Abstract
Fms-related tyrosine kinase 1 (Flt1), the receptor of VEGF/PIGF, is associated with cancer angiogenesis and tumorigenesis. Although the high expression of Flt1 in glioma is identified, its regulatory mechanism remains unclear. In the present study, we demonstrate that miR-139-5p inhibits Flt1 expression mediated by binding its 3′ untranslated region (3′UTR) to regulate the progression of human glioma. We found miR-139-5p was downregulated in glioma tissues compared with normal brain tissues whereas a converse expression level of Flt1 was observed. Additionally we proved that miR-139-5p directly integrated with the 3′UTR of Flt1 via luciferase activity assay and cells transfected with miR-139-5p characterized with a low expression of Flt1 in mRNA and protein levels. Furthermore, we validated that miR-139-5p enforced its biological modulation via targeting Flt1 through rescue experiments. miR-139-5p suppressed and Flt1 stimulated the malignant activities of glioma cells. We demonstrated that miR-139-5p inhibited the Flt1-mediated Wnt/β-catenin signaling pathway in glioma cells. Conclusively, our study indicated that miR-139-5p can counteract the malignant phenotypes of glioma cells by the inhibitory effect of the Flt1-mediated Wnt/β-catenin signaling pathway.
Keywords: microRNAs, miR-139-5p, Fms-related tyrosine kinase 1, Wnt/β-catenin, glioma
Introduction
Glioma, one of the most common primary brain tumors, presents particularly aggressive activities and highly fatal prognosis. The malignancy is the characteristic of glioma, which including rapid tumor growth, metastasis and angiogenesis (1). Although accumulated advanced techniques contribute to the diagnosis and treatment of glioma, the median survival is still from 9 to 12 months (2). Given the high incidence, refractoriness and poor prognosis, novel therapeutic strategies based on molecular interaction networks must be achieved for glioma (3).
Fms-related tyrosine kinase 1 (Flt1) is a full-length tyrosine kinase receptor characterized by extracellular domain, which is known to be expressed on vessel endothelial cells and presents a critical point in angiogenesis and subsequent cancer progression (4). Recent studies revealed that Flt1 is also widly expressed on tumor cells (5) and the effect of these Flt1 on tumor progression has been investigated (6–8). However, the detailed mechanism of Flt1 in glioma progression are still barely known. Wnt/β-catenin signaling pathway is a conservative pathway, which has been observed to have a vital role in many cancers containing glioma (9–12). The Wnt/β-catenin is involved in tumor growth, metastasis and angiogenesis, while the activation of Flt1 may be a basis of Wnt/β-catenin activity in malignant glioma progression.
Recently, microRNAs (miRNAs) have been related to participation in the biological process of tumor initiation and progression. These small noncoding single-stranded RNAs serve as tumor-suppressors or oncogenes by completely or incompletely binding the 3′ untranslated region (3′UTR) of its target gene to result in either the degradation of messenger RNA (mRNA) or the reduction of protein expression in numerous cancers (13). Downregulation of certain miRNAs correlated with various tumorigenic processes (14). Among these, miR-139-5p has been validated to be decreased in several tumors (15,16) and restoration of miR-139-5p contributes to the inhibitory impact on tumor malignancies. To date, miR-139-5p was identified to be responsible for target genes, such as NOTCH1, IGF1R, ZEB1, ZEB2 and Mcl-1 (15–18). However, whether Flt1 is drawn into the epigenetic alteration in miR-139-5p-mediated modulation of glioma progression remains unclear.
In the present study, we found miR-139-5p was dysregulated and lead to abrogation of the malignant phenotypes of glioma cells by directly pairing with the 3′UTR of Flt1. Additionally, we proved that high expression of Flt1 may be involved in modulating the malignancy of glioma in vitro. Furthermore, miR-139-5p suppressed Flt1-mediated Wnt/β-catenin signaling pathway in the progression of glioma. Together, our finding may provide a potential supplementary therapeutic strategy concerning the molecular mechanism of glioma.
Materials and methods
Patient samples and cell culture
Human glioma tissues and normal brain tissues were obtained from Tianjin Huanhu Hospital, including 6 grade I-II tumors, 6 grade III tumors, 14 grade IV tumors and 12 normal brain tissues. Tissues were collected during surgery and immediately snap-frozen in liquid nitrogen and storing at −80°C. This study was approved by the hospital institutional review board and written informed consent was obtained from all the patients.
Human glioma cell lines U87, SNB19, U251, LN308 and LN229 were all obtained from the Peking Union Medical College cell library (Beijing, China) which were cultured in complete medium containing Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (both from Gibco, Los Angeles, CA, USA) and 100 U/ml penicillin/streptomycin and maintained in a humidified atmosphere with 5% CO2 at 37°C.
Plasmid construction, oligonucleotides and cell transfection
Hsa-miR-139-5p mimics, Flt1 siRNA, were both chemically artificialized by GenePharma (Shanghai, China) as well as their relative negative control. The sequences are as follows: miR-139-5p, 5′-UCUACAGUGCACGUGUCUCCAGU-3′; miR-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′; si-Flt1, 5′-GGACGUAACUGAAGAGGAUTT-3′; si-NC, 5′-UUCUCC GAACGUGUCACGUTT-3′. The cDNA encoding Flt1 (GV230-Flt1) was purchased from Genechem (Shanghai, China). Cell transfection was with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) per the manufacturer’s manual.
RNA extraction and qRT-PCR
TRIzol reagent (Invitrogen) was applied to extract total RNA according to the manufacturer’s instructions. Specific stem-loop primer and oligod(T) primer were respectively used to measure the levels of miR-139-5p and Flt1 through PrimeScript RT reagent kit (Invitrogen) as described previously (19). U6 and β-actin served as an endogenous control. Then the cDNAs were further amplified by qRT-PCR using SYBR Premix Ex Taq™ II kit (Takara, Dalian, China) and the changes were symbolized by relative quantification. The primers used are shown in Table I.
Table I.
