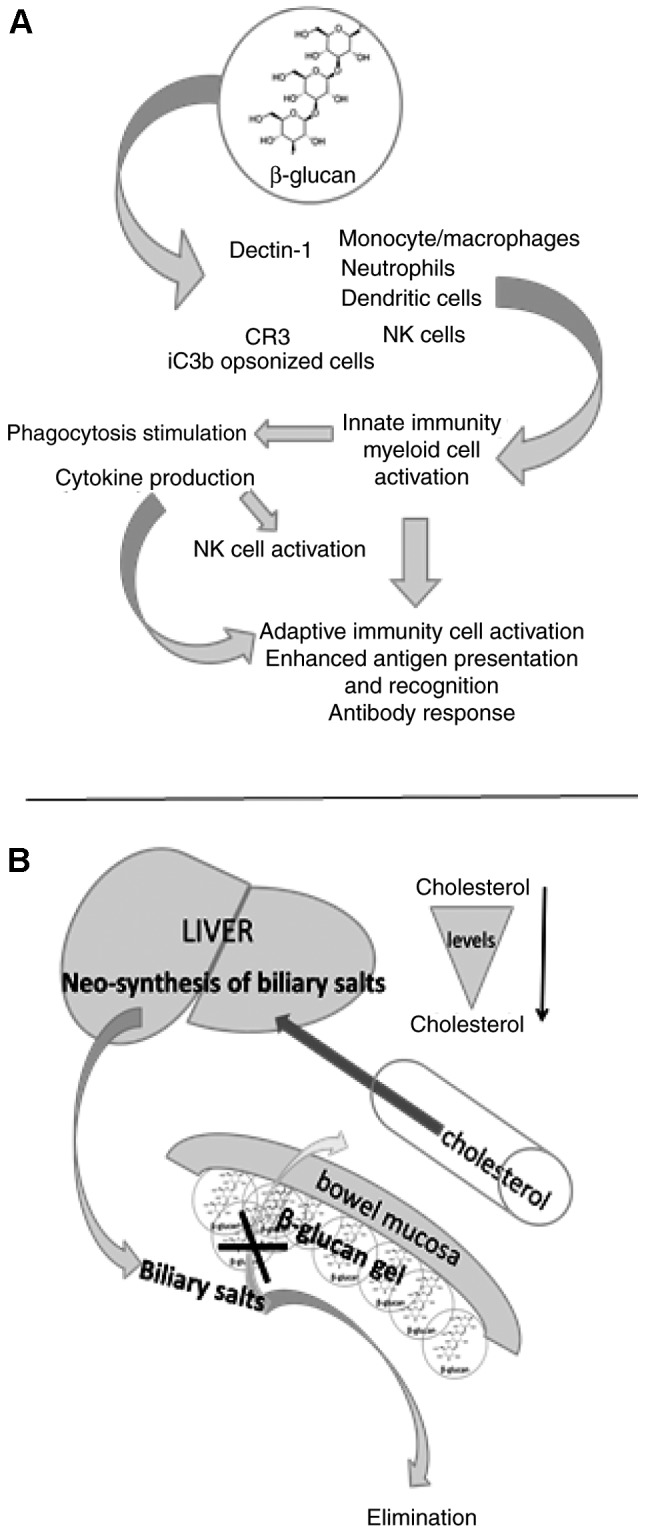
Simplified representation of the effects of β-glucan on immunity and cholesterol balance. (A) β-glucans interact with receptors expressed on the surface of innate immune cells and with iC3b/CR3 complement complex of opsonized cells, resulting in immune stimulation that involves innate and adaptive immunity. (B) It is hypothesized that, acting as fibers, β-glucans can form a gel on the mucosal surface of the bowel. This inhibits resorption of the biliary salts, stimulating biliary salt neo-synthesis in the liver. Increased biliary salts activate utilization of circulating cholesterol, thus reducing its levels in the blood. NK, natural killer cells; CR3, complement receptor type.
Initially, studies investigating the effects of β-glucans assessed insoluble β-glucans; studies evaluating β-glucan-receptor interactions were conducted later. Among the most important receptors that interact with β-glucans are dectin-1, complement receptor type 3 [CR3; also known as cluster of differentiation (CD)11b/CD18] and langerin. Dectin-1 is a type II transmembrane protein present in leukocytes, with the highest levels of expression in neutrophils, macrophages and dendritic cells (57). Langerin is composed of two main parts: A carbohydrate-recognizing domain and a region that can form trimmers and this receptor is most commonly expressed in Langerhans cells (58). CR3 is a receptor that forms part of the integrin β2 family. It is mostly found on neutrophils, macrophages and natural killer cells, but is also present on bone marrow progenitor cells (59). The special configuration assumed by the CR3 receptor following interaction with complement iC3b on opsonized target cells mediates cytotoxic activation (60). The importance of CR3 in β-glucan binding and action was confirmed by the resistance of CR3-knockout mice to β-glucan (61). More detailed information regarding the molecular interactions of β-glucans with their receptors has been documented in a recent comprehensive review (62).
Experimental studies investigating the signaling pathways activated following β-glucan-cell interaction indicated that nuclear factor NF-κB was activated via inhibitor of NF-κB kinase β activity and following alteration of the phosphorylation and degradation of factor of k light polypeptide gene enhancer in B-cells inhibitor a (IkBa) (63). β-glucan may alter the response to polymicrobial sepsis via modulation of IKK3 kinase activity with subsequent decreases in the phosphorylation of IkBa and activation of NF-κB. In addition, the involvement of tyrosine-protein kinase SYK (Syk) was confirmed on the dectin-1 receptor. β-glucan binding is followed by the phosphorylation of dectin-1 by proto-oncogene tyrosine-protein kinase Src. As a result, Syk is activated and subsequently activates the CARD9-Bc110-Malt1 complex (64). It was demonstrated that Syk mediates the activation of downstream signaling; incusing the extracellular-regulated kinase 1/2 and p38a mitogen-activated protein kinase (MAPK) cascade; activating mitogen and stress activated protein kinases 1 and 2 and subsequently phosphorylating the cAMP response element binding protein. The involvement of the extracellular-regulated kinase 1/2 and p38a mitogen-activated protein kinase (MAPK) pathways was identified (65). Furthermore, β-glucan and its associated signaling pathways have been evaluated previously (66). However, current understanding of the intracellular signaling that occurs following β-glucan binding remains incomplete. There may either be too many competing receptors able to bind β-glucan or different types of β-glucans isolated from several different sources may have been used in previous studies, complicating the results.
