Abstract
Background
Lymphatic metastasis is regulated in multiple steps including the transit of tumor cells via the lymphatic vessels and the successful seeding in draining lymph nodes. Thus, several molecular signals and cellular changes must be involved in this complex process to facilitate tumor cell entry, colonization, and survival in the lymph node. To our knowledge, the present work explores, for the first time in the literature, the redox status (oxidative stress parameters and enzymatic and non-enzymatic antioxidant defense systems) in the sentinel lymph node (SLN) of women with breast cancer.
Patients and methods
SLNs from 75 women with breast cancer were identified using the one-step nucleic acid amplification (OSNA) method as negative (n = 43), with micrometastases (n = 13), or with macrometastases (n = 19). It will allow us to gain knowledge about the pro-oxidant/antioxidant mechanisms involved in the processes of distant metastases in breast cancer and also to assess whether these parameters may be alternative techniques for staging.
Results
We found different levels of lipid peroxidation in SLNs with micrometastases (increased) and macrometastases (decreased), a decrease in carbonyl group content in SLNs with macrometastases only, and an increase in total antioxidant capacity (TAC) in SNLs with micrometastases and macrometastases. A decrease in the levels of reduced glutathione (GSH) also appears in the SLNs with macrometastases only. Finally, we show increased levels of superoxide dismutase (SOD) and catalase (CAT) activity in SLNs with micrometastases and macrometastases, and decreased levels of glutathione peroxidase (GPx) activity in SNLs with macrometastases but not with micrometastases.
Conclusions
Redox status of lymph node microenvironment participates in the progression of metastatic breast cancer.
Keywords: Breast cancer, catalase, glutathione peroxidase, GSH, OSNA, sentinel lymph node, superoxide dismutase, total antioxidant capacity
Introduction
Cancer metastasis is a multistep process that involves the dissemination of cancer cells from the primary tumor to distant organs (1,2). In this process, little is known about lymphatic metastasis. In contrast to a previous hypothesis which indicated that lymphatic metastasis is an entirely passive process, several recent reports have indicated that it is regulated in multiple steps including the transit of tumor cells via the lymphatic vessels and successful seeding in draining lymph nodes (2,3).
Metastatic status in the axillary lymph nodes is the most important prognostic factor for patients with breast cancer. However, axillary lymph node dissection is associated with significant morbidity. In contrast, sentinel lymph node (SLN) biopsy is a minimally invasive procedure that also allows accurate axillary nodal staging with less morbidity (4). In fact, SLN biopsy has been validated in early breast cancer to reflect the status of the remaining lymph nodes in the draining nodal basin, and patients with negative SLNs avoid axillary lymph node dissection (5). Intraoperative one-step nucleic acid amplification (OSNA) analysis for sentinel node biopsy in breast cancer is beginning to be used to increase the sensitivity of surgical staging through the discovery of microscopic or even cellular metastases missed on routine pathologic review (6). Therefore, increasing recognition of the importance of lymph node metastasis in cancer biology has prompted studies to unravel the cellular and molecular events involved in this complex process.
Several studies support findings that reactive species are involved in the etiology and progression of breast cancer because certain markers of oxidative stress, including lipid peroxidation products such as malondialdehyde (MDA) (7) and 8-isoprostanes (8), protein oxidation products such as carbonyls and diene conjugates (9,10), and DNA adducts (11), are frequently identified in breast cancer patients (12). In contrast, endogenous antioxidants constitute the defense mechanisms that scavenge reactive species in cells, which include non-enzyme and enzyme antioxidant defense systems such as reduced glutathione (GSH), alpha-lipoic acid, coenzyme Q, ferritin, uric acid, bilirubin, metallothionein, l-carnitine, melatonin and enzymatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), respectively. Of them, SOD, CAT, and GPx are the enzymes that normally act to prevent or decrease the tissue damage caused by free radicals. SOD metabolizes free radicals and dismutates superoxide anions (O2•-) to H2O2 and protects cells against O2•--mediated lipid peroxidation, CAT converts H2O2 into H2O and O2, and GPx reduces H2O2 and other organic peroxides. Several studies have also shown significant changes in both enzymatic and non-enzymatic antioxidant systems in women with breast cancer (12,13). However, little is known about the role of oxidative stress in lymph node metastasis. Thus, it has been described in colorectal cancer cells that the increase of intracellular reactive species is first associated with cell growth and invasion. However, a further increase inhibits cancer cell proliferation, whereas any decrease in reactive species needs to stimulate lymph node metastasis (14). On the other hand, it has been also described that breast cancer cell metastasis is due to elevated levels of reactive species (15). Therefore, the knowledge of the redox status of the SLN in women with breast cancer represents a new way to study the role of redox processes and pro-oxidant/antioxidant mechanisms involved in the mechanisms of distant metastases. Also, due to the great success of selective SLN biopsy as a diagnostic staging method, oxidative stress parameters and/or the enzymatic and non-enzymatic antioxidant defense systems involved could be useful as alternative/complementary techniques of staging in women with breast cancer. To that end, in the present report we evaluate a wide range of well known and widely validated oxidative stress parameters (lipid peroxidation, protein oxidation, and total antioxidant capacity), as well as non-enzyme (GSH) and enzyme (SOD, CAT, and GPx) antioxidant defense systems in the SLN of women with breast cancer determined as negative, with micrometastases, or with macrometastases by the OSNA method. An alteration of the normal redox balance to an antioxidant state will be described in the metastatic SLN of women with breast cancer.
Subjects and methods
Subjects and study design
A total of 75 women with breast cancer were recruited at the Unit of Breast Pathology at the University Hospital of Jaén. Enrollment criteria included patients with T2–3 N0 breast cancer who were not previously treated with neoadjuvant chemotherapy. Patients were evaluated before surgery and were included in the study if the axilla was negative clinically and by echography, using a 7–12 MHz lineal probe. When a suspicious node appeared, core biopsy was performed. Patients signed an informed consent for the SLN procedure. This study was approved by the Ethical Committee of the University Hospital of Jaén, and all patients signed a term of free, informed consent. Patient characterization included age at diagnosis, tumor size, tumor histology, pathologic T classification, Scarff–Bloom–Richardson grade, hormonal and HER-2/neu (human epidermal growth factor receptor 2) status, and molecular subtype.
