Abstract
Cancer cells have defects in regulatory mechanisms that usually control cell proliferation and homeostasis. Different cancer cells share crucial alterations in cell physiology, which lead to malignant growth. Tumorigenesis or tumor growth requires a series of events that include constant cell proliferation, promotion of metastasis and invasion, stimulation of angiogenesis, evasion of tumor suppressor factors, as well as avoidance of cell death pathways. All these events in tumor progression may be regulated by growth factors produced by normal or malignant cells. The growth factor progranulin has significant biological effects in different types of cancer. This protein is a regulator of tumorigenesis because it stimulates cell proliferation, migration, invasion, angiogenesis, malignant transformation, resistance to anticancer drugs, and immune evasion. This review focuses on the biological effects of progranulin in several cancer models and provides evidence that this growth factor should be considered as a potential biomarker and target in cancer treatment.
Keywords: Progranulin, Cancer, Biomarker
Introduction
Progranulin (PGRN) is a protein also known as acrogranin, granulin/epithelin precursor (GEP), proepithelin (PEPI), PC cell-derived growth factor (PCDGF) and 88 kDa glycoprotein (GP88). These names were originated by the different groups that independently identified the parent glycoprotein or its derivative fragments in specific tissues or cells. PGRN is a 67–88 kDa glycoprotein identified in 1990 by Anakwe and Gerton in the sperm of the guinea-pig and, because it was present in the acrosome granule, they called it “acrogranin”. Later, they established its participation in the biogenesis of guinea-pig acrosome [1, 2]. Simultaneously, two other research groups isolated and characterized small cell growth-modulating proteins or peptides of approximately 6 kDa. The first group purified peptides from rat kidney, which they called “epithelins” [3]. The second research team utilized rat bone marrow and human peripheral leukocytes as sources, naming the peptides “granulins” [4]. Rat epithelins and rat and human granulins were subsequently recognized as homologous and derived from a common precursor named “proepithelin” [5] or PGRN [6], respectively, which were also homologous to acrogranin [2]. Another name for this autocrine growth factor, which is homologous to PGRN, is PCDGF since it was purified from PC cells derived from adipogenic teratoma cells (1246-3A cell line) [7].
The granulin precursor (GRN) gene encoding human PGRN, contains 13 exons and is located on the long arm of chromosome 17, specifically in the cytogenetic band 17q21.23, while the murine gene is found in a syntenic region on chromosome 11 [8, 9]. Human preprogranulin consists of 593 amino acids, of which about 15% of the residues correspond to cysteines; its sequence forms seven and a half repetitions in tandem of a highly conserved motif called the granulin domain [6, 9].
After secretion, PGRN may be proteolytically cleaved between the granulin domains to produce fragments or individual granulin peptides. Neutrophil secreted elastase, proteinase 3, matrix metalloproteinase 9 (MMP-9), MMP-12, and MMP-14, as well as a disintegrin-like and metalloproteinase domain 7 (ADAMTS-7), are proteases capable of processing PGRN [10–15]. Furthermore, a recent study demonstrated that cathepsin L (Cat L), a lysosomal protease, proteolytically processes and degrades intracellular PGRN in the lysosome [16]. In contrast, secretory leucocyte protease inhibitor protein (SLPI) and high-density lipoprotein/apolipoprotein A-I (HDL/Apo A-I) protect PGRN from proteolysis by two mechanisms: (1) binding to the inter-granulin linker regions to block accessibility to proteases and (2) inhibiting the converting protease directly [10, 17] (Figure 1).
Figure 1.
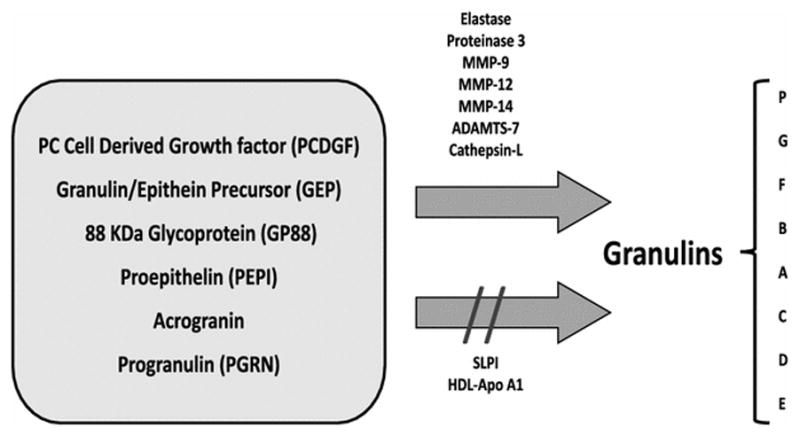
Granulin Family. Granulins are small peptides derived from a larger precursor (PC cell-derived growth factor, granulin/epithein precursor, 88 kDa glycoprotein, proepithelin, acrogranin, or progranulin), which can be cleaved by several enzymes. PGRN cleavage releases seven full-length granulin domains (G, F, B, A, C, D, E) and one half-length paragranulin domain (P). Secretory leukocytes protease inhibitor (SLPI) or high-density lipoprotein/apolipoprotein A-1 (HDL-Apo A1) binding to the full-length PGRN prevents its proteolytic process.
Intact PGRN protein is a concatenation of 7 granulin domains (G-F-B-A-C-D-E) preceded by a half granulin domain, “paragranulin” (P) (Figure 1). The intact protein sequence (P-G-F-B-A-C-D-E) is encoded by 12 out of the 13 exons of the GRN gene. PGRN actions depend on the cellular target. As mentioned above, PGRN can be cleaved into bioactive fragments or individual granulin peptides. Generally, PGRN is considered anti-inflammatory; however, when PGRN is fragmented, the resulting granulin peptides take on new bioactivities. For example, some granulin peptides are pro-inflammatory. In other cases, granulin peptides are toxic; in the case of Caenorhabditis elegans, certain granulin peptides increase TDP-43 levels via a post-translational mechanism and exacerbate TDP-43 toxicity [18]. Rollinson et al. [19] expressed recombinant PGRN and each of the individual granulin peptides; they demonstrated that short-duration treatments with PGRN result in the down-regulation of several critical transcripts. Gene ontology analysis supports the regulation of biological processes such as the spliceosome and proteasome in response to PGRN treatment, as well as lysosomal pathway constituents. On the other hand, granulin peptide treatments alter the regulation of numerous non-coding RNAs.