4. Cholesterol and bile acids as essential molecules
Cholesterol is a source of biologically active substances important for the survival of organisms and serves a pivotal role in the body; therefore, cholesterol should not be unilaterally condemned. Together with other phospholipid substances, cholesterol forms the basic structural components of cell membranes and participates in their biochemical activity. To a lesser extent, cholesterol is also located in membranes of intracellular organelles and in lipoproteins found in the blood plasma, where 70% of cholesterol is esterified with fatty acids to form cholesteryl esters. The brain tissue contains ~25% of cholesterol in the body (67). Cholesterol is synthetized in the liver and is essential for the formation of bile acids, steroid hormones and vitamin D (68). It then becomes involved in regulating energy metabolism and overall energy expenditure; for example, it promotes adaptive thermogenesis following exposure to cold via its conversion to bile acids and by shaping the gut microbiome (69). Primary bile salts are produced in the liver via the oxidization of cholesterol to produce cholic acid and chenodeoxycholic acid. These products are then conjugated with taurine and glycine to produce taurocholic and glycocholic acids, and once they are in the bile, they are utilized in lipid metabolism and energy expenditure, together with pancreatic lipase (70).
Exogenous cholesterol enters the body with food of animal origin. It binds to LDL and enters the cells via receptor endocytosis, which involves the binding of LDL-C to cell surface receptors, which are subsequently engulfed (71). The enterohepatic circulation of bile acids is physiologically significant. Bile acids are efficiently reabsorbed from the intestine into the liver and only 5–20% of these bile acids are lost in the feces. If larger quantities of bile acids are lost, a corresponding amount must be synthesized de novo in the liver (72) and as a source of newly synthetized bile acids, it lowers blood cholesterol levels. Therefore, it is important to bear in mind that all processes leading to the increased fecal excretion of bile acids reduce blood cholesterol levels. The enterohepatic circulation of bile acids is decreased by means of free calcium ions, primarily found in milk, which form insoluble compounds with intestinal bile acids, known as 'soap substances'. This leads to the potential lowering of circulating cholesterol levels, resulting in the neo-synthesis of bile acids in the liver (73). It has been proposed that dietary fibers of various origins, including β-glucans, bind to enteral bile acids and thereby promote their excretion into feces. However, their link to cholesterol is based on a different principle (Fig. 2B).
5. Forms of cholesterol
Cholesterol is a molecule essential for all animal life; therefore every cell in the body is able to synthesize cholesterol via a complex process starting with the mevalonate pathway and ending with lanosterol conversion. Cholesterol is an important substance that exhibits beneficial biological properties, including as a constituent of cellular membranes and a base for steroid hormones and biliary salt synthesis. There are more forms of cholesterol that exist in the organism but the most important molecules regarding health are LDL-C and HDL-C. Other types of lipoproteins present in the blood include chylomicrons, very-low-density lipoproteins (VLDL) and intermediate-density lipoproteins (IDL) (74). The cholesterol found in different lipoproteins is identical, although some exists in its native free alcohol form, whereas others exist as fatty acyl esters. Lipoprotein particles are organized by complex apolipoproteins, which are bound by specific receptors expressed on cell membranes (75). It is important to note that, whereas cholesterol and particularly LDL are usually considered to be the most harmful molecules, non-HDL-C has become a commonly used marker to identify a blood lipid pattern associated with an increased risk of heart disease (76). Non-HDL-C is inversely correlated with HDL-C in children and adults (77,78). Indeed, it has been suggested that non-HDL-C may replace LDL-C as the primary therapeutic target to treat patients with hypercholesterolemia (79). The National Cholesterol Education Program currently recommends that reducing non-HDL-C levels in patients with hypercholesterolemia should be a secondary lipid-lowering target (80). Reducing non-HDL-C levels is becoming an increasingly important target, as the incidence of visceral obesity, diabetes mellitus and metabolic syndrome continues to increase. HDL levels are inversely correlated with atherosclerotic disease; therefore efforts are underway to increase HDL-C and major apolipoprotein of HDL (apoAI) levels. Potential new treatments, including inhibiting the p38 MAPK pathway or administrating apolipoprotein mimetics, were found to be effective at lowering cholesterol in mice and humans (81,82).
Cholesterol can only harm organisms when its concentrations are elevated in the blood, particularly when levels of its oxidized LDL form (ox-LDL) are elevated (83). This may cause long-term chronic inflammation in the artery wall, resulting in the increased storage of cholesterol. HDL, which transports cholesterol to the liver, has the opposite effect and reduces the atherogenic action of ox-LDL (84). In humans (85) and some animals (86,87), atherosclerosis is a disease that evolves following the induction of focal lesions, resulting in atheroma plaque formation at the arterial level in different areas of the body. It is an important cause of coronary vessel obstruction and coronary artery disease, also known as ischemic heart disease (88,89). Two reviews by Gistera and Hansson (90), and by Jonsson and Backhed (91) discuss the complexity of atheroma lesion development, including its sequence of events and underlying mechanisms of action, such as chronic inflammation and macrophage activity fueled by increased cholesterol levels, as well as the influence of the microbiome on atherogenesis.
6. Hypocholesterolemic effects of dietary fibers
None of the cholesterol lowering drugs currently used are free of side effects. By contrast, it is generally accepted that different types of dietary fiber, including β-glucans, have positive effects on health without inducing significant side effects (92). The results of experimental studies in animals and clinical trials in humans have demonstrated that dietary fiber supplements not only improve general health but also reduce the risk of various chronic diseases associated with lifestyle, including CVD, cancer and type 2 diabetes (93), which all have a large impact on public health and society in general. Due to their variety, accurately defining the different types of dietary fibers has been challenging. This is exemplified by the 10-year process that was required to obtain an international legal definition for dietary fiber (https://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/us-codex-alimentarius/recent-delegation-reports/delegate-report-31st-session-ccnfsdu/ct_index) by the Codex Alimentarius Commission (94).
Previously, it was considered that soluble dietary fiber lowered levels of lipids and cholesterol in the blood and that insoluble dietary fiber only contributed to fecal bulking. However, it has now been demonstrated that these effects depend on the degree of viscosity and fermentation capability of fiber (95). The definition of dietary fiber has therefore been extended to include all types of carbohydrates and other resistant substances, such as starches, with similar properties. Soluble dietary fiber that resists enzymatic digestion in the small intestine is not absorbed; it is partially or fully fermented in the large intestine (38,96).
The hypocholesterolemic effects of dietary fiber are still not fully understood. It has been attributed to the ability of soluble dietary fiber to form viscous solutions that prolong gastric emptying, inhibit the transport of triglycerides and cholesterol across the intestine and reduce total LDL-lipoprotein concentrations (97). The consequences of the increased viscosity of the luminal contents manifest via the amplification of the thickness of the water layer and in the decrease of cholesterol uptake from the intestinal lumen (98).
Dietary fiber is able to bind to bile acids, monoglycerides, free fatty acids and cholesterol. Dietary fiber also decreases absorption and increases the fecal excretion of these chemical substances (99,100). The structure of insoluble dietary fiber enables them to directly bind to bile acids and they may lower blood cholesterol levels in this manner, whereas soluble dietary fiber may increase the viscosity of the chyme, thus reducing bile acid diffusion (101). Dietary fiber fractions from mushrooms are also able to modulate cholesterol-related gene expression (34). Furthermore, it has been suggested that treatment with β-glucans prevents the development of fatty liver (102).
