Abstract
Background
Impairments of hand function make it difficult to perform daily life activities and to return to work. The aim of this study was to investigate the effect of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) combined with task-oriented mirror therapy (TOMT) on hand rehabilitation in acute stroke patients.
Material/Methods
Twenty subacute stroke patients in the initial stages (<3 months) participated in the study. Subjects were allocated to 2 groups: the experimental group received HF-rTMS + TOMT and the control group received HF-rTMS. TOMT training was conducted in 10 sessions over 2 weeks for 30 min. rTMS was applied at a 20 Hz frequency over the hand motor area in the cortex of the affected hemisphere for 15 min. Outcomes, including motor-evoked potential (MEP), pinch grip, hand grip, and box and block test, were measured before and after training.
Results
Significant improvements in the MEP and hand function variables were observed in both groups (p<0.05). In particular, hand functions (pinch grip and box and block test) were significantly different between the 2 groups (p<0.05).
Conclusions
HF-rTMS combined with TOMT had a positive effect on hand function and can be used for the rehabilitation of precise hand movements in acute stroke patients.
MeSH Keywords: Hand, Stroke, Transcranial Magnetic Stimulation
Background
Repetitive transcranial magnetic stimulation (rTMS) temporarily or continuously induces magnetic stimulation to a local area of the cerebral cortex to induce neurobiological changes in the brain through excitation and inhibition of nerves. Such changes have a positive influence on the treatment of patients who have undergone nerve grafting and psychiatric and rehabilitation therapies. Further, together with functional magnetic resonance imaging (fMRI), electroencephalography, and positron emission tomography, rTMS is applied in various research fields, including the balance of excitation and inhibition within the cerebral cortex; connectivity and interaction among the cortico-cortical fibers, cortex, and sub-cortex; and brain plasticity [1,2].
Recent studies have shown that in brain stroke patients, stimulating certain parts of the brain with rTMS had a positive effect on motor nerve function recovery, pain, dysphagia, aphasia, and neglect [3,4]. Particularly, rTMS has received attention because it is an outstanding intervention method for the recovery of upper-limb motor nerve function [5]. The motor areas in both the left and right cerebral hemispheres of the normal brain without neurological problems are paired functionally and work competitively to maintain balance. However, in the case of a brain stroke in which 1 of the cerebral hemispheres is damaged, there is reorganization of brain cells and changes in the sensitivity balance in the sensory and motor areas of the cerebral cortex due to changes in the sensitivity in both cerebral hemispheres. Therefore, there is an increase in the sensitivity of the primary motor cortex (M1) in the non-damaged hemisphere and a decrease in the sensitivity of the M1 in the damaged hemisphere [6–8]. This competitive recovery model of the M1 in both cerebral hemispheres has a positive influence on upper-limb motor nerve function recovery [5]. In other words, this competitive relationship between the cerebral cortices enables the balancing of sensitivity between the 2 cerebral hemispheres by increasing the sensitivity of the damaged cerebral hemisphere and lowering the sensitivity of the non-damaged cerebral hemisphere to enhance upper-limb motor nerve function in the paralyzed side [9–11].
The recovery of upper-limb function by rTMS is induced via 2 stimulation frequency modes: high-frequency and low-frequency. When high-frequency stimulation is applied to the damaged cerebral hemisphere, it increases the sensitivity of the cerebral cortex. Low-frequency stimulation is applied to the non-damaged cerebral hemisphere to reduce the sensitivity of the cerebral cortex. The application of the 2 frequency modes, depending on the condition of the patient, enhances the functional recovery of the upper limb [12,13]. It has been reported that in a random experiment on brain stroke patients, positive outcomes with regard to enhancement of upper-limb motor function in the paralyzed side were observed with the application of high-frequency stimulation as compared to low-frequency stimulation [14]. Further, combining rTMS with rehabilitation and medication in the initial stages after a stroke is reported to induce a significant improvement in upper-limb function in brain stroke patients [13]. Moreover, in a study which compared the result of applying low-frequency stimulation for upper-limb physical therapy after application of rTMS and applying rTMS after upper-limb physical therapy with chronic brain stroke patients as its subject, the most significant change was presented in a group which received physical therapy after application of rTMS [15]. In addition, in recent times, extensive studies on the application of rTMS have been conducted.
