Abstract
Introduction
HIV affects more women than any other life‐threatening infectious agent, and most infections are sexually transmitted. HIV must breach the female genital tract mucosal barrier to establish systemic infection, and clinical studies indicate virus more easily evades this barrier in women using depot‐medroxyprogesterone acetate (DMPA) and other injectable progestins for contraception. Identifying a potential mechanism for this association, we learned DMPA promotes susceptibility of wild‐type mice to genital herpes simplex virus type 2 (HSV‐2) infection by reducing genital tissue expression of the cell‐cell adhesion molecule desmoglein‐1 (DSG‐1) and increasing genital mucosal permeability. Conversely, DMPA‐mediated increases in genital mucosal permeability and HSV‐2 susceptibility were eliminated in mice concomitantly administered exogenous oestrogen (E). To confirm and extend these findings, herein we used humanized mice to define effects of systemic DMPA and intravaginal (ivag) E administration on susceptibility to genital infection with cell‐associated HIV‐1.
Methods
Effects of DMPA or an intravaginal (ivag) E cream on engraftment of NOD‐scid‐IL‐2Rgcnull (NSG) mice with human peripheral blood mononuclear cells (hPBMCs) were defined with flow cytometry. Confocal microscopy was used to evaluate effects of DMPA, DMPA and E cream, or DMPA and the pharmacologically active component of the cream on vaginal tissue DSG‐1 expression and genital mucosal permeability to low molecular weight (LMW) molecules and hPBMCs. In other studies, hPBMC‐engrafted NSG mice (hPBMC‐NSG) received DMPA or DMPA and ivag E cream before genital inoculation with 106 HIV‐1‐infected hPBMCs. Mice were euthanized 10 days after infection, and plasma HIV‐1 load quantified by qRT‐PCR and splenocytes used to detect HIV‐1 p24 antigen via immunohistochemistry and infectious virus via TZM‐bl luciferase assay.
Results
Whereas hPBMC engraftment was unaffected by DMPA or E treatment, mice administered DMPA and E (cream or the pharmacologically active cream component) displayed greater vaginal tissue expression of DSG‐1 protein and decreased vaginal mucosal permeability to LMW molecules and hPBMCs versus DMPA‐treated mice. DMPA‐treated hPBMC‐NSG mice were also uniformly susceptible to genital transmission of cell‐associated HIV‐1, while no animal concomitantly administered DMPA and E cream acquired systemic HIV‐1 infection.
Conclusion
Exogenous E administration reduces susceptibility of DMPA‐treated humanized mice to genital HIV‐1 infection.
Keywords: DMPA, genital HIV transmission, HIV prevention, humanized mice, oestrogen
1. Introduction
Women aged 15 to 24 years in sub‐Saharan Africa are especially vulnerable to HIV, and currently represent 25% of the new infections in the region 1. Reasons for this are unclear but certainly multi‐factorial, with gender‐based social, economic, political and cultural disparities possibly contributing 2. High prevalence of intergenerational sexual partnerships may play an additional role 3, and certain hormonal contraceptives appear to at least modestly increase the risk of HIV acquisition 4, 5, 6. The injectable progestins depot‐medroxyprogesterone acetate (DMPA) and norethisterone enanthate are commonly used in sub‐Saharan Africa, and women using these agents were found twice as likely to acquire HIV as women using no form of hormonal contraception 7.
Most likely, cell‐free and cell‐associated HIV‐1 are sexually transmitted, but the exact transmission frequency of each is unknown 8. Transmission of cell‐free HIV has been the more thoroughly explored, and most non‐human primate transmission studies with simian immunodeficiency virus (SIV) utilized cell‐free virus. However, in humans, HIV‐1 is more often isolated from seminal cells than seminal fluid, and cell‐associated HIV‐1 is detected in the seminal fluid of men receiving highly active antiretroviral therapy 9, 10, 11, 12, 13. Seminal fluid from healthy men contains about 105 leukocytes/ml, and these numbers are markedly increased by genital infection 13, 14. These observations suggest that transmission of cell‐associated HIV‐1 can cause systemic infection 15, 16; a possibility supported in animal models. Female cats were genitally infected with cell‐associated feline immunodeficiency virus (another lentivirus with T‐cell tropism) 17, and female macaques were genitally infected with cell‐associated SIV 18. Cell‐associated HIV‐1 was also transmitted to severe combined immunodeficient (SCID) mice reconstituted with human peripheral blood leukocytes, whereas productive infection did not result from inoculating these mice with cell‐free HIV‐1 19.
It is interesting to note that multiple non‐human primate and murine models of genital HIV infection administer DMPA prior to infection to achieve uniform infectivity 20, 21. Although mechanisms responsible for this effect were not fully defined, DMPA is known to similarly enhance mouse susceptibly to genital infection with human papilloma virus and Chlamydia 22, 23. Offering mechanistic insight into this observation, we saw DMPA and levonorgestrel (LNG), another exogenous progestin used for long‐acting reversible contraception, increase genital mucosal permeability and susceptibility of wild‐type mice to intravaginal (ivag) infection with herpes simplex virus type 2 (HSV‐2) 24. We also found these progestin‐mediated increases in genital mucosal permeability and HSV‐2 susceptibility were abolished in mice administered DMPA and exogenous oestrogen (E) prior to infection 24. Herein, we sought to further explore these findings by comparing genital mucosal permeability and transmission of cell‐associated HIV‐1 in humanized mice administered DMPA or DMPA and E.