Dietary fiber influences the expression of genes that regulate lipid and carbohydrate metabolism. It has been demonstrated that resistant starch, fructans, inulin and β-glucans affect the phosphorylation of AMP-activated protein kinase (AMPK), which is a key enzyme involved in energy exchange (103,104). Furthermore, it is well known that higher concentrations of blood HDL protect against CVD. ApoAI activates AMPK in the arterial endothelia and increases the phosphorylation and activity of nitrogen oxide synthase (105).
Anaerobic gut bacteria in the cecum and large intestine produce short chain fatty acids (SCFAs) as the end products of the fermentation of non-digestible carbohydrates that pass through the small intestine unaffected (106,107). These gut-generated SCFAs exert multiple effects on energy metabolism and on immunity (108–110). SCFAs influence the immune system via G-protein-coupled free fatty acid receptors (FFAs) 2 and 3. FFA2 is expressed primarily in leukocytes and colonic L-cells and also partially expressed in adipocytes, whereas FFA3 is primarily expressed in adipocytes (111). β-glucans may interact directly with immunocompetent cells, such as mucosal macrophages and dendritic cells, which exhibit pattern recognition receptors with carbohydrate-binding domains, subsequently inducing a decrease in IL-12 and increase in IL-10 production by modulation of toll-like receptor signaling (110,112); this is consistent with an anti-inflammatory phenotype. By contrast, the influence of dietary fiber on metabolism and immunity may be indirect; for example, they may act as a source of SCFAs (28,113). The uptake of cholesterol together with fatty acids in the gut into the enterocytes may be accomplished by passive transport and by means of transporter proteins (114,115). Three key transcription factors, known as sterol regulatory element binding proteins 1a, 1c and 2, determine the expression of genes that regulate the synthesis of cholesterol and fatty acids (116,117). Dietary fiber may downregulate the expression of genes for these transporter proteins: Sterol regulatory element binding transcription factor (SREBF)1, which regulates lipid metabolism and SREBF2, which regulates cholesterol synthesis (36,118,119). Therefore, among the potential mechanisms involved in lowering LDL levels in the blood by dietary fiber, the reduced adsorption of biliary salts from the bowel by β-glucans, either by direct glucanbiliary salt complexes or the formation of a viscous layer inhibiting absorption, are supported by the majority of studies (40,41,119). Indeed, the neosynthesis of biliary salts by the liver induces the utilization of the hepatic free cholesterol. Lowering cholesterol levels induces the upregulation of 7-α-hydroxylase, 3-hydroxy-3-methylglutaryl coenzyme A reductase and LDL-C receptors to restore the original conditions (120). This may explain the lowering of LDL by β-glucans and other dietary fibers reported in various studies, although some meta-analyses report that β-glucans affect HDL and LDL cholesterol (35,118–121). At the same time, β-glucans may downregulate the proinflammatory signals elicited by altered levels of cholesterol.
7. Animal studies
To more effectively understand the mechanisms that underline human CVD, suitable animal models are required. Although there are currently no ideal animal models, the comparative investigation of existing models may improve understanding of the sequential onset of the pathological alterations; from the initiation of the disease to the final stages of atherosclerotic plaque development. It has been demonstrated that increasing dietary fiber intake of diabetic animals may reduce the intestinal absorption of nutrients, such as glucose (97,122). Kirby et al (123) indicated that serum cholesterol concentration was reduced by 13% and plasma LDL-C levels were reduced by 14%, whereas HDL-C concentrations remained unchanged in hypercholesterolemic men on a diet supplemented with oat bran. The hypocholesterolemic effects of β-glucans have been confirmed in broiler chicks, which were fed a diet supplemented with barley (124). Furthermore, feeding diabetic rats a high fiber diet supplemented with barley significantly improved plasma glucose concentrations and decreased cholesterol levels compared with rats fed low and very low fiber diets consisting of rice and cornstarch, respectively (125). Golden Syrian hamsters are a suitable animal model to study the vascular changes that occur during atherogenesis (126). These hamsters have a number of advantages: As in humans, their primary cholesterol carrier in the plasma is LDL, their lipoprotein metabolism is similar to that of humans 84 and their LDL receptor gene is similar to the LDL gene in humans. Lim et al (127) applied β-glucans from yeast-like fungus Aureobasidium pullulans, known as polycan, to a hamster experimental animal model of hyperlipemia induced by a high-fat diet. The authors concluded that polycan decreased the atherosclerotic effects, hyperlipemia and hepatic damage induced by a high-fat diet. Previous studies on a hamster model of hypercholesterolemia using β-glucans isolated from oat and barley also identified that they induce beneficial hypolipidemic and anti-atherosclerotic effects (128,129). Another study found that β-glucan treatment reduced the inflammation induced by a diet high in cholesterol (130). In a rat experimental model, β-glucans reduced total cholesterol, triglyceride and malondialdehyde levels by 42% (131). Furthermore, using hamsters as a model, Tong et al (132) found that dietary β-glucans reduced the concentration of plasma LDL-C by promoting the excretion of fecal lipids and regulating the activities of 3′-hydroxy-3-methyl-glutaryl-coenzyme A reductase and CEP7A1. In a study by the current authors, yeast-derived β-glucan 300 markedly lowered total cholesterol levels in mice with experimentally-induced hypercholesterolemia. Supplementation with three other β-glucans, Krestin, ImmunoFiber and Now glucan, induced similar, albeit decreased effects (133). To the best of our knowledge, this study was the first to directly compare the effects of several different β-glucans. As the most effective glucan was yeast-derived, it supports the claim that such results following increased fiber intake may be explained by a subsequent decrease in bile acid absorption (134). To the best of our knowledge, no studies have yet investigated the effects of β-glucans on cholesterol levels in apolipoprotein-E knockout mice (135,136).
8. Human studies
Long-term clinical studies investigating the effects of soluble forms of β-glucans in men with hypercholesterolemia demonstrated that they decrease blood cholesterol levels (137–139). In 1989, Newman et al (140) demonstrated that certain barley cultivars, which are rich in water-soluble fiber, have a hypocholesterolemic effect in men. Several volunteer men were randomly assigned to add wheat or barley bran diet supplements to their normal diets for 28 days.
Those who consumed wheat supplements exhibited significantly increased serum total cholesterol and LDL-C levels compared with pretreatment. The cholesterol levels of subjects that received barley supplements who had average cholesterol levels pretreatment were not significantly altered; however, triglyceride and LDL-C levels were reduced in subjects that had increased cholesterol levels pretreatment. Studies published over the past 20 years have demonstrated that LDL-C levels are reduced following the supplementation of diets with β-glucans in humans with increased blood concentrations of triglycerides and cholesterol (119,120,141–151); however, it has not been confirmed that these effects are caused by β-glucans (152–154). Ho et al (35) published a systematic review and meta-analysis of 14 trials involving 615 healthy and hypercholesterolemic participants, summarizing the evidence that barley β-glucans decrease LDL-C but not HDL-C levels. Diets supplemented with a median dose of 6.5 and 6.9 g/day β-glucans decreased LDL-C and non-HDL-C levels by 7% compared with control diets. Two previous meta-analyses have confirmed the cholesterol-lowering effect of barley β-glucans. One reported a reduction in LDL-C levels by 0.26 mmol.l−1 following a median β-glucan dose of 7 g/day based on the results of seven studies (155). A second meta-analysis, published in 2010, demonstrated that LDL-C was lowered by 0.27 mmol.l−1 following a median β-glucan dose of 5 g/day (156).