PGRN and granulins produce significant biological effects, which are mediated through a cell-surface receptor. A unique PGRN-specific receptor has not yet been identified. Up to now, PGRN has been demonstrated to interact with different binding proteins depending on the cell type. PGRN is able to bind to the beta propeller region of sortilin through its C-terminal tail. This protein is a single-pass transmembrane protein in the vacuolar protein sorting/targeting protein 10 (VPS10) family, which regulates intracellular protein trafficking and acts as a cell surface receptor. In the brain, sortilin can regulate PGRN levels through endocytosis [20]. In castration-resistant prostate cancer cells, sortilin loss may contribute to prostate cancer enhancing PGRN effects. Therefore, extracellular PGRN levels depend on PGRN-sortilin interactions [21]. PGRN and granulin interactions with tumor necrosis factor receptor (TNFR) 1 and 2 has also been demonstrated. In these receptors, PGRN binds directly to the extracellular CRD2 and CRD3 domains. In mouse inflammatory models, binding of PGRN to TNF-receptors inhibits TNF-α binding and its biological effects, which may explain the anti-inflammatory properties of PGRN [22]. More recently, EphA2, a member of a large family of receptor tyrosine kinases (RTKs), was identified as a functional signal receptor for PGRN in a human urinary bladder cancer cell line. The interaction between EphA2 and PGRN provides the opportunity to understand the mechanisms through which PGRN activates tumorigenic signaling pathways [23].
In vitro and in vivo studies have demonstrated the important role of PGRN in diverse physiological processes. This protein participates in the growth stimulation of preimplantation mammalian embryo development [24], in early embryogenesis [25], in wound healing [26], inflammation [11, 27], angiogenesis [26, 28], and bone and cartilage development [29, 30]. It is also an adipokine implicated in obesity, insulin resistance, and rheumatic disease [31]. The role of PGRN in several neural pathologies led to an explosion of GRN genetic studies [32–35]. PGRN is a neurotropic and neuroprotective factor that shields neural tissue from inflammation and degeneration. Loss of function mutations in the GRN gene has been associated with neurological and neurodegenerative diseases as in frontotemporal dementia, Parkinson’s disease, Creutzfeldt-Jakob disease, motor neuron disease, and Alzheimer’s disease [36, 37].
PGRN overexpression has been observed in many different types of cancer as described in Figure 2. In these tissues, high expression of PGRN drives tumor progression since it promotes cellular responses such as cell proliferation, migration, invasion, angiogenesis, malignant transformation, resistance to anticancer drugs, and immune evasion (Figure 3). Numerous studies over the last two decades have pointed to the importance of PGRN in cancer, and this review focuses on the biological effects of PGRN in several cancer models. Furthermore, the uses of PGRN as a biomarker and as a target in cancer treatment are addressed.
Figure 2.
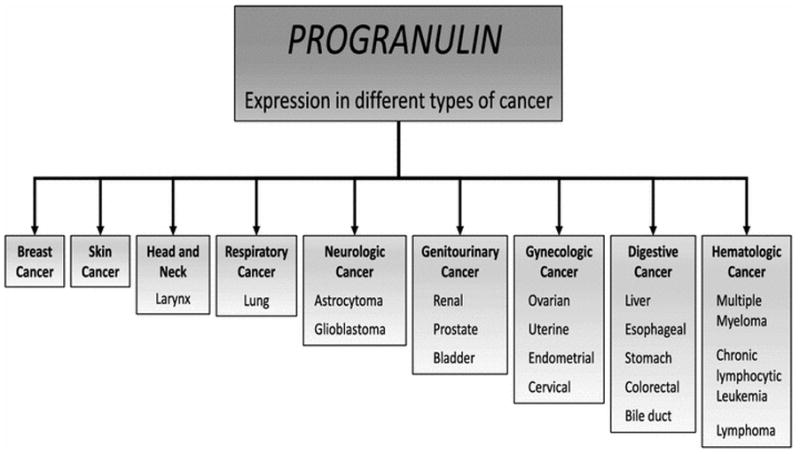
Progranulin expression in cancer. Increased expression of PGRN has been detected in cancer tissue from different organs.
Figure 3.
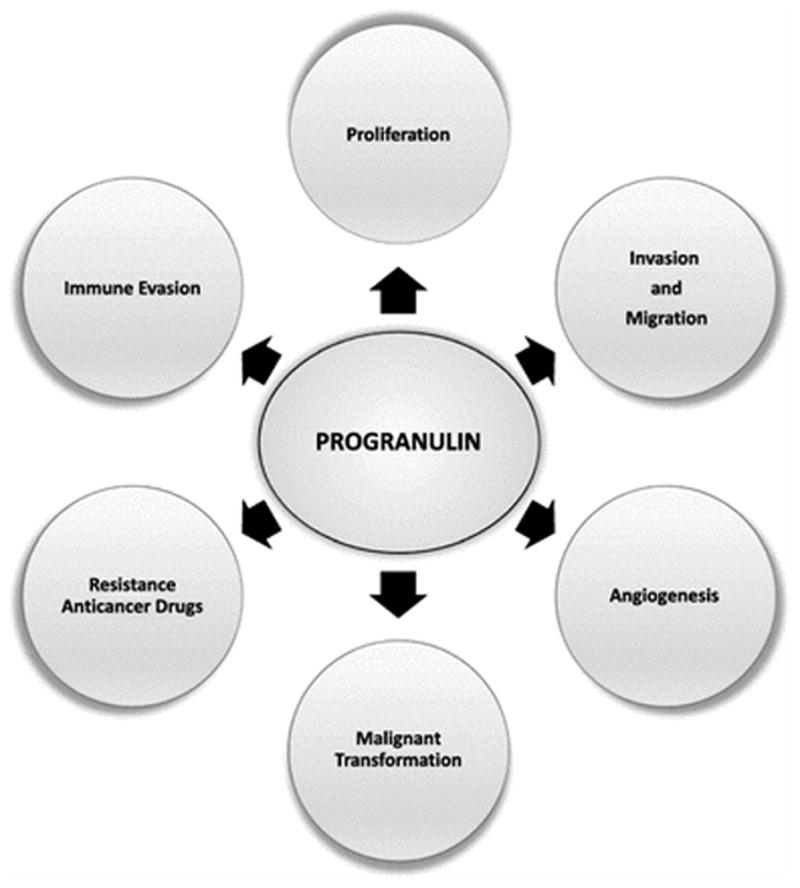
Biological effects of PGRN. Increased expression of PGRN promotes different responses that can lead to the development of cancer.
Biological effects of progranulin in cancer
Cellular Proliferation
PGRN promotes proliferation of normal epithelial cells and several cancer cell lines. Cellular proliferation was first demonstrated by the PGRN-derivative epithelin 1 (granulin A) on BALB/MK mouse epidermal keratinocytes [3]. Later, a study in mouse embryos revealed that exogenous PGRN added to cultures increases the number of trophectoderm cells compared to the controls, besides exhibiting other important effects on embryos [24, 38]. Moreover, to emphasize how important this protein is as a growth factor, PGRN purified from the culture medium of the PC cell line (highly tumorigenic cells) promotes proliferation of 3T3 mouse embryo fibroblast cells [7]. The 3T3 cells generally need two or more growth factors to proliferate in vitro; however, PGRN by itself can function as a mitogen in these cells. Furthermore, PGRN stimulates cell division in 3T3 cells lacking expression of insulin-like growth factor receptor 1 (IGF-1R), which is required for other growth factors [Insulin-like growth factor (IGF-1), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF)] to promote proliferation [39, 40]. Additional evidence supporting PGRN proliferative effects comes from studies in which the inhibition of PGRN expression by transfection of a short hairpin RNA (shRNA) into the cell line derived from colorectal cancer SW1116 decreases Ki67, whereas overexpression of PGRN increases it [41].