2. Methods
2.1. Human peripheral blood mononuclear cells
Human peripheral blood mononuclear cells (hPBMCs) were isolated by density gradient centrifugation 25, 26 from healthy donor buffy coats obtained from the Central‐Southeast Ohio Region American Red Cross. hPBMCs used to reconstitute mice in this study were determined by TZM‐bl cell assay 27, 28, 29, 30, 31, 32 to not contain infectious HIV‐1 particles.
2.2. Humanized mice
Prior to animal experimentation, ethical approval was obtained from The Ohio State University IACUC, and in vivo procedures were performed from December 2015 to September 2016 in compliance with the principles of the Guide for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research. Six‐ to eight‐week‐old NOD‐scid‐IL‐2Rgcnull (NSG) female mice (n = 65) were acquired from Jackson Laboratory (Bar Harbor, ME, USA), and kept under a 12 hour to 12 hour light–dark cycle with ad libitum access to food and water. For NSG mouse engraftment, hPBMCs were thawed, washed and re‐suspended in PBS, and intravenously (i.v.) administered (107 cells per mouse). Fourteen and 24 days after hPBMC injection, hPBMC engraftment was assessed using peripheral blood. Blood was incubated with RBC lysis buffer (eBioscience, San Diego, CA, USA), and cells stained with Live/Dead Fixable near‐IR (Invitrogen, Eugene, OR, USA) and anti‐human CD45 BV510 (H130), anti‐mouse CD45 PerCP (30‐F11) (BioLegend, San Diego, CA, USA) and anti‐human CD3 FITC (UCHT1) (BD Biosciences, San Jose, CA, USA) antibodies. Cells were fixed in CytofixTM buffer (BD Biosciences), and collected by FACSCanto II flow cytometer (BD Biosciences). Data were acquired by FACSDiva (BD Biosciences), and analysed with FlowJo software (Tree Star Inc., Ashland OR, USA). Unlike engrafted bone marrow, liver and thymus (BLT) humanized mice, no human cells are seen in lower genital tract mucosal tissues of hPBMC‐engrafted NSG (hPBMC‐NSG) mice 33. These mice (rather than BLT mice) therefore were used in the current study to explore the impact of exogenous sex steroids on vaginal mucosal integrity and genital HIV transmission, as the absence of HIV‐1 target cells in the vaginal mucosal epithelium of NSG mice minimizes the potential for confounding created by exogenous steroid‐mediated effects on human immune cell infiltration or function in the lower genital tract.
2.3. Exogenous steroid administration
hPBMC‐NSG mice were subcutaneously (s.c.) injected with 1 mg of DMPA (Depo‐Provera®; Pharmacia and Upjohn Co., New York, NY, USA) 5 days before mucosal permeability assays were performed or HIV‐1 transmission studies initiated (Figure 1a). This dose achieves serum levels in mice that approximate peak serum concentrations measured in women that initiate DMPA 34. As indicated, mice were ivag administered a commercially available E cream (Premarin®; Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc., Philadelphia PA, USA) or its active component (Pfizer Inc.) daily in the 3 days before permeability assays were performed or transmission studies initiated (Figure 1a). As indicated, untreated NSG mice in the oestrus stage of the oestrus cycle provided controls for experiments used to assess genital mucosal permeability and integrity.
Figure 1.
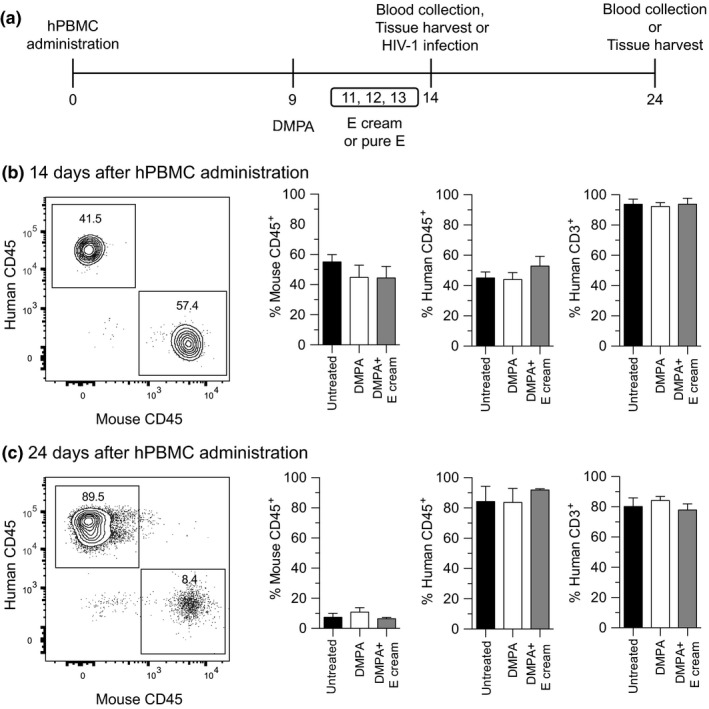
Exogenous DMPA or E did not affect hPBMC engraftment of NSG mice. (a) Schematic of the study design used to assess effects of administering DMPA and E on hPBMC engraftment, genital mucosal permeability and HIV‐1 susceptibility of NSG mice. To define effects of DMPA and E on engraftment, peripheral blood was obtained 14 days and 24 days after hPBMC administration (untreated NSG mice provided controls). (b,c) Flow cytometric studies identified no between‐group differences in the percentages of murine CD45+ cells and human CD45+ CD3+ cells after hPBMC administration; left panels show representative contour plots; quadrant numbers denote population percentages. Data are from 2 independent experiments with 3 animals per group (bars denote mean ± SD). Statistical analyses performed using one‐way ANOVA with Dunnett's multiple comparisons test. DMPA, depot medroxyprogesterone acetate; E, ivag oestrogen cream; hPBMC‐NSG (hPBMC‐engrafted NOD‐scid‐IL‐2Rgcnull) mice.