It is generally accepted that barley β-glucans reduce apolipoprotein B levels in patients with hypercholesterolemia (157). However, further studies are required to investigate the effects of β-glucans in patients with different metabolic phenotypes, as well as on the content of β-glucans in barley, which depends on the genotype of the plant, and on the environmental conditions and composition of the soil the plant grows in (158). Treatment with an average dose osf 6.5 g/day barley β-glucan for ≥4 weeks may have beneficial effects in individuals with and without elevated blood concentrations of cholesterol (35,159). A further source of β-glucans suitable for lowering blood cholesterol concentrations may include different species of fungi. The underlying mechanisms of β-glucans from mushrooms have not yet been explained. It has been repeatedly confirmed that the consumption of fungi decreases blood cholesterol levels in animals (160,161) and the increased intake of fungi may reduce CVD risk. Clinical trials have also confirmed that the consumption of soluble β-glucans reduces total cholesterol levels, including LDL-C, without affecting HDL-C and triglyceride levels (38,39,118). A previous study conducted in a murine model (162) demonstrated that the novel insoluble dietary fiber chitin-glucan, which is extracted from the cell wall of fungi and combined with olive oil, associated with the Mediterranean diet, which is known for its beneficial effects on health (163), substantially reduces levels of Ox-LDL suggesting that this combination may be used as a therapeutic strategy to treat patients with CVD. Furthermore, in patients with mild hypercholesterolemia it has been demonstrated that high-molecular weight β-glucans decrease serum cholesterol levels differently than low-molecular weight β-glucans (164). Beside differences in viscosity, CYP7A1rs3808607 polymorphism also contributed to the different effects of these two tested glucans (164).
9. Conclusions
Over the past several decades, it has been suggested that increasing levels of fiber, including β-glucans, in the diet leads to a reduction in cholesterol levels. However, the precise mechanisms of this action remain unclear. Further research is required to more specifically define the role of β-glucan in modulating the immunological aspects of atherosclerosis. The current review strengthens the hypotheses that of all forms of dietary fiber, natural β-glucan molecules are the most promising to use as a method of treating patients with dyslipidemia. It remains unclear whether increasing β-glucan intake should be recommended to patients with severe hypercholesterolemia, to be used in addition to or even as an alternative to statins, as there have been very few direct comparisons between the effects of using β-glucans and statins on such patients. However, β-glucans may more effective as they have a different mechanism of action than statins; while statins block the action of the liver enzyme responsible for producing cholesterol, β-glucans promote a more physiologically-based rebalancing of cholesterol levels.
Acknowledgments
The authors wish to acknowledge the support of the Institute of Microbiology (CZ; grant no. RVO 61388971) and UniCredit Bank (CZ) and ARPA Foundation, Pisa (IT) donations.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.World Health Organization . Cardiovascular disease. 2016. [Google Scholar]
- 2.World Health Organization . Obesity and overweight fact sheet. 2017. [Google Scholar]
- 3.World Health Organization . Obesity and overweight fact sheet. 2016. [Google Scholar]
- 4.CDC, National Center for Chronic Disease Prevention and Health Promotion and Division for Heart Disease and Stroke Prevention . Heart Disease Facts and Statistics. CDC; [Google Scholar]
- 5.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 6.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: A meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Brancati FL. The challenge of obesity-related chronic diseases. J Gen Intern Med. 2000;15:828–829. doi: 10.1046/j.1525-1497.2000.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oreopoulos A, Ezekowitz JA, McAlister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, Johnson JA, Padwal RS. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85:609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose KS, Gupta SK, Vyas P. Adipocytokine levels in genetically high risk for type 2 diabetes in the Indian population: A cross-sectional study. Exp Diabetes Res. 2012;2012:386524. doi: 10.1155/2012/386524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson NC, Callas PW, Hanley AJ, Festa A, Haffner SM, Wagenknecht LE, Tracy RP. Circulating levels of TNF-α are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: The insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2012;97:1032–1040. doi: 10.1210/jc.2011-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 12.Emanuela F, Grazia M, Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harland JI. Food combinations for cholesterol lowering. Nutr Res Rev. 2012;25:249–266. doi: 10.1017/S0954422412000170. [DOI] [PubMed] [Google Scholar]
- 14.Johnston TP, Korolenko TA, Pirro M, Sahebkar A. Preventing cardiovascular heart disease: Promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res. 2017;120:219–225. doi: 10.1016/j.phrs.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: Implications for risk assessment, prevention and treatment. Drug Saf. 2010;33:171–187. doi: 10.2165/11319380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Camerino GM, Musumeci O, Conte E, Musaraj K, Fonzino A, Barca E, Marino M, Rodolico C, Tricarico D, Camerino C, et al. Risk of myopathy in patients in therapy with statins: Identification of biological markers in a pilot study. Front Pharmacol. 2017;8:500. doi: 10.3389/fphar.2017.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caparros-Martin JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, Ward NC, Croft KD, Newsholme P, Hughes JD, et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. doi: 10.1186/s40168-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhaegh BP, de Vries F, Masclee AA, Keshavarzian A, de Boer A, Souverein PC, Pierik MJ, Jonkers DM. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:1004–1013. doi: 10.1111/apt.13583. [DOI] [PubMed] [Google Scholar]
- 19.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A, et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–1495. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- 21.Mao QQ, Lin YW, Chen H, Qin J, Zheng XY, Xu X, Xie LP. Dietary fiber intake is inversely associated with risk of pancreatic cancer: A meta-analysis. Asia Pac J Clin Nutr. 2017;26:89–96. doi: 10.6133/apjcn.102015.03. [DOI] [PubMed] [Google Scholar]