Mirror therapy is an economical, effective, and simple rehabilitation treatment method for the recovery of upper-limb function. Mirror therapy improves the mobility of damaged limbs via visual stimuli induced by viewing the movement of the non-damaged limb in the mirror and overlaps with limbs of the damaged side [16]. The mirror neuron principle can be explained by the mirror therapy theory. The mirror neuron system was first discovered at the back of the lower frontal lobe of the front motor cortex and later it was also found to exist at the front of the inferior parietal lobe. The frontal lobe and parietal lobe are connected by numerous nerves. The frontal lobe is responsible for providing motor commands and the parietal lobe integrates the senses; thus, the nerves that connect the frontal lobe and the parietal lobe integrate senses and motion. Therefore, a mirror neuron is not described as a cell but is referred to as the mirror neuron system because it is a nerve system in which the motor area of the frontal lobe receives a sensory signal from the parietal lobe [17]. According to a study by Cheng et al. (2008), although there was a difference in the concentration of the shadow on fMRI when examining the brain activity of subjects who were observing certain motions versus executing those motions in reverse, omnidirectional brain activity was noted only when subjects were observing the motion [18]. In many recent studies, mirror therapy has been shown to improve motor function in patients with upper-limb function impairments due to orthopedic and neurologic issues. Yavuzer et al. (2008) reported an improvement in motor techniques related to hand function in the mirror treatment group as compared to the sham mirror treatment group in a random assignment experiment with 36 acute brain stroke patients [19]. However, mirror therapy is a repetitive and simple program that is difficult to sustain as a continuous treatment method as patients lose interest. Because task-oriented therapy uses common daily life motions, it is reported to have an effect on function recovery in brain stroke patients as the motions are more enjoyable, influence the reorganization of the cerebral cortex, and contribute to brain plasticity [20]. A study in which mirror therapy was performed using a task-oriented method with daily life tasks to make treatment more interesting and long-lasting reported a higher treatment effect in the task-oriented mirror therapy (TOMT) group than in the simple mirror therapy group [21].
Brain stroke patients experience difficulties performing daily life activities due to upper-limb function impairments coupled with muscle weakness and stiffness, sensory abnormalities and imbalance, and tension. It has been reported that changes in hand grip strength are closely related to the motor capacity of brain stroke patients and are indicative of the outcomes [22]. Particularly, impairments of hand function that is required for performing delicate movements make it difficult to perform daily life activities (e.g., dressing, eating, and writing) and to return to work [23]. In the case of brain stroke patients, although recovery depends on the location and extent of the damage, recovery can be expedited if treatment is provided during the initial 3 months after the stroke. Thereafter, the recovery time increases with the passage of time, and neurological recovery stagnates after 1 year [24]. Therefore, providing rehabilitation treatment in the initial stage immediately after the stroke is very important. This study aimed to examine the improvements in hand function recovery in acute brain stroke patients by combining TOMT, which is an economical, simple, and effective therapy for upper-limb function recovery, with rTMS, which is widely used and known to be the most effective intervention method for upper-limb motor nerve function recovery.
Material and Methods
Subjects
This study included 39 acute stroke patients who were in the initial stages (<3 months). The patients were transferred to the Department of Rehabilitative Medicine for rehabilitation treatment among patients who had entered the stabilization stage after undergoing surgery and treatment after a diagnosis of brain stroke at the Department of Neurosurgery and Neurology at G University Hospital of Incheon. The inclusion criteria were: (1) symptoms of unilateral hemiparesis in ischemic and hemorrhagic stroke patients, (2) a Korean Mini-Mental State Examination score of >25, and (3) absence of psychological and emotional abnormalities, (4) Inpatients within 3 months from the day of diagnosis of stroke, (5) Manual muscle testing grade of upper extremity on paralyzed side is less than F-grade, and (6) Those who agree to want to participate in the research. Exclusion criteria were: (1) patients with pacemakers or metal objects implanted in the head, (2) patients with neglect symptoms or vision impairment, (3) History of seizures, (4) Absence of a motor-evoked potential (MEP) response upon applying rTMS to the damaged cerebral hemisphere, and (5) Upper-limb function impairments on both sides due to orthopedic or neurologic causes. Twenty-four subjects, excluding 15 people who did not satisfy the selection criteria, among 39 subjects were selected.
All experimental protocols and procedures were explained to the subjects and were approved by the Institutional Review Board of Sahmyook University, Korea. All subjects provided written informed consent prior to study enrollment.