2.4. HIV‐1 infection
106 hPBMCs/ml were plated in RPMI‐1640 with 10% FBS, 2 mM L‐glutamine, 1 mM sodium pyruvate, non‐essential amino acids, 50 μM 2‐ME, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml gentamycin (Mediatech, Manassas, VA, USA) (hereafter termed complete media). Cells were stimulated for 48 hour in complete media containing 5 μg/ml of phytohaemagglutinin (PHA) (Sigma‐Aldrich, St. Louis, MO, USA). Cells were centrifuged, re‐suspended (2 x 106 cells/ml) in complete media supplemented with 10 IU/ml recombinant human IL‐2 (rhIL‐2) (PeproTech, Rocky Hill, NJ, USA), and incubated another 5 days. hPBMCs were inoculated with 600 TCID50 of HIV‐1 BaL 35 for 24 hour, and re‐suspended in PBS (108 cells/ml) for in vivo infections (portions of the HIV‐1‐infected hPBMC culture were used in a luciferase gene reporter assay to confirm HIV‐1 infectivity). For infection, hPBMC‐NSG mice were anaesthetized with xylazine and ketamine hydrochloride 36, and ivag inoculated with 106 (10 μl) of HIV‐1‐infected huPBMCs. Mice were euthanized 10 days later to assess HIV‐1 infection status. Of note, mice were euthanized 24 days after hPBMC administration, while typical onset of clinical signs and mortality from graft versus host disease in NSG mice occurs no sooner than 25 days and 40 days after hPBMC engraftment respectively 37. At euthanasia, plasma was separated from blood and stored at −80°C. Approximately 2/3 of the spleen was transferred to chilled complete media, with the rest placed in buffered 4% formaldehyde for 24 hour (Thermo Scientific, Rockford, IL, USA). Splenic tissues placed in media were processed into single‐cell suspension, and cultured in complete media (106 cells/ml) supplemented with rhIL‐2 (media replenished every 3 days). After 8 days, supernatants were incubated with TZM‐bl indicator cells to detect infectious HIV‐1 particles. In these assays, splenocytes from uninfected mice provided negative controls and HIV‐1 BaL diluted in complete media served as positive controls. Plasma HIV‐1 load was quantified at an OSU clinical laboratory using Abbott's real‐time PCR assay, a FDA‐approved test for HIV RNA viral load. All assays used to evaluate HIV infection status in our study were performed by investigators unaware of mouse treatment group assignment.
2.5. Mucosal permeability assays
To assess genital mucosal permeability to low molecular weight (LMW) molecules, sedated mice were ivag administered a 10 μl PBS solution containing 70 kDa Texas‐Red dextran and Lucifer yellow CH lithium (Invitrogen, Carlsbad, CA, USA). Mice were euthanized 45 minutes later, and fluorescent molecule penetration into vaginal tissue defined using FV1000 spectral confocal microscope system (Olympus, Center Valley, PA, USA) and ImageJ software 24, 38. To evaluate hPBMC entry into vaginal tissue, uninfected hPBMCs were activated as described above. Eight days later, cells were labelled with 5 μM of carboxyfluorescein succinimidyl ester (CellTrace CFSE; Life Technologies, Carlsbad, CA, USA), re‐suspended in PBS (108 cells/ml), and mice ivag inoculated with 10 μl of this suspension. After 15 hour, mice were euthanized, vaginas excised, and tissues fixed in formaldehyde, agarose‐embedded and DAPI stained. The fluorescent signal of CSFE‐labelled hPBMCs was used to assess depth of leukocyte infiltration into vaginal submucosal tissue using confocal microscopy and ImageJ software. Confocal images were acquired by sequential scanning to prevent fluorescence crossover.