- 22.Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in hut microbiota defensive players for inflammation and atherosclerosis. J Atheroscler Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Rodas MC, Valenzuela R, Videla LA. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci. 2015;16:25168–25198. doi: 10.3390/ijms161025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton-Freeman B, Liyanage D, Rahman S, Edirisinghe I. Ratios of soluble and insoluble dietary fibers on satiety and energy intake in overweight pre- and postmenopausal women. Nutr Healthy Aging. 2017;4:157–168. doi: 10.3233/NHA-160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam CL, Thomson LM, Williams PA, Ross AW. Soluble fermentable dietary fibre (Pectin) decreases caloric intake, adiposity and lipidaemia in high-fat diet-induced obese rats. PLoS One. 2015;10:e0140392. doi: 10.1371/journal.pone.0140392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torcello-Gómez A, Fernández Fraguas C, Ridout MJ, Woodward NC, Wilde PJ, Foster TJ. Effect of substituent pattern and molecular weight of cellulose ethers on interactions with different bile salts. Food Funct. 2015;6:730–739. doi: 10.1039/C5FO00099H. [DOI] [PubMed] [Google Scholar]
- 27.Meneses ME, Martinez-Carrera D, Torres N, Sánchez-Tapia M, Aguilar-López M, Morales P, Sobal M, Bernabé T, Escudero H, Granados-Portillo O, Tovar AR. Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 mice. PLoS One. 2016;11:e0159631. doi: 10.1371/journal.pone.0159631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung TV, Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J Nutr. 2016;146:1970–1979. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Y, Marungruang N, Fak F, Nyman M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br J Nutr. 2015;113:1558–1570. doi: 10.1017/S0007114515000793. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Zhang L, Li H, Smidt H, Wright AG, Zhang K, Ding X, Zeng Q, Bai S, Wang J, et al. Different types of dietary fibers trigger specific alterations in composition and predicted functions of colonic bacterial communities in BALB/c mice. Front Microbiol. 2017;8:966. doi: 10.3389/fmicb.2017.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winglee K, Fodor AA. Intrinsic association between diet and the gut microbiome: Current evidence. Nutr Diet Suppl. 2015;7:69–76. doi: 10.2147/NDS.S62362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476. doi: 10.1371/journal.pone.0080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vannucci L, Krizan J, Sima P, Stakheev D, Caja F, Rajsiglova L, Horak V, Saieh M. Immunostimulatory properties and anti-tumor activities of glucans (Review) Int J Oncol. 2013;43:357–364. doi: 10.3892/ijo.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chanput W, Reitsma M, Kleinjans L, Mes JJ, Savelkoul HF, Wichers HJ. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol Nutr Food Res. 2012;56:822–833. doi: 10.1002/mnfr.201100715. [DOI] [PubMed] [Google Scholar]
- 35.Ho HV, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, Jenkins AL, Vuksan V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomized-controlled trials. Br J Nutr. 2016;116:1369–1382. doi: 10.1017/S000711451600341X. [DOI] [PubMed] [Google Scholar]
- 36.Caz V, Gil-Ramirez A, Largo C, Tabernero M, Santamaría M, Martín-Hernández R, Marín FR, Reglero G, Soler-Rivas C. Modulation of cholesterol-related gene expression by dietary fiber fractions from edible mushrooms. J Agric Food Chem. 2015;63:7371–7380. doi: 10.1021/acs.jafc.5b02942. [DOI] [PubMed] [Google Scholar]
- 37.Barsanti L, Passarelli V, Evangelista V, Frassanito AM, Gualtieri P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat Prod Rep. 2011;28:457–466. doi: 10.1039/c0np00018c. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JW, Baird P, Davis RH, Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Horn L, McCoin M, Kris-Etherton PM, Burke F, Carson JA, Champagne CM, Karmally W, Sikand G. The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc. 2008;108:287–331. doi: 10.1016/j.jada.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 40.Vetvicka V, Vetvickova J. Effects of yeast-derived beta-glucans on blood cholesterol and macrophage functionality. J Immunotoxicol. 2009;6:30–35. doi: 10.1080/15476910802604317. [DOI] [PubMed] [Google Scholar]
- 41.de Groot A, Luyken R, Pikaar NA. Cholesterol-lowering effect of rolled oats. Lancet. 1963;2:303–304. doi: 10.1016/S0140-6736(63)90210-1. [DOI] [PubMed] [Google Scholar]
- 42.Czop JK. The role of beta-glucan receptors on blood and tissue leukocytes in phagocytosis and metabolic activation. Pathol Immunopathol Res. 1986;5:286–296. doi: 10.1159/000157022. [DOI] [PubMed] [Google Scholar]
- 43.Estrada A, Yun CH, Van Kessel A, Li B, Hauta S, Laarveld B. Immunomodulatory activities of oat beta-glucan in vitro and in vivo. Microbiol Immunol. 1997;41:991–998. doi: 10.1111/j.1348-0421.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 44.Torrence PF. Biological response modifiers: New approaches to disease intervention. Academic Press; Orlando: 1985. p. 397. [Google Scholar]
- 45.Novak M, Vetvicka V. Beta-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008;5:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 46.Vetvicka V, Vetvickova J. β1,3-Glucan: Silver bullet or hot air. Open Glycoscience. 2010;3:1–6. [Google Scholar]
- 47.Vetvicka V, Vetvickova J. Comparison of immunological effects of commercially available β-glucans: Part III. Int J Clin Pathol. 2006;2 doi: 10.15406/icpjl.2016.02.00046. [DOI] [Google Scholar]
- 48.Würsch P, Pi-Sunyer FX. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care. 1997;20:1774–1780. doi: 10.2337/diacare.20.11.1774. [DOI] [PubMed] [Google Scholar]
- 49.Mosikanon K, Arthan D, Kettawan A, Tungtrongchitr R, Prangthip P. Yeast β-glucan modulates inflammation and waist circumference in overweight and obese subjects. J Diet Suppl. 2017;14:173–185. doi: 10.1080/19390211.2016.1207005. [DOI] [PubMed] [Google Scholar]
- 50.Browder W, Williams D, Lucore P, Pretus H, Jones E, McNamee R. Effect of enhanced macrophage function on early wound healing. Surgery. 1988;104:224–230. [PubMed] [Google Scholar]
- 51.Vetvicka V, Vetvickova J. Anti-stress action of an orally-given combination of resveratrol, β-glucan, and vitamin C. Molecules. 2014;19:13724–13734. doi: 10.3390/molecules190913724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vetvicka V, Vetvickova J. β-glucan attenuates chronic fatigue syndrome in murine model. J Nat Sci. 2015;1:e112. [Google Scholar]
- 53.Sima P, Vannucci L, Vetvicka V. Glucans and cancer: Historical perspective. Cancer Translational Med. 2015;1:209–214. doi: 10.4103/2395-3977.172860. [DOI] [Google Scholar]
- 54.Barbieri A, Quagliariello V, Del Vecchio V, Falco M, Luciano A, Amruthraj NJ, Nasti G, Ottaiano A, Berretta M, Iaffaioli RV, Arra C. Anticancer and anti-inflammatory properties of gano-derma lucidum extract effects on melanoma and triple-negative breast cancer treatment. Nutrients. 2017;9:pii:E210. doi: 10.3390/nu9030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter J, Kral V, Svozil V, Rajnohova DL, Pohorska JI, Vetvicka V. Effects of transfer point glucan #300 supplementation on children exposed to passive smoking-placebo-driven double-blind clinical trials. J Nutr Health. 2014;1:105. [Google Scholar]
