Abstract
Introduction
Achieving viral suppression is key in the global strategy to end the HIV epidemic. However, the levels of viral suppression have yet to be described in many resource‐limited settings.
Methods
We investigated the time to virologic failure (VF; defined as a viral load of ≥1000 copies/ml) and changes in CD4 counts since starting antiretroviral therapy (ART) in a cohort of HIV‐infected adults in Hanoi, Vietnam. Factors related to the time to VF and impaired early immune recovery (defined as not attaining an increase in 100 cells/mm3 in CD4 counts at 24 months) were further analysed.
Results
From 1806 participants, 225 were identified as having VF at a median of 50 months of first‐line ART. The viral suppression rate at 12 months was 95.5% and survival without VF was maintained above 90% until 42 months. An increase in CD4 counts from the baseline was greater in groups with lower baseline CD4 counts. A younger age (multivariate hazard ratio (HR) 0.75, vs. <30), hepatitis C (HCV)‐antibody positivity (HR 1.43), and stavudine (d4T)‐containing regimens (HR 1.4, vs. zidovudine (AZT)) were associated with earlier VF. Factors associated with impaired early immune recovery included the male sex (odds ratio (OR) 1.78), HCV‐antibody positivity (OR 1.72), d4T‐based regimens (OR 0.51, vs. AZT), and nevirapine‐based regimens (OR 0.53, vs. efavirenz) after controlling for baseline CD4 counts.
Conclusion
Durable high‐rate viral suppression was observed in the cohort of patients on first‐line ART in Vietnam. Our results highlight the need to increase adherence support among injection drug users and HCV co‐infected patients.
Keywords: HIV, viral load, injection drug use, hepatitis C, antiretroviral therapy, Vietnam
1. Introduction
The increased accessibility to antiretroviral therapy (ART) has dramatically reduced the mortality and morbidity of HIV‐infected individuals globally. Recent studies have also demonstrated the significant benefits of early ART initiation and preventive ART use 1, 2, which had accelerated ART promotion. In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) set the “90‐90‐90” goal, which aims to diagnose 90% of all HIV‐infected individuals, provide ART to 90% of diagnosed individuals, and achieve undetectable HIV RNA levels in 90% of individuals receiving ART by 2020 3. According to the 90‐90‐90 strategy, the plasma HIV viral load (VL) test is considered an essential tool to evaluate the progress of the third goal. Routine VL monitoring has been the gold standard to detect treatment failure since the mid‐1990s in resource‐rich countries, and the World Health Organization (WHO) recommends VL monitoring after six months of ART and then every 12 months whenever possible 2, 4, 5. However, VL monitoring is still unavailable for many ART programmes in resource‐limited countries, given the procedure's cost and complexity, which has limited information regarding viral suppression rates in these regions.
VL monitoring identifies individuals who need additional support to adhere to their ART regimen or when to switch from the current regimen to salvage ART, which can facilitate effective resource allocation and investment. Vietnam has recently expanded ART programme with a coverage rate of 68% by the end of 2013 6, although most patient groups have not experienced the benefits of VL monitoring. The HIV epidemic in Vietnam is strongly associated with injection drug use (IDU; 45%) and transmission to their sexual partners 6, and the overall virologic outcome of ART may be influenced by the social and epidemiological characteristics of these high‐risk groups. Several previous studies, including three Vietnamese studies, have revealed suboptimal adherence to ART among drug users 7, 8, 9, 10, 11, although those studies only examined small target populations during short study periods 11, 12, 13, 14, 15, 16. As ART requires lifelong optimal adherence to experience its full benefits, it would be preferable to examine long‐term virologic outcomes that are related to treatment failure, such as death and loss to follow‐up. Therefore, we aimed to determine the time to virologic failure (VF), which was defined as having an HIV VL of ≥1000 copies/ml during first‐line ART 4, as well as longitudinal immune recovery, and their related risk.
2. Methods
2.1. Study setting and population
Analyses were conducted with a longitudinal dataset from the Hanoi HIV Cohort Study 17, which was established in October 2007 in two large hospitals in urban Hanoi, Vietnam, namely National Hospital of Tropical Diseases (NHTD) and Bach Mai Hospital (BMH); both of these hospitals are teaching and referral hospitals and provide free ART programmes. HIV‐infected individuals aged 18 years or above were consecutively enrolled from October 2007 to April 2013 and followed up until April 2015.
For this study, we enrolled patients who were on ART since October 2007 to March 2012 and extended the eligibility to untreated patients in April 2012. Retrospective and prospective data were obtained at the time of enrolment and every six months during ART and included all ART histories and reasons for ART change. Free VL testing had been provided as part of the programme, using the COBAS® AmpliPrep/COBAS® TaqMan® HIV‐1 Test system (Roche Diagnostics Ltd., Rotkruez, Switzerland). The VL testing was performed every six months until an undetectable VL was observed, and was subsequently performed at least annually thereafter. Genotypic drug resistance testing was performed for participants with a VL of >1000 copies/ml. The test results were returned to the treating physicians, and the ART was switched to a salvage regimen for participants with a VL of >1000 copies/ml with any drug resistance mutation or two consecutive VLs of >1000 copies/ml. Patients who had no VL test results during first‐line ART, were on ART for less than 7 days, or had started with mono/dual therapy or regimens that contained protease inhibitor or nucleos(t)ide reverse transcriptase inhibitor (NRTI) combinations other than zidovudine (AZT), stavudine (d4T), or tenofovir (TDF) plus lamivudine (3TC) or emtricitabine (FTC), were excluded from the analyses. Diagnosis, prophylaxis, and treatment of opportunistic infections and indication and selection of antiretroviral drugs were decided based on the Vietnamese national guidelines 18, 19, 20, which were updated twice during the study period according to changes in the WHO guidelines 4, 21, 22. The CD4 count for ART indication was increased from 200 to 250 cells/mm3 in 2009 19 and to 350 cells/mm3 in 2011 20. AZT and d4T were replaced with TDF as the preferred first‐line drug in 2011 20.