Identification of the sentinel lymph node, evaluation of the axilla status, and surgery
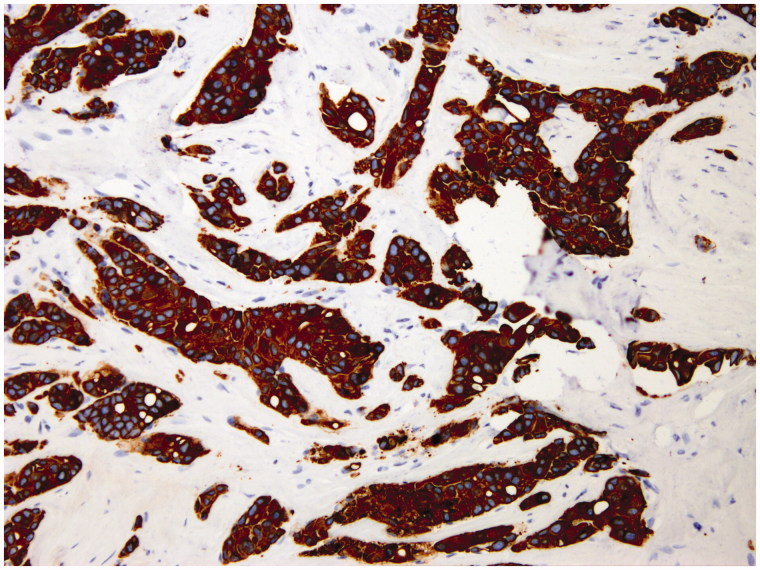
The sentinel procedure was performed for all patients using only a radioisotope. On the day before the surgery, lymphatic mapping was performed using 4.0 mCi of technetium-99 nanocolloid (Nanocoll, Amersham, UK) injected subareolarly. Preoperative lymphoscintigraphy was performed on all patients 1 h after the injection, and the drainage pattern was recorded. A hand-held gamma detection probe (Gamma Finder II, World of Medicine AG, Ludwigsstadt, Germany) was used to identify areas of increased activity in the axilla and nodal basins the day after the injection, usually approximately 20–24 h after the injection. The sentinel node was considered if it was radioactive or palpable. All nodes were detected at axillary level. Each sentinel node was excised, sent to the pathology department, and subjected to OSNA analysis (16). Previously, the expression of cytokeratin 19 (CK19) was confirmed in 100% of the tumor cells by large-core needle biopsy (Figure 1). The OSNA protocol consisted of homogenization of tissue in an mRNA-stabilizing solution (Lynorhag, pH 3.5; Sysmex, Barcelona, Spain) and subsequent isothermal (65 °C) amplification of CK19 using a Lynoamp amplification kit (Sysmex) through a reverse transcriptase loop-mediated isothermal amplification assay (RT-LAMP) in a gene amplification detector RD-100i (Sysmex) in compliance with the protocol described above. The technique uses six primers, which increases the specificity and speed of the reaction. Tissue homogenates from each lymph node were kept frozen at −80 °C as a back-up for possible future studies. All cases were classified according to the tumor–node–metastasis (TNM) classification of malignant tumor staging.
Figure 1.
Monoclonal antibody staining for cytokeratin 19 in large-core needle biopsy of breast carcinoma obtained for diagnosis. Note the intense diffuse staining at membrane and intracytoplasmic level in 100% of tumor cells.
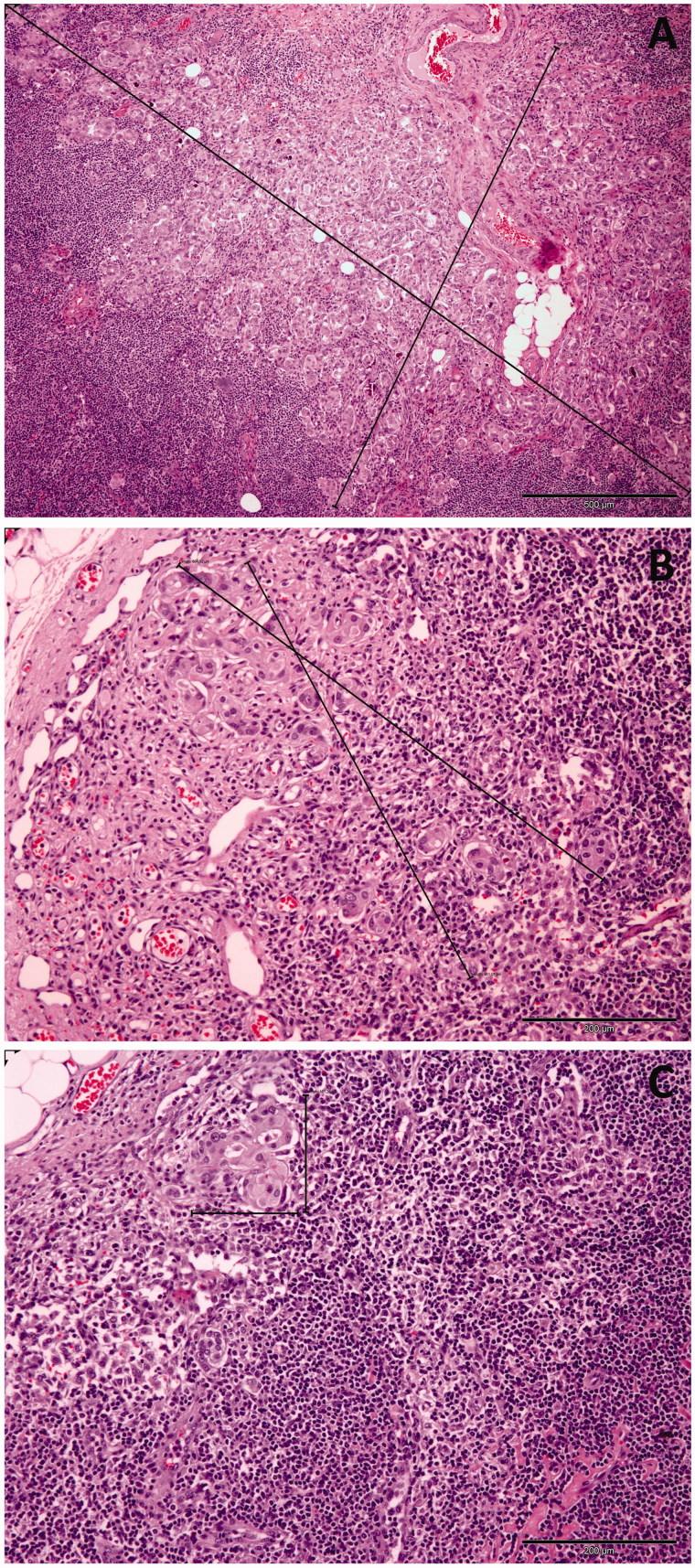
In the OSNA assay, cases showing mRNA CK19 levels >250 copies/µL were considered positive and were classified as micrometastases (number of copies >250 copies/µL, <5,000 copies/µL) or macrometastases (number of copies >5,000 copies/µL) following system specifications based on previous calculations. Cases identified as ‘negative’ (<250 copies/µL) by the system were classified further as isolated tumor cells (ITCs) (number of copies/µL >100, but fewer than 250) or true negative if the number of copies/µL was <100. A complete axillary node dissection was performed in those patients with macrometastases only. Lymph nodes submitted as part of the axillary dissection were evaluated using standard H&E staining (Figure 2). Breast surgery with conservative treatment (palpable or roll lumpectomy) or mastectomy (simple, skin sparing, or nipple sparing) with immediate reconstruction was performed as planned. Results of the intraoperative assessment were recorded, and patients were followed every six months after surgery clinically and by echography.