- 56.Vetvicka V, Richter J, Svozil V, Rajnohová Dobiášová L, Král V. Placebo-driven clinical trials of yeast-derived β-(1-3) glucan in children with chronic respiratory problems. Ann Transl Med. 2013;1:26. doi: 10.3978/j.issn.2305-5839.2013.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 58.Stambach NS, Taylor ME. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- 59.Allendorf DJ, Ostroff GR, BAran JT, et al. BTR 2003: Unified Science & Technology for Reducing Biological Threats & Counterin Terrorism. Albuquesrque: University of New Mexico; 2003. Oral WGP beta glucan treatment accelerates myeloid recovery and survival after irradiation wxposure; pp. 104–113. [Google Scholar]
- 60.Ross GD, Vĕtvicka V. CR3 (CD11b. CD18): A phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Cell Exp Immunol. 1993;92:181–184. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia Y, Vetvicka V, Yan J, Hanikyrová M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 62.Legentil L, Paris F, Ballet C, Trouvelot S, Daire X, Vetvicka V, Ferrières V. Molecular interactions of β-(1→>3)-glucans with their receptors. Molecules. 2015;20:9745–9766. doi: 10.3390/molecules20069745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams DL, Ha T, Li C, Laffan J, Kalbfleisch J, Browder W. Inhibition of LPS-induced NFkappaB activation by a glucan ligand involves down-regulation of IKKbeta kinase activity and altered phosphorylation and degradation of IkappaBalpha. Shock. 2000;13:446–452. doi: 10.1097/00024382-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8:e60086. doi: 10.1371/journal.pone.0060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Větvička V. (Beta)-glucans as natural biological response modifiers. Nova Science Publishers Inc; New York: 2013. [Google Scholar]
- 67.Orth M, Bellosta S. Cholesterol: Its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T, Chiang JY. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Res. 2009;2009:501739. doi: 10.1155/2009/501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Worthmann A, John C, Rühlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23:839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 70.Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159–165. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown MS, Goldstein JL. How LDL receptors influence cholesterol and atherosclerosis. Sci Am. 1984;251:58–66. doi: 10.1038/scientificamerican1184-58. [DOI] [PubMed] [Google Scholar]
- 72.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 73.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 74.Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density-lipoprotein. Biochem Soc Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- 75.Davidson WS, Hilliard GM. The spatial organization of apolipoprotein A-I on the edge of discoidal high density lipo-protein particles. J Biol Chem. 2003;278:27199–27207. doi: 10.1074/jbc.M302764200. [DOI] [PubMed] [Google Scholar]
- 76.de Oliveira Alvim R, Mourao-Junior CA, Magalhaes GL, de Oliveira CM, Krieger JE, Mill JG, Pereira AC. Non-HDL cholesterol is a good predictor of the risk of incfeased arterial stiffness in postmenopausal women in an urban Brazilian population. Clinics (Sao Paulo) 2017;72:106–110. doi: 10.6061/clinics/2017(02)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.CIR.79.1.8. [DOI] [PubMed] [Google Scholar]
- 78.Srinivasan SR, Myers L, Berenson GS. Distribution and correlates of non-high-density lipoprotein cholesterol in children: The Bogalusa heart study. Pediatrics. 2002;110:e29. doi: 10.1542/peds.110.3.e29. [DOI] [PubMed] [Google Scholar]
- 79.Hoenig MR. Implications of the obesity epidemic for lipid-lowering therapy: Non-HDL cholesterol should replace LDL cholesterol as the primary therapeutic target. Vasc Health Risk Manag. 2008;4:143–156. doi: 10.2147/VHRM.S2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The National Cholesterol Education Program. https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf.
- 81.Masson D, Koseki M, Ishibashi M, Larson CJ, Miller SG, King BD, Tall AR. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler Thromb Vasc Biol. 2009;29:2054–2060. doi: 10.1161/ATVBAHA.109.191320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White CR, Garber DW, Anantharamaiah GM. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: A review. J Lipid Res. 2014;55:2007–2021. doi: 10.1194/jlr.R051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parathasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of high-density lipoprotein: Alternations in cardiovascular disease. EMBO Mol Med. 2012;4:251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stary HC. The sequence of cell and matrix changes in athero-sclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990;11(Suppl E):S3–S19. doi: 10.1093/eurheartj/11.suppl_E.3. [DOI] [PubMed] [Google Scholar]
- 86.Sullivan MP, Cerda JJ, Robbins FL, Burgin CW, Beatty RJ. The gerbil, hamster, and guinea pig as rodent models for hyper-lipidemia. Lab Anim Sci. 1993;43:575–578. [PubMed] [Google Scholar]
- 87.Sima A, Stancu C, Constantinescu E, Ologeanu L, Simionescu M. The hyperlipemic hamster-a model for testing the anti-atherogenic effect of amlodipine. J Cell Mol Med. 2001;5:153–162. doi: 10.1111/j.1582-4934.2001.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohmura H, Fukushima Y, Mizuno A, Niwa K, Kobayashi Y, Ebina T, Kimura K, Ishibashi S, Daida H. Research Committee on Primary Hyperlipidemia of the Ministry of Health and Welfare of Japan: Estimated prevalence of heterozygous familial hypercholesterolemia in patients with acute coronary syndrome. Int Heart J. 2017;58:88–94. doi: 10.1536/ihj.16-188. [DOI] [PubMed] [Google Scholar]
- 89.Rosenson RS, Brewer HB, Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gistera A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 91.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 92.Babicek K, Cehová I, Simon RR, Harwood M, Cox DJ. Toxicological assesment of a particulate yeast (1,3/1,6)-beta-D-glucan in rats. Food Chem Toxicol. 2007;45:1719–1730. doi: 10.1016/j.fct.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 93.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 94.Phillips GO, Cui SW. An introduction: Evolution and finalisation of the regulatory definition of dietary fibre. Food Hydrocolloids. 2011;25:139–143. doi: 10.1016/j.foodhyd.2010.04.011. [DOI] [Google Scholar]
- 95.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411, 418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 96.Cheung PCK. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci Human Wellness. 2013;2:162–166. doi: 10.1016/j.fshw.2013.08.001. [DOI] [Google Scholar]