Mitogenic effects of PGRN also have been observed in other cell lines such as the human epithelial adrenal carcinoma cell line SW-13, multiple myeloma ARP-1 and RPMI 8226 cells, lung carcinoma A549 cells, cervical cancer SiHa cells, prostate carcinoma DU145 cells, laryngeal carcinoma Hep-2 cells and breast cancer cell line SKBR3. Cell proliferation promoted by PGRN was through the activation of the signaling transduction pathways of MAPK/ERK 1/2 and PI3K/AKT/mTOR [42–48].
Molecular mechanisms by which PGRN induces proliferation are still poorly understood. However, studies in breast cancer cells have revealed that PGRN regulates the expression of cyclin D1 and B [49, 50]. Additionally, another study demonstrated a significant correlation between PGRN and cyclin D1 expression in cutaneous squamous cell carcinoma tissues compared with control samples [51]. Additional mechanisms that have been described include phosphorylation of c-myc in MCF-7 breast cancer cells that overexpress the human epidermal growth factor receptor 2 (HER2 receptor) [48], and inhibition of senescence in cervical cancer cell lines [46].
Migration and Invasion
Activation of the epithelium-mesenchymal transition (EMT) process, which can occur as tumorigenesis progresses, is important for cell migration and invasiveness. The EMT process allows cells to escape from the primary tumor after the epithelial cells acquire characteristics of mobile mesenchymal cells [52]. Several studies suggest that PGRN promotes migration and invasion in various cell lines such as breast cancer MCF-7 cells, colorectal cancer SW1116 cells, bladder cancer 5637 cells, prostate cancer LNCaP and DU145 cells, human epithelial adrenal carcinoma SW-13 cells, hepatocellular carcinoma Hep 3B cells, and ovarian carcinoma SW626 and A2780 cells [41, 42, 45, 49, 53–55].
PGRN-promoted migration and invasion of cancer cells can occur by multiple mechanisms. One is through the EMT process, since PGRN overexpression in ovarian cancer cell line A2780 increases mesenchymal marker expression, such as vimentin and twist, and decreases epithelial markers such as E-cadherin and cytokeratin, thereby increasing cell migration and invasiveness [56].
PGRN also increases the invasive capacity of ovarian and breast cancer cells through activation of MMP-2 and upregulation of MMP-9, respectively [49, 53]. In bladder cancer cells, PGRN activates focal adhesion kinase (FAK) through paxillin and ERK phosphorylation. Also, PGRN interacts with the F-actin protein drebrin and induces actin cytoskeleton remodeling. Both effects induce invasion and migration of malignant bladder cells [54]. In contrast to the effects of intact PGRN, the proteolysis-derived granulin A binds to α-enolase of human HepG-2 cancer cells to inhibit growth, glucose uptake, migration, and invasion [57].
Angiogenesis
The earliest relationship between PGRN and angiogenesis was established for wound healing, where PGRN induces human microvascular endothelial cell proliferation and promotes tube-like structures [26]. Subsequently, in the MCF-7 breast cancer cell line, elevated PGRN concentrations are associated with high endothelial growth factor (VEGF) expression [53]. Additionally, PGRN and its derivatives isolated from mesothelioma cells induce VEGF-independent angiogenesis [58]. Other studies have shown a correlation between high PGRN expression with elevated VEGF expression as well as high microvessel density in tissues from breast cancer tumors, esophageal squamous cell carcinoma, and colorectal tumors [41, 59, 60].
Downregulation or null PGRN expression by transfection of shRNA in the cell line SW1116 derived from colorectal carcinoma decreases expression of angiogenic factors such as VEGF-A, VEGF-C, and FGF-1 (fibroblast growth factor 1), and reduces secreted VEGF-A levels in the culture medium of the cells. Interestingly, PGRN overexpression increases the same angiogenic factors. VEGF-A expression induced by PGRN is mediated by tumor necrosis factor receptor 2 (TNFR2)/Akt and the ERK signal transduction pathways [41]. Another mechanism by which PGRN mediates angiogenesis is by promoting proliferation, migration and tubular structure formation by HUVEC cells (human umbilical vein endothelial cells). Interesting, all these events are mediated through the interaction between PGRN and midkine protein (MK), a heparin-binding growth factor [61].
Malignant Cell Transformation
Malignant transformation is the unsolved process by which cells acquire the properties of cancer. Efficient anchorage-independent growth, which is the ability of cells to grow independently of a solid surface, and tumor production in nude mice are commonly considered to indicate a highly transformed phenotype. The earliest study to demonstrate that PGRN can promote cell transformation was performed by He et al. [42], in which overexpression of this growth factor in non-transformed renal epithelial MDCK cells and human adrenal carcinoma SW-13 cells enhanced clonogenicity in semisolid agar. Also, in this study overproduction of PGRN in SW-13 cells markedly increases their tumorigenicity in nude mice. Later, another study with immortalized uterine smooth muscle cells transfected either with PGRN alone or in combination with human telomerase reverse transcriptase (hTERT) and Simian Virus 40 Early Region (SV40ER) genes, showed that PGRN transfection gene increases colony formation in soft agar; while co-expression of PGRN, hTERT, and SV40ER resulted in more extensive anchorage-independent growth and tumor formation in nude mice. These results suggest that co-expression of these three genes is necessary and sufficient to transform uterine smooth muscle cells [62]. Similarly, transfection of PGRN gene into ovarian surface epithelial cells immortalized by co-transfection with the hTERT and Simian Virus 40 large T antigen (SV40LT) genes revealed that PGRN overexpression increases colonogenicity in soft agar and tumorigenicity in nude mice [63]. Moreover, in human cervical mucosa epithelial H8 cells, PGRN overexpression by gene transfection and cell culture treatment with human recombinant PGRN promotes malignant transformation in vitro and in vivo as illustrated by increases in cell proliferation and tumor formation in nude mice, respectively [64].
Progranulin and resistance to anticancer drugs
Resistance to anti-neoplastic drugs is a recurrent problem in the treatment of many types of cancers and is the major obstacle to survival in patients with metastatic chemoresistant cancer. The precise cause of this problem is unknown, but growth factor overexpression and their receptors are suspected to be involved. One study has reported that cancer cells that become resistant to anticancer drugs can influence the tumor microenvironment, since cells initially chemosensitive within a tumor, may become chemoresistant due to the secretion of soluble factors released by chemoresistant cells. PGRN is one of the factors identified in the conditioned medium of chemoresistant colorectal cancer cells, and it can influence the survival in otherwise chemosensitive tumor cells through the activation of growth and survival signaling pathways [65].