This study was a single-blind, randomized, controlled trial. By using random allocation software (version 1.0), 24 patients who ultimately satisfied the inclusion criteria were divided into 2 groups [25]. The first group was the HF-rTMS group (control group, n=12) and the second group was the HF-rTMS + TOMT group (experimental group, n=12). Toward the end of the experiment, the HF-rTMS + TOMT group included only 8 patients because 4 patients dropped out due to scalp pain or personal reasons.
Outcome measures
Recording of cortical excitability – MEP
Cortical excitability was measured by determining the latency, amplitude, and RMT of the MEP, which is a parameter of transcranial magnetic stimulation [26]. The MEP is used to prognosticate corticospinal and motor capacity recovery after brain stroke [27,28]. To measure the MEPs, silver-chloride electrodes were attached to the skin over the tendon of the abductor pollicis brevis muscle belly, which is the target muscle, with the patient lying comfortably in supine position on the bed, and electromyography was conducted by using KEYPOINT®.NET and software. Then, the spot where movement of the target muscle was observed with the naked eye when a single coil was used to apply TMS at an interval of 1 cm to the region of the scalp, which corresponds to M1 of both hemispheres, was identified. This point is referred to as the hot spot. RMT was set as 0.05-mV higher from max value to min value of amplitude in electromyography in case of increasing and applying the intensity of TMS at the hot spot. The minimum RMT value was set after 5 consecutive responses from 10 trials. The smaller the RMT value after the experiment as compared to the value before the experiment, the greater was the positive effect on upper-limb motor function. With regard to amplitude and latency, measurement was conducted with 100% RMT stimulation as the reference, considering the reliability of the measurement in study subjects. When transcranial stimulation was applied to the hot spot, the response of the abductor pollicis brevis muscle was presented in the form of an X-Y graph on the computer monitor. The max and min values on the y-axis in the graph were calculated and referred to as the amplitude value. The higher the amplitude value, the greater is the positive influence it has on hand motor function recovery. Further, the latency value refers to the point where the amplitude response begins. In other words, it refers to the time until the response of the abductor pollicis brevis muscle is observed after TMS [29,30]. In this case, the shorter the time, the greater is the positive influence on hand motor function recovery. For measurement accuracy, we ensured that no impedance was exerted on the target muscle by any external force during the experiment.
Measurement of hand motor function
Box and block test
The box and block test is a simple method that evaluates simple hand motor function and involves subjects moving 1-inch wooden blocks from one box to another within 1 min [31]. A higher number of moved blocks indicates a greater improvement in precise hand movement. This test has been shown to have high intra-rater and inter-rater reliability in previous studies [32,33], and it is described as a method with high reliability among the available tests used to evaluate precise hand motor function, regardless of type of study subjects [34].
Hand grip and pinch grip strength
Hand grip strength is widely used as a measurement in adults and is considered to be representative of total body strength. The hand grip strength and lateral pinch grip force of both the paretic and nonparetic hands were measured in kilograms-force using a JAMAR® Hand Evaluation Kit (a hydraulic dynamometer and a hydraulic pinch gauge). During the measurements, patients were seated with their shoulder adducted and neutrally rotated, elbow flexed at 90 degrees, forearm in a neutral position, and wrist between 0 and 30 degrees dorsiflexion and between 0 and 15 degrees ulnar deviation [35]. During each trial, on the therapist’s cue, the patients were encouraged to apply maximal force while holding the equipment. The scores of 3 successive trials were recorded and averaged.
Procedures
In both groups, either HF-rTMS or HF-rTMS + TOMT was applied for 5 days/week for 2 weeks, excluding weekends, at the Neurology Lab and Exercise Therapy Center of G University hospital. The HF-rTMS + TOMT program included 20 min of general exercise therapy at the Exercise Therapy Center, 15 min of HF-rTMS (20 Hz) at the Neurology Lab, and 20 min of TOMT at the Exercise Therapy Center. The program for the HF-rTMS group included 20 min of general exercise therapy at the Exercise Therapy Center and 15 min of HF-rTMS (20 Hz) at the Neurology Lab. HF-rTMS was carried out by a rehabilitation medicine physician. TOMT and general exercise therapy were conducted by an experienced physical therapist.
Evaluation was performed twice: before (baseline) and after 2 weeks of intervention. Evaluations were performed by an assessor who was blinded to the study details (Figure 1).