Primers used in the polymerase chain reaction.
| Primer names | Primer sequences |
|---|---|
| miR-139-5p RT primer | 5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACACTGGAG-3′ |
| U6 RT primer | 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC-3′ |
| miR-139-5p forward | 5′-TGCGGTCTACAGTGCACGTGTCTC-3′ |
| U6 forward | 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ |
| U6 reverse | 5′-CCAGTGCAGGGTCCGAGGT-3′ |
| Flt1 forward | 5′-CCACCATCTGAACGTGGTTA-3′ |
| Flt1 reverse | 5′-GCTGCATCCTTGTTGAGAAA-3′ |
| β-actin forward | 5′-CGTGACATTAAGGAGAAGCTG-3′ |
| β-actin reverse | 5′-CTAGAAGCATTTGCGGTGGAC-3′ |
Proliferation assay and colony-formation analysis
The description of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and colony-formation assay were reported previously (19).
Cell cycle assay via flow cytometry
Flow cytometry for cell cycle assay were described previously (16). The results were analyzed with FlowJo (BD Pharmingen, San Diego, CA, USA).
Tumorigenicity in the nude mouse model
All procedures were forced as the protocols approved by the Animal Care and Use Committee of Tianjin Medical University. The U87 subcutaneous model was constructed as mentioned previously (19). Ten days later the male nude mice (4 weeks old) were randomly divided into two treatment groups, which were injected with 400 pmol miR-139-5p or miR-NC mimics (20 nmol/l×20 μl) in Lipofectamine 2000 into xenograft model via a multisite injection every 3 days as well as the measurement of tumor volume [(volume=length x width2)/2]. The xenograft tumor growth curves were calculated and the tumors were removed for the immunohistochemical assay after 18 days of treatment.
Migration and invasion assays
The Transwell assay was carried out with the chambers (8 μm pore size; Corning, Cambridge, MA, USA) covered with or without Matrigel (BD Biosciences, San Diego, CA, USA) respectively. Detail processes of the Transwell assay were previously described (15).
Vasculogenic mimicry formation assay
Growth factor-reduced Matrigel (50 μl/well; BD Biosciences) was precoated on the 96-well plate at 37°C for 60 min to solidify, which was seeded with the transfected U87 cell suspension (2×104/well) and incubated at 37°C for 12 h, and then was analyzed directly under a microscope. Each closed tube was numbered.
Luciferase reporter assay
The 3′UTR of Flt1 reporter was created containing one putative miR-139-5p targeting site and one randomly scrambled sequence inserted into the pGL3 vector (Promega, Madison, WI, USA). U87 cells were cotransfected with the wild or mutant plasmid and miR-139-5p or miR-NC. Following 48 h cultivation, luciferase activity was determined with the Dual-Luciferase reporter system (Promega).
Immunocytochemical staining
Immunocytochemical analysis was used to visualize the expression of Flt1 in glioma tissues, isolated tumors and cells as described previously (19,20).
Western blot analysis
The detailed process of western blot analysis can be found in a previous study (19). The antibodies used were: Flt1, cyclin D1, p21, matrix metalloproteinase 2 (MMP2), MMP9, E-cadherin, vimentin, VE-cadherin, Wnt1, β-catenin, GSK-3β, p-GSK-3β and β-actin were all from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Statistical analysis
Each experiment was executed three times and quantitative data are shown as mean ± SD and all statistical analysis were carried out with SPSS 17.0. The significance was calculated through two-tailed Student’s t-test. P<0.05 was defined as statistical significance.
Results
miR-139-5p expression is frequently downregulated in human glioma tissues and cell lines
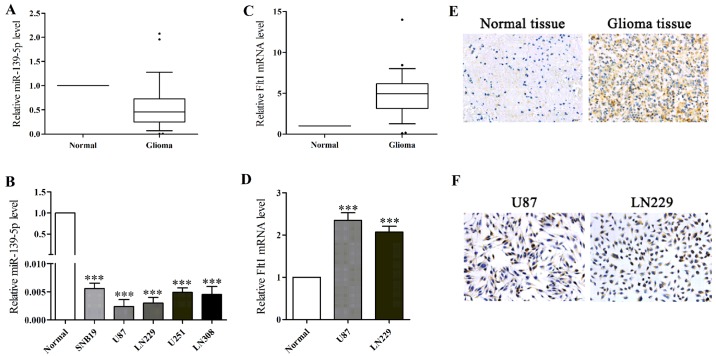
To evaluate the action of miR-139-5p in glioma, we first analyzed the expression levels of normal brain specimens and glioma specimens as well as five glioma cell lines via qRT-PCR. The results showed miR-139-5p expression levels were generally lower in glioma specimens and glioma cell lines (Fig. 1A and B). According to results, the two lowest level glioma cells (U87 and LN229 cells) were selected for the following experiments.
Figure 1.
miR-139-5p inversely correlated the expression of Fms-related tyrosine kinase 1 (Flt1) in glioma. (A) The relative expression levels of miR-139-5p in 26 glioma clinical tissue specimens and 12 normal brain tissues were assessed by qRT-PCR. Data were normalized to U6 control. (B) Relative miR-139-5p levels in five glioma cells compared to normal brain tissues. U6 was used as internal control. (C) The relative expression levels of Flt1 in human glioma tissues and normal brain tissues. Data were normalized to β-actin control. (D) Relative Flt1 levels in U87 and LN229 glioma cells compared to normal brain tissues. β-actin was used as internal control. (E) The relative Flt1 expression in glioma tissues. Representative IHC images (×200) showing the magnitude of Flt1 in glioma tissues compared with normal brain tissues. (F) Membrane-bound and cytoplasmic-positive staining of Flt1 was observed in U87 and LN229 cells by IHC (×200). *p<0.05, **p<0.01 and ***p<0.001.