The study protocol was approved by the ethics committee in the Vietnamese Ministry of Health (No: 1666/QD‐BYT) and the institutional ethical review boards in BMH, NHTD, and the National Center for Global Health and Medicine in Tokyo, Japan (NCGM‐G‐001074‐01). All study participants provided written informed consent before study enrolment.
2.2. Statistical analysis
The time from ART initiation to treatment failure was analysed based on VF or a combined clinical endpoint, which was defined as the first episode of death, or change in ART drug due to lack of efficacy or VF after ≥6 months of first‐line ART. First‐line ART was defined as the ART prescribed for the first time in the patient's life and was considered to be continued until the last clinic visit or a change in ART due to lack of efficacy. Loss to follow‐up was defined as cases of patients whose data for 12 months before the last day of data collection in the database were not found, and the date of the last clinic visit was used as the date of loss to follow‐up. For both endpoints, the patients were censored at the end of the observation period or when they were transferred to another clinic. In addition, patients were censored in the VF analysis in cases of death, loss to follow‐up, or change in the ART. The baseline CD4 cell count at ART initiation was defined as the closest CD4 measurement prior to and within three months of ART initiation. The mean CD4 count and mean CD4 count change trajectories were estimated using linear regression with the outcome as a function of time (in years). For the latter, to ensure flexibility in the estimated shape of the trajectories, we used a natural cubic spline with three knots at six months, three years, and six years 23, 24.
We analysed the risk factors for early VF using the Cox proportional hazards model, and a logistic regression model was used to analyse the risk factors for failed early immune recovery, which was defined as not achieving an increase in CD4 counts of 100 cells/mm3 at 24 months. Both analyses included the following covariates: age at ART initiation, sex at birth, AIDS history before ART, baseline CD4 count, previous IDU, a positive hepatitis B virus surface antigen (HBs antigen) result, a positive anti‐hepatitis C virus antibody (HCV‐antibody) result, and antiretroviral categories of NRTIs (AZT, d4T, or TDF) and non‐nucleoside reverse‐transcriptase inhibitors (nevirapine (NVP) or efavirenz (EFV)). Variables that were significant for the univariate analysis (p < 0.10) were chosen for the multivariate analysis and considered statistically significant at p < 0.05 in the final model. The chi‐square test was used to evaluate the association between IDU history and HCV‐antibody positivity. All statistical analyses were performed using STATA software (version 12; StataCorp LP, College Station, TX, USA) and R Statistical Software (version 3.2.0; Foundation for Statistical Computing, Vienna, Austria) 23.
3. Results
3.1. Baseline characteristics
Between October 2007 and May 2013, 2156 HIV‐infected individuals were enrolled in the Hanoi HIV Cohort Study. This study excluded 350 patients, including 185 patients who never underwent ART, 48 patients who underwent ART for less than six months, 96 patients whose initial ART did not match the study criteria, 15 patients without VL results during their first‐line ART, five patients whose ART start date was unknown, and one patient who was ≤18 years old at the start of ART. The remaining 1806 patients provided 7752 person‐years of follow‐up and were assigned to the survival analysis with virologic outcome categories. Data of 1441 patients with available baseline CD4 counts were used for CD4 count and CD4 count change trajectories, and 1013 of the 1441 patients who had CD4 results at 24 months were assigned to the immune recovery analysis category. The characteristics of study participants are summarized in Table 1. Overall, 64% of the participants were men and the median age was 31 years (range, 18–75). Thirty‐two percent declared previous IDU, and 45% had HCV co‐infection, which strongly indicates exposure to sharing multiple needles. The median baseline CD4 count was greater after 2011, compared to before 2011, which was related to the updated ART indication in the national guidelines (before 2011 median 100 cells/mm3 (ranged 1–671) vs. after 2,011,158 cells/mm3 (ranged 1–693)).
Table 1.
Characteristics of study participants
| Total | Immune recovery study | |
|---|---|---|
| Number of participants | 1806 | 1013 |
| Median age at starting ART | 31 (18–75) | 34 (18–75) |
| <30 years old | 668 (37) | 352 (35) |
| 30 to 39 years old | 845 (47) | 495 (49) |
| ≥40 years old | 293 (16) | 166 (16) |
| Male sex | 1156 (64) | 642 (63) |
| HIV risk factors (multiple possible) | ||
| Sexual contact | 1334 (74) | 755 (75) |
| Injection drug use, n (%) | 583 (32) | 308 (30) |
| Other/unknown | 126 (7) | 74 (7.3) |
| HBs antigen | ||
| Positive | 242 (14) | 135 (14) |
| Negative | 1507 (86) | 863 (86) |
| Missing | 57 | 15 |
| Anti‐HCV antibody | ||
| Positive | 737 (45) | 385 (42) |
| Negative | 897 (55) | 527 (58) |
| Missing | 172 | 101 |
| AIDS before ART | 307 (17) | 202 (20) |
| Median baseline CD4 count (/mm3) | 101 (1 to 693) | 81 (1 to 693) |
| <100 | 712 (39) | 556 (55) |
| 100 to 199 | 377 (21) | 266 (26) |
| ≥200 | 352 (19) | 191 (19) |
| Missing | 365 | ‐ |
| Median time on ART, months | 50 (6–152) | 53.5 (7–119) |
| Year of ART initiation | ||
| ≤2008 | 514 (29) | 203 (20) |
| 2009 to 2010 | 518 (28) | 384 (38) |
| ≥2011 | 774 (43) | 426 (42) |
| NRTI | ||
| AZT + 3TC or FTC | 832 (46) | 523 (52) |
| d4T + 3TC or FTC | 608 (34) | 349 (34) |
| TDF + 3TC or FTC | 366 (20) | 141 (14) |
| NNRTI | ||
| NVP | 958 (53) | 556 (55) |
| EFV | 848 (47) | 457 (45) |
Data are reported as number (%) or median (range). ART, antiretroviral therapy; HBs antigen, hepatitis B surface antigen; HCV, hepatitis C virus; NRTI, nucleos(t)ide reverse transcriptase inhibitors; AZT, zidovudine; d4T, stavudine; TDF, tenofovir; 3TC, lamivudine; FTC, emtricitabine; NNRTI, non‐nucleoside reverse‐transcriptase inhibitors; NVP, nevirapine; EFV, efavirenz.