Figure 1.
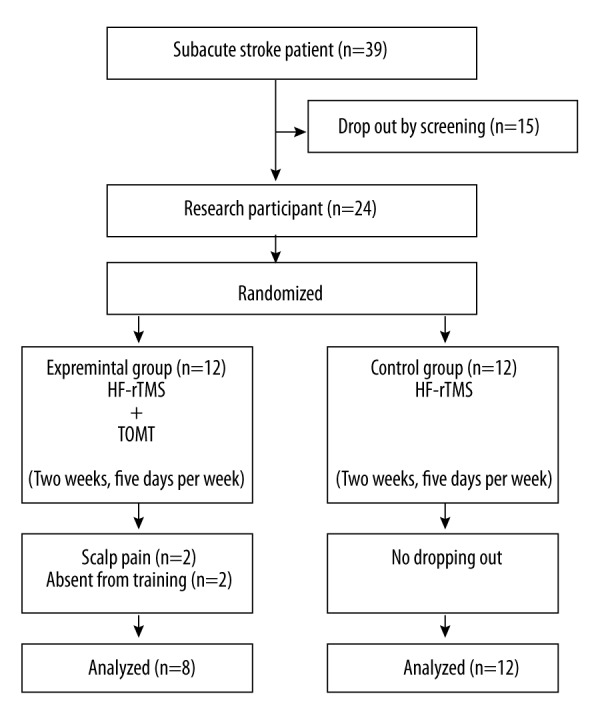
Flow diagram of the experimental procedure.
Interventions
The general exercise therapy involved joint mobility, eccentric and concentric contraction, and strengthening by automated full-body workout machine (Super Dynamic, Korea).
HF-rTMS was applied while patients were in supine position on the bed. By using the MAG PRO butterfly coil (MCF-B65) and MAG PRO R30 (Medtronic, Inc.), HF-rTMS was applied with a 45° angle between the application part and coil at the front and back. The hot spot was set by locating the spot where movement of the target muscle (i.e., the abductor pollicis brevis) due to the MEP could be viewed most clearly with the naked eye upon transcranial magnetic stimulation to the region of the scalp corresponding to the hand M1 in the damaged cerebral hemisphere.
High frequency (20 Hz/min as a standard, 100 pulses were applied for 5 s at 20 pulses/s followed by a resting period of 55 s; 1500 pulses were applied in 15 cycles) was applied by determining the resting motor threshold (RMT) value for each patient for this part and properly adjusting the stimulation intensity according to the patient condition such that the intensity does not exceed 90% of the threshold during application. To ensure that the location of the hot spots remained unchanged during the 10 treatments, they were marked on the scalp by using a permanent marker pen and photographs were taken with a camera. HF-rTMS was always performed by the same doctor.
The mirror box for TOMT was made of black polyvinyl chloride Formax, and its dimensions were as follows: width, 15 cm; length, 40 cm; height, 30 cm; and thickness, 1 T. Further, to ensure patient safety, an acrylic mirror was attached to the mirror box instead of a glass mirror. Patients were instructed to sit on a height-adjustable Bobath mattress without back support. The mirror box was placed such that it tilted toward the damaged side along the center line of the patient’s trunk on a steady table. They were instructed to put the hand of the damaged side in the mirror box so that it could not be seen and to place the hand of the non-damaged side in front of the mirror. At a specific moment, patients were instructed to voluntarily adjust the position of the hand reflected in the mirror according to the position of the hand inside the mirror box. Patients were instructed to gaze attentively at the image of the hand reflected in the mirror at the start of the program. TOMT involved 5 motions, including the box and block test, picking up sand, inserting a coin into a savings box, scooping sand with a spoon, and wiping with a towel; training was conducted for 30 min under the supervision of a physical therapist.
Statistical analysis
Data were analyzed using SPSS version 18.0 for Windows (SPSS Inc., Madison, WI, USA). To compare the pre- and post-test data for the 2 groups, a repeated measures analysis of variance (2×2) with a between-subjects factor was used. To compare the pre- and post-intervention differences between the 2 groups, a paired t test was used. Independent t tests were performed to compare the pre- and post-test scores and the difference by time for the 2 groups. A p value less than 0.05 was considered to indicate a statistically significant difference.
Results
There were no significant differences in the general characteristics of participants in the experimental and control groups (Table 1).