2.6. DSG‐1 protein and HIV‐1 p24 antigen
To assess desmoglein‐1 (DSG‐1) protein expression, vaginal tissue excised from euthanized mice was fixed in buffered formaldehyde. DSG‐1 expression was quantified as described earlier 24. Using methods described previously, HIV‐1 p24 protein expression was defined in splenic tissue sections after de‐paraffinization and antigen retrieval, overnight blocking with 5% BSA, and incubation with rabbit monoclonal anti‐CD45 (EP322Y) and goat polyclonal anti‐HIV p24 (ab53841) (Abcam, Cambridge, MA, USA) 24. Samples were processed for confocal microscopy analysis as detailed above.
2.7. Statistical considerations
All statistical analyses were performed using Prism 6 software (GraphPad, La Jolla, CA, USA), with normality assessed using evaluation of the residuals. For comparisons between 2 groups, unpaired Student's t‐tests were used. For comparisons between multiple groups, one‐way ANOVA with Dunnett's post hoc test or Kruskal–Wallis test with Dunn's post hoc test were used (depending on data distribution) (p ≤ 0.05 were deemed statistically significant).
3. Results
3.1. DMPA and E administration did not affect hPBMC engraftment of NSG mice
While the primary objective of this study was to define the effects of treatment with DMPA and E on susceptibility of hPBMC‐NSG mice to genital HIV‐1 infection, we first needed to determine if these compounds altered hPBMC engraftment. Using peripheral blood from untreated controls, DMPA‐treated and DMPA‐ and E cream‐treated NSG mice 14 days and 24 days after hPBMC administration (Figure 1a), our flow cytometric analyses identified more robust engraftment of human CD45+ CD3+ cells at the latter time point. However, comparing untreated controls and mice administered systemic DMPA or DMPA and ivag E, neither time point was associated with statistically significant differences hPBMC engraftment (Figure 1b,c).
3.2. Exogenous E prevented DMPA‐mediated loss of genital mucosal barrier function
Because DMPA and E did not affect hPBMC engraftment of NSG mice, we explored the effects of these compounds on genital mucosal barrier function. We first defined effects of DMPA and E on vaginal expression of DSG‐1, a cell‐cell adhesion molecule needed to maintain barrier function in cutaneous and intestinal epithelium 39, 40. In earlier work with wild‐type mice, we established that systemic DMPA treatment significantly reduced vaginal tissue expression of DSG‐1 without affecting levels of other cell‐cell adhesion molecules expressed in genital mucosa, including tight junction protein 1, claudin‐1 and occludin 24. In the current study, we measured vaginal tissue expression of DSG‐1 protein in hPBMC‐NSG mice that were: oestrus stage and untreated with exogenous sex steroids; DMPA‐treated; or administered DMPA and ivag E cream. To exclude the possibility that physical properties of the E cream produced any observed effect, other hPBMC‐NSG mice received DMPA injection and ivag administration of the pharmacologically active component of E cream (i.e. pure E). These studies revealed comparable DSG‐1 protein expression in untreated control mice and hPBMC‐NSG mice administered DMPA and oestrogen (E cream or pure E), but significantly reduced DSG‐1 expression in vaginal tissue of mice treated with DMPA alone (Figure 2).
Figure 2.
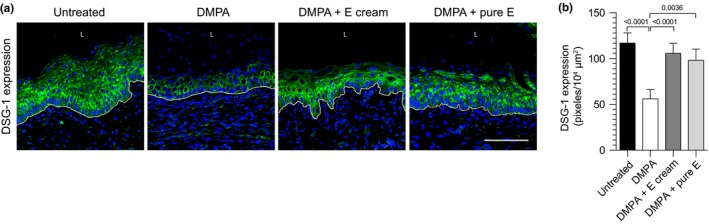
DMPA‐mediated reduction in vaginal DSG‐1 protein expression was abrogated in hPBMC‐NSG mice administered DMPA and E. hPBMC‐NSG mice were untreated or treated with DMPA, DMPA and ivag E cream, or DMPA and ivag pure E. As detailed in Methods, vaginal tissue was collected from euthanized mice to quantify DSG‐1 protein expression. (a) Representative confocal microscopic images of vaginal DSG‐1 protein expression; L (vaginal lumen); DAPI (Blue); DSG‐1 (green); white line delimits the vaginal mucosal epithelium; scale bar denotes 100 μm. (b) Quantification of DSG‐1 protein expression showed significantly reduced levels in hPBMC‐NSG mice administered DMPA alone. Data from 2 independent experiments with 3 animals per group (bars denote mean ± SD). Statistical analyses performed using one‐way ANOVA with Dunnett's multiple comparisons test. hPBMC‐NSG (hPBMC‐engrafted NOD‐scid‐IL‐2Rgcnull) mice; DMPA, depot medroxyprogesterone acetate; E, vaginal oestrogen cream; pure E, pharmacologically active component of E cream; DSG‐1, desmoglein‐1.