Figure 2.
Macrometastasis, micrometastasis, and isolated tumor cells in sentinel lymph nodes of women with breast cancer. (A) H&E section (×40) shows abundant clusters of epithelial cells on a fibrous stroma at an intranodal location. The clusters are larger than 2 mm. (B) H&E section (×100) shows a focus of epithelial cells on a fibrous stroma at an intranodal location measuring more than 0.2 mm but less than 2 mm. (C) H&E section (×100) demonstrates a small cluster of epithelial cells in the subcapsular region. The cluster measures less than 0.2 mm.
Oxidative stress parameter assays
Lipid peroxidation assay
Lipid peroxidation was measured by analyzing the amount of thiobarbituric acid reactive substances (TBARS) as previously described by Ramírez-Expósito et al. (12). Briefly, 25 µL of each sample was mixed with 100 µL of ice-cold 20% trichloroacetic acid (TCA). After centrifugation, a volume of supernatant was added to an equal volume of 0.67% 4,6-dihydroxypyrimidine-2-thiol (TBA), and the mixture was kept in a boiling water bath for 15 min. Samples were cooled to room temperature, and the absorbance at 532 nm was recorded after subtracting blanks containing TCA and TBA in an equal volume. The signal was read against a MDA standard curve, and the results were expressed as ng of MDA per mg of protein.
Protein oxidation assay
Protein oxidation was measured by analyzing the carbonyl group content of proteins as previously described by Ramírez-Expósito et al. (12). Briefly, 25 µL of sample was mixed with 100 µL of ice-cold 20% TCA and centrifuged. Protein precipitates were left to react with 10 mM 2,4-dinitrophenylhydrazine for an hour at room temperature in the dark. After the reaction, proteins were precipitated with 20% TCA, and unreacted dye was washed twice with 10% TCA. The pellets were dissolved in 1 M NaOH, and absorbance was recorded at 360 nm. The results were expressed as nmol per mg of protein using an extinction coefficient of 2.1 × 104 M−1 cm−1.
Total antioxidant capacity (TAC) assay
TAC was measured using copper(II)-neocuproine as chromogenic oxidant, as previously described by Apak et al. (17) as the CUPRAC method. Results were compared with a standard curve obtained with Trolox and were expressed in nmol of Trolox equivalents per mg of protein.
Non-enzymatic antioxidant defense system
Determination of reduced glutathione (GSH)
GSH levels were measured using a glutathione colorimetric assay kit from BioVision (Deltaclon, Madrid, Spain), according to the manufacturer’s instructions. Data were presented as ng of GSH per mg of protein.
Enzymatic antioxidant defense systems
Superoxide dismutase (SOD) assay
SOD activity was measured according to Paoletti et al. (18): a 10 µL sample was mixed with reaction buffer containing 100 mM triethanolamide–diethanolamide buffer (TDB) pH 7.4, 7.5 mM β-nicotinamide adenine dinucleotide (NADH) and ethylenediaminetetraacetic acid (EDTA)/MnCl2 at a ratio of 1 : 2 (v/v). To start the reaction, 25 µL of 10 mM β-mercaptoethanol was added. The absorbance was recorded at 340 nm for 2–15 min. One unit of SOD activity was defined as the amount of enzyme necessary to produce a 50% inhibition of the NADH oxidation rate under the assay conditions.
Catalase (CAT) activity assay
Samples were processed and analyzed for CAT activity as described by Aebi (19) with slight modifications by Cohen et al. (20). Briefly, 10 µL of sample was added to 10 mM H2O2 in 20 mM potassium phosphate buffer (pH 7.0) and incubated at 30 °C for 1 min. Initial reaction rate was measured from the decrease in absorbance at 240 nm. CAT activity was expressed in nmol of H2O2 decomposed per minute per mg of protein under the assay conditions.
Glutathione peroxidase (GPx) activity assay
GPx activity was measured according to Ellerby and Bredesen (21). The reaction mixture was formed by 50 mM potassium phosphate (pH 7.4), 25 mM β-nicotinamide adenine dinucleotide phosphate (NADPH), 1 mM of GSH, and 100 U/mL of yeast glutathione reductase; 10 µL of sample was added and mixed with the reaction mixture in a 96-well dish. The hydroperoxide-independent NADPH consumption rate was recorded for 3 min at 37 °C at 340 nm. Then, 2.5 µL of tert-butyl hydroperoxide was added to start the reaction, mixed, and the overall rate at 340 nm was recorded. The same procedure was carried out in the same reaction volume without the sample. This allowed subtraction of the non-enzymatic rate of GSH oxidation. GPx activity was expressed as µmol of NADH oxidized per minute per mg of protein under the assay conditions.
Statistical analysis
All values represent the mean ± standard error of the mean (SEM). Data were analyzed by analysis of variance (ANOVA) plus least significant difference (LSD) post-hoc test, using IBM SPSS v.19 software. Values of P < 0.05 were considered significant.
Results
Subject population
This study involves a population sample characterized by the clinicopathological parameters presented in Table 1. Table 2 shows the characteristics of the SLN and lymph nodes obtained after axillary dissection.
Table 1.