Table 1.
General characteristics of the participants.
| Experimental group (n=8) | Control group (n=12) | P | |
|---|---|---|---|
| Age (year) | 51±2.98 | 74.11±2.88 | 0.10 |
| Height (cm) | 155.16±5.57 | 153.64±4.68 | 0.21 |
| Weight (kg) | 54.21±5.01 | 53.15±3.97 | 0.32 |
| Time since stroke (months) | 1.63±0.74 | 1.75±0.62 | 0.68 |
| Sex (male/female) | 4/4 | 4/8 | |
| Paretic side (right/left) | 6/2 | 7/5 | |
| Ischemic/hemorrhagic | 5/3 | 7/5 |
Values are presented as mean ± standard deviation.
Cortical excitability
Resting motor threshold
The results that indicate brain recovery are shown in Table 2. Comparison of the threshold before and after HF-rTMS + TOMT and HF-rTMS revealed a significant difference in both groups (p < 0.05). There was no significant difference between the groups (p>0.05) (Table 2).
Table 2.
Pre- and post-program changes in cortical excitability.
| Experimental group (n=8) | Control group (n=12) | Difference (post-pre) | t(p) | |||
|---|---|---|---|---|---|---|
| Experimental group | Control group | |||||
| Threshold (%) | Pre | 36.25±21.33 | 29.09±20.22 | 11.25±9.91 | 11.81±9.81 | −0.124 (0.903) |
| Post | 47.50±20.52 | 40.90±21.65 | ||||
| t(p) | −3.211 (0.015) | −3.993 (0.003) | ||||
| Latency (m/s) | Pre | 26.29±4.45 | 25.04±8.93 | −3.05±3.37 | −3.35±3.94 | 0.172 (0.865) |
| Post | 23.24±5.85 | 21.69±8.08 | ||||
| t(p) | 2.559 (0.038) | 2.818 (0.018) | ||||
| Amplitude (m/v) | Pre | 0.93±0.83 | 0.82±1.82 | 0.70±0.63 | 0.66±0.71 | 0.115 (0.910) |
| Post | 1.63±1.10 | 1.49±2.21 | ||||
| t(p) | −3.119 (0.017) | −3.093 (0.011) | ||||
Values are presented as mean ± standard deviation.
Latency
A significant difference was observed in the latency before and after HF-rTMS + TOMT and HF-rTMS in both groups (p<0.05). In the comparison of the difference between the groups, we found no significant difference (p>0.05) (Table 2).
Amplitude
A significant difference in the amplitude before and after HF-rTMS + TOMT and HF-rTMS was observed in both groups (p < 0.05). Comparison of the difference between the groups revealed no significant difference (p > 0.05) (Table 2).
Hand motor function
Hand grip strength
The results of the hand function tests are shown in Table 3. Comparison of hand grip strength before and after HF-rTMS + TOMT and HF-rTMS revealed a significant difference in both groups (p<0.05). Comparison of the difference in hand grip strength revealed no significant difference between the groups (p>0.05) (Table 3).
Table 3.
Pre- and post-program changes in hand motor function.
| Experimental group (n=8) | Control group (n=12) | Difference (post-pre) | t(p) | |||
|---|---|---|---|---|---|---|
| Experimental group | Control group | |||||
| Hand grip strength (kg) | Pre | 5.43±4.15 | 5.45±6.87 | 9.93±8.36 | 5.36±6.42 | 1.351 (0.194) |
| Post | 15.37±8.46 | 10.81±10.43 | ||||
| t(p) | −3.359 (0.012) | −2.770 (0.020) | ||||
| Pinch grip (kg) | Pre | 2.18±4.42 | 1.06±1.06 | 1.81±0.70 | 0.61±0.75 | 3.550 (0.003) |
| Post | 4.00±4.55 | 1.68±1.47 | ||||
| t(p) | −7.28 (0.000) | −2.731 (0.021) | ||||
| Box & block test (ea/min) | Pre | 8.25±9.40 | 14.81±15.74 | 22.62±16.37 | 5.63±5.69 | 2.814 (0.022) |
| Post | 30.87±14.32 | 20.45±20.07 | ||||
| t(p) | −3.909 (0.006) | −3.281 (0.008) | ||||
Values are presented as mean ± standard deviation.