In accordance with previous studies, we found the average expression of Flt1 mRNA was notably higher in human glioma tissues and in glioma cell lines (Fig. 1C and D). Moreover, we performed immunohistochemical examination to observe the expression of Flt1, and the results showed that Flt1 expression was more commonly positive in glioma tissues compared normal brain tissues (Fig. 1E). Immunohistochemistry (ICH) was performed to further locate the expression of Flt1 in U87 and LN229 cells, and as shown in Fig. 1F, Flt1 was found highly located on cytomembrane and in the cytoplasm. Then we observed the inverse relevance between the levels of miR-139-5p and Flt1 based on the data above.
miR-139-5p potently targets Flt1 gene to decrease its expression
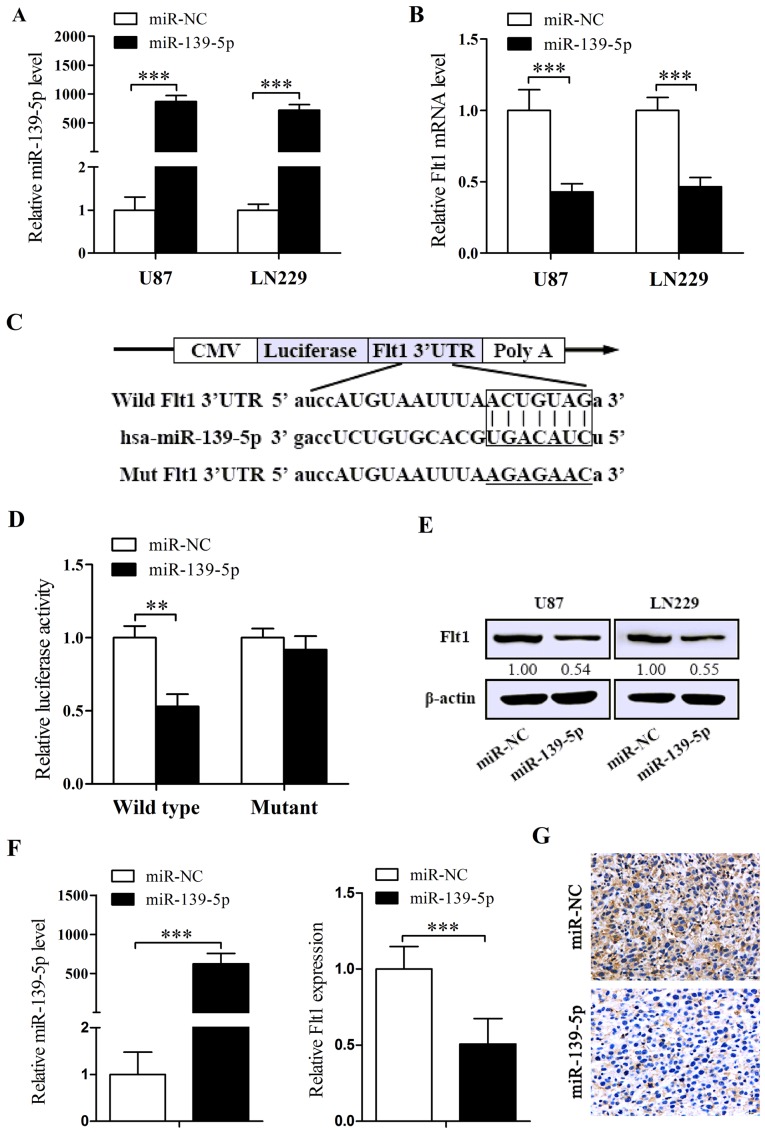
In order to explain whether the lower levels of endogenous miR-139-5p implies that miR-139-5p is a potential tumor suppressor, chemosynthesised miR-139-5p mimics were transiently transfected into U87 and LN229 cells. The efficiency was checked by qRT-PCR. As revealed in Fig. 2A, the levels of miR-139-5p are significantly increased after the transient transfection of miR-139-5p mimics.
Figure 2.
miR-139-5p directly downregulates the expression of Fms-related tyrosine kinase 1 (Flt1) in glioma cells. (A) The efficiency of miR-139-5p mimics in U87 and LN229 was confirmed by qRT-PCR. (B) The levels of Flt1 mRNA in U87 and LN229 cells transfected with either miR-139-5p mimics or miR-NC. (C) The predicted matching sites for miR-139-5p in the 3′ untranslated region (3′UTR) of Flt1 and the mutations in the matching sites are shown. (D) Luciferase constructs Flt1 wild-type or mutant were transfected into U87 cells with miR-139-5p mimics. Luciferase activity was measured 48 h after transfection. (E) Western blot analysis was performed to analyze the Flt1 protein level in U87 and LN229 cells transfected with either miR-139-5p mimics or miR-NC. (F) qRT-PCR for the levels of miR-139-5p and Flt1 mRNA in miR-139-5p mimics-treated or miR-NC-treated U87 tumor tissues isolated from nude mice. (G) IHC images (×200) showing the expression levels of Flt1 in U87 tumor xenograft tissues administered with either miR-139-5p mimics or miR-NC. *p<0.05, **p<0.01 and ***p<0.001.
To comprehensively explore the mechanism of miR-139-5p in glioma, considerate analysis of 2 bioinformatics algorithms, namely miRanda and RNA22, were performed to predict a putative target gene. Next we detected Flt1 expression levels in cells treated with miR-139-5p mimics and found reducted expression of Flt1 both in mRNA and in protein levels (Fig. 2B and E). To further confirm whether the decrease of Flt1 is related to the agent that miR-139-5p directly targets its binding sites (Fig. 2C), we cotransfected U87 cells with the vectors containing wild-type or mutant Flt1 3′UTR binding areas together with miR-139-5p or miR-NC. The results indicated that miR-139-5p induced a remarkable depression in luciferase intensity of the wild-type Flt1 3′UTR, while the mutant-3′UTR showed no distinct changes (Fig. 2D). Furthermore, we detected the expression of miR-139-5p and Flt1 in the tumor tissues removed from the xenograft models dosed with miR-139-5p mimics or miR-NC by qRT-PCR and ICH, and the results indicated evident higher levels of miR-139-5p and apparent lower levels of Flt1 in miR-139-5p mimics injecting tumors (Fig. 2F and G). To conclude, we demonstrated that miR-139-5p directly target Flt1 transcription, and potentially induce the downregulation of Flt1.