Tangkeangsirisin et al. [66] were one of the first groups to demonstrate that PGRN confers resistance to anticancer drugs. In their study, they observed that overexpression of PGRN in breast cancer cells MCF-7 prevents tamoxifen (anti-estrogen)-induced apoptosis, and promotes tumor growth and angiogenesis in vivo, even in the presence of tamoxifen. Subsequently, Wang and Serrero [67] revealed that PGRN overexpression in multiple myeloma cells leads to the development of resistance to dexamethasone, a conventional drug for treatment of patients with multiple myeloma. Furthermore, Kim and Serrero [68] showed that trastuzumab, a monoclonal antibody directed against HER2, does not have a significant growth-inhibitory effect on breast cancer cells overexpressing HER2 and PGRN. More recently, Abrhale et al. [69] described that PGRN overexpression in MCF-7 breast cancer cells confers resistance to letrozole, an aromatase inhibitor used in the treatment of estrogen receptor-positive (ER+) breast cancer, in a dose- and time-dependent manner. Conversely, PGRN silencing reduces cell viability and restores sensitivity to letrozole. Finally, chemoresistance to cisplatin has been shown in ovarian cancer cell lines overexpressing PGRN [70].
The mechanisms by which PGRN promotes drug-resistance in these types of cancer is unknown. Nevertheless, decreased expression of the anti-apoptotic Bcl-2 protein induced by letrozole and cisplatin does not occur in the presence of PGRN [69, 70]. Similarly, this same mechanism, where PGRN avoids down-regulation of Bcl-2, is involved in the development of resistance to tamoxifen and trastuzumab in breast cancer cells [50, 66, 68]. Another possible mechanism involved in progranulin-conferred chemoresistance includes the inhibition of the poly (ADP-ribose) polymerase (PARP) cleavage induced by tamoxifen and dexamethasone, a hallmark of caspase-dependent apoptosis [66, 67].
Increased levels of PGRN have also been associated with chemoresistance to doxorubicin and cisplatin, and with a reduced survival time of patients with hepatocellular carcinoma. The mechanism of this association seems to be mediated by the expression of adenosine triphosphate-dependent binding cassette (ABC)B5 drug transporter in cells that also express the hepatic cancer stem cell markers CD133 and epithelial cell adhesion molecule (EpCAM) [71, 72].
In a more recent study with glioblastoma multiforme cells, PGRN overproduction induced resistance to temozolomide (alkylating agent) by upregulation of DNA repair genes PARP, serine/threonine kinase ATM, BRCA1, Rad51, and X-ray repair cross-complementing gene 1 (XRCC1) as well as enhanced expression of cancer stemness genes CD133, CD44 and ABCG2, through the activation of AP-1 transcription factor, specifically cFos/JunB [73].
Although it has been shown that PGRN confers resistance to anticancer drugs, more studies are warranted to further investigate other chemoresistance mechanisms in different types of cancer.
Progranulin and Immunoresponse
Immune evasion is a strategy used by tumors to escape the host immune response, enabling the tumor to continue growing. Cheung et al. [74] reported that PGRN renders hepatocellular carcinoma cells resistant to natural killer (NK) cell-mediated cytotoxicity. The resistance to NK cell lysis induced by PGRN seems to be due to downregulation of MHC class I chain-related molecule A (MICA), which is a ligand for NK stimulatory receptor NK group 2-member D (NKG2D), and upregulation of human leukocyte antigen-E (HLA-E), a ligand for NK inhibitory receptor CG94/NKG2A. Interestingly, inhibition of PGRN activity by neutralizing antibody in hepatocellular carcinoma cells enhanced sensitivity to NK cytotoxicity.
Progranulin as cancer biomarker
A cancer biomarker is a biomolecule such as DNA, RNA or protein that can be measured in tissues or body fluids and can assist in cancer diagnosis, design of treatment modalities, treatment effectiveness, or cancer recurrence detection. Therefore, based on their usage, cancer biomarkers can be diagnostic, predictive, and prognostic [75]. For its important role in carcinogenesis, PGRN has been considered to be a diagnostic, predictive, and prognostic biomarker for some types of cancer.
In epithelial ovarian cancer, PGRN levels in sera of patients with advanced stages of disease can predict who will have recurrence within 18 months of completion of chemotherapy and who will exhibit significantly shortened progression free-survival and overall survival [76]. Furthermore, a correlation between high expression of PGRN mRNA in tissues from malignant ovarian tumors and shorter patient overall survival was observed by Cuevas-Antonio et al. [77]. According to the previous studies, Carlson et al. [78] demonstrated that PGRN serum concentrations are significantly elevated in patients with advanced stages (III and IV) of ovarian epithelial cancer but not in the earlier stages I and II. Also in this study, high PGRN concentrations were associated with decreased in overall patients’ survival. Therefore, these studies demonstrate the promise in using PGRN as a biomarker of ovarian cancer.
Also, in breast cancer, PGRN levels in serum samples from patients with stage I–IV are significantly higher than in healthy women; nonetheless, no differences were observed between the diverse stages of this malignancy [79]. A cohort study of 697 newly diagnosed breast cancer patients who underwent curative surgery, evaluated the association between preoperative PGRN levels and breast cancer recurrence. This study revealed that preoperative serum PGRN levels are clinically significant for predicting recurrence in patients with hormone receptor-positive tumors; however, these data have several limitations that are important to consider when interpreting the study’s results [80]. Serrero et al. [81] also analyzed PGRN expression by immunohistochemistry in paraffin-embedded breast tumor sections from patients with estrogen receptor-positive invasive ductal carcinoma. Their results showed that high PGRN expression was associated with a decrease in disease-free and overall survival as well as a 5.9-fold higher hazard of disease recurrence and a 2.5-higher mortality risk compared to patients with low PGRN expression in the tumor tissue. Taken together, all these data suggest that PGRN could be a predictive and prognostic marker in breast cancer. Nevertheless, more studies are required to determine whether PGRN could be a useful biomarker in other types of breast cancer that are receptor-positive or -negative for estrogen, HER2, and progesterone.
Tumor samples (n=210) surgically obtained from astrocytoma patients were analyzed to determine PGRN expression and its association with tumor grade and overall survival of the patients. PGRN expression is up-regulated in astrocytoma cells and tumor blood vessels as compared with normal brain and positively correlated with pathological grading. In grade II astrocytoma, strong vascular PGRN expression is closely related to tumor recurrence. Moreover, in glioblastoma patients, the increased PGRN expression correlates with decreased patient survival [82].