Pinch grip
There was a significant difference in the pinch grip before and after HF-rTMS + TOMT and HF-rTMS in both groups (p<0.05). Further, there was a significant difference in the pinch grip between the groups (p<0.05) (Table 3).
Box and block test
With regard to the box and block test, a significant difference was observed before and after HF-rTMS + TOMT and HF-rTMS in both groups (p<0.05). Comparison of the differences between the groups revealed a significant difference (p<0.05) (Table 3).
Discussion
Brain stroke is generally caused by damage to 1 cerebral hemisphere, and brings about physiological changes in brain cells. There is an abnormal increase in transcallosal inhibition in the damaged cerebral hemisphere, whereas transcallosal inhibition decreases in the non-damaged hemisphere. In other words, there is an imbalance in the parallel relationship between sensitivity, which is excitation, and inhibition in the left and right hemispheres, and this leads to motor function impairments [36]. The treatment intervention method rTMS produces outstanding effects with regard to motor function recovery in brain stroke patients through non-invasive control of excitation and inhibition of brain cells [14]. In addition, TOMT is a treatment method that focuses on the recovery of more precise and accurate motor function through task-oriented trainings based on the mirror neuron system [21]. Therefore, the purpose of this study was to examine the influence of rTMS and TOMT on the recovery of motor nerves and precise and accurate hand function in brain stroke patients in the initial stages after the stroke.
Twenty-four brain stroke patients in the initial stages after the stroke who met the selection criteria were divided into 2 groups, and treatments were conducted for 5 days/week for 2 weeks. The study was completed with 20 subjects after 4 subjects dropped out. There was a significant improvement in the RMT, amplitude, and latency of corticospinal excitability in both the experiment and control groups (p<0.05). Additionally, there was a significant improvement in hand function in both the experiment and control groups (p<0.05). Particularly, there was significant difference between the groups with regard to the pinch grip strength and box and block test outcomes (p<0.05).
Corticospinal excitability is a response presented during the MEP test. In the MEP test, the graph of the hot spot where response of the target muscle (abductor pollicis brevis in this study) is clearly visible on applying transcranial magnetic stimulation to M1 of the brain is analyzed. The data obtained are used to prognosticate motor nerve recovery of brain stroke patients. HF-rTMS plays a major role in increasing the excitability of the damaged cerebral hemisphere that has decreased excitability [37]. It positively influences the recovery of damaged motor nerves by balancing the excitation and inhibition of both hemispheres [38]. Previous studies have reported that an increase in the excitability of the damaged motor cortex through rTMS reorganizes the damaged motor cortex and generates an environment suitable for motor learning to enhance motor function [38]. Takeuchi et al. (2005) reported that the application of rTMS in brain stroke patients not only increases the excitation of residual nerve cells in M1, but also influences other neural networks that are far from the epicenter to bring about an improvement in motor function [39]. Accordingly, a statistically significant influence on all the variables of corticospinal excitability, including the RMT, amplitude, and latency, were observed in both groups on application of HF-rTMS (p<0.05).
There was a statistically significant difference between the 2 groups (p<0.05) in changes in grip strength, pinch grip, and box and block test outcomes relevant to hand function before and after the intervention. There was also a statistically significant difference between the 2 groups with regard to the amount of change in the pinch grip and the box and block test outcomes, which are the most important factors in this study (p<0.05). Higgins et al. (2013) reported statistically significant results for the box and block test, which is used to evaluate precise hand movement, and motor excitability in chronic brain stroke patients with hand function impairments. They reported that motor excitability can predict grip strength, pinch grip, and motor recovery in the low-frequency-repetitive transcranial magnetic stimulation (LF-rTMS) group as compared to the sham LF-rTMS group upon the application of task-oriented training with the use of daily life movements that require use of the hands [5]. Further, training to enhance precise and accurate upper-limb motor function in brain stroke patients by combining task-oriented training, which is an intervention method to improve function by affecting neuroplasticity through repetitive performance of daily life movements, and mirror therapy, which is an intervention method to enhance function by exerting positive influences on brain activity with the use of a mirror, produced greater improvements in the Fugl-Meyer Assessment motor function, box and block, cube carry, and card turning tests, which evaluate delicate hand function [21]. Accordingly, a statistically significant influence on the pinch grip and box and block test outcomes, which specifically require delicate hand movements, was observed in this study.