Patient and tumor characteristics.
| Sentinel lymph node status |
||||||
|---|---|---|---|---|---|---|
| Negative |
Micrometastases |
Macrometastases |
||||
| Characteristic | n = 43 | % | n = 13 | % | n = 19 | % |
| Age (years) | ||||||
| Mean | 50.3 ± 1.6 | 50.7 ± 3.4 | 51.8 ± 2.5 | |||
| Median | 48.0 | 45.0 | 50.0 | |||
| Range | 31–76 | 35–75 | 35–74 | |||
| Tumor histology | ||||||
| Ductal | 39 | 90.7 | 10 | 76.9 | 16 | 84.2 |
| Lobular | 3 | 6.9 | 1 | 7.7 | 3 | 15.8 |
| Other | 1 | 2.3 | 2 | 15.4 | 0 | 0.0 |
| Molecular subtypes | ||||||
| Luminal A | 24 | 55.8 | 12 | 92.3 | 15 | 78.9 |
| Luminal B | 7 | 16.3 | 1 | 7.7 | 4 | 21.1 |
| Her-2 | 3 | 6.9 | 0 | 0.0 | 0 | 0.0 |
| Triple negative | 9 | 20.9 | 0 | 0.0 | 0 | 0.0 |
| Pathologic tumor size (cm) | ||||||
| Mean ± SEM | 3.65 ± 0.17 | 3.69 ± 0.36 | 3.42 ± 0.28 | |||
| Median | 3.5 | 3.4 | 3.0 | |||
| Range | 2–8 | 2.4–7 | 2.4–7.5 | |||
| Pathologic T classification | ||||||
| 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 2 | 39 | 90.7 | 11 | 84.6 | 17 | 89.5 |
| 3 | 4 | 9.3 | 2 | 15.4 | 2 | 10.5 |
| Scarff–Bloom–Richardson grade | ||||||
| I | 7 | 16.3 | 3 | 23.1 | 6 | 31.6 |
| II | 19 | 44.2 | 7 | 53.8 | 12 | 63.1 |
| III | 17 | 39.5 | 3 | 23.1 | 1 | 5.3 |
| Hormonal status | ||||||
| ER+ | 31 | 72.1 | 13 | 100 | 19 | 100 |
| ER− | 12 | 27.9 | 0 | 0.0 | 0 | 0.0 |
| PgR+ | 29 | 67.4 | 13 | 100 | 18 | 94.7 |
| PgR− | 14 | 32.5 | 0 | 0.0 | 1 | 5.3 |
| HER-2/neu status | ||||||
| Negative | 34 | 79.1 | 12 | 92.3 | 16 | 84.2 |
| Positive | 9 | 20.9 | 1 | 7.7 | 3 | 15.8 |
Table 2.
Sentinel and axillary lymph node dissection characterization.
| Characteristic | Sentinel lymph node status |
||
|---|---|---|---|
| Negative | Micrometastases | Macrometastases | |
| n = 43 | n = 13 | n = 19 | |
| Sentinel lymph node removed | |||
| Mean ± SEM | 1.19 ± 0.60 | 1.38 ± 0.18 | 1.74 ± 0.25 |
| Median | 1 | 1 | 1 |
| Range | 1–2 | 1–3 | 1–4 |
| Axillary nodes removed | |||
| Mean ± SEM | 15.40 ± 2.29 | 16.16 ± 1.60 | |
| Median | 17.5 | 15.0 | |
| Range | 5–30 | 6–33 | |
| Axillary nodes positive | |||
| Mean ± SEM | 2.90 ± 1.60 | 2.74 ± 1.02 | |
| Median | 1 | 1 | |
| Range | 0–17 | 0–18 | |
Oxidative stress parameters
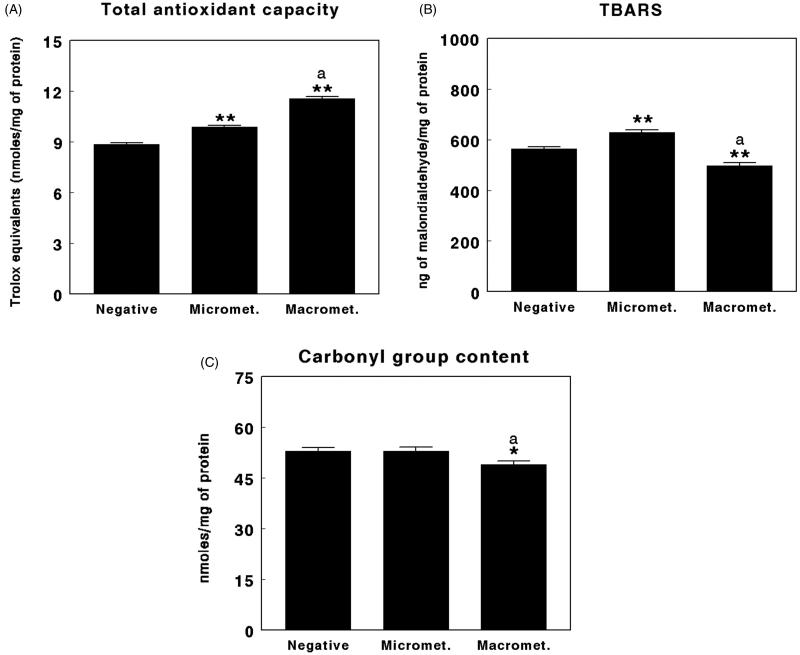
Table 3 and Figures 3–5 show oxidative stress parameters found in the SLNs of women with breast cancer evaluated as negative, SLNs with micrometastases, or SLNs with macrometastases. Lipid peroxidation, analyzed as TBARS levels, showed significantly higher (P < 0.01) levels in the SLNs with micrometastases compared with negative SLNs, whereas the levels were significantly lower (P < 0.01) in the SLNs with macrometastases compared with negative SLNs. There were also significant differences (P < 0.01) between SLNs with micrometastases and macrometastases, the highest TBARS values appearing in the SLNs with micrometastases and the lowest in the SLNs with macrometastases (Figure 3(A)).
Table 3.
Oxidative stress parameters, non-enzyme and enzyme antioxidant defense systems in the sentinel lymph node (SLN) of women with breast cancer.
| Negative SLN | SLN with micrometastases | SLN with macrometastases | |
|---|---|---|---|
| Oxidative stress parameters | |||
| Total antioxidant capacity (nmol of Trolox equivalents/mg of protein) | 8.86 ± 0.09 | 9.88 ± 0.1 | 11.57 ± 0.1 |
| Lipid peroxidation (ng of malondialdehyde/mg of protein) | 564.8 ± 7.1 | 629.4 ± 10.2 | 497.5 ± 11.9 |
| Protein oxidation (nmol per mg of protein) | 53.0 ± 0.9 | 53.1 ± 1.06 | 48.9 ± 1.02 |
| Non-enzymatic antioxidant defenses | |||
| GSH (ng per mg of protein) | 9.4 ± 0.1 | 9.38 ± 0.1 | 8.9 ± 1.1 |
| Enzymatic antioxidant defenses | |||
| Superoxide dismutase (percentage of inhibition) | 58.9 ± 0.3 | 71.5 ± 0.6 | 72.6 ± 0.4 |
| Catalase (nmol/min/mg of protein) | 56.8 ± 0.2 | 72.7 ± 0.4 | 74.1 ± 0.5 |
| Glutathione peroxidase (µmol/min/mg of protein) | 1.63 ± 0.01 | 1.63 ± 0.01 | 1.45 ± 0.009 |
Figure 3.