One study with tissue samples from patients with prostate cancer demonstrated that PGRN expression is detected in more than 50% of cells in all samples of high-grade prostatic intraepithelial neoplasia and invasive cancer. In normal prostate tissues, PGRN expression is low and less than 10% of cells express the protein [83]. Likewise, PGRN levels in malignant renal tissue detected by western blot analysis were significantly higher as compared to benign renal tissue. Also, in the same study, tissue samples from patients with renal cell carcinoma show higher levels of PGRN expression compared with low-grade renal cell carcinoma and normal tissue [84]. In patients with bladder cancer, urinary levels of PGRN are significantly increased relative to control samples; in addition, high PGRN levels correlate with the stage and pathological cancer grade [85]. Unfortunately, the small sample size of this study is a limitation; however, the results suggest that urine PGRN levels may serve as a simple test as part of a bladder cancer work-up. Other studies have reached the same conclusions but also with critical limitations; however, it is important to note that PGRN has been included in a panel of urinary diagnostic markers for non-invasive detection of primary and recurrent urothelial urinary bladder carcinoma. So far, the analyses that have been performed are inconclusive and it is necessary to carry out larger or multicenter studies to substantiate the benefits of including PGRN in a diagnostic panel [86].
A cohort study of 131 patients with chronic lymphocytic leukemia demonstrated that PGRN concentrations measured in plasma are significantly increased compared to normal controls. Moreover, the high PGRN plasma levels are strongly associated with adverse risk factors including unmutated immunoglobulin heavy chain variable (IGHV) gene status, expression of CD38, and levels of ZAP-70. Therefore, PGRN, as well as the other risk factors, is a prognostic marker for establishing the time to first treatment and overall survival in these patients [87].
Edelman et al. [88] demonstrated the utility of PGRN as a biomarker for non-small cell lung carcinoma because the blood levels of patients with stages III and IV are higher in comparison with healthy patients and correlate with a low progression-free survival.
In a retrospective study of tissues from patients with advanced biliary tract cancer who received palliative chemotherapy, PGRN expression was evaluated by immunohistochemistry. A poorer response to chemotherapy in patients with PGRN-positive tumors is observed and also PGRN overexpression is significantly associated with poor progression-free survival [89]. In conclusion, PGRN could find application in predicting treatment outcomes.
Recently, a study of 254 patients with untreated malignant lymphoma showed that PGRN concentrations measured in sera of patients are significantly higher than levels found in the healthy control group [90]. In addition, the group of patients with high PGRN concentrations show a poor prognosis with respect to overall survival and progressive-free survival.
Collectively, the studies presented in this section show that PGRN concentrations in serum, urine or its expression in tissues can be useful for monitoring the clinical course of tumors and patient prognosis. Therefore, PGRN could be a worthy biomarker for some types of cancers.
Progranulin inhibition as treatment for cancer
Overexpression of PGRN in different types of cancer produces significant effects in carcinogenesis. Several studies have demonstrated that inhibition of PGRN expression with small interfering RNA (siRNA), short hairpin (shRNA), anti-sense cDNA, or specific neutralizing antibodies minimize its participation in tumorigenesis.
Strong inhibition of tumorigenicity in vivo was first documented using PC cells derived from adipogenic teratoma cells, where PGRN expression was inhibited by transfection of antisense PGRN cDNA [91]. The results of this study showed that tumors develop promptly when empty-vector control PC cells are injected into mice; while tumor growth is significantly inhibited if the PC cells are transfected with antisense PGRN cDNA prior to injection into mouse hosts.
Treatment of MDA-MB-468 breast cancer cells with anti-PGRN neutralizing antibody resulted in dose-dependent decrease of their proliferation [92]. Additionally, inhibition of PGRN expression by antisense PGRN cDNA transfection also reduces proliferation and colony formation; moreover, tumor formation in vivo is significantly inhibited. These results demonstrated that PGRN acts as an autocrine factor and is essential for the tumorigenicity of breast carcinomas.
In several ovarian cancer cell lines, inhibition of PGRN by antisense cDNA transfection decreases cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2 [49]. Other studies using the ovarian cancer cell lines HEY-A8 and OVCAR-3 show that PGRN production and secretion can be regulated by endothelin 1 (ET-1), lysophosphatidic acid (LPA), and cAMP through the signal transduction pathway of cAMP-EPAC-ERK1/2. Treatment of these cells with neutralizing PGRN antibody inhibits basal as well as LPA, ET-1, and cAMP-induced proliferation. Moreover, apoptosis is induced by this treatment, as demonstrated by the presence of caspase-3 and PARP cleavage, DNA fragmentation, and nuclear condensation [93].
PGRN levels are significantly increased in gastric cancer tissue compared with healthy tissue. Helicobacter pylori infection is the primary cause of gastric cancer and is associated with an increased gastric epithelial cell proliferation and migration. A recent study demonstrated that infection of gastric epithelial cells by H. pylori significantly increase PGRN expression and induces cell proliferation and migration. Furthermore, downregulation of PGRN by specific siRNA reduces H. pylori-induced cell proliferation and migration in gastric epithelial cells [94].
Several studies using hepatocellular carcinoma cells and orthotopic liver tumor models demonstrated that PGRN neutralization by anti-PGRN antibody inhibited growth of hepatoma cells in vitro without significant effect on normal liver cells. In the nude mice model, neutralizing antibody therapy decreases PGRN serum levels, inhibits in a dose-dependent manner the growth of established tumors, and decreases tumor angiogenesis by reducing microvessel density and VEGF levels. It seems that these effects are at least partially mediated via p44/42 MAPK/Akt signal transduction pathway [95]. In another study, chemoresistant cells from hepatocellular carcinoma were generated by cisplatin and doxorubicin treatment [96]. These subpopulations express higher cancer stem cell markers such as CD133, PGRN and ABCB5 multidrug transporter as compared with the parental cell lines. Also, their ability to form colonies and spheroids is enhanced. Interestingly, incubation in the presence of anti-PGRN antibody sensitized these chemoresistant subpopulation and their parental cell lines to apoptosis induced by chemotherapy. The mechanism behind this enhanced chemotherapy response seems to be due to a decrease in ABCB5 expression, and cell survival signaling mediated via Akt and Bcl-2. In the same study, the combined effects of PGRN neutralizing antibody and cisplatin in vivo were evaluated in a orthotopic liver tumor model. Anti-PGRN treatment alone inhibited tumor growth, while combination with cisplatin eliminated the intrahepatic tumor in three weeks [96]. A recent study designed to identify effective and non-invasive diagnostic biomarkers for hepatocellular carcinoma by oncoproteogenomics technology (integration of proteomics, genomics, and transcriptomic analyses) revealed a significantly higher co-amplification and co-expression of PGRN and S100A9 in tumor and urine samples from patients compared to the controls [97].
Finally, endogenous depletion of PGRN by shRNA in UMUC-3 urothelial cancer cells inhibits cell proliferation, migration, invasion, and anchorage-independent growth as well as reduced tumor cell growth in vivo in both xenograft and orthotopic tumor models. Furthermore, suppression of PGRN expression sensitized bladder cancer cells to cisplatin [98].
Collectively, the discussed evidence supports the hypothesis that PGRN neutralization may be an important target for cancer therapy. Thus, development of new anti-PGRN neutralizing antibodies for clinical use are warranted.