3.2. Time to VF and combined clinical endpoints
In a median time of 50 months of first‐line ART, 225 individuals (12.4%) experienced VF, which corresponded to an incidence of 2.9/100 person‐years. Among these 225 individuals, 47 individuals were identified as having a VL ≥1000 copies/ml within 12 months and 18 individuals subsequently achieved an undetectable VL. The combined clinical endpoint was detected for 311 individuals, which corresponded to an incidence of 4.0 cases/100 person‐years. These cases included 225 cases of VF, 36 deaths, and 50 cases of loss to follow‐up. By dividing the group that received a VL test by the group with VLs below each threshold, we observed that the proportion of viral suppression at 12 months was calculated to be 95.5% (1329/1391) for a threshold of 1000, 92.9% (1292/1391) for a threshold of 200, and 80% (1112/1391) for a threshold of 1000. Figure 1 illustrates the survival curves with neither VF nor the combined clinical failure. The probability of survival without VF was maintained above 90% until 42 months of first‐line ART, with probabilities of 86% at five years and 78% at ten years. The probability of survival without the combined clinical endpoint was 80% at five years and 70% at ten years.
Figure 1.
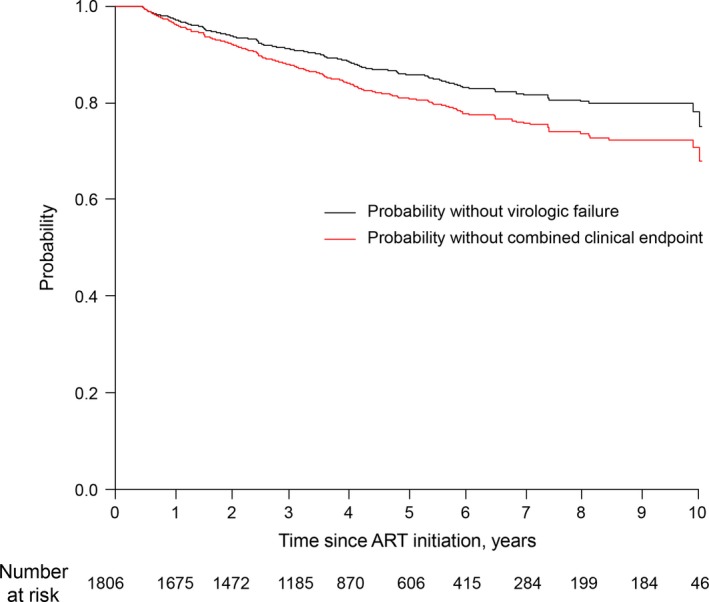
Time to virologic failure (VF) or the combined clinical endpoint during the first‐line ART. The black line indicates the probability of not achieving virologic endpoint and the red line indicates the probability of not achieving the combined clinical endpoint. The virologic endpoint was defined as the first episode of VF, (a viral load of ≥1000 copies/ml after starting ART. The combined clinical endpoint was defined as the first episode of either VF, death, loss to follow‐up or switching to a salvage regimen. ART, antiretroviral therapy.
In the univariate analyses, earlier VF was associated with IDU, HCV‐antibody positivity, and d4T use were associated with an earlier VF. However, protective effects were observed for age ≥30 years, HBs antigen positivity, a baseline CD4 count of ≥200 cells/mm3, and an EFV‐based regimen showed significant protective effects (Table 2). Of these factors, we found HCV‐antibody positivity was strongly associated with IDU history (p < 0.001) with 83% concordance, which may have affected the result of the multivariate analysis when both factors were integrated into the same multivariate model. As individuals with IDU may have been reluctant to disclose their IDU, we considered the presence of antibodies to HCV a better indicator of sharing needs, compared to a self‐reported history of IDU. Thus, we developed two multivariate models, with one that included all significant factors in the univariate model (Model 1‐1) and another that included all significant factors except IDU (Model 1‐2). Model 1‐1 revealed a significant result for age of >30 years, while Model 1‐2 also revealed a significant result for antibodies to HCV.
Table 2.