Total antioxidant capacity (TAC) (A), thiobarbituric acid reactive substances (TBARS) (B), and carbonyl group content (C) in negative sentinel lymph nodes (SLNs), SLNs with micrometastasis, and SLNs with macrometastasis of women with breast cancer. Results are expressed in nmol of Trolox equivalents per mg of protein for TAC, in ng of malondialdehyde per mg of protein for TBARS, and in nmol per mg of protein for protein carbonyls (mean ± SEM; n = 13–43; *P < 0.05; **P < 0.01; aP < 0.05 micrometastasis versus macrometastasis).
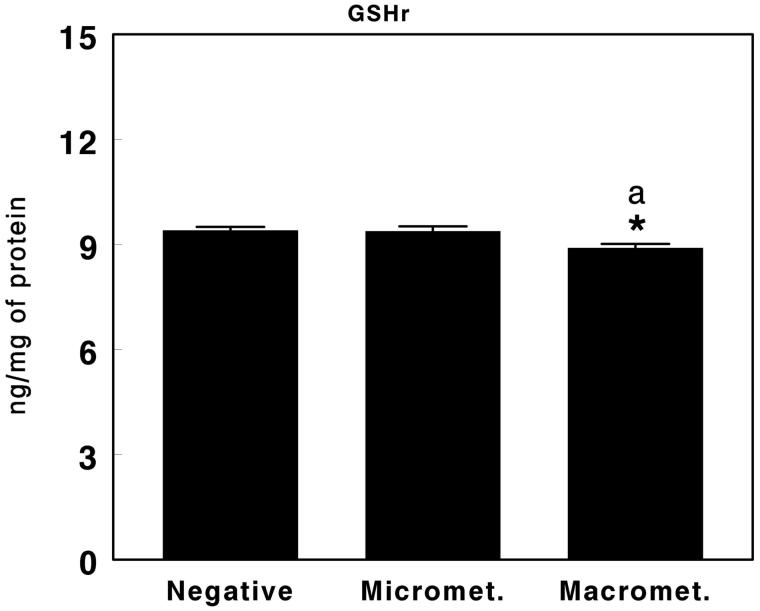
Figure 4.
Reduced glutathione (GSH) in negative sentinel lymph nodes (SLNs), SLNs with micrometastasis, and SLNs with macrometastasis of women with breast cancer. Results are expressed in ng per mg of protein (mean ± SEM; n = 13–43; *P < 0.05; aP < 0.05 micrometastasis versus macrometastasis).
Figure 5.
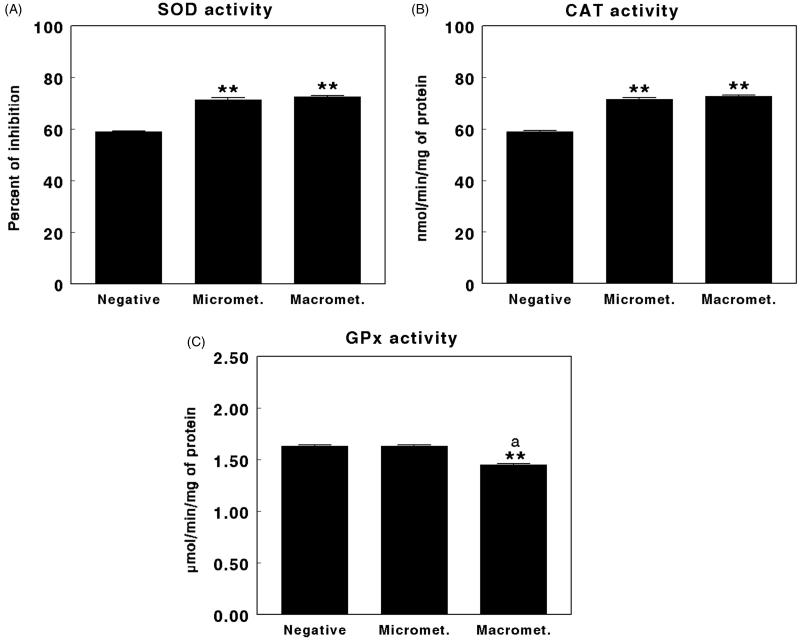
Superoxide dismutase (SOD) (A), catalase (CAT) (B), and glutathione peroxidase (GPx) (C) activity in negative sentinel lymph nodes (SLNs), SLNs with micrometastasis, and SLNs with macrometastasis of women with breast cancer. Results are expressed as percentage inhibition for SOD, in nmol per min and per mg of protein for CAT, and in µmol per min per mg of protein for GPx (mean ± SEM; n = 13–43; **P < 0.01; aP > 0.05 micrometastasis versus macrometastasis).
Protein oxidation, analyzed as carbonyl group content, showed significantly lower levels (P < 0.05) in the SLNs with macrometastases when compared with negative SLNs. There were also significant differences (P < 0.05) between the SLNs with micrometastases and macrometastases, lower values of protein oxidation appearing in the SLNs with macrometastases (Figure 3(B)).
Regarding TAC, the results showed significantly higher levels (P < 0.01) in the SLNs with micrometastases compared with negative SLNs. Similarly, the levels were also significantly higher (P < 0.01) in the SLNs with macrometastases compared with negative SLNs. There were also significant differences (P < 0.01) between the SLNs with micrometastases and macrometastases, higher values of TAC appearing in the SLNs with macrometastases (Figure 3(C)).
Non-enzymatic antioxidant defense system
Figure 4 shows the levels of reduced glutathione (GSH) found in the SLNs of women with breast cancer evaluated as negative, with micrometastases, and with macrometastases. The results showed significantly lower levels (P < 0.05) of GSH in the SLNs with macrometastases compared with negative SLNs or SLNs with micrometastases. There were no significant differences between the levels of GSH in negative SLNs and SLNs with micrometastases (Figure 4).