The main limit of this research is the relatively small number of subjects. Despite being performed in a university hospital, few patients wanted treatment with the latest technology introduced. Moreover, it was difficult to recruit subjects due to the high cost of treatment. Accordingly, further prospective investigations with more patients are needed to confirm our findings. In addition, in this experiment, we treated 10 sections for 2 weeks, but in the next study we are considering ways to increase the treatment period.
Conclusions
In brain stroke patients in the initial stages after the stroke, HF-rTMS and TOMT had a positive effect on hand function and on variables of corticospinal excitability, including RMT, amplitude, and latency, by positively influencing the recovery of damaged motor nerves via the HF-rTMS-induced reorganization of the damaged motor cortex by increasing the excitability of the damaged motor cortex and balancing the excitation and inhibition of both cerebral hemispheres. A significant improvement was observed after the application of TOMT, which is a method for retraining of more accurate and precise movements, surpassing the improvements in consistent motor function, when compared to the group that did not perform the box and block and pinch grip tests, which requires more accurate and precise movements. Based on these results, we propose the use of HF-rTMS along with other treatment methods such as TOMT as an effective rehabilitation therapy for brain stroke patients to bring about more effective improvements in accurate and precise movements.
Footnotes
Source of support: This study was supported by Sahmyook University
References
- 1.Naro A, Bramanti A, Leo A, et al. Effects of cerebellar transcranial alternating current stimulation on motor cortex excitability and motor function. Brain Struct Funct. 2017;222(6):2891–906. doi: 10.1007/s00429-016-1355-1. [DOI] [PubMed] [Google Scholar]
- 2.Gueugneau N, Grosprêtre S, Stapley P, Lepers R. High-frequency neuromuscular electrical stimulation modulates interhemispheric inhibition in healthy humans. J Neurophysiol. 2017;117(1):467–75. doi: 10.1152/jn.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park E, Kim MS, Chang WH, et al. Effects of bilateral repetitive transcranial magnetic stimulation on post-stroke dysphagia. Brain Stimul. 2017;10(1):75–82. doi: 10.1016/j.brs.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt J, Godecke E, Johnson L, Langhorne P. Early rehabilitation after stroke. Curr Opin Neurol. 2017;30(1):48–54. doi: 10.1097/WCO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 5.Higgins J, Koski L, Xie H. Combining rTMS and task-oriented training in the rehabilitation of the arm after stroke: A pilot randomized controlled trial. Stroke Res Treat. 2013;2013:539146. doi: 10.1155/2013/539146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Xing G, Fan Y, et al. Short-and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: A systematic review and meta-analysis. Clin Rehabil. 2017;31(9):1137–53. doi: 10.1177/0269215517692386. [DOI] [PubMed] [Google Scholar]
- 7.Schrader LM, Sadeghinejad S, Sadeghinejad J, et al. Comparison of low frequency repetitive transcranial magnetic stimulation parameters on motor cortex excitability in normal subjects. Int J Epilepsy. 2016;3(1):2–6. [Google Scholar]
- 8.Makovac E, Thayer JF, Ottaviani C. A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain-heart pathways to cardiovascular disease. Neurosci Biobehav Rev. 2017;74:330–41. doi: 10.1016/j.neubiorev.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Karabanov A, Ziemann U, Hamada M, et al. Consensus paper: Probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 2015;8(5):993–1006. doi: 10.1016/j.brs.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Volz LJ, Rehme AK, Michely J, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex. 2016;26(6):2882–94. doi: 10.1093/cercor/bhw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etoh S, Noma T, Ikeda K, et al. Effects of repetitive trascranial magnetic stimulation on repetitive facilitation exercises of the hemiplegic hand in chronic stroke patients. J Rehab Med. 2013;45(9):843–47. doi: 10.2340/16501977-1175. [DOI] [PubMed] [Google Scholar]
- 12.Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Dimitri D, De Filippis D, Galetto V, Zettin M. Evaluation of the effectiveness of transcranial direct current stimulation (tDCS) and psychosensory stimulation through DOCS scale in a minimally conscious subject. Neurocase. 2017;23(2):96–104. doi: 10.1080/13554794.2017.1305112. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high-and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013;22(4):413–18. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Avenanti A, Coccia M, Ladavas E, et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke A randomized trial. Neurology. 2012;78(4):256–64. doi: 10.1212/WNL.0b013e3182436558. [DOI] [PubMed] [Google Scholar]