Factors associated with time to treatment failure during the first‐line ART
| n = 1806 | VFa | Combined clinical endpointb | ||||
|---|---|---|---|---|---|---|
| Univariate, HR (95% CI), p | Multivariate, HR (95% CI), p | Univariate, HR (95% CI), p | Multivariate, HR (95% CI), p | |||
| Model 1‐1 | Model 1‐2 | Model 2‐1 | Model 2‐2 | |||
| Age at starting ART (years) | ||||||
| <30 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 30 to 39 | 0.63 (0.47 to 0.83), 0.001 | 0.69 (0.49 to 0.99), 0.04 | 0.69 (0.49 to 0.99), 0.04 | 0.73 (0.58 to 0.92), 0.007 | 0.77 (0.56 to 1.04), 0.08 | 0.77 (0.56 to 1.04), 0.09 |
| ≥40 | 0.64 (0.42 to 0.98), 0.039 | 0.71 (0.41 to 1.22), 0.21 | 0.70 (0.41 to 1.21), 0.20 | 1.09 (0.80 to 1.48), 0.60 | 1.02 (0.67 to 1.58), 0.94 | 1.02 (0.66 to 1.57), 0.94 |
| Male sex | 1.11 (0.84 to 1.47), 0.45 | 0.79 (0.51 to 1.24), 0.31 | 0.83 (0.55 to 1.25), 0.38 | 1.41 (1.10 to 1.81), 0.007 | 0.85 (0.57 to 1.25), 0.40 | 0.88 (0.61 to 1.27), 0.50 |
| Injection drug use | 1.44 (1.11 to 1.88), 0.006 | 1.15 (0.71 to 1.87), 0.57 | 1.62 (1.29 to 2.02), <0.001 | 1.15 (0.76 to 1.73), 0.51 | ||
| HBs antigen | 0.64 (0.40 to 1.01), 0.058 | 0.58 (0.31 to 1.05), 0.07 | 0.58 (0.31 to 1.06), 0.07 | 0.73 (0.50 to 1.05), 0.09 | 0.78 (0.49 to 1.20), 0.26 | 0.78 (0.50 to 1.21), 0.28 |
| Anti‐HCV antibodies | 1.42 (1.09 to 1.87), 0.01 | 1.53 (0.95 to 2.46), 0.08 | 1.64 (1.06 to 2.12), 0.015 | 1.60 (1.27 to 2.02), <0.001 | 1.62 (1.09 to 2.43), 0.02 | 1.74 (1.24 to 2.44), 0.001 |
| AIDS before ART | 1.00 (0.70 to 1.43), 0.99 | 1.08 (0.81 to 1.46), 0.60 | ||||
| Baseline CD4 count (/mm3) | ||||||
| <100 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 100–199 | 1.03 (0.73 to 1.45), 0.87 | 0.99 (0.69 to 1.42), 0.95 | 0.99 (0.69 to 1.43), 0.97 | 0.90 (0.67 to 1.22), 0.52 | 0.91 (0.66 to 1.26), 0.58 | 0.92 (0.66 to 1.27), 0.60 |
| ≥200 | 0.56 (0.34 to 0.93), 0.03 | 0.63 (0.37 to 1.07), 0.09 | 0.63 (0.37 to 1.07), 0.09 | 0.58 (0.38 to 0.88), 0.011 | 0.74 (0.47 to 1.15), 0.18 | 0.72 (0.47 to 1.15), 0.18 |
| NRTI | ||||||
| AZT + 3TC or FTC | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| d4T + 3TC or FTC | 1.35 (1.03 to 1.78), 0.03 | 1.39 (1.01 to 1.97), 0.06 | 1.40 (0.99 to 1.97), 0.06 | 1.38 (1.09 to 1.74), 0.007 | 1.51 (1.12 to 2.03), 0.007 | 1.51 (1.12 to 2.04), 0.006 |
| TDF + 3TC or FTC | 0.80 (0.48 to 1.34), 0.40 | 1.16 (0.62 to 2.18), 0.65 | 1.15 (0.61 to 2.16), 0.66 | 0.87 (0.57 to 1.34), 0.63 | 0.94 (0.55 to 1.58), 0.80 | 0.93 (0.55 to 1.57), 0.78 |
| NNRTI | ||||||
| NVP | 1.00 | 1.00 | 1.00 | 1.00 | ||
| EFV | 0.72 (0.55 to 0.96), 0.025 | 0.77 (0.52 to 1.14), 0.20 | 0.77 (0.52 to 1.14), 0.19 | 1.00 (0.80 to 1.26), 0.99 | ||
HR, hazard ratio; CI, confidence interval; ART, antiretroviral therapy; HBs antigen, hepatitis B surface antigen; HCV, hepatitis C virus; NRTI, nucleos(t)ide reverse transcriptase inhibitors; AZT, zidovudine; d4T, stavudine; TDF, tenofovir; 3TC, lamivudine; FTC, emtricitabine; NNRTI, non‐nucleoside reverse‐transcriptase inhibitors; NVP, nevirapine; EFV, efavirenz VF, virologic failure.
VF was defined as the first episode of a viral load of ≥1000 copies/ml after the initiation of ART.
The combined clinical endpoint was defined as the first episode of either VF, death, loss to follow‐up, or switching to a salvage regimen. Factors with a p < 0.1 in the univariate model were included in the multivariate models. Confidence intervals that did not overlap the null value of (HR = 1) are shown in bold.
In the univariate analyses, the combined clinical endpoint was associated with the male sex, IDU, HCV‐antibody positivity, and d4T. However, protective effects were observed for age of >30 years and a baseline CD4 count of ≥200 cells/mm3. We also developed two multivariate models for the combined clinical endpoint (Model 2‐1 and Model 2‐2) and both models provided similar results, with shorter survival being significantly predicted by antibodies to HCV and d4T use.
3.3. Immune recovery during first‐line ART
Figure 2 shows that the absolute CD4 counts in 1441 patients increased over time regardless of their baseline CD4 count categories <100 cells/mm3, 100 to 199 cells/mm3, or ≥200 cells/mm3. Although 95% confidence intervals are not shown in Figure 2, the three baseline category‐specific intervals crossed at approximately 6.5 years. The mean change in CD4 count was 155 cells/mm3 in the first year and 255 cells/mm3 at 24 months (Figure 2b) with 167 of 1013 (16.5%) individuals failing to achieve early immune recovery.
Figure 2.