Conclusions
In the last two decades, the study of PGRN in cancer has advanced considerably. Research has been conducted to establish a role for PGRN in many forms of cancer, as well as to determine the mechanism of action for its biological effects. So far, several proteins have been proposed to act as the PGRN receptor. However, binding to these proteins does not fully explain PGRN growth factor or neuroprotective effects; therefore, it is thought that PGRN must have its own receptor. Signaling pathways activated by PGRN have already been described, and they are essential pathways in cancer development and progression. According to the data in the literature, it is clear that PGRN overexpression induces proliferation and migration in several cancer cells. Also, modulation of PGRN levels by RNA silencing or inhibition of PGRN by treatment with neutralizing antibodies counteracts these effects. All this evidence and the fact that PGRN is a unique secretory glycoprotein highlight its potential use as a biomarker or as a therapeutic target in cancer. The information that currently exists regarding PGRN is very exciting, but more clinical studies, with a larger patient base, are warranted to cement possible uses for this interesting growth factor in diagnosis and treatment.
Footnotes
Conflict of interest
All authors declare that they do not have conflict of interest.
References
- 1.Anakwe OO, Gerton GL. Acrosome biogenesis begins during meiosis: evidence from the synthesis and distribution of an acrosomal glycoprotein, acrogranin, during guinea pig …. Biology of reproduction. 1990 doi: 10.1095/biolreprod42.2.317. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Hoff HB, Nemoto H, et al. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–243. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- 3.Shoyab M, McDonald VL, Byles… C. Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proceedings of the …. 1990 doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman A, Belcourt D, Bennett H, Lazure… C. Granulins, a novel class of peptide from leukocytes. Biochemical and …. 1990 doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 5.Plowman GD, Green JM, Neubauer… MG. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. Journal of Biological …. 1992 [PubMed] [Google Scholar]
- 6.Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Gao G, Crabb JW, Serrero G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 8.Bhandari V, Bateman A. Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun. 1992;188:57–63. doi: 10.1016/0006-291x(92)92349-3. [DOI] [PubMed] [Google Scholar]
- 9.Baba T, Nemoto H, Watanabe K, Arai Y, Gerton GL. Exon/intron organization of the gene encoding the mouse epithelin/granulin precursor (acrogranin) FEBS Lett. 1993;322:89–94. doi: 10.1016/0014-5793(93)81544-a. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl… SM. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002 doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 11.Kessenbrock K, Fröhlich L, Sixt… M. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. The Journal of …. 2008 doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, Kessler BM. Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics. 2008;7:2215–2228. doi: 10.1074/mcp.M800095-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh HS, Choi N, Tarassishin L, Lee SC. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12) PLoS One. 2012;7:e35115. doi: 10.1371/journal.pone.0035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai XH, Wang DW, Kong L, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–4219. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CW, Stankowski JN, Chew J, et al. The lysosomal protein cathepsin L is a progranulin protease. Mol Neurodegener. 2017;12:55. doi: 10.1186/s13024-017-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okura H, Yamashita S, Ohama T, et al. HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J Atheroscler Thromb. 2010;17:568–577. doi: 10.5551/jat.3921. [DOI] [PubMed] [Google Scholar]
- 18.Salazar DA, Butler VJ, Argouarch AR, et al. The Progranulin Cleavage Products, Granulins, Exacerbate TDP-43 Toxicity and Increase TDP-43 Levels. J Neurosci. 2015;35:9315–9328. doi: 10.1523/JNEUROSCI.4808-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollinson S, Young K, Bennion-Callister J, Pickering-Brown SM. Identification of biological pathways regulated by PGRN and GRN peptide treatments using transcriptome analysis. Eur J Neurosci. 2016;44:2214–2225. doi: 10.1111/ejn.13297. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Brady OA, Meng PS, Mao Y, Hu F. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One. 2011;6:e21023. doi: 10.1371/journal.pone.0021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanimoto R, Morcavallo A, Terracciano M, et al. Sortilin regulates progranulin action in castration-resistant prostate cancer cells. Endocrinology. 2015;156:58–70. doi: 10.1210/en.2014-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang BC, Liu H, Talwar A, Jian J. New discovery rarely runs smooth: an update on progranulin/TNFR interactions. Protein Cell. 2015;6:792–803. doi: 10.1007/s13238-015-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neill T, Buraschi S, Goyal A, et al. EphA2 is a functional receptor for the growth factor progranulin. J Cell Biol. 2016;215:687–703. doi: 10.1083/jcb.201603079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz-Cueto L, Stein P, Jacobs A, Schultz… RM. Modulation of mouse preimplantation embryo development by acrogranin (epithelin/granulin precursor) Developmental …. 2000 doi: 10.1006/dbio.1999.9564. [DOI] [PubMed] [Google Scholar]
- 25.Daniel R, He Z, Carmichael KP, Halper… J. Cellular localization of gene expression for progranulin. … of Histochemistry & …. 2000 doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Ong CHP, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nature medicine. 2003 doi: 10.1038/nm816. [DOI] [PubMed]
- 27.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu… GY. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice…. 2011 doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toh H, Cao M, Daniels E, Bateman A. Expression of the growth factor progranulin in endothelial cells influences growth and development of blood vessels: a novel mouse model. PloS one. 2013 doi: 10.1371/journal.pone.0064989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng JQ, Guo FJ, Jiang BC, Zhang Y, Frenkel… S. Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. The FASEB Journal. 2010 doi: 10.1096/fj.09-144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu K, Zhang Y, Ilalov K, Carlson CS, Feng… JQ. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. Journal of Biological …. 2007 doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 31.Abella V, Pino J, Scotece M, et al. Progranulin as a biomarker and potential therapeutic agent. Drug Discov Today. 2017 doi: 10.1016/j.drudis.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Antonell A, Gil S, Sánchez-Valle R, et al. Serum progranulin levels in patients with frontotemporal lobar degeneration and Alzheimer’s disease: detection of GRN mutations in a Spanish cohort. J Alzheimers Dis. 2012;31:581–591. doi: 10.3233/JAD-2012-112120. [DOI] [PubMed] [Google Scholar]