- 97.Jenkins DJ, Kendall CW, Axelsen M, Augustin LS, Vuksan V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol. 2000;11:49–56. doi: 10.1097/00041433-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Gee JM, Blackburn NA, Johnson IT. The influence of guar gum on intestinal cholesterol transport in the rat. Br J Nutr. 1983;50:215–224. doi: 10.1079/BJN19830091. [DOI] [PubMed] [Google Scholar]
- 99.Chau CF, Huang YL. Effects of the insoluble fiber derived from Passiflora edulis seed on plasma and hepatic lipids and fecal output. Mol Nutr Food Res. 2005;49:786–790. doi: 10.1002/mnfr.200500060. [DOI] [PubMed] [Google Scholar]
- 100.Cho IJ, Lee C, Ha TY. Hypolipidemic effect of soluble fiber isolated from seeds of Cassia tora Linn. In rats fed a high-cholesterol diet. J Agric Food Chem. 2007;55:1592–1596. doi: 10.1021/jf0622127. [DOI] [PubMed] [Google Scholar]
- 101.Zacherl C, Eisner P, Engel KH. In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibres. Food Chem. 2011;126:423–428. doi: 10.1016/j.foodchem.2010.10.113. [DOI] [Google Scholar]
- 102.Aoki S, Iwai A, Kawata K, Muramatsu D, Uchiyama H, Okabe M, Ikesue M, Maeda N, Uede T. Oral administration of the Aureobasidium pullulans-derived β-glucan effectively prevents the development of high fat diet-induced fatty liver in mice. Sci Rep. 2015;5:10457. doi: 10.1038/srep10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 104.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: Ready for prime time. Nutr Clin Pract. 2006;21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 107.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010;1801:1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Wismar R, Brix S, Frøkiaer H, Laerke HN. Dietary fibers as immunoregulatory compounds in health and disease. Ann NY Acad Sci. 2010;1190:70–85. doi: 10.1111/j.1749-6632.2009.05256.x. [DOI] [PubMed] [Google Scholar]
- 111.Vangaveti V, Shashidhar V, Jarrod G, Baune BT, Kennedy RL. Free fatty acid receptors: Emerging targets for treatment of diabetes and its complications. Ther Adv Endocrinol Metab. 2010;1:165–175. doi: 10.1177/2042018810381066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–175. doi: 10.1111/j.1749-6632.2008.03985.x. [DOI] [PubMed] [Google Scholar]
- 113.Cosola C, De Angelis M, Rocchetti MT, Montemurno E, Maranzano V, Dalfino G, Manno C, Zito A, Gesualdo M, Ciccone MM, et al. Beta-glucans supplementation associates with reduction in P-Cresyl sulfate levels and improved endo-thelial vascular reactivity in healthy individuals. PLoS One. 2017;12:e0169635. doi: 10.1371/journal.pone.0169635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mansbach CM, II, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645–G650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 115.Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: Cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293:G519–G524. doi: 10.1152/ajpgi.00189.2007. [DOI] [PubMed] [Google Scholar]
- 116.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 117.Sato R. Sterol metabolism and SREBP activation. Arch Biochem Biophys. 2010;501:177–181. doi: 10.1016/j.abb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 118.Drozdowski LA, Reimer RA, Temelli F, Bell RC, Vasanthan T, Thomson AB. Beta-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J Nutr Biochem. 2010;21:695–701. doi: 10.1016/j.jnutbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen J, Huang XF. The effects of diets enriched in beta-glucans on blood lipoprotein concentrations. J Clin Lipidol. 2009;3:154–158. doi: 10.1016/j.jacl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 120.Rondanelli M, Opizzi A, Monteferrario F, Klersy C, Cazzola R, Cestaro B. Beta-glucan- or rice bran-enriched foods: A comparative crossover clinical trial on lipidic pattern in mildly hypercholesterolemic men. Eur J Clin Nutr. 2011;65:864–871. doi: 10.1038/ejcn.2011.48. [DOI] [PubMed] [Google Scholar]
- 121.Wolever TM, Tosh SM, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Thomson BA, Duss R, Wood PJ. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am J Clin Nutr. 2010;92:723–732. doi: 10.3945/ajcn.2010.29174. [DOI] [PubMed] [Google Scholar]
- 122.Johnson IT, Gee JM. Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut. 1981;22:398–403. doi: 10.1136/gut.22.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirby RW, Anderson JW, Sieling B, Rees ED, Chen WJ, Miller RE, Kay RM. Oat-bran intake selectively lowers serum low-density lipoprotein cholesterol concentrations of hypercholesterolemic men. Am J Clin Nutr. 1981;34:824–829. doi: 10.1093/ajcn/34.5.824. [DOI] [PubMed] [Google Scholar]
- 124.Fadel JG, Newman RK, Newman CW, Barnes AE. Hypocholesterolemic effects of beta-glucans in different barley diets fed to broiler chicks. Nutr Rep Int. 1987;35:1049–1058. [Google Scholar]
- 125.Li J, Kaneko T, Qin LQ, Wang J, Wang Y, Sato A. Long-term effects of high dietary fiber intake on glucose tolerance and lipid metabolism in GK rats: Comparison among barley, rice, and cornstarch. Metabolism. 2003;52:1206–1210. doi: 10.1016/S0026-0495(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 126.Sima A, Bulla A, Simionescu N. Experimental obstructive coronary atherosclerosis in the hyperlipidemic hamster. J Submicrosc Cytol Pathol. 1990;22:1–16. [PubMed] [Google Scholar]
- 127.Lim MK, Ku SK, Choi JS, Kim JW. Effect of polycan, a β-glucan originating from Aureobasidium, on a high-fat diet-induced hyperlipemic hamster model. Exp Ther Med. 2015;9:1369–1378. doi: 10.3892/etm.2015.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delaney B, Nicolosi RJ, Wilson TA, Carlson T, Frazer S, Zheng GH, Hess R, Ostergren K, Haworth J, Knutson N. Beta-glucan fractions from barley and oats are similarly anti-atherogenic in hypercholesterolemic Syrian golden hamsters. J Nutr. 2003;133:468–475. doi: 10.1093/jn/133.2.468. [DOI] [PubMed] [Google Scholar]
- 129.Wilson TA, Nicolosi RJ, Delaney B, Chadwell K, Moolchandani V, Kotyla T, Ponduru S, Zheng GH, Hess R, Knutson N, et al. Reduced and high molecular weight barley beta-glucans decrease plasma total and non-HDL-cholesterol in hypercholesterolemic Syrian golden hamsters. J Nutr. 2004;134:2617–2622. doi: 10.1093/jn/134.10.2617. [DOI] [PubMed] [Google Scholar]
- 130.Wu YS, Ho SY, Nan FH, Chen SN. Ganoderma lucidum beta 1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement Altern Med. 2016;16:500. doi: 10.1186/s12906-016-1476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kusmiati, Dhewantara FX. Cholesterol-lowering effect of beta glucan extracted from Saccharomyces cerevisiae in rats. Sci Pharm. 2016;84:153–165. doi: 10.3797/scipharm.ISP.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tong LT, Zhong K, Liu L, Zhou X, Qiu J, Zhou S. Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem. 2015;169:344–349. doi: 10.1016/j.foodchem.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 133.Vetvicka V, Vetvickova J. Physiological effects of different types of beta-glucan. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:225–231. doi: 10.5507/bp.2007.038. [DOI] [PubMed] [Google Scholar]