Enzymatic antioxidant defense systems
Figure 5 shows the levels of antioxidant enzymes found in the SLNs of women with breast cancer evaluated as negative, with micrometastases, and with macrometastases. SOD activity showed significantly higher levels (P < 0.01) in the SLNs with micrometastases compared with negative SLNs. Similarly, the levels were also significantly higher (P < 0.01) in the SLNs with macrometastases compared with negative SLNs. No significant differences were found between the levels of SOD in SLNs with micrometastases and macrometastases (Figure 5(A)).
Similarly, CAT activity showed significantly higher levels (P < 0.01) in the SLNs with micrometastases compared with negative SLNs. The levels were also significantly higher (P < 0.01) in the SLNs with macrometastases compared with negative SLNs. There were no significant differences between the levels of CAT among SLNs with micrometastases and macrometastases (Figure 5(B)).
Finally, GPx activity showed no significant difference between the SLNs with micrometastases when compared with negative SLNs. However, GPx levels were significantly lower (P < 0.01) in the SLNs with macrometastases when compared with negative SLNs or SLNs with micrometastases (Figure 5(C)). None of the parameters evaluated was useful for predicting the number of lymph nodes affected.
Discussion
Metastasis is a complex phenomenon with multiple stages, responsible for over 90% of cancer mortality (22,23). It consists of a series of biological processes that result in the spread of tumor cells from the primary tumor to distant organs. To do this, tumor cells have to perform local invasion and intravasation, survive in blood and lymphatic circulation, reach some distant organ, perform extravasation, formation of micrometastases, and metastatic colonization, and eventually form macrometastases. All these processes are managed by different genetic and/or epigenetic tumor cell alterations (24–26). Free radicals seem to be involved in almost all of these processes, modifying one or more signaling pathways (27,28). Although it is well known that high levels of free radicals can kill tumor cells—in fact, chemotherapy is based largely on the production of free radicals—often tumor cells can use the free radicals for their own benefit (29). Thus, free radicals can help processes such as cytoskeletal reorganization, regulation of signaling pathways and transcriptional processes acting in favor of cell survival, proliferation and metastasis, including the promotion of chemoresistance (30,31). Nevertheless, the metastatic process remains one of the least known aspects of cancer. In fact, prevention and therapeutic methods are mainly directed to the removal of the primary tumor and rarely against metastasis, and the SLN represents one of the first potential structures to be colonized by tumor cells.
To our knowledge, this paper analyzes for the first time in the literature the redox status (oxidative stress parameters and enzymatic and non-enzymatic antioxidant defense systems) in the SLNs of women with breast cancer, identified by the OSNA method as negative SLNs, SLNs with macrometastases, or SLNs with micrometastasis, in order to understand the pro-oxidant/antioxidant mechanisms involved in the processes of distant metastases in breast cancer and also whether these parameters could be useful as alternate or complementary staging techniques.
Thus, we have described different levels of lipid peroxidation in SLNs with micrometastases (increased) and macrometastases (decreased), a decrease in carbonyl group content in SLNs with macrometastases only, and an increase in TAC in SLNs with micrometastases and macrometastases. A decrease in the levels of reduced glutathione (GSH) also appeared in the SLNs with macrometastases only. Finally, we have also described increased levels of SOD and CAT activity in SLNs with micrometastases and macrometastases and decreased levels of GPx activity in SLNs with macrometastases but not with micrometastases. Thus, it is confirmed that cancer in general and the metastatic processes in particular are associated with the redox status and the antioxidant defense systems, indicating that different levels of oxidative stress can promote different behaviors by tumor cells. As previously indicated, relatively low oxidative damage (i.e. a sublethal level) triggers the activation of cell signaling mechanisms that promote proliferation, migration, and invasion by tumor cells, whereas only very high levels of oxidative damage would cause cell death (32–35).
Although this study shows for the first time the redox status, analyzing oxidative stress parameters and enzymatic and non-enzymatic antioxidant defense system levels, in the SLNs of women with breast cancer, we have previously evaluated the plasma levels of these parameters (12). The results show the existence of discrepancies in both locations. Thus, in the plasma of patients with breast cancer, an increase in both lipid peroxidation and protein oxidation and a decrease in TAC were observed. These data agree with several studies that also found increased markers of oxidative stress related to lipid peroxidation (7,8,11,36,37) and protein oxidation (10,38) and a reduction in TAC (10,39) in patients with breast cancer at serum, plasma, whole blood, and red cell levels (40–43) but also in the microenvironment of the breast tumor (8,9). Therefore, our results indicate that the SLN mainly shows an antioxidant status whereas plasma reflects a pro-oxidant one. Thus, the oxidant/antioxidant mechanisms in the SLN would reflect the redox status mainly locally as a consequence of metastatic processes. Also, in the SLN, the antioxidant status promoted by metastases, as reflected by the increased TAC in micro- and macrometastases, could be due to the increase in enzymatic antioxidant defenses (SOD and CAT), which may also explain the decreases found in lipid peroxidation and protein oxidation levels in the SLNs with macrometastases. The increase in TBARS found in the SLNs with micrometastases may be due to an effect of oxidative stress in the earliest stages of metastatic colonization of the SLN, promoted by the immune response to tumor cells (33) or other metabolic events (15).
As for non-enzymatic antioxidant defense systems, a significant decrease in GSH levels has been also described in the plasma of patients with breast cancer (10,12,44). In the SLN, we also found a decrease in GSH levels with macrometastases. In general, breast cancer seems to be accompanied by a decrease of the key compound of non-enzymatic antioxidant defense systems, GSH.
Lastly, the enzymatic antioxidant defense systems are also affected in the SLN. As we mentioned above, SOD and CAT activity is increased in both micrometastases and macrometastases, which could be responsible for the increased levels in TAC, and would be the response to increased production of free radicals promoted by metastasis (33,45,46). In contrast, previous analysis in plasma (12,39,47), erythrocytes, and whole blood (41,43,48) of women with breast cancer showed decreased SOD, CAT, and GPx activity, being therefore insufficient for antioxidant protection (43,49). However, GPx activity was also reduced in the SLNs with macrometastases, which also explains the low GSH levels found at this location. Therefore, the decreased antioxidant potential that occurs in the SLN caused by reduced levels of GPx, and therefore GSH, may result from an attempt by alternate mechanisms to avoid or at least not to promote the metastatic process. In fact, it has been postulated that within the range of biomolecules damaged by reactive species, GPx may be particularly sensitive to this damage (44). In any case, low levels of GSH, both circulating and in the SLN of breast cancer patients, appear to support the hypothesis that GSH is inversely related to malignant transformation (50). Unfortunately, none of the parameters measured here could predict the number of nodes affected or proved useful for nodal staging. However, the results obtained for GPx allow us to propose it as a potential alternate biomarker for nodal staging. Due to the existence of several types of GPx which can be differentiated at a molecular level, further studies must discriminate the importance of each of them in the SLN.