- 16.McInnes K, Friesen C, Boe S. Specific brain lesions impair explicit motor imagery ability: A systematic review of the evidence. Arch Phys Med Rehabil. 2016;97(3):478–89.e1. doi: 10.1016/j.apmr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Tramacere A, Pievani T, Ferrari PF. Mirror neurons in the tree of life: Mosaic evolution, plasticity and exaptation of sensorimotor matching responses. Biol Rev Camb Philos Soc. 2017;92(3):1819–41. doi: 10.1111/brv.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong TT-J, Cunnington R, Williams MA, et al. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol. 2008;18(20):1576–80. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yavuzer G, Selles R, Sezer N, et al. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2008;89(3):393–98. doi: 10.1016/j.apmr.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 20.Carrico C, Chelette KC, Westgate PM, et al. Nerve stimulation enhances task-oriented training in chronic, severe motor deficit after stroke. Stroke. 2016;47(7):1879–84. doi: 10.1161/STROKEAHA.116.012671. [DOI] [PubMed] [Google Scholar]
- 21.Paik Y-R, Kim S-K, Lee J-S, Jeon B-J. Simple and task-oriented mirror therapy for upper extremity function in stroke patients: A pilot study. Hong Kong Journal of Occupational Therapy. 2014;24(1):6–12. [Google Scholar]
- 22.Hartman K, Altschuler EL. Mirror therapy for hemiparesis following stroke: A review. Current Physical Medicine and Rehabilitation Reports. 2016;4(4):237–48. [Google Scholar]
- 23.Jones TA. Motor compensation and its effects on neural reorganization after stroke. Nat Rev Neurosci. 2017;18(5):267–80. doi: 10.1038/nrn.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Mendoza EH, Hermann DM. Correlates of post-stroke brain plasticity, relationship to pathophysiological settings and implications for human proof-of-concept studies. Front Cell Neurosci. 2016;10:196. doi: 10.3389/fncel.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simis M, Adeyemo BO, Medeiros LF, et al. Motor cortex-induced plasticity by noninvasive brain stimulation: A comparison between transcranial direct current stimulation and transcranial magnetic stimulation. Neuroreport. 2013;24(17):973–75. doi: 10.1097/WNR.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 27.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery. Stroke. 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 28.Cakar E, Akyuz G, Durmus O, et al. The relationships of motor-evoked potentials to hand dexterity, motor function, and spasticity in chronic stroke patients: A transcranial magnetic stimulation study. Acta Neurol Belg. 2016;116(4):481–87. doi: 10.1007/s13760-016-0633-2. [DOI] [PubMed] [Google Scholar]
- 29.Alia C, Spalletti C, Lai S, et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front Cell Neurosci. 2017;11:76. doi: 10.3389/fncel.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas FS, Franklin C, Narayana S, et al. Repetitive transcranial magnetic stimulation educes frequency-specific causal relationships in the motor network. Brain Stimul. 2016;9(3):406–14. doi: 10.1016/j.brs.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canny ML, Thompson JM, Wheeler MJ. Reliability of the box and block test of manual dexterity for use with patients with fibromyalgia. Am J Occup Ther. 2009;63(4):506–10. doi: 10.5014/ajot.63.4.506. [DOI] [PubMed] [Google Scholar]
- 32.Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin Rehabil. 2005;19(4):404–11. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 33.Desrosiers J, Bravo G, Hébert R, et al. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75(7):751–55. [PubMed] [Google Scholar]
- 34.Lundquist CB, Maribo T. The Fugl-Meyer assessment of the upper extremity: Reliability, responsiveness and validity of the Danish version. Disabil Rehabil. 2017;39(9):934–39. doi: 10.3109/09638288.2016.1163422. [DOI] [PubMed] [Google Scholar]
- 35.Lopes J, Grams ST, da Silva EF, et al. Reference equations for handgrip strength: Normative values in young adult and middle-aged subjects. Clin Nutrit. 2017 doi: 10.1016/j.clnu.2017.03.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 37.Wang R-Y, Tseng H-Y, Liao K-K, et al. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke a randomized trial. Neurorehabil Neural Repair. 2012;26(3):222–30. doi: 10.1177/1545968311423265. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi N, Tada T, Toshima M, et al. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40(4):298–303. doi: 10.2340/16501977-0181. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi N, Chuma T, Matsuo Y, et al. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–886. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]