In follow‐up studies, we used identically treated groups of mice to delineate the effects of systemic DMPA and ivag E administration on vaginal mucosal permeability to LMW molecules and activated human leukocytes. Compared to DMPA‐treated hPBMC‐NSG mice, these studies identified reduced mucosal penetration of fluorescent LMW molecules and leukocytes in untreated mice and hPBMC‐NSG mice administered DMPA and ivag E or DMPA and pure E (Figure 3). Because we identified similar vaginal tissue expression of DSG‐1 protein and comparably reduced vaginal tissue penetration of LMW molecules and activated human leukocytes among hPBMC‐NSG mice treated with DMPA and E cream or pure E (vs. mice treated with DMPA alone), these studies also resolved E itself was responsible for strengthening genital mucosal barrier function.
Figure 3.
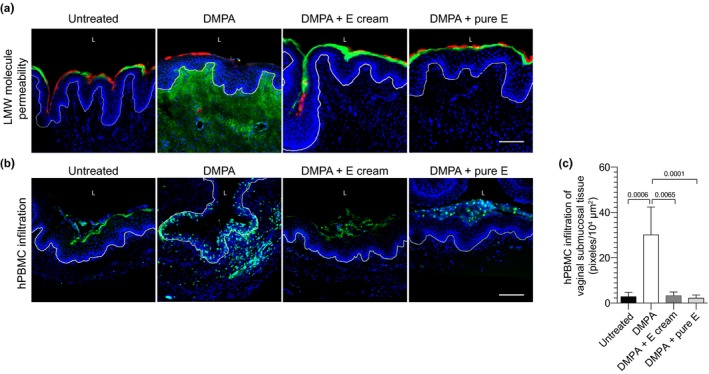
Combined treatment with DMPA and exogenous E obviated DMPA‐mediated increases in vaginal mucosal permeability. hPBMC‐NSG mice remained untreated or were treated as described in Figure 2. As detailed in Methods, vaginal tissue was excised from euthanized mice to assess permeability to LMW molecules or CFSE‐labelled hPBMCs. (a, b) Representative images illustrate increased permeability to LMW molecules and activated human leukocytes in hPBMC‐NSG mice administered DMPA vs. untreated controls or animals administered DMPA and ivag E; scale bars denote 100 μm. (a) L (vaginal lumen); DAPI (blue) Lucifer Yellow (green); 70 kDa Texas‐Red dextran (red), and (b) L (vaginal lumen); DAPI (blue); CFSE‐labelled hPBMCs (green); white line delimits vaginal mucosal epithelium. (c) Quantifying depth of hPBMC infiltration into vaginal submucosal tissue identified significantly deeper infiltration in mice administered DMPA alone. Displayed data from 2 independent experiments with 3 animals per group (bars denote mean ± SD). Statistical analyses performed using one‐way ANOVA with Dunnett's multiple comparisons test. hPBMC‐NSG (hPBMC‐engrafted NOD‐scid‐IL‐2Rgcnull) mice; DMPA, depot medroxyprogesterone acetate; CFSE, carboxyfluorescein succinimidyl ester; E, oestrogen cream; pure E, pharmacologically active component of the E cream.
3.3. Exogenous E abrogated susceptibility of DMPA‐treated humanized mice to genital transmission of cell‐associated HIV
Because ivag E or pure E restored DMPA‐mediated loss of mucosal barrier function, we hypothesized exogenous E eliminates the susceptibility of hPBMC‐NSG mice to genital transmission of cell‐associated HIV‐1. To explore this hypothesis, hPBMC‐NSG mice were systemically treated with DMPA or DMPA and ivag E cream (Figure 1a). All mice were genitally inoculated with 106 HIV‐1‐infected hPBMCs, and euthanized 10 days later to assess HIV‐1 infection status. Immunohistochemical tests for HIV‐1 p24 antigen detected this protein only in the spleens of DMPA‐treated hPBMC‐NSG mice (Figure 4a). Using a TZM‐bl luciferase assay to qualitatively detect the presence of infectious HIV‐1 particles, we detected significantly increased signal in the spleens of DMPA‐treated mice versus mice treated with DMPA and the ivag E cream (Figure 4b). These results indicated that systemic HIV‐1 infection was prevented by combined DMPA and ivag E treatment. Offering further support for this conclusion, HIV‐1 virus copies were detected by qRT‐PCR assay in the plasma of hPBMC‐NSG mice administered DMPA, but not in the plasma of hPBMC‐NSG mice treated with DMPA and E (Figure 4c).
Figure 4.