Overexpression of miR-139-5p affects glioma cell growth in vitro and in vivo and blocks the cell cycle in G0/G1 progress
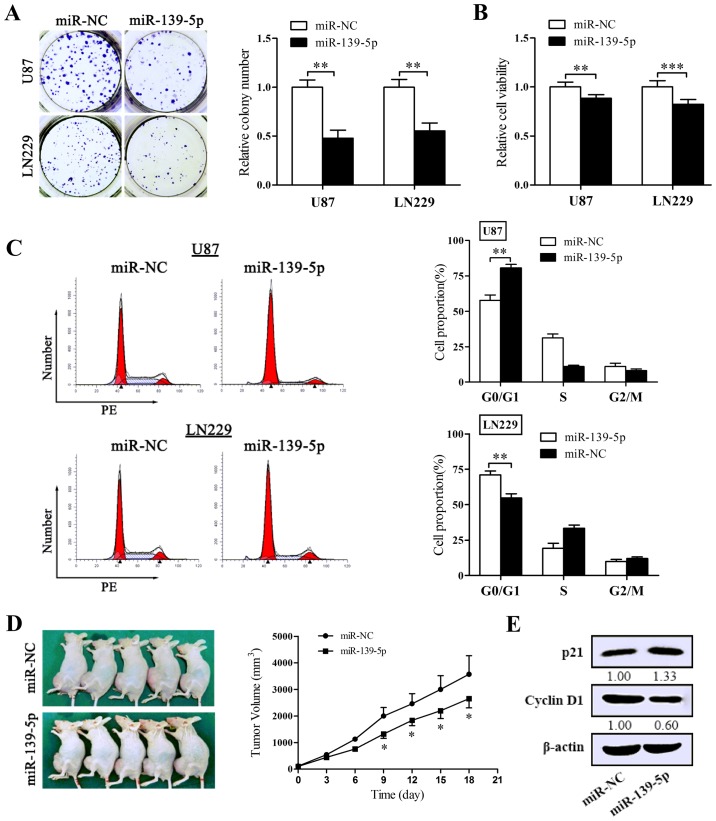
To identify the effect on the proliferation of miR-139-5p, colony formation assay and MTT assay were performed in U87 and LN229 cells. Reintroduction of miR-139-5p degraded colony formation numbers of U87 and LN229 cells (Fig. 3A). Coincidentally, forced expression of miR-139-5p induced a remarkable diminution in cell viability (Fig. 3B). To affirm the inhibitory impact of miR-139-5p on tumorigenesis in vivo, the xenograft tumor models were conducted. As shown in Fig. 3D, the multilocus delivery of miR-139-5p mimics led to a prohibitive reaction to xenograft tumor growth.
Figure 3.
Restoration of miR-139-5p expression inhibits tumor proliferation in vitro and in vivo and blocks the cell cycle progression. (A) Colony formation assay was performed to examine the impact of miR-139-5p on proliferation of U87 and LN229 cells. Cells were cultivated for 14 days before being fixed and stained. (B) Cellular viability assay. The cell viability of transfected U87 and LN229 cells was determined via MTT assay 72 h after transfection. (C) The cell cycle progression of U87 and LN229 cells was determined by flow cytometry. The DNA content was measured via propidium iodide (PI) staining. The charts (right) show the population of cells in G0/G1, S and G2/M phase. (D) Xenograft nude mouse model (n=5) was obtained, following which miR-139-5p mimics or miR-NC were intratumorally delivered every 3 days. Tumor volumes following miR-139-5p administration were significantly reduced. Photography of the xenograft tumor-bearing mice and the tumor growth curve are shown. (E) The influence of miR-139-5p on the protein levels of proliferation-associated molecules (p21, cyclin D1) were determined by western blotting. *p<0.05, **p<0.01 and ***p<0.001.
To determine whether miR-139-5p accommodates the cell cycle processes, we performed flow cytometry and obtained results that miR-139-5p accumulated the number of cells in G0/G1 phase and suppressed the G1/S transition both in U87 and LN229 cells (Fig. 3C). Then we tested the expression of p21 and cyclin D1 in U87 cells to explain the molecular levels in proliferation. As pictured in Fig. 3E, the levels of cyclin D1 declined in cells overexpressing miR-139-5p and in contrast p21 increased. Thus, these data suggest that miR-139-5p prohibits the proliferation of glioma cells both in vitro and in vivo via blocking the transition process of G1/S of glioma cells.
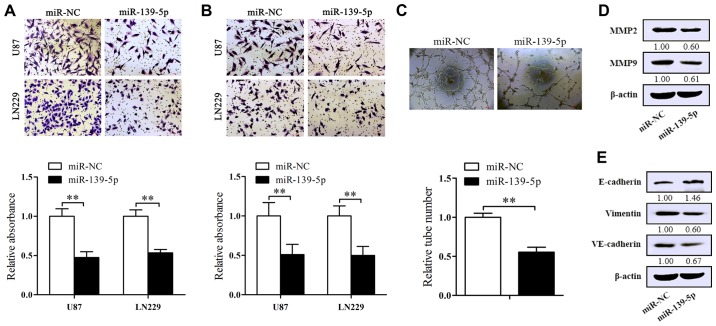
Forced-expression of miR-139-5p suppresses the migration, invasion and vasculogenic mimicry (VM) of glioma cells in vitro and altered the expression of EMT-associated protein
To identify the influence of miR-139-5p on glioma cell migration and invasion qualities, the inserts were covered with or without Matrigel. As expected, forced-expression of miR-139-5p suppressed the migrated cells compared to control ones (Fig. 4A). At equal place, decreased invasion of U87 and LN229 cells were also obtained in the inserts covered with Matrigel (Fig. 4B). To further explore the mechanism behind the inhibition, we examined the levels of MMP2 and MMP9 which both are related to migration/invasion processes. The results of western blot analysis show a significantly decrease of MMP2 and MMP9 levels in miR-139-5p overexpression cells (Fig. 4D).
Figure 4.
Overexpression of miR-139-5p suppresses migration, invasion, vasculogenic mimicry and EMT in vitro. (A) Transwell migration assays were performed in U87 and LN229 cells transfected with miR-139-5p mimics or miR-NC. Migratory cells were stained and the average number of cells was counted. (B) Transwell invasion assays were performed in U87 and LN229 cells transfected with miR-139-5p mimics or miR-NC. The invasive cells were stained and the average number of cells was counted. (C) The impact of miR-139-5p on vasculogenic mimicry. U87 cells were used to examine the impact of overexpression of miR-139-5p on vasculogenic mimicry through three-dimensional Matrigel culture assay. (D) The migration/invasion marker protein expression of matrix metalloproteinase 2 (MMP2) and MMP9 in U87 cells were determined by western blotting. (E) The influence of miR-139-5p on the protein levels of EMT-associated molecules (E-cadherin and vimentin) and of a key vasculogenic mimicry (VM) molecule (VE-cadherin) were examined via western blotting. *p<0.05, **p<0.01.