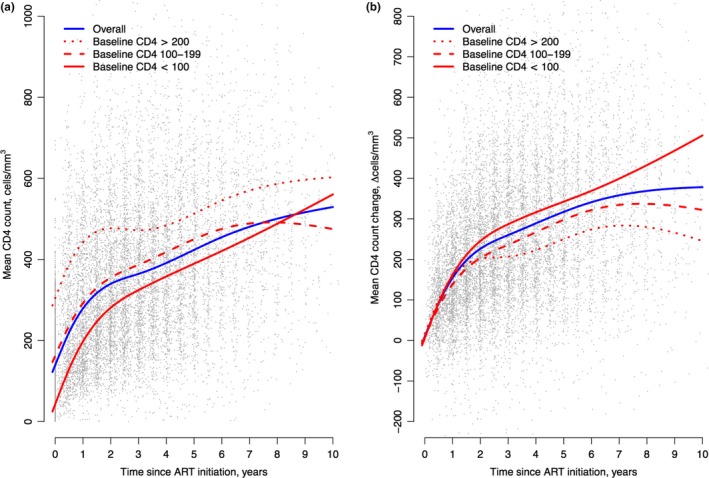
CD4 count trajectory during the first‐line ART (a) The mean absolute CD4 counts during the first‐line ART, (b) The mean CD4 count change from baseline during the first‐line ART. ART, antiretroviral therapy.
In the univariate model, impaired early immune recovery was associated with the male sex, IDU, and HCV‐antibody positivity. Protective effects were observed for d4T and EFV (Table 3) other than baseline CD4 categories. Given the strong association between HCV‐antibody positivity and IDU mentioned above, we also developed models for impaired early immune recovery that did and did not incorporate IDU history (Model 3‐1 and Model 3‐2). Both models provided similar results compared to the univariate models, although IDU was a significant factor in Model 3‐1.
Table 3.
Risk factors for impaired early immune recovery after 24 months of the first‐line ART
| n = 1013 | Univariate, OR (95% CI), p | Multivariate, OR (95% CI), p | |
|---|---|---|---|
| Model 3‐1 | Model 3‐2 | ||
| Age at starting ART (years old) | |||
| <30 | 1.00 | 1.00 | 1.00 |
| 30 to 39 | 1.21 (0.83 to 1.76), 0.33 | 1.36 (0.90 to 2.06), 0.15 | 1.36 (0.90 to 2.06), 0.15 |
| ≥40 | 1.30 (0.79 to 2.13), 0.30 | 1.31 (0.74 to 2.31), 0.36 | 1.30 (0.73 to 2.28), 0.38 |
| Gender male | 1.50 (1.04 to 2.16), 0.03 | 1.78 (1.10 to 2.87), 0.017 | 1.86 (1.19 to 2.93), 0.007 |
| Injection drug use | 1.43 (1.00 to 2.02), 0.046 | 1.19 (0.69 to 2.06), 0.53 | |
| HBs antigen positive | 0.66 (0.38 to 1.15), 0.14 | ||
| Anti‐HCV antibody positive | 1.46 (1.03 to 2.07), 0.03 | 1.72 (1.01 to 2.91), 0.04 | 1.89 (1.22 to 2.92), 0.004 |
| AIDS before ART | 0.71 (0.46 to 1.12), 0.14 | ||
| Baseline CD4 count (/mm3) | |||
| <100 | 1.00 | 1.00 | 1.00 |
| 100 to 199 | 2.95 (1.97 to 4.41), <0.001 | 2.95 (1.90 to 4.59), <0.001 | 2.95 (1.90 to 4.60), <0.001 |
| ≥200 | 3.53 (2.30 to 5.42), <0.001 | 3.49 (2.15 to 5.65), <0.001 | 3.48 (2.15 to 5.65), <0.001 |
| NRTI | |||
| AZT + 3TC or FTC | 1.00 | 1.00 | 1.00 |
| d4T + 3TC or FTC | 0.45 (0.29 to 0.67), <0.001 | 0.51 (0.32 to 0.82), 0.005 | 0.52 (0.33 to 0.83), 0.003 |
| TDF + 3TC or FTC | 0.86 (0.53 to 1.39), 0.53 | 1.30 (0.74 to 2.29), 0.36 | 1.30 (0.74 to 2.28), 0.37 |
| Key drug | |||
| NVP | 1.00 | 1.00 | 1.00 |
| EFV | 0.59 (0.42 to 0.84), 0.003 | 0.53 (0.28 to 0.70), <0.001 | 0.44 (0.28 to 0.70), <0.001 |
Impaired immune recovery was defined as failing to attain 100/μl increase in CD4 count in 24 months of the first‐line ART. Factors provide p > 0.1 in univariate model were included in the multivariate models. Factors with p‐value less than 0.1 in the univariate model were included in the multivariate models. Confidential intervals that do not overlap the null value of HR=1 are shown in bold. OR, odds ratio; CI, confidential interval; ART, antiretroviral therapy; HBs antigen, hepatitis B surface antigen; HCV, hepatitis C virus; NRTI, nucleos(t)ide reverse transcriptase inhibitors; AZT, zidovudine; d4T, stavudine; TDF, tenofovir; 3TC, lamivudine; FTC, emtricitabine; NNRTI, non‐nucleoside reverse‐transcriptase inhibitors; NVP, nevirapine; EFV, efavirenz.
4. Discussion
This study is the first to address the long‐term virologic efficacy of first‐line ART in Vietnam, and to explore factors that were related to VF and early immune recovery. The results revealed high‐level durable viral suppression and favourable CD4+ recovery. A younger age and HCV co‐infection were associated with early VF and immune recovery at 24 months was more likely to be impaired among men and individuals with HCV co‐infection. The currently recommended EFV‐containing regimen was associated with a lower VF rate and higher early immune recovery rate, compared to the NVP‐containing regimen, and d4T was associated with a higher VF rate than AZT.