- 134.Malkki Y. Oat fiber. In: Cho S, Dreher ML, editors. Food Science and Technology: Handbook of Dietary Fiber. M. Dekker; New York: 2001. pp. 497–512. [Google Scholar]
- 135.Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in Apoe−/− and Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2001;21:1567–1570. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 136.Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: Atherosclerosis, metabolic syndrome, and beyond. J Lipid Res. 2009;50(Suppl):S178–S182. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson JW, Story L, Sieling B, Chen WJ, Petro MS, Story J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am J Clin Nutr. 1984;40:1146–1155. doi: 10.1093/ajcn/40.6.1146. [DOI] [PubMed] [Google Scholar]
- 138.Anderson JW, Gustafson NJ. Hypocholesterolemic effects of oat and bean products. Am J Clin Nutr. 1988;48(Suppl 3):S749–S753. doi: 10.1093/ajcn/48.3.749. [DOI] [PubMed] [Google Scholar]
- 139.Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P, Collins MW. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur J Clin Nutr. 1994;48:465–474. [PubMed] [Google Scholar]
- 140.Newman RK, Lewis SE, Newman CW, Boik RJ, Ramage RT. Hypocholesterolemic effect of barley foods on healthy men. Nutr Rep Int. 1989;39:749–760. [Google Scholar]
- 141.McIntosh GH, Whyte J, McArthur R, Nestel PJ. Barley and wheat foods: Influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr. 1991;53:1205–1209. doi: 10.1093/ajcn/53.5.1205. [DOI] [PubMed] [Google Scholar]
- 142.Lupton JR, Robinson MC, Morin JL. Cholesterol-lowering effect of barley bran flour and oil. J Am Diet Assoc. 1994;94:65–70. doi: 10.1016/0002-8223(94)92044-3. [DOI] [PubMed] [Google Scholar]
- 143.Lia A, Hallmans G, Sandberg AS, Sundberg B, Aman P, Anderson H. Oat beta-glucan increases bile excretion and a fiber rich barley fracton increases cholesterol excretion in ileostomy subjects. Am J Clin Nutr. 1995;62:1245–1251. doi: 10.1093/ajcn/62.6.1245. [DOI] [PubMed] [Google Scholar]
- 144.Bell S, Goldman VM, Bistrian BR, Arnold AH, Ostroff G, Forse RA. Effect of beta-glucan from oats and yeast on serum lipids. Crit Rev Food Sci Nutr. 1999;39:189–202. doi: 10.1080/10408399908500493. [DOI] [PubMed] [Google Scholar]
- 145.Li J, Kaneko T, Qin LQ, Wang J, Wang Y. Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition. 2003;19:926–929. doi: 10.1016/S0899-9007(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 146.Behall KM, Scholfield DJ, Hallfrisch J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am J Clin Nutr. 2004;80:1185–1193. doi: 10.1093/ajcn/80.5.1185. [DOI] [PubMed] [Google Scholar]
- 147.Behall KM, Scholfield DJ, Hallfrisch J. Lipids significantly reduced by diets containing barley in moderately hypercholes-terolemic men. J Am Coll Nutr. 2004;23:55–62. doi: 10.1080/07315724.2004.10719343. [DOI] [PubMed] [Google Scholar]
- 148.Keenan JM, Goulson M, Shamliyan T, Knutson N, Kolberg L, Curry L. The effects of concentrated barley beta-glucan on blood lipids in a population of hypercholesterolaemic men and women. Br J Nutr. 2007;97:1162–1168. doi: 10.1017/S0007114507682968. [DOI] [PubMed] [Google Scholar]
- 149.Shimizu C, Kihara M, Aoe S, Araki S, Ito K, Hayashi K, Watari J, Sakata Y, Ikegami S. Effect of high beta-glucan barley on serum cholesterol concentrations and visceral fat area in Japanese men-a randomized, double-blinded, placebo-controlled trial. Plant Foods Hum Nutr. 2008;63:21–25. doi: 10.1007/s11130-007-0064-6. [DOI] [PubMed] [Google Scholar]
- 150.Sundberg B. Cholesterol lowering effects of a barley fibre flake product. Agro Food Industry Hi-Tech. 2008;19:14–17. [Google Scholar]
- 151.Zhu X, Sun X, Wang M, Zhang C, Cao Y, Mo G, Liang J, Zhu S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr Metab Cardiovasc Dis. 2015;25:714–723. doi: 10.1016/j.numecd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 152.Keogh GF, Cooper GJ, Mulvey TB, McArdle BH, Coles GD, Monro JA, Poppitt SD. Randomized controlled crossover study of the effect of a highly beta-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterol-emic men. Am J Clin Nutr. 2003;78:711–718. doi: 10.1093/ajcn/78.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Biörklund M, van Rees A, Mensink RP, Onning G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with beta-glucans from oats or barley: A randomised dose-controlled trial. Eur J Clin Nutr 5. 2005;9:1272–1281. doi: 10.1038/sj.ejcn.1602240. [DOI] [PubMed] [Google Scholar]
- 154.Ibrügger S, Kristensen M, Poulsen MW, Mikkelsen MS, Ejsing J, Jespersen BM, Dragsted LO, Engelsen SB, Bügel S. Extracted oat and barley β-glucans do not affect cholesterol metabolism in young healthy adults. J Nutr. 2013;143:1579–1585. doi: 10.3945/jn.112.173054. [DOI] [PubMed] [Google Scholar]
- 155.Talati R, Baker WL, Pabilonia MS, White CM, Coleman CI. The effects of barley-derived soluble fiber on serum lipids. Ann Fam Med. 2009;7:157–163. doi: 10.1370/afm.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.AbuMweis SS, Jew S, Ames NP. β-glucan from barley and its lipid-lowering capacity: A meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 2010;64:1472–1480. doi: 10.1038/ejcn.2010.178. [DOI] [PubMed] [Google Scholar]
- 157.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 158.Newman RK, Newman CW. Genetics and Nutrient Composition. John Wiley & Sons; Hoboken, NJ: 2008. Barley for food and health: Science, technology, and products; pp. 56–94. [Google Scholar]
- 159.Ho HV, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, Jenkins AL, Vuksan V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley β-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reductioni-iv. Eur J Clin Nutr. 2016;70:1340. doi: 10.1038/ejcn.2016.129. [DOI] [PubMed] [Google Scholar]
- 160.Mori K, Kobayashi C, Tomita T, Inatomi S, Ikeda M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr Res. 2008;28:335–342. doi: 10.1016/j.nutres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 161.Sun JE, Ao ZH, Lu ZM, Xu HY, Zhang XM, Dou WF, Xu ZH. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol. 2008;118:7–13. doi: 10.1016/j.jep.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 162.Bays HE, Evans JL, Maki KC, Evans M, Maquet V, Cooper R, Anderson JW. Chitin-glucan fiber effects on oxidized low-density lipoprotein: A randomized controlled trial. Eur J Clin Nutr. 2013;67:2–7. doi: 10.1038/ejcn.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr Rev. 2006;64(Suppl):S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y, Harding SV, Eck P, Thandapilly SJ, Gamel TH, Abdel-Aal el-SM, Crow GH, Tosh SM, Jones PJ, Ames NP. High-molecular-weight β-glucan decreases serum cholesterol differentially based on the CYP7A1 rs3808607 polymorphism in mildly hypercholesterolemic adults. J Nutr. 2016;146:720–727. doi: 10.3945/jn.115.223206. [DOI] [PubMed] [Google Scholar]