Finally, we must indicate that it is not known if radiation caused by the injection of the isotope the day before the operation could influence the parameters under study. Changes have been described in several antioxidant defense systems in human and laboratory animals promoted by both ionizing and non-ionizing radiation (51), with great variability depending of the source of radiation, dose, or time of administration. However, all patients in our study received the same procedure, minimizing these putative side-effects. In any case, future research using naive lymph nodes collected from axillary clearance without isotope injection could be an interesting approach to elucidate this question.
We can conclude that in the SLN of women with breast cancer the normal redox balance is altered to an antioxidant state, shown by an increase in TAC and decreased levels of lipid peroxidation and protein oxidation, especially in SLNs with macrometastases. This antioxidant status is promoted by an increase in enzymatic antioxidant defense systems mediated by SOD and CAT, which could reflect an attempt to prevent lymph node colonization by tumor cells. The decreased levels found for GSH and GPx support the hypothesis that these antioxidant systems may be highly related to the process of metastatic invasion. Finally, although the analysis of the redox state of the SLN by simple procedures does not appear to be useful for nodal staging due to their low sensitivity and specificity, it has been demonstrated to be very useful for understanding the participation of free radicals and antioxidant systems in the metastatic process in breast cancer. We also propose molecular analysis and/or expression levels of GPx as an alternative marker suitable for lymph node staging, which needs further studies for verification.
Funding Statement
This work was supported by Junta de Andalucía through PAIDI CTS-1039 [formerly BIO-296].
Disclosure statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Notes on contributors
María Jesús Ramírez-Expósito is Associate Professor at the Department of Health Sciences of University of Jaén.
Nieves Urbano-Polo is a Ph.D. student in the Experimental and Clinical Physiopathology Research Group.
Basilio Dueñas is Surgeon at the Unit of Breast Pathology at the Jaén Hospital Complex.
Joaquín Navarro-Cecilia is Surgeon at the Unit of Breast Pathology at Jaén Hospital Complex.
Cesar Ramírez-Tortosa is the Head of the Pathology Unit at the Jaén Hospital Complex.
María Dolores Martín-Salvago is Senior Consultant of Pathology at the Jaén Hospital Complex.
José Manuel Martínez-Martos is Associate Professor at the Department of Health Sciences of University of Jaén. He is also the Head of the Experimental and Clinical Physiopathology Research Group.
References
- 1.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. [DOI] [PubMed] [Google Scholar]
- 2.Pereira ER, Jones D, Jung K, Padera TP.. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol. 2015;38:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podgrabinska S, Skobe M.. Role of lymphatic vasculature in regional and distant metastases. Microvasc Res. 2014;95:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al.; American Society of Clinical Oncology . American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Cecilia J, Duenas-Rodriguez B, Luque-Lopez C, Ramírez-Expósito MJ, Martínez-Ferrol J, Ruíz-Mateas A, et al. . Intraoperative sentinel node biopsy by one-step nucleic acid amplification (OSNA) avoids axillary lymphadenectomy in women with breast cancer treated with neoadjuvant chemotherapy. Eur J Surg Oncol. 2013;39:873–9. [DOI] [PubMed] [Google Scholar]
- 6.Bernet L, Cano R, Martinez M, Dueñas B, Matias-Guiu X, Morell L, et al. . Diagnosis of the sentinel lymph node in breast cancer: a reproducible molecular method: a multicentric Spanish study. Histopathology. 2011;58:863–9. [DOI] [PubMed] [Google Scholar]
- 7.Gonenc A, Erten D, Aslan S, Akinci M, Simşek B, Torun M.. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int. 2006;30:376–80. [DOI] [PubMed] [Google Scholar]
- 8.Mannello F, Tonti GA, Pagliarani S, Benedetti S, Canestrari F, Zhu W, et al. . The 8-epimer of prostaglandin F(2alpha), a marker of lipid peroxidation and oxidative stress, is decreased in the nipple aspirate fluid of women with breast cancer. Int J Cancer. 2007;120:1971–6. [DOI] [PubMed] [Google Scholar]
- 9.Mannello F, Tonti GA, Medda V.. Protein oxidation in breast microenvironment: nipple aspirate fluid collected from breast cancer women contains increased protein carbonyl concentration. Cell Oncol. 2009;31:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC, et al. . Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133:881–8. [DOI] [PubMed] [Google Scholar]
- 11.Tas F, Hansel H, Belce A, Ilvan S, Argon A, Camlica H, et al. . Oxidative stress in breast cancer. Med Oncol. 2005;22:11–15. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez-Exposito MJ, Sanchez-Lopez E, Cueto-Urena C, Duenas B, Carrera-Gonzalez P, Navarro-Cecilia J, et al. . Circulating oxidative stress parameters in pre- and post-menopausal healthy women and in women suffering from breast cancer treated or not with neoadjuvant chemotherapy. Exp Gerontol. 2014;58:34–42. [DOI] [PubMed] [Google Scholar]
- 13.Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME.. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–90. [DOI] [PubMed] [Google Scholar]
- 14.Inokuma T, Haraguchi M, Fujita F, Torashima Y, Eguchi S, Kanematsu T.. Suppression of reactive oxygen species develops lymph node metastasis in colorectal cancer. Hepatogastroenterology. 2012;59:2480–3. [DOI] [PubMed] [Google Scholar]
- 15.Li LD, Sun HF, Liu XX, Gao SP, Jiang HL, Hu X, et al. . Down-regulation of NDUFB9 promotes breast cancer cell proliferation, metastasis by mediating mitochondrial metabolism. PLoS One. 2015;10:e0144441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozmen V, Unal ES, Muslumanoglu ME, Igci A, Canbay E, Ozcinar B, et al. . Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol. 2010;36:23–9. [DOI] [PubMed] [Google Scholar]