The viral suppression rate at 12 months in this study (95.5%) was comparable to that of a previous study (93.5%) 15 and was higher than that of previous small studies in Vietnam (70% to 78%) 12, 13, 14, 15, 16. Furthermore, the 80% suppression at 12 months based on a threshold VL of 50 copies/ml was comparable to that observed in resource‐rich countries 25. Consistently favourable outcomes were even observed for the combined clinical endpoint, which accounted for death/loss to follow‐up and eliminated bias caused by early censoring of these outcomes 25. Similarly, our previous studies that revealed a high retention rate in care and low mortality in this cohort 17, 26. The excellent virologic outcome in our study strongly supports the achievability of Vietnam's goal to end AIDS by 2030 6 in addition to the UNAIDS 90‐90‐90 goal 3.
We found that VF was associated with age of <30 years and antibodies to HCV, although self‐reported IDU was not significantly associated with VF. Given the legal consequences of IDU in Vietnam and the strong link between IDU and HCV acquisition, it is highly probable that IDU was underreported by the patients and should still be considered a risk for VF. Previous studies have also reported that younger age and IDU were associated with suboptimal adherence to ART 15, 26. Our results indicated that these populations need more support for ART adherence. In addition, we unexpectedly found that individuals with HBs antigen were less likely to develop VF, which may be related to the management of hepatitis in the national ART guidelines 19, 20, 28, which state that cessation of an anti‐HBV drug in an ART regimen may have motivated physicians and patients not to miss a dose. Furthermore, a lower baseline CD4 count (<200 cells/mm3) was associated with a higher risk of VF in the univariate model, which has also been observed in previous studies 15. A lower baseline CD4 count might be the result of a combination of social and clinical factors. For example, a late diagnosis of HIV, behaviour wherein individuals reduced likelihood of seeking care, barriers to accessing healthcare, and lack of appropriate knowledge among healthcare providers on when to start ART are plausible risk factors for VF among those with lower baseline CD4 counts. However, having AIDS prior to ART was not associated with VF, although most AIDS patients in this cohort had tuberculosis (TB) and the mortality of HIV/TB co‐infection was relatively low, compared to that in other resource‐limited countries 17. Since TB treatment also requires strict adherence, the additional involvement of a TB specialist may increase the likelihood that the patient adheres to the ART.
This study revealed that d4T use was associated with a higher probability of VF than AZT, even though previous clinical trials had revealed minimal difference in virologic efficacy between d4T and AZT 29. However, EFV use was associated with a lower probability of VF compared to NVP in the univariate model, which was further supported by data for both in vitro potency 30 and from previous clinical trials 31, 32, 33. One possible reason for the inferiority of d4T in this study might be suboptimal adherence, which could be caused by side effects that are related to mitochondrial toxicity. Nevertheless, it is difficult to assess the actual effects of a single antiretroviral agent on virologic outcome in real‐world settings. The Vietnamese national guideline previously recommended d4T/3TC/NVP for first‐line ART regimens 18, although the recommendations were changed to AZT and EFV in 2009 19 and then the combination of TDF/EFV plus 3TC 20 in 2011. Moreover, d4T has been very rapidly phased out of use since 2011 based on global concerns regarding mitochondrial toxicity 22. Thus, d4T was more frequently used with NVP than EFV, while TDF, which has a high genetic barrier 34, was exclusively used with EFV. Therefore, although it is difficult to evaluate the efficacy of every specific regimen, our data may suggest that the current recommendation of TDF/3TC or FTC/EFV is more beneficial than d4T‐ or NVP‐containing regimens.
We examined the longitudinal immune recovery that occurs during first‐line ART and explored factors related to early immune recovery. A relatively large change in CD4 counts was observed early during the ART, which agrees with the findings of other studies 35, 36. However, the rate of increase in CD4 counts was slightly faster in the groups with lower baseline CD4 counts. Our analyses revealed that it took approximately 6.5 years for the mean CD4 count trajectories to become indistinguishable based on overlapping the 95% confidence, which supports the recent global trend toward early ART initiation 2, 27.
We found that male sex, IDU, and HCV‐antibody positivity were related to impaired early immune recovery, which agrees with the findings of previous studies 37, 38. In this context, chronic immune activation by HCV co‐infection may lead to further immune dysfunction 38 and liver cirrhosis may contribute to low leukocyte counts. This relationship is particularly important in Vietnam, where HCV co‐infection is distinctly frequent among male HIV‐infected individuals. In addition, AZT use was linked to impaired early immune recovery, while EFV use was associated with a more favourable early immune recovery. The mechanisms whereby AZT‐containing regimens cause impaired immune recovery remain unclear, although the relationship is commonly accepted and generally explained by AZT‐related bone marrow suppression. Although the immune recovery in EFV‐based regimens h is generally considered similar to that in NVP‐based regimens 39, 40, there remains controversy regarding which drug provides the better CD4 response h. Nevertheless, EFV is preferred for Vietnamese patients who are co‐infected with HCV than NVP based on concerns regarding the hepatic toxicity of NVP 19, 20, 27, which may eventually provide an immune recovery benefit among HCV co‐infected patients.
This study had limitations. First, there was a lack of information about adherence to ART and other factors that could affect it such as alcohol consumption and mental health status 9. Second, the baseline VL was not obtainable for all participants, and a high baseline VL is a known risk factor for VF. Third, the baseline CD4+ count was also unavailable for 365 patients (20%), which may obscure the precise effect of the baseline CD4+ count in our analyses. Fourth, the duration of ART at cohort enrolment was diverse, especially when the enrolment was started in 2007. Thus, the cohort may not include patients who died shortly after starting ART before 2007 and the survival outcome could be overestimated. Finally, both participating clinics were located in large urban referral hospitals in Hanoi, and it remains unclear whether our results are generalizable to other centres.