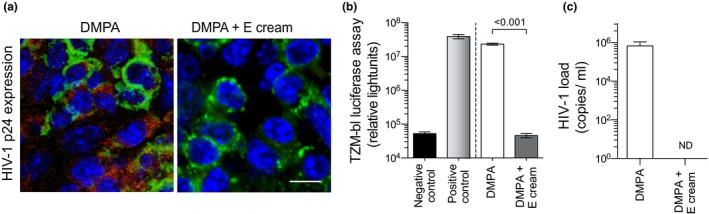
Exogenous E reversed susceptibility of DMPA‐treated humanized mice to genital transmission of cell‐associated HIV‐1. hPBMC‐NSG mice were treated with DMPA or DMPA and ivag E cream and genitally inoculated with cell‐associated HIV‐1 (as described in Methods and depicted in Figure 1a). Ten days after genital inoculation, mice were euthanized to assess HIV‐1 infection status. (a) Representative images of immunostaining for HIV‐1 p24 antigen in the spleens of euthanized mice show this viral protein detected only in mice treated with DMPA alone; DAPI (blue); anti‐human CD45 (green); anti‐HIV‐1 p24 antigen (red); scale bar denotes 20 μm. (b) TZM‐bl luciferase assay identified infectious HIV‐1 particles only in spleens from DMPA‐treated mice (splenocytes from uninfected mice and HIV‐1 BaL diluted in media provided negative and positive controls respectively). (c) A qRT‐PCR assay detected HIV‐1 virus only in the serum of hPBMC‐NSG mice administered DMPA alone (ND denotes no virus was detected). Data displayed are from 2 independent experiments with 5 animals per group (bars denote mean ± SD). Statistical analyses were performed using the unpaired Student's t‐test. DMPA, depot medroxyprogesterone acetate; E, vaginal oestrogen cream; hPBMC‐NSG (NOD‐scid‐IL‐2Rgcnull) mice.
4. Discussion
Male‐to‐female HIV transmission rates approximate 0.12% per sex act 41, implying the virus must overcome host defences in the female genital tract to establish systemic infection. As examples, virus particles that avoid entrapment in epithelial surface mucus must breach the mucosal epithelium to interact with submucosal tissue target cells 42, 43. While pathogen‐induced ulcers and coital abrasions may help HIV‐1 evade genital mucosal barriers 44, 45, current findings suggest DMPA‐mediated increases in genital mucosal permeability also promote virus transmission. Compared to hPBMC‐NSG mice administered DMPA and E, vaginal tissue of mice treated with DMPA displayed reduced expression of DSG‐1 protein and impaired mucosal barrier function, and DMPA‐treated hPBMC‐NSG mice were highly susceptible to genital transmission of cell‐associated HIV‐1. These findings thus corroborate results in which DMPA‐ and LNG‐treated wild‐type mice displayed increased genital mucosal permeability and susceptibility to cell‐free HSV‐2 infection 24. Current findings also corroborate clinical data in which ectocervical DSG‐1 expression and mucosal permeability were analogously altered in women initiating use of DMPA or a LNG‐releasing intra‐uterine system 24, 46.
While our results may identify an underlying mechanism by which exogenous progestins enhance HIV susceptibility, they cannot exclude contributions from other mechanisms. Several clinical studies saw greater inflammation in genital tissue after women initiated DMPA 24, 47, 48, and it is possible that DMPA‐mediated increases in genital inflammation can enhance HIV susceptibility. On the other hand, mouse model findings reveal that DMPA‐mediated increases in genital inflammation occurred downstrearm of DMPA‐mediated increases in genital mucosal permeability that facilitated tissue invasion by endogenous microbiota 24. Moreover, while DMPA makes hPBMC‐NSG mice uniformly susceptibly to genital HIV‐1 transmission (Figure 4), genital tract submucosal tissue in these mice does not contain human immune cells 33, 49. This implies that the enhanced HIV susceptibility of DMPA‐treated hPBMC‐NSG mice was not created by DMPA‐mediated inflammatory responses that increased the frequency of HIV target cells. While current results offer novel indication that DMPA‐mediated impairment of genital mucosal barrier function promotes HIV transmission, actual contribution of this effect to similarly enhancing HIV susceptibility of women using DMPA awaits further investigation.
On the other hand, DMPA‐mediated enhanced susceptibility of hPBMC‐NSG mice to cell‐associated HIV‐1 infection is congruent with previous reports that DMPA increased susceptibility of hPBMC‐SCID mice and non‐human primates to atraumatic genital inoculation with cell‐associated HIV‐1 and SIV, respectively 19, 50. Our current findings provide important extension of these results, showing that treatment of mice with ivag E improves genital mucosal barrier function and protects DMPA‐treated mice from cell‐associated HIV‐1 acquisition. While non‐human primate studies identified that exogenous E reduced susceptibility to genital SIV infection 51, 52, our findings appear to be the first to demonstrate that exogenous E abrogates HIV‐1 susceptibility in DMPA‐treated animals. Current results further establish the enhanced barrier protection was a direct effect of E, as ivag administration of an E cream (Premarin®) and pure Premarin® substance similarly increased vaginal expression of DSG‐1 protein and reduced genital mucosal permeability to LMW molecules and hPBMCs. These findings imply the ability of ivag E cream to protect DMPA‐treated hPBMC‐NSG mice from cell‐associated HIV‐1 infection was not an artefact of cream impeding access of HIV‐infected hPBMCs to the genital epithelial surface. Considered in combination with prior results 24, the capability of exogenous E to protect progestin‐treated mice from cell‐free and cell‐associated genital virus infection implies that exogenous E and progestin may be components of a hormonal contraceptive platform that is less compromising of genital mucosal barrier function than those that deliver progestin unopposed. However, as there are important differences in the genital tract of mice and women, including the fact that rodent vaginal epithelium is keratinized and human vaginal epithelium is not 53, it will be critical to define efficacy of contraceptive platforms releasing exogenous E and progestin in highly relevant clinical models, including non‐human primates.