VM, a kind of non-endothelial cell blood vessels composed by solid tumor cells, has already been reported in human glioblastoma tissues (21). Here we used U87 cells to assess whether miR-139-5p affects the formation of VM. The results we attained proclaimed that the tube-formed number was decreased in miR-139-5p overexpressing U87 cells (Fig. 4C). Furthermore, western blot analysis was executed to explore the vital molecular markers in VM and EMT influenced by miR-139-5p mimics. As shown in Fig. 4E, the elevated level of miR-139-5p resulted in the reduction of VE-cadherin and vimentin and the increase of E-cadherin in U87 cells. These data revealed the inhibitory impression of miR-139-5p on EMT and VM of glioma cells.
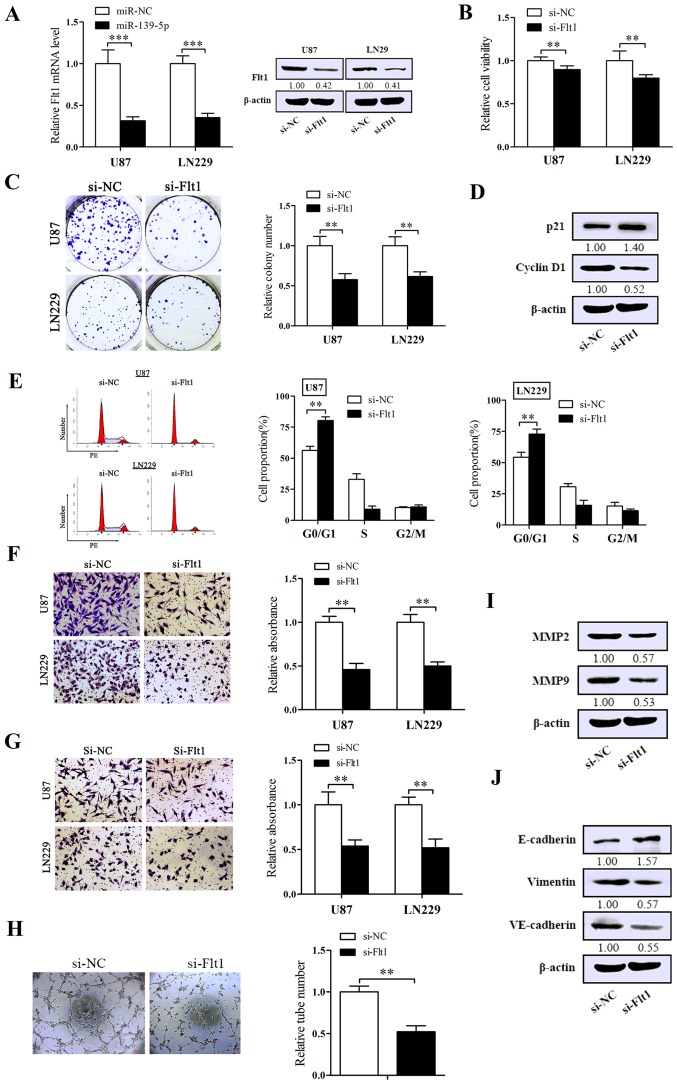
Suppression of Flt1 performs the inhibitory effect on the malignant phenotype of glioma cells
To further investigate the concrete biological impression of Flt1 on glioma cells, we chemically synthesized a specific siRNA against Flt1. U87 and LN229 cells was transfected with the siRNA of Flt1, then we validated the efficiency of silencing Flt1. As expected, the expression of Flt1 was obviously decreased in both mRNA and protein levels (Fig. 5A). As shown in Fig. 5B and C, knockdown of Flt1 induced the suppression of cell viability and the capacity of colony-formation. Flow cytometry was performed to assess the impact of Flt1 on cell cycle. As shown in Fig. 5E, downregulation of Flt1 markedly arrested cell cycle in G0/G1 phase. The migration/invasion assay pointed out that silencing of Flt1 remarkably abated both migration and invasion (Fig. 5F and G). Moreover, silencing Flt1 also reduced the shaping of VM in U87 cells (Fig. 5H).
Figure 5.
Suppression of Fms-related tyrosine kinase 1 (Flt1) has inhibitory effect on the malignant phenotype of glioma cells. (A) The efficiency of si-Flt1 in U87 and LN229 cells was examined in the mRNA and protein levels via qRT-PCR and western blot analysis. (B and C) Cellular viability assay and Colony formation assay were performed to determine the cell proliferation of transfected U87 and LN229 cells. (D) The impact of Flt1 on the protein levels of proliferation-associated molecules (p21, cyclin D1) were determined by western blotting. (E) The effect of Flt1 on the cell cycle progression of U87 and LN229 cells was determined by flow cytometry. (F and G) Transwell migration and invasion assays were used to test the effect of Flt1 on the migration (F) and invasion (G) ability of U87 and LN229 cells. (H) The impact of miR-139-5p on vasculogenic mimicry in U87 cells by three-dimensional Matrigel culture. (I and J) The influence of Flt1 on the protein levels of migration/invasion-associated molecules [matrix metalloproteinase 9 (MMP9) and MMP2] and of certain key molecules in EMT (E-cadherin and vimentin) and vasculogenic mimicry (VM) (VE-cadherin). *p<0.05, **p<0.01 and ***p<0.001.
Furthermore, U87 cells were used to examine the representative molecules related to the malignant phenotypes following the silencing of Flt1 by western blot analysis. Cell proliferation ability showed lower level of cyclin D1 and higher level of p21 (Fig. 5D) in si-Flt1-transfected U87 cells, in which we also obtained the upregulation levels of MMP2 and MMP9 (Fig. 5I). Coincidentally with the effect in EMT and VM, interfering Flt1 decreased the expression levels of VE-cadherin and vimentin and increased the E-cadherin level compared with control cells (Fig. 5J). In conclusion, Flt1 may serve as an oncogene in glioma.