In conclusion, our study is the first study to describe the long‐term probability of viral suppression and immune recovery during first‐line ART in Vietnam. The viral suppression rate was 95.5% at 12 months and the survival rate without VF was maintained at 90% for 42 months. Younger age and HCV‐antibody positivity were associated with early VF, while male sex, IDU, and HCV co‐infection were associated with impaired immune recovery at 24 months. EFV‐containing regimens provided\ better virologic outcomes and greater early CD4 recovery, compared to d4T‐containing regimens, which had a higher rate of VF. Although we observed durable viral suppression, our results suggest that increased support is needed to help patients with IDU or HCV co‐infection adhere to their ART. Our findings also support the current global trend toward wide‐spread routine VL testing, which can facilitate more effective adherence support interventions.
Competing interests
The authors have declared that no competing interests exist.
Authors' Contributions
Study concept and design: JT, SM, SH, KNV, SO; Collection and interpretation of data: JT, SM, DNT, HDNT, CDD, VVT, TTP, TNV. Drafting the manuscript: JT, SH; Statistical analysis: JT, SH; Obtained funding: SO.
Funding
This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases from the Japan Agency for Medical Research and Development (15fm0108001h0001; http://www.amed.go.jp/en/).
Acknowledgements
We thank Ms. Nguyen Thi Yen, Ms. Nguyen Thi Tien, Ms. Nguyen Lan Phuong, Ms. Le Thi Hoa, Mr. Pham Hong Hai, Dr. Nguyen Thi Ngoc Chi, and Dr. Dang Thi Bich for collecting data. We also thank Ms. Kyoko Ishigaki, Ms. Keiko Saito and Ms. Nguyen Thi Huyen for their assistance in performing the study.
Tanuma, J. , Matsumoto, S. , Haneuse, S. , Cuong, D. D. , Vu, T. V. , Thuy, P. T. T. , Dung, N. T. , Dung, N. T. H. , Trung, N. V. , Kinh, N. V. and Oka, S. Long‐term viral suppression and immune recovery during first‐line antiretroviral therapy: a study of an HIV‐infected adult cohort in Hanoi, Vietnam. J Int AIDS Soc. 2017; 20(4):e25030
References
- 1. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV‐1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. What's New. Geneva: WHO Press; 2015. Available from: http://www.who.int/hiv/pub/arv/15249_HIVTreatementandCare_PolicybriefforWEB.pdf. Accessed 27 July 2016 [Google Scholar]
- 3. UNAIDS . 90‐90‐90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed 27 July 2016 [Google Scholar]
- 4. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO Press; 2013. Available from: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. Accessed 27 July 2016 [Google Scholar]
- 5. World Health Organization . Technical Operational Considerations Implementing HIV Viral Load Testing Guide. Geneva: WHO Press; 2014. Available from: http://apps.who.int/iris/bitstream/10665/128121/1/9789241507578_eng.pdf?ua=1&ua=1. Accessed 27 July 2016 [Google Scholar]
- 6. Ministry of Health Socialist Republic of VietNam . Optimizing Viet Nam's HIV Response: An Investment Case; 2014. Available from: http://www.unaids.org/sites/default/files/country/documents/VNM_narrative_report_2015.pdf. Accessed 27 July 2016
- 7. Jiamsakul A, Kumarasamy N, Ditangco R, Li PC, Phanuphak P, Sirisanthana T, et al. Factors associated with suboptimal adherence to antiretroviral therapy in Asia. J Int AIDS Soc. 2014;17:18911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joseph B, Kerr T, Puskas CM, Montaner J, Wood E, Milloy MJ. Factors linked to transitions in adherence to antiretroviral therapy among HIV‐infected illicit drug users in a Canadian setting. AIDS Care. 2015;27:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Do HM, Dunne MP, Kato M, Pham CV, Nguyen KV. Factors associated with suboptimal adherence to antiretroviral therapy in Viet Nam: a cross‐sectional study using audio computer‐assisted self‐interview (ACASI). BMC Infect Dis. 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordan MR, Obeng‐Aduasare Y, Sheehan H, Hong SY, Terrin N, Duong DV, et al. Correlates of non‐adherence to antiretroviral therapy in a cohort of HIV‐positive drug users receiving antiretroviral therapy in Hanoi, Vietnam. Int J STD AIDS. 2014;25:662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tran DA, Ngo AD, Shakeshaft A, Wilson DP, Doran C, Zhang L. Trends in and determinants of loss to follow up and early mortality in a rapid expansion of the antiretroviral treatment program in Vietnam: findings from 13 outpatient clinics. PLoS One. 2013;8:e73181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan MR, La H, Nguyen HD, Sheehan H, Lien TT, Duong DV, et al. Correlates of HIV‐1 viral suppression in a cohort of HIV‐positive drug users receiving antiretroviral therapy in Hanoi, Vietnam. Int J STD AIDS. 2009;20:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trinh TT, Montague BT, Flanigan TP, Gerard HM. HIV suppression among patients on treatment in Vietnam: a review of HIV viral load testing in a public Urban Clinic in Ho Chi Minh City. AIDS Res Treat. 2011;2011:230953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pham QD, Huynh TK, Luong TT, Tran T, Vu TX, Truong LX. HIV‐1 drug resistance and associated factors among adults failing first‐line highly active antiretroviral therapy in Ho Chi Minh City, Vietnam. HIV Clin Trials. 2013;14:34–44. [DOI] [PubMed] [Google Scholar]
- 15. Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Doran C, Zhang L. Determinants of virological failure after 1 year's antiretroviral therapy in Vietnamese people with HIV: findings from a retrospective cohort of 13 outpatient clinics in six provinces. Sex Transm Infect. 2014;90:538–44. [DOI] [PubMed] [Google Scholar]
- 16. Do DC, Agneskog E, Nguyen TKC, Santacatterina M, Sönnerborg A, Larsson M, et al. Monitoring the efficacy of antiretroviral therapy by a simple reverse transcriptase assay in HIV‐infected adults in rural Vietnam. Future Virol. 2012;7:923–31. [Google Scholar]
- 17. Tanuma J, Lee KH, Haneuse S, Matsumoto S, Nguyen DT, Nguyen DT, et al. Incidence of AIDS‐defining opportunistic infections and mortality during antiretroviral therapy in a cohort of adult HIV‐infected individuals in Hanoi, 2007‐2014. PLoS One. 2016;11:e0150781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ministry of Health Socialist Republic of VietNam . Antiretroviral treatment protocol for people living with HIV/AIDS. 2006 (No: 2051/QD‐BYT). 2006.