5. Conclusion
Our studies show that DMPA weakens genital mucosal barrier function. As barrier function is such a fundamental anti‐virus host defence, any factor that weakens this protection may represent an important HIV risk factor. Our studies also provide new biological plausibility for the putative link between DMPA and HIV susceptibility, and suggest that use of unopposed progestins for hormonal contraception may impede efforts to curb the HIV pandemic. On the other hand, we identified that exogenous E reverses DMPA‐mediated increases in mucosal permeability and HIV susceptibility. Based on the ability of exogenous E to eliminate susceptibly of DMPA‐treated humanized mice to genital HIV‐1 infection, combined use of exogenous progestin and E may provide basis for hormonal contraceptive approaches among women at higher risk for HIV acquisition, particularly in more resource‐limited settings. However, defining the safety and efficacy of contraceptive platforms that use exogenous E to boost genital mucosal barrier function requires new clinical study and further exploration of clinically relevant animal models.
Competing interests
Authors have no competing interests to declare.
Authors’ contributions
Study design and data acquisition, analysis and interpretation performed by N.E.Q.C., R.D.V.M., J.J.K, and T.L.C.; M.E.G. and J.M.G. performed and analysed some experiments; manuscript originally drafted by N.E.Q.C, with all authors contributing to final version.
Funding
Funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01HD072663), OSU College of Medicine, and Stanford University School of Medicine.
Acknowledgements
We acknowledge support provided by the Central‐Southeast Ohio Region American Red Cross and OSU's Comparative Pathology and Mouse Phenotyping Shared Resource, Campus Microscopy and Imaging Facility, Clinical Microbiology and Special Functions Laboratories, and University Laboratory Animal Resources. We further acknowledge Pfizer Inc. (New York, NY) for providing reagents via their compound transfer programme, and the NIH AIDS Reagent Programme for providing HIV‐1Ba‐L (Suzanne Gartner, Mikulas Popovic and Robert Gallo) and TZM‐bl (John C. Kappes, Xiaoyun Wu, and Tranzyme Inc).
Quispe Calla N. E., Vicetti Miguel R. D., Glick M. E., Kwiek J. J., Gabriel J. M. and Cherpes T. L.. Exogenous oestrogen inhibits genital transmission of cell‐associated HIV‐1 in DMPA‐treated humanized mice. J Int AIDS Soc. 2018; 21(1):e25063
References
- 1. HIV/AIDS JUNPo . Global AIDS Update 2016. UNAIDS Switzerland. 2017. [cited 2017 June 1]. Available from: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016.
- 2. Ramjee G, Daniels B. Women and HIV in Sub‐Saharan Africa. AIDS Res Therapy. 2013;10(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leclerc‐Madlala S. Age‐disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25. [DOI] [PubMed] [Google Scholar]
- 4. Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–90. [DOI] [PubMed] [Google Scholar]
- 5. Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS. 2016;30(17):2665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Hormonal contraceptive use and risk of HIV‐1 transmission: a prospective cohort analysis. Lancet Infect Dis. 2012;12(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sagar M. Origin of the transmitted virus in HIV infection: infected cells versus cell‐free virus. J Infect Dis. 2014;210(Suppl 3):S667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Politch JA, Marathe J, Anderson DJ. Characteristics and quantities of HIV host cells in human genital tract secretions. J Infect Dis. 2014;210(Suppl 3):S609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maldarelli F. HIV‐infected cells are frequently clonally expanded after prolonged antiretroviral therapy: implications for HIV persistence. J Virus Erad. 2015;1(4):237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maldarelli F. The role of HIV integration in viral persistence: no more whistling past the proviral graveyard. J Clin Invest. 2016;126(2):438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houzet L, Matusali G, Dejucq‐Rainsford N. Origins of HIV‐infected leukocytes and virions in semen. J Infect Dis. 2014;210(Suppl 3):S622–30. [DOI] [PubMed] [Google Scholar]
- 13. Doncel GF, Anderson S, Zalenskaya I. Role of semen in modulating the female genital tract microenvironment–implications for HIV transmission. Am J Reprod Immunol. 2014;71(6):564–74. [DOI] [PubMed] [Google Scholar]
- 14. Whaley KJ, Mayer KH. Strategies for preventing mucosal cell‐associated HIV transmission. J Infect Dis. 2014;210(Suppl 3):S674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gummuluru S, Pina Ramirez NG, Akiyama H. CD169‐dependent cell‐associated HIV‐1 transmission: a driver of virus dissemination. J Infect Dis. 2014;210(Suppl 3):S641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting trojan horse leukocytes for HIV prevention. AIDS. 2010;24(2):163–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moench TR. Cell‐associated transmission of HIV type 1 and other lentiviruses in small‐animal models. J Infect Dis. 2014;210(Suppl 3):S654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, et al. Infection of macaques after vaginal exposure to cell‐associated simian immunodeficiency virus. J Infect Dis. 2010;202(3):337–44. [DOI] [PubMed] [Google Scholar]