- 17.Apak R, Guclu K, Ozyurek M, Karademir SE, Altun M.. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: the CUPRAC method. Free Radic Res. 2005;39:949–61. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti F, Aldinucci D, Mocali A, Caparrini A.. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem. 1986;154:536–41. [DOI] [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. [DOI] [PubMed] [Google Scholar]
- 20.Cohen G, Kim M, Ogwu V.. A modified catalase assay suitable for a plate reader and for the analysis of brain cell cultures. J Neurosci Methods. 1996;67:53–6. [PubMed] [Google Scholar]
- 21.Ellerby LM, Bredesen DE.. Measurement of cellular oxidation, reactive oxygen species, and antioxidant enzymes during apoptosis. Methods Enzymol. 2000;322:413–21. [DOI] [PubMed] [Google Scholar]
- 22.Chaffer CL, Weinberg RA.. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. [DOI] [PubMed] [Google Scholar]
- 23.Wirtz D, Konstantopoulos K, Searson PC.. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedl P, Wolf K.. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. [DOI] [PubMed] [Google Scholar]
- 25.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. [DOI] [PubMed] [Google Scholar]
- 26.Valastyan S, Weinberg RA.. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurd TR, DeGennaro M, Lehmann R.. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012;22:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tochhawng L, Deng S, Pervaiz S, Yap CT.. Redox regulation of cancer cell migration and invasion. Mitochondrion. 2013;13:246–53. [DOI] [PubMed] [Google Scholar]
- 29.Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K, et al. . High glucose promotes tumor invasion and increases metastasis-associated protein expression in human lung epithelial cells by upregulating heme oxygenase-1 via reactive oxygen species or the TGF-beta1/PI3K/Akt signaling pathway. Cell Physiol Biochem. 2015;35:1008–22. [DOI] [PubMed] [Google Scholar]
- 30.Landriscina M, Maddalena F, Laudiero G, Esposito F.. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–16. [DOI] [PubMed] [Google Scholar]
- 31.Pervaiz S. Pro-oxidant milieu blunts scissors: insight into tumor progression, drug resistance, and novel druggable targets. Curr Pharm Des. 2006;12:4469–77. [DOI] [PubMed] [Google Scholar]
- 32.Benhar M, Engelberg D, Levitzki A.. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz B, Courtneidge SA.. Redox signaling at invasive microdomains in cancer cells. Free Radic Biol Med. 2012;52:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gloire G, Legrand-Poels S, Piette J.. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–505. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–9. [DOI] [PubMed] [Google Scholar]
- 36.Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P.. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 2008;49:640–3. [PubMed] [Google Scholar]
- 37.Sanchez-Rodriguez MA, Zacarias-Flores M, Arronte-Rosales A, Correa-Munoz E, Mendoza-Nunez VM.. Menopause as risk factor for oxidative stress. Menopause. 2011;19:361–7. [DOI] [PubMed] [Google Scholar]
- 38.Badid N, Ahmed FZ, Merzouk H, Belbraouet S, Mokhtari N, Merzouk SA, et al. . Oxidant/antioxidant status, lipids and hormonal profile in overweight women with breast cancer. Pathol Oncol Res. 2010;16:159–67. [DOI] [PubMed] [Google Scholar]
- 39.Kasapovic J, Pejic S, Todorovic A, Stojiljković V, Pajović SB.. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages. Cell Biochem Funct. 2008;26:723–30. [DOI] [PubMed] [Google Scholar]
- 40.Bhuvarahamurthy V, Balasubramanian N, Govindasamy S.. Effect of radiotherapy and chemoradiotherapy on circulating antioxidant system of human uterine cervical carcinoma. Mol Cell Biochem. 1996;158:17–23. [DOI] [PubMed] [Google Scholar]
- 41.Erhola M, Kellokumpu-Lehtinen P, Metsa-Ketela T, Alanko K, Nieminen MM.. Effects of anthracyclin-based chemotherapy on total plasma antioxidant capacity in small cell lung cancer patients. Free Radic Biol Med. 1996;21:383–90. [DOI] [PubMed] [Google Scholar]
- 42.Faure H, Coudray C, Mousseau M, Ducros V, Douki T, Bianchini F, et al. . 5-Hydroxymethyluracil excretion, plasma TBARS and plasma antioxidant vitamins in adriamycin-treated patients. Free Radic Biol Med. 1996;20:979–83. [DOI] [PubMed] [Google Scholar]
- 43.Sharma A, Rajappa M, Saxena A, Sharma M.. Antioxidant status in advanced cervical cancer patients undergoing neoadjuvant chemoradiation. Br J Biomed Sci. 2007;64:23–7. [DOI] [PubMed] [Google Scholar]
- 44.Salo DC, Lin SW, Pacifici RE, Davies KJ.. Superoxide dismutase is preferentially degraded by a proteolytic system from red blood cells following oxidative modification by hydrogen peroxide. Free Radic Biol Med. 1988;5:335–9. [DOI] [PubMed] [Google Scholar]
- 45.Chen ZX, Pervaiz S.. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408–20. [DOI] [PubMed] [Google Scholar]
- 46.Nourazarian AR, Kangari P, Salmaninejad A.. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev. 2014;15:4745–51. [DOI] [PubMed] [Google Scholar]
- 47.Kasapovic J, Pejic S, Stojiljkovic V, Todorović A, Radošević-Jelić L, Saičić ZS, et al. . Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clin Biochem. 2010;43:1287–93. [DOI] [PubMed] [Google Scholar]
- 48.Kopanski Z, Grabowska M, Kosiniak-Kamysz A, Bertrandt J, Kołodziejski L, Opoka W, et al. . The influence of antineoplastic chemotherapy on the glutathione enzymes activity in the blood. Biofactors. 2004;22:79–82. [DOI] [PubMed] [Google Scholar]
- 49.Stachowicz-Stencel T, Synakiewicz A, Owczarzak A, Aleksandrowicz-Wrona E, Sliwinska A, Lysiak-Szydlowska W, et al. . The antioxidant status and response to therapy in children with soft tissue sarcomas and neuroblastoma. Pediatr Blood Cancer. 2011;57:561–8. [DOI] [PubMed] [Google Scholar]
- 50.Kumaraguruparan R, Subapriya R, Kabalimoorthy J, Nagini S.. Antioxidant profile in the circulation of patients with fibroadenoma and adenocarcinoma of the breast. Clin Biochem. 2002;35:275–9. [DOI] [PubMed] [Google Scholar]
- 51.Rendic S, Guengerich FP.. Summary of information on the effects of ionizing and non-ionizing radiation on cytochrome P450 and other drug metabolizing enzymes and transporters. Curr Drug Metab. 2012;13:787–814. [DOI] [PMC free article] [PubMed] [Google Scholar]