- 19. Ministry of Health Socialist Republic of VietNam . Guidelines for diagnosis and treatment of HIV/AIDS. 2009 (No. 3003/QĐ‐BYT). 2009.
- 20. Ministry of Health Socialist Republic of VietNam . Guidelines for diagnosis and treatment of HIV/AIDS. 2011 (No: 4139/ QĐ‐BYT). 2011.
- 21. World Health Organization . Antiretroviral Therapy for HIV infection in Adults and Adolescents: recommendations for a public health approach. 2006 rev. Geneva: WHO Press; 2006. Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. Accessed 27 July 2016 [Google Scholar]
- 22. World Health Organization . Antiretroviral Therapy for HIV Infection in Adults and Adolescents: recommendations for a public health approach. 2010 rev. Geneva: WHO Press; 2010. Available from: http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. Accessed 27 July 2016 [PubMed] [Google Scholar]
- 23. Friedman J, Trevor H, Robert T. The Elements of Statistical Learning. Vol. 1. Berlin: Springer; Springer series in statistics, 2001. [Google Scholar]
- 24. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing; 2011. Available from: http://www.R-project.org/. Accessed 27 July 2016 [Google Scholar]
- 25. McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low‐ and middle‐income countries: a systematic review. Bull World Health Organ. 2013;91:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsumoto S, Tanuma J, Mizushima D, Nguyen NC, Pham TT, Do CD, et al. High treatment retention rate in HIV‐infected patients receiving antiretroviral therapy at two large HIV clinics in Hanoi, Vietnam. PLoS One. 2015;10:e0139594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cesar C, Jenkins CA, Shepherd BE, Padgett D, Mejía F, Ribeiro SR, et al. Incidence of virological failure and major regimen change of initial combination antiretroviral therapy in the Latin America and the Caribbean: an observational cohort study. Lancet HIV. 2015;2:e492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ministry of Health Socialist Republic of VietNam . Guidelines for diagnosis and treatment of HIV/AIDS. 2015 (No: 3047/QĐ‐BYT). 2015.
- 29. Spaulding A, Rutherford GW, Siegfried N. Stavudine or zidovudine in three‐drug combination therapy for initial treatment of HIV infection in antiretroviral‐naive individuals. Cochrane Database Syst Rev. 2010;8:Cd008651. [DOI] [PubMed] [Google Scholar]
- 30. Sluis‐Cremer N, Tachedjian G. Mechanisms of inhibition of HIV replication by non‐nucleoside reverse transcriptase inhibitors. Virus Res. 2008;134:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Velen K, Lewis JJ, Charalambous S, Grant AD, Churchyard GJ, Hoffmann CJ. Comparison of tenofovir, zidovudine, or stavudine as part of first‐line antiretroviral therapy in a resource‐limited‐setting: a cohort study. PLoS One. 2013;8:e64459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV‐1 infection in adults: a systematic review and meta‐analysis of 114 studies with up to 144 weeks' follow‐up. PLoS One. 2014;9:e97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang MW, Kanki PJ, Shafer RW. A review of the virological efficacy of the 4 World Health Organization‐recommended tenofovir‐containing regimens for initial HIV therapy. Clin Infect Dis. 2012;54:862–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brenner BG, Coutsinos D. The K65R mutation in HIV‐1 reverse transcriptase: genetic barriers, resistance profile and clinical implications. HIV Ther. 2009;3:583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T‐lymphocyte recovery in individuals with advanced HIV‐1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95. [DOI] [PubMed] [Google Scholar]
- 36. Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al. Predictors of trend in CD4‐positive T‐cell count and mortality among HIV‐1‐infected individuals with virological failure to all three antiretroviral‐drug classes. Lancet. 2004;364:51–62. [DOI] [PubMed] [Google Scholar]
- 37. Marcus JL, Leyden WA, Chao CR, Xu L, Quesenberry CP Jr, Tien PC, et al. Differences in response to antiretroviral therapy by sex and hepatitis C infection status. AIDS Patient Care STDS. 2015;29:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Operskalski EA, Kovacs A. HIV/HCV co‐infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Berg‐Wolf M, Hullsiek KH, Peng G, Kozal MJ, Novak RM, Chen L, et al. Virologic, immunologic, clinical, safety, and resistance outcomes from a long‐term comparison of efavirenz‐based versus nevirapine‐based antiretroviral regimens as initial therapy in HIV‐1‐infected persons. HIV Clin Trials. 2008;9:324–36. [DOI] [PubMed] [Google Scholar]
- 40. Pillay P, Ford N, Shubber Z, Ferrand RA. Outcomes for efavirenz versus nevirapine‐containing regimens for treatment of HIV‐1 infection: a systematic review and meta‐analysis. PLoS One. 2013;8:e68995. [DOI] [PMC free article] [PubMed] [Google Scholar]