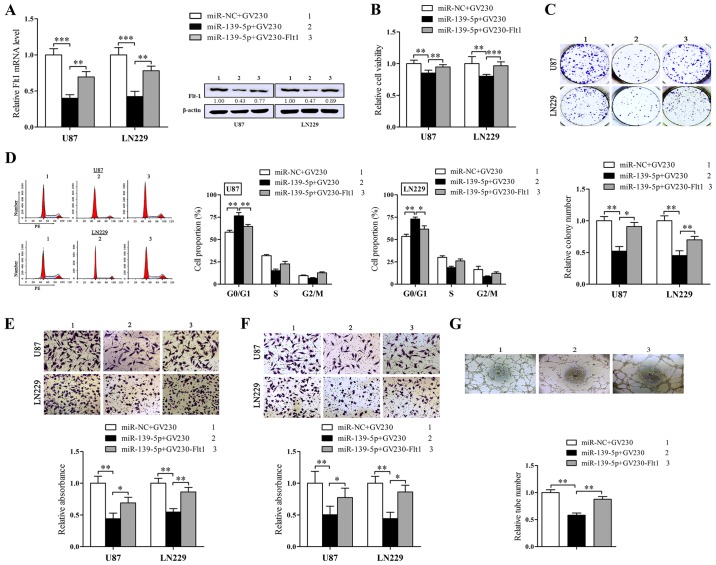
Restoration of Flt1 neutralizes the function of miR-139-5p in glioma cells in vitro
Given the observations above uncovered that miR-139-5p and Flt1 served as converse agents in regulating malignant phenotypes of human glioma cells, we doubted whether the function of miR-139-5p on glioma cells was mediated through its inhibitory impression on Flt1 expression. Then the rescue experiments were conducted to dispose it. We constructed an Flt1 expression plasmid without its 3′UTR and cotransfected it with miR-139-5p mimics. Western blot analysis was forced to validate whether the Flt1 protein levels reduced by miR-139-5p may be rescued via the overexpression of Flt1 (Fig. 6A).
Figure 6.
Restoration of Fms-related tyrosine kinase 1 (Flt1) counteracts the function of miR-139-5p resulted in glioma cells. (A) U87 and LN229 cells were transfected with GV230-Flt1 or miR-139-5p or control vector followed by qRT-PCR and western blot analysis to examine the expression of Flt1 in the mRNA and protein levels. (B–D) The proliferation ability of transfected U87 and LN229 cells were determined via MTT assay (B), flow cytometry to measure the cell cycle progression (C) and colony formation assay (D). (E–G) The transfected cells were submitted to Transwell-migration (E), invasion assay (F) and vasculogenic mimicry assay (G). *p<0.05, **p<0.01 and ***p<0.001.
Then we further executed the rescue experiment in different phenotypes of glioma cells. As anticipated (Fig. 6B), the restoration of Flt1 could validly neutralize the suppressive influence of miR-139-5p on the cell viability and colony formation (Fig. 6D) as well as the cell cycle block in G0/G1 (Fig. 6C). In addition, overexpression of Flt1 also overturned the inhibition of migration/invasion and VM that miR-139-5p induced in glioma cells (Fig. 6E–6G).
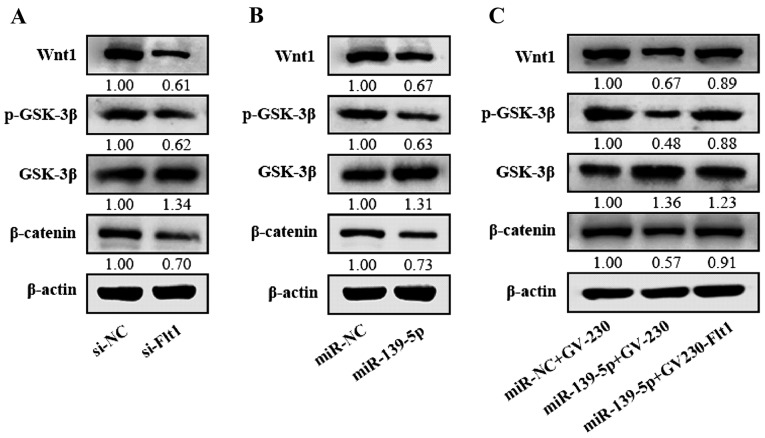
miR-139-5p inhibits the Wnt/β-catenin pathway via targeting Flt1 in glioma cells
In spite of confirming the effect of Flt1 in the malignance of glioma cells, we poorly clarified the extensional mechanism among these. Flt1 is usually treated with VEGF as a whole involved in VEGF/Flt1, which may serve as a potential synthetic lethality to Wnt/β-catenin signaling pathway (22). In this study, we proved that knockdown of Flt1 induced the restrain of the Wnt/β-catenin pathway in glioma cells (Fig. 7A). Together with our above results, we presumed that miR-139-5p may suppress the Wnt/β-catenin signaling pathway by targeting Flt1 in glioma. Then we verified the impression of miR-139-5p on the levels of Wnt1, p-GSK-3β, GSK-3β and β-catenin, and the results indicated that the Wnt1, β-catenin and the phosphorylated GSK-3β levels in miR-139-5p-overexpressing cells were downregulated while the GSK-3β was inversely upregulated (Fig. 7B), which indicates that miR-139-5p may inhibit the Wnt/β-catenin pathway. To further verify the influence of miR-139-5p on regulating the Wnt/β-catenin signaling pathway mediated by Flt1, miR-139-5p mimics and Flt1 expression plasmid without 3′UTR were cotransfected in U87 cells. Our results showed that Flt1 can rescue the inhibition of Wnt/β-catenin pathway induced by miR-139-5p (Fig. 7C). All the data indicate that miR-139-5p may downregulate the Wnt/β-catenin pathway through its target Flt1.
Figure 7.
miR-139-5p inhibits the Wnt/β-catenin pathway via targeting Fms-related tyrosine kinase 1 (Flt1) in glioma cells. (A) Western blot assays were performed to measure the impact of Flt1 on the levels of Wnt1, GSK-3β, p-GSK-3β and β-catenin in U87 cells. (B) Western blot assays were performed to examine the influence of miR-139-5p on the levels of Wnt1, GSK-3β, p-GSK-3β and β-catenin in U87 cells. (C) Western blot assays were performed to determine the restoration of Flt1 reverse effect of miR-139-5p on the levels of Wnt1, GSK-3β, p-GSK-3β and β-catenin in U87 cells.