- 33.Kämäläinen A, Viswanathan J, Natunen T, et al. GRN variant rs5848 reduces plasma and brain levels of granulin in Alzheimer’s disease patients. J Alzheimers Dis. 2013;33:23–27. doi: 10.3233/JAD-2012-120946. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson AM, Finch NA, Rademakers R. Human genetics as a tool to identify progranulin regulators. J Mol Neurosci. 2011;45:532–537. doi: 10.1007/s12031-011-9554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toh H, Chitramuthu BP, Bennett HP, Bateman A. Structure, function, and mechanism of progranulin; the brain and beyond. J Mol Neurosci. 2011;45:538–548. doi: 10.1007/s12031-011-9569-4. [DOI] [PubMed] [Google Scholar]
- 36.Petkau TL, Leavitt BR. Progranulin in neurodegenerative disease. Trends Neurosci. 2014;37:388–398. doi: 10.1016/j.tins.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Kao AW, McKay A, Singh PP, Brunet A, Huang EJ. Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci. 2017;18:325–333. doi: 10.1038/nrn.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin J, Díaz-Cueto L, Schwarze JE, et al. Effects of progranulin on blastocyst hatching and subsequent adhesion and outgrowth in the mouse. Biol Reprod. 2005;73:434–442. doi: 10.1095/biolreprod.105.040030. [DOI] [PubMed] [Google Scholar]
- 39.Xu SQ, Tang D, Chamberlain S, et al. The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem. 1998;273:20078–20083. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 40.Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 41.Yang D, Wang LL, Dong TT, et al. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am J Cancer Res. 2015;5:3085–3097. [PMC free article] [PubMed] [Google Scholar]
- 42.He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- 43.Wang W, Hayashi J, Kim WE, Serrero G. PC cell-derived growth factor (granulin precursor) expression and action in human multiple myeloma. Clin Cancer Res. 2003;9:2221–2228. [PubMed] [Google Scholar]
- 44.Kong WJ, Zhang SL, Chen X, et al. PC cell-derived growth factor overexpression promotes proliferation and survival of laryngeal carcinoma. Anticancer Drugs. 2007;18:29–40. doi: 10.1097/01.cad.0000236315.96574.58. [DOI] [PubMed] [Google Scholar]
- 45.Monami G, Emiliozzi V, Bitto A, Lovat F, Xu… SQ. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. The American journal of …. 2009 doi: 10.2353/ajpath.2009.080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XY, Pan ZX, Liu H, et al. Effect of progranulin (PGRN) on the proliferation and senescence of cervical cancer cells. Genet Mol Res. 2015;14:14331–14338. doi: 10.4238/2015.November.13.18. [DOI] [PubMed] [Google Scholar]
- 47.Feng T, Zheng L, Liu F, et al. Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget. 2016;7:58381–58395. doi: 10.18632/oncotarget.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim WE, Yue B, Serrero G. Signaling Pathway of GP88 (Progranulin) in Breast Cancer Cells: Upregulation and Phosphorylation of c-myc by GP88/Progranulin in Her2-Overexpressing Breast Cancer Cells. Breast Cancer (Auckl) 2015;9:71–77. doi: 10.4137/BCBCR.S29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Xi L, Liao G, et al. Inhibition of PC cell-derived growth factor (PCDGF)/granulin-epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2. BMC Cancer. 2007;7:22. doi: 10.1186/1471-2407-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu R, Serrero G. Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor) Proc Natl Acad Sci U S A. 2001;98:142–147. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang K, Huang C, Shan K, Chen J, Li H. Significance of PC cell-derived growth factor and cyclin D1 expression in cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2012;37:411–417. doi: 10.1111/j.1365-2230.2011.04275.x. [DOI] [PubMed] [Google Scholar]
- 52.Prieto-García E, Díaz-García CV, García-Ruiz I, Agulló-Ortuño MT. Epithelial-to-mesenchymal transition in tumor progression. Med Oncol. 2017;34:122. doi: 10.1007/s12032-017-0980-8. [DOI] [PubMed] [Google Scholar]
- 53.Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 54.Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 55.Cheung ST, Wong SY, Leung KL, et al. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res. 2004;10:7629–7636. doi: 10.1158/1078-0432.CCR-04-0960. [DOI] [PubMed] [Google Scholar]
- 56.Dong T, Yang D, Li R, et al. PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp Mol Pathol. 2016;100:17–25. doi: 10.1016/j.yexmp.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Xu H, Wu N, et al. Interaction between granulin A and enolase 1 attenuates the migration and invasion of human hepatoma cells. Oncotarget. 2017;8:30305–30316. doi: 10.18632/oncotarget.16328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eguchi R, Nakano T, Wakabayashi I. Progranulin and granulin-like protein as novel VEGF-independent angiogenic factors derived from human mesothelioma cells. Oncogene. 2017;36:714–722. doi: 10.1038/onc.2016.226. [DOI] [PubMed] [Google Scholar]
- 59.Chen XY, Li JS, Liang QP, He DZ, Zhao J. Expression of PC cell-derived growth factor and vascular endothelial growth factor in esophageal squamous cell carcinoma and their clinicopathologic significance. Chin Med J (Engl) 2008;121:881–886. [PubMed] [Google Scholar]
- 60.Li LQ, Huang HL, Ping JL, Wang XH, Zhong J, Dai LC. Clinicopathologic and prognostic implications of progranulin in breast carcinoma. Chin Med J (Engl) 2011;124:2045–2050. [PubMed] [Google Scholar]
- 61.Huang H, Li J, Lu Y, Min L, Li D, Dai L. Role of midkine-progranulin interaction during angiogenesis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:8809–8820. [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumura N, Mandai M, Miyanishi M, et al. Oncogenic property of acrogranin in human uterine leiomyosarcoma: direct evidence of genetic contribution in in vivo tumorigenesis. Clin Cancer Res. 2006;12:1402–1411. doi: 10.1158/1078-0432.CCR-05-2003. [DOI] [PubMed] [Google Scholar]
- 63.Miyanishi M, Mandai M, Matsumura N, et al. Immortalized ovarian surface epithelial cells acquire tumorigenicity by Acrogranin gene overexpression. Oncol Rep. 2007;17:329–333. [PubMed] [Google Scholar]
- 64.Lu Y, Zheng L, Zhang W, et al. Growth factor progranulin contributes to cervical cancer cell proliferation and transformation in vivo and in vitro. Gynecol Oncol. 2014;134:364–371. doi: 10.1016/j.ygyno.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Bose D, Zimmerman LJ, Pierobon M, et al. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. Br J Cancer. 2011;105:1759–1767. doi: 10.1038/bjc.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tangkeangsirisin W, Hayashi J, Serrero G. PC cell-derived growth factor mediates tamoxifen resistance and promotes tumor growth of human breast cancer cells. Cancer Res. 2004;64:1737–1743. doi: 10.1158/0008-5472.can-03-2364. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Hayashi J, Serrero G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin Cancer Res. 2006;12:49–56. doi: 10.1158/1078-0432.CCR-05-0929. [DOI] [PubMed] [Google Scholar]
- 68.Kim WE, Serrero G. PC cell-derived growth factor stimulates proliferation and confers Trastuzumab resistance to Her-2-overexpressing breast cancer cells. Clin Cancer Res. 2006;12:4192–4199. doi: 10.1158/1078-0432.CCR-05-2663. [DOI] [PubMed] [Google Scholar]