- 19. Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, et al. Vaginal transmission of cell‐associated HIV‐1 in the mouse is blocked by a topical, membrane‐modifying agent. J Clin Invest. 2002;109(2):205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017;13(7):e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherpes TL, Harvey SA, Phillips JM, Vicetti Miguel RD, Melan MA, Quispe Calla NE, et al. Use of transcriptional profiling to delineate the initial response of mice to intravaginal herpes simplex virus type 2 infection. Viral Immunol. 2013;26(3):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175(11):7536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol‐9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–61. [DOI] [PubMed] [Google Scholar]
- 24. Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, Hall‐Stoodley L, Kaur B, Trout W, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal Immunol. 2016;9(6):1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quispe Calla NE, Ghonime MG, Cherpes TL, Vicetti Miguel ND. Medroxyprogesterone acetate impairs human dendritic cell activation and function. Hum Reprod. 2015;30(5):1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quispe Calla NE, Vicetti Miguel RD, Mei A, Fan S, Gilmore JR, Cherpes TL. Dendritic cell function and pathogen‐specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection. Sci Rep. 2016;6:37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. Evidence that ecotropic murine leukemia virus contamination in TZM‐bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol. 2009;83(16):8289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeuchi Y, McClure MO, Pizzato M. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol. 2008;82(24):12585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T‐20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T‐20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell ES, Kwiek JJ, Keys J, Barton K, Mwapasa V, Montefiori DC, et al. The genetic bottleneck in vertical transmission of subtype C HIV‐1 is not driven by selection of especially neutralization‐resistant virus from the maternal viral population. J Virol. 2011;85(16):8253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoddart CA, Maidji E, Galkina SA, Kosikova G, Rivera JM, Moreno ME, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD‐scid IL‐2Rgamma(‐/‐) (NSG) BLT mice. Virology. 2011;417(1):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vicetti Miguel RD, Hendricks RL, Aguirre AJ, Melan MA, Harvey SA, Terry‐Allison T, et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J Immunol. 2012;189(7):3449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV‐III/LAV infection. Science. 1986;233(4760):215–9. [DOI] [PubMed] [Google Scholar]
- 36. Vicetti Miguel RD, Quispe Calla NE, Pavelko SD, Cherpes TL. Intravaginal chlamydia trachomatis challenge infection elicits TH1 and TH17 immune responses in mice that promote pathogen clearance and genital tract damage. PLoS ONE. 2016;11(9):e0162445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ali N, Flutter B, Sanchez Rodriguez R, Sharif‐Paghaleh E, Barber LD, Lombardi G, et al. Xenogeneic graft‐versus‐host‐disease in NOD‐scid IL‐2Rgammanull mice display a T‐effector memory phenotype. PLoS ONE. 2012;7(8):e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein‐1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7(3):718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV‐1 infection per sexual act: systematic review and meta‐analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neidleman JA, Chen JC, Kohgadai N, Muller JA, Laustsen A, Thavachelvam K, et al. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathog. 2017;13(2):e1006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barreto‐de‐Souza V, Arakelyan A, Margolis L, Vanpouille C. HIV‐1 vaginal transmission: cell‐free or cell‐associated virus? Am J Reprod Immunol. 2014;71(6):589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keogan S, Siegert K, Wigdahl B, Krebs FC. Critical review: immunomodulation by seminal factors and implications for male‐to‐female HIV‐1 transmission. J Acquir Immune Defic Syndr. 2015;69(2):131–7. [DOI] [PubMed] [Google Scholar]
- 45. Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV‐1 infection. Nat Rev Microbiol. 2003;1(1):25–34. [DOI] [PubMed] [Google Scholar]
- 46. Quispe Calla NE, Vicetti Miguel RD, Trout W, Cherpes TL. HIV and hormonal contraception: bench and bedside. J Acquir Immune Defic Syndr. 2017;74(3):e85–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldfien GA, Barragan F, Chen J, Takeda M, Irwin JC, Perry J, et al. Progestin‐containing contraceptives alter expression of host defense‐related genes of the endometrium and cervix. Reprod Sci. 2015;22(7):814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deese J, Masson L, Miller W, Cohen M, Morrison C, Wang M, et al. Injectable progestin‐only contraception is associated with increased levels of pro‐inflammatory cytokines in the female genital tract. Am J Reprod Immunol. 2015;74(4):357–67. [DOI] [PubMed] [Google Scholar]
- 49. Shultz LD, Brehm MA, Garcia‐Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell‐free and cell‐associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–23. [PubMed] [Google Scholar]
- 51. Smith SM, Mefford M, Sodora D, Klase Z, Singh M, Alexander N, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18(12):1637–43. [DOI] [PubMed] [Google Scholar]
- 52. Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182(3):708–15. [DOI] [PubMed] [Google Scholar]
- 53. Boyd KL, Muehlenbachs A, Rendi MH. Female reproductive system In: Comparative Anatomy and Histology, 2nd edn Treuting P, Dintzis S, Montine KS. (eds). London: Academic Press; 2017. [Google Scholar]