Discussion
Accumulated information concerning the molecular mechanisms tightly interrelated to the initiation and progression of glioma have been reported in recent years (23). MicroRNAs, mostly worked as a tumor-suppressor agent in carcinoma, is commonly abrogated due to the frequent hypermethylation of CpG islands of its primary transcripts (24,25). miRNAs are involved in glioma tumorigenesis, metastasis or angiogenesis, including that in glioma cell biological processes in vitro. Recent studies have indicated that miR-139-5p promotes apoptosis in cooperation with TMZ (16) and inhibited the migration and invasion (15) of glioma cells in vitro. Our study further proved that miR-139-5p worked as a tumor inhibitory factor, which mediated inhibition of proliferation, migration, invasion, EMT and VM of glioma cells. However, the molecular mechanism and the epigenetic influence of miR-139-5p on human glioma remain unclear.
Recent studies have demonstrated that VEGF pathway significantly contributed to glioblastoma angiogenesis and tumor growth (26) and anti-VEGF/VEGFRs therapeutics may be a new potential treatment for glioma (27,28). Flt1, also named VEGFR-1, is one of VEGF receptors, which have been previously regarded as a vascular-endothelial ligand of VEGF (29). A recent study reported that Flt1 was also expressed in many tumor cells. Lee et al found that Flt1 located on breast cancer cell membrane, which intracrined VEGF blinding as a survival factor (30). Lichtenberger et al proved that VEGF interacted with Flt1 on tumor cells to enhance tumor metastasis (31). Flt1 has also been detected on human ovarian carcinoma cells exerting a crucial function in cancer cell invasion (6). Given that Flt1 has recently been reported highly expressed in glioblastoma (26), the cumulative expression level of Flt1 was examined in glioma tissues and two glioma cell lines in our study. Then we further located the expression of Flt1 on U87 and LN229 cell membrane. The function of tumor cell membrane-bound Flt1 can be observed in several previous studies. Wei et al confirmed that Flt1 located in colorectal cancer cells was important to CRC progression (7). Studies enforced to evaluate the tumor cell effects of Flt1 in lung adenocarcinoma cells revealed that Flt1 occupied a considerable role in cell proliferation and migration/invasion (32). In the present study, the function of Flt1 in glioma malignancy was affirmed with Flt1-siRNA. Based upon in vitro research, we obtained similar results in the suppression of aggressive phenotypes of glioma cells, including proliferation, migration/invasion, EMT and VM. These outcomes validated the inhibitive effect of Flt1-specific siRNA in glioma progression, but the explicit regulation mechanisms of Flt1 require exploration of the epigenetic aspects.
In the present study, we determined the expression level of Flt1 at transcriptional level and found the level of miR-139-5p was evidently decreased in negative correlation with increased expression of Flt1 in glioma tissues and cells. Then we predicted that miR-139-5p may serve as a significant agent negatively regulated by Flt1 in the field of cell malignant phenotypes in glioma. Subsequently, bioinformatics analysis was used and luciferase reporter analysis to validate that miR-139-5p explicitly binds the 3′UTR region of Flt1. As expected, we verified that the level of Flt1 was decreased in cells treated with miR-139-5p mimics in both mRNA and protein levels. Thereafter, based on the data above we considered that miR-139-5p may restain the malignant phenotypes via decreasing Flt1 level. Consistent with our proposal, forced-expression of miR-139-5p suppressed the proliferation, migration/invasion, EMT and VM in glioma cells. Furthermore, the results that overexpression of Flt1 without the 3′UTR greatly reversed the suppressing influence of miR-139-5p on the malignant phenotypes were likewise observed in cotransfected glioma cells. Taken together, these results confirm that Flt1 is the direct downstream miR-139-5p target to regulate the development of glioma.
Our study observed the effects of Flt1 on the malignancy on glioma cells, but the mechanism is poorly understood. Flt1 is normally involved in VEGF/Flt1 pathway, which has been reported in many studies to be a critical fraction interaction with several signaling pathway, such as PI3K/Akt-Rac1 pathway (33) and ERK/MAPK pathway (34). PIGF (53% homology to VEGF) is another ligand for Flt1 and PIGF/Flt1 plays a key role in colorectal cancer progression through p38-MMP9 pathway (7). The Wnt signaling is activated in numerous cancers and Wnt/β-catenin pathway is the canonical function group of Wnt signals contributing to tumor progression including proliferation, metastasis and angiogenesis (9–12). β-catenin was accumulated in the cytoplasm when GSK-3β is phosphorylated due to the activation of Wnt1, and then transfers into the nucleus to interwork with the transcription factors of TCF/Lef-1, which further modulate the levels of corresponding target genes to regulate the malignancy of cancer cells, such as proliferation, migration/invasion and VM. Inhibition of Wnt/β-catenin has been administered to be part of cancer therapeutic strategies, and Wnt/β-catenin inhibition may reverse the resistance to the inhibitor of PI3K/Akt treatment (35). A previous study declared that the excitation of Wnt/β-catenin relies on Flt1 activity in colorectal cancer cells (36), which means Flt1 may be one of the significant regulators of Wnt/β-catenin signals. In this study, we proved that miR-139-5p inhibits the Wnt/β-catenin pathway and decreased the phosphorylation of GSK-3β through altering Flt1 expression, which can be rescued by the overexpression of Flt1. Together with the impression of miR-139-5p/Flt1 regulatory axis on the malignancy on glioma cells, the Flt1/Wnt/β-catenin pathway may be a mechanism that is involved in miR-139-5p regulating the development of glioma.
In conclusion, we have confirmed a connection between miR-139-5p and Flt1, and further reveal that miR-139-5p serves as a tumor-suppressor partially through downregulating the expression of Flt1 and inhibiting the Wnt/β-catenin pathway in glioma. Despite the gradual comprehension of the various molecular mechanisms involved in glioma, the therapy of glioma remains a difficult clinical challenge. Given that anti- VEGFR drugs have been a novel trial therapeutic for glioma, our results contribute to present an Flt1-associated miRNA for potential diagnostic and therapeutic strategies in glioma.
Acknowledgments
The study was supported by the National Key Technology Support Program (no. 2014BAI04B00), the National Key disciplines Fund of the Ministry of Health of the People’s Republic of China, the Foundation of Tianjin Science and Technology Committee (no. 14JCZDJC35600).