- 69.Abrhale T, Brodie A, Sabnis G, et al. GP88 (PC-Cell Derived Growth Factor, progranulin) stimulates proliferation and confers letrozole resistance to aromatase overexpressing breast cancer cells. BMC Cancer. 2011;11:231. doi: 10.1186/1471-2407-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pizarro GO, Zhou XC, Koch A, et al. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int J Cancer. 2007;120:2339–2343. doi: 10.1002/ijc.22559. [DOI] [PubMed] [Google Scholar]
- 71.Cheung ST, Cheung PF, Cheng CK, Wong NC, Fan ST. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–355. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 72.Cheung PF, Cheng CK, Wong NC, et al. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PLoS One. 2011;6:e28246. doi: 10.1371/journal.pone.0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bandey I, Chiou SH, Huang AP, Tsai JC, Tu PH. Progranulin promotes Temozolomide resistance of glioblastoma by orchestrating DNA repair and tumor stemness. Oncogene. 2015;34:1853–1864. doi: 10.1038/onc.2014.92. [DOI] [PubMed] [Google Scholar]
- 74.Cheung PF, Yip CW, Wong NC, et al. Granulin-epithelin precursor renders hepatocellular carcinoma cells resistant to natural killer cytotoxicity. Cancer Immunol Res. 2014;2:1209–1219. doi: 10.1158/2326-6066.CIR-14-0096. [DOI] [PubMed] [Google Scholar]
- 75.Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4:256–269. doi: 10.3978/j.issn.2218-676X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han JJ, Yu M, Houston N, Steinberg SM, Kohn EC. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol Oncol. 2011;120:5–10. doi: 10.1016/j.ygyno.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cuevas-Antonio R, Cancino C, Arechavaleta-Velasco F, et al. Expression of progranulin (Acrogranin/PCDGF/Granulin-Epithelin Precursor) in benign and malignant ovarian tumors and activation of MAPK signaling in ovarian cancer cell line. Cancer Invest. 2010;28:452–458. doi: 10.3109/07357900903346455. [DOI] [PubMed] [Google Scholar]
- 78.Carlson AM, Maurer MJ, Goergen KM, et al. Utility of progranulin and serum leukocyte protease inhibitor as diagnostic and prognostic biomarkers in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1730–1735. doi: 10.1158/1055-9965.EPI-12-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tkaczuk KR, Yue B, Zhan M, et al. Increased Circulating Level of the Survival Factor GP88 (Progranulin) in the Serum of Breast Cancer Patients When Compared to Healthy Subjects. Breast Cancer (Auckl) 2011;5:155–162. doi: 10.4137/BCBCR.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koo DH, Park CY, Lee ES, Ro J, Oh SW. Progranulin as a prognostic biomarker for breast cancer recurrence in patients who had hormone receptor-positive tumors: a cohort study. PLoS One. 2012;7:e39880. doi: 10.1371/journal.pone.0039880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serrero G, Hawkins DM, Yue B, et al. Progranulin (GP88) tumor tissue expression is associated with increased risk of recurrence in breast cancer patients diagnosed with estrogen receptor positive invasive ductal carcinoma. Breast Cancer Res. 2012;14:R26. doi: 10.1186/bcr3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M, Li G, Yin J, Lin T, Zhang J. Progranulin overexpression predicts overall survival in patients with glioblastoma. Med Oncol. 2012;29:2423–2431. doi: 10.1007/s12032-011-0131-6. [DOI] [PubMed] [Google Scholar]
- 83.Pan CX, Kinch MS, Kiener PA, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res. 2004;10:1333–1337. doi: 10.1158/1078-0432.ccr-1123-03. [DOI] [PubMed] [Google Scholar]
- 84.Donald CD, Laddu A, Chandham P, et al. Expression of progranulin and the epithelin/granulin precursor acrogranin correlates with neoplastic state in renal epithelium. Anticancer Res. 2001;21:3739–3742. [PubMed] [Google Scholar]
- 85.Selmy MA, Ibrahim GH, El Serafi TI, Ghobeish AA. Evaluation of urinary proepithelin as a potential biomarker for bladder cancer detection and prognosis in Egyptian patients. Cancer Biomark. 2010;7:163–170. doi: 10.3233/CBM-2010-0186. [DOI] [PubMed] [Google Scholar]
- 86.Soukup V, Kalousová M, Capoun O, et al. Panel of Urinary Diagnostic Markers for Non-Invasive Detection of Primary and Recurrent Urothelial Urinary Bladder Carcinoma. Urol Int. 2015;95:56–64. doi: 10.1159/000368166. [DOI] [PubMed] [Google Scholar]
- 87.Göbel M, Eisele L, Möllmann M, et al. Progranulin is a novel independent predictor of disease progression and overall survival in chronic lymphocytic leukemia. PLoS One. 2013;8:e72107. doi: 10.1371/journal.pone.0072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edelman MJ, Feliciano J, Yue B, et al. GP88 (progranulin): a novel tissue and circulating biomarker for non-small cell lung carcinoma. Hum Pathol. 2014;45:1893–1899. doi: 10.1016/j.humpath.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JH, Do IG, Kim K, et al. Progranulin as a predictive factor of response to chemotherapy in advanced biliary tract carcinoma. Cancer Chemother Pharmacol. 2016;78:1085–1092. doi: 10.1007/s00280-016-3170-z. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto Y, Goto N, Takemura M, et al. Association between increased serum GP88 (progranulin) concentrations and prognosis in patients with malignant lymphomas. Clin Chim Acta. 2017 doi: 10.1016/j.cca.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 91.Zhang H, Serrero G. Inhibition of tumorigenicity of the teratoma PC cell line by transfection with antisense cDNA for PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) Proc Natl Acad Sci U S A. 1998;95:14202–14207. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu R, Serrero G. Inhibition of PC cell-derived growth factor (PCDGF, epithelin/granulin precursor) expression by antisense PCDGF cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-468. Proc Natl Acad Sci U S A. 2000;97:3993–3998. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamrava M, Simpkins F, Alejandro E, Michener C, Meltzer E, Kohn EC. Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin-epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene. 2005;24:7084–7093. doi: 10.1038/sj.onc.1208857. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Sun Y, Liu S, et al. Upregulation of progranulin by Helicobacter pylori in human gastric epithelial cells via p38MAPK and MEK1/2 signaling pathway: role in epithelial cell proliferation and migration. FEMS Immunol Med Microbiol. 2011;63:82–92. doi: 10.1111/j.1574-695X.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 95.Ho JC, Ip YC, Cheung ST, et al. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology. 2008;47:1524–1532. doi: 10.1002/hep.22191. [DOI] [PubMed] [Google Scholar]
- 96.Wong NC, Cheung PF, Yip CW, et al. Antibody against granulin-epithelin precursor sensitizes hepatocellular carcinoma to chemotherapeutic agents. Mol Cancer Ther. 2014;13:3001–3012. doi: 10.1158/1535-7163.MCT-14-0012. [DOI] [PubMed] [Google Scholar]
- 97.Huang CH, Kuo CJ, Liang SS, et al. Onco-proteogenomics identifies urinary S100A9 and GRN as potential combinatorial biomarkers for early diagnosis of hepatocellular carcinoma. BBA Clin. 2015;3:205–213. doi: 10.1016/j.bbacli.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buraschi S, Xu SQ, Stefanello M, et al. Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin. Oncotarget. 2016;7:39980–39995. doi: 10.18632/oncotarget.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]