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 3.Takano S, Yamashita T, Ohneda O. Molecular therapeutic targets for glioma angiogenesis. J Oncol. 2010;2010:351908. doi: 10.1155/2010/351908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Herold-Mende C, Steiner HH, Andl T, Riede D, Buttler A, Reisser C, Fusenig NE, Mueller MM. Expression and functional significance of vascular endothelial growth factor receptors in human tumor cells. Lab Invest. 1999;79:1573–1582. [PubMed] [Google Scholar]
- 6.Li J, Li L, Li Z, Gong G, Chen P, Liu H, Wang J, Liu Y, Wu X. The role of miR-205 in the VEGF-mediated promotion of human ovarian cancer cell invasion. Gynecol Oncol. 2015;137:125–133. doi: 10.1016/j.ygyno.2015.01.531. [DOI] [PubMed] [Google Scholar]
- 7.Wei SC, Tsao PN, Weng MT, Cao Z, Wong JM. Flt-1 in colorectal cancer cells is required for the tumor invasive effect of placental growth factor through a p38-MMP9 pathway. J Biomed Sci. 2013;20:39. doi: 10.1186/1423-0127-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragoso R, Pereira T, Wu Y, Zhu Z, Cabeçadas J, Dias S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood. 2006;107:1608–1616. doi: 10.1182/blood-2005-06-2530. [DOI] [PubMed] [Google Scholar]
- 9.Pierzynski JA, Hildebrandt MA, Kamat AM, Lin J, Ye Y, Dinney CP, Wu X. Genetic variants in the Wnt/β-catenin signaling pathway as indicators of bladder cancer risk. J Urol. 2015;194:1771–1776. doi: 10.1016/j.juro.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mir R, Pradhan SJ, Patil P, Mulherkar R, Galande S. Wnt/beta-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene. 2016;35:1679–1691. doi: 10.1038/onc.2015.232. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Zhao Y, Zheng Y. MiR-122/Wnt/β-catenin regulatory circuitry sustains glioma progression. Tumour Biol. 2014;35:8565–8572. doi: 10.1007/s13277-014-2089-4. [DOI] [PubMed] [Google Scholar]
- 12.Yan Z, Che S, Wang J, Jiao Y, Wang C, Meng Q. miR-155 contributes to the progression of glioma by enhancing Wnt/beta-catenin pathway. Tumour Biol. 2015;36:5323–5331. doi: 10.1007/s13277-015-3193-9. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 15.Yue S, Wang L, Zhang H, Min Y, Lou Y, Sun H, Jiang Y, Zhang W, Liang A, Guo Y, et al. miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol. 2015;36:6741–6749. doi: 10.1007/s13277-015-3372-8. [DOI] [PubMed] [Google Scholar]
- 16.Li RY, Chen LC, Zhang HY, Du WZ, Feng Y, Wang HB, Wen JQ, Liu X, Li XF, Sun Y, et al. miR-139 inhibits Mcl-1 expression and potentiates TMZ-induced apoptosis in glioma. CNS Neurosci Ther. 2013;19:477–483. doi: 10.1111/cns.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu CW, Wang K, Zheng S, Ng SS, Chan FK, et al. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol Cancer. 2014;13:124. doi: 10.1186/1476-4598-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Hang M, Yuan CY, Wu FL, Chen SB, Xue K. MicroRNA-139-5p inhibits cell proliferation and invasion by targeting insulin-like growth factor 1 receptor in human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:3864–3870. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Tong X, Gao H, Yan X, Xu X, Sun S, Wang Q, Wang J. Silencing HIWI suppresses the growth, invasion and migration of glioma cells. Int J Oncol. 2014;45:2385–2392. doi: 10.3892/ijo.2014.2673. [DOI] [PubMed] [Google Scholar]
- 20.Pin AL, Houle F, Fournier P, Guillonneau M, Paquet ÉR, Simard MJ, Royal I, Huot J. Annexin-1-mediated endothelial cell migration and angiogenesis are regulated by vascular endothelial growth factor (VEGF)-induced inhibition of miR-196a expression. J Biol Chem. 2012;287:30541–30551. doi: 10.1074/jbc.M112.393561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, et al. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik S, Dothager RS, Marasa J, Lewis CL, Piwnica-Worms D. Vascular endothelial growth factor receptor-1 is synthetic lethal to aberrant {beta}-catenin activation in colon cancer. Clin Cancer Res. 2009;15:7529–7537. doi: 10.1158/1078-0432.CCR-09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marumoto T, Saya H. Molecular biology of glioma. Adv Exp Med Biol. 2012;746:2–11. doi: 10.1007/978-1-4614-3146-6_1. [DOI] [PubMed] [Google Scholar]
- 24.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setién F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Tang H, Wang Z, Zhang B, Liu W, Lu H, Xiao L, Liu X, Wang R, Li X, et al. miR-185 targets the DNA methyl-transferases 1 and regulates global DNA methylation in human glioma. Mol Cancer. 2011;10:124. doi: 10.1186/1476-4598-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R. Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27:371–377. doi: 10.1179/016164105X39833. [DOI] [PubMed] [Google Scholar]
- 27.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira JM, Ruhrberg C, Schwarz Q. VEGF receptor signaling in vertebrate development. Organogenesis. 2010;6:97–106. doi: 10.4161/org.6.2.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 32.Roybal JD, Zang Y, Ahn YH, Yang Y, Gibbons DL, Baird BN, Alvarez C, Thilaganathan N, Liu DD, Saintigny P, et al. miR-200 inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol Cancer Res. 2011;9:25–35. doi: 10.1158/1541-7786.MCR-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Yamauchi M, Muramatsu M, Osawa T, Tsuchida R, Shibuya M. RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3K/Akt pathway. J Biol Chem. 2011;286:9097–9106. doi: 10.1074/jbc.M110.165605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi M, Matsui A, Inao M, Mochida S, Fujiwara K. ERK/MAPK-dependent PI3K/Akt phosphorylation through VEGFR-1 after VEGF stimulation in activated hepatic stellate cells. Hepatol Res. 2003;26:232–236. doi: 10.1016/S1386-6346(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 35.Arqués O, Chicote I, Puig I, Tenbaum SP, Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J, Silberschmidt D, et al. Tankyrase inhibition blocks Wnt/beta-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22:644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 36.Zeitlin BD, Ellis LM, Nor JE. Inhibition of vascular endothelial growth factor receptor-1/Wnt/{beta}-catenin crosstalk leads to tumor cell death. Clin Cancer Res. 2009;15:7453–7455. doi: 10.1158/1078-0432.CCR-09-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]