Abstract
Background
A hypoxic microenvironment is associated with resistance to tyrosine kinase inhibitors (TKIs) and a poor prognosis in chronic myeloid leukemia (CML). The E3 ubiquitin ligase Siah2 plays a vital role in the regulation of hypoxia response, as well as in leukemogenesis. However, the role of Siah2 in CML resistance is unclear, and it is unknown whether vitaminK3 (a Siah2 inhibitor) can improve the chemo-sensitivity of CML cells in a hypoxic microenvironment.
Material/Methods
The expression of Siah2 was detected in CML patients (CML-CP and CML-BC), K562 cells, and K562-imatinib-resistant cells (K562-R cells). We measured the expression of PHD3, HIF-1α, and VEGF in both cell lines under normoxia and hypoxic conditions, and the degree of leukemic sensitivity to imatinib and VitaminK3 were evaluated.
Results
Siah2 was overexpressed in CML-BC patients (n=9) as compared to CML-CP patients (n=13). Similarly, K562-imatinib-resistant cells (K562-R cells) showed a significantly higher expression of Siah2 as compared to K562 cells in a hypoxic microenvironment. Compared to normoxia, under hypoxic conditions, both cell lines had lower PHD3, higher HIF-1α, and higher VEGF expression. Additionally, Vitamin K3 (an inhibitor of Siah2) reversed these changes and promoted a higher degree of leukemic sensitivity to imatinib.
Conclusions
Our findings indicate that the Siah2-PHD3- HIF-1α-VEGF axis is an important hypoxic signaling pathway in a leukemic microenvironment. An inhibitor of Siah2, combined with TKIs, might be a promising therapy for relapsing and refractory CML patients.
MeSH Keywords: Hypoxia-Inducible Factor 1, alpha Subunit; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Prolyl-Hydroxylase Inhibitors; Ubiquitin-Protein Ligases; Vascular Endothelial Growth Factors; Vitamin K3
Background
Hypoxia usually refers to a state of oxygen depletion, which can be found in the microenvironments of tumors and in normal tissues. A hypoxic microenvironment in a tumor results from uncontrolled tumor growth that depletes the original oxygen supply. In hematology, these low-oxygen conditions are prevalent in the bone marrow. This is called the hypoxic niche. The hypoxic niche aids hematopoietic stem cells in maintaining dormancy prior to a stimulus and their ability to proliferate in the future. Several studies have indicated that hypoxic tumor cells are more resistant to existing anti-tumor treatments, including chemotherapy, radiotherapy, and, indirectly, surgery [1,2]. There are many reasons for hypoxia-induced tumor protection. Firstly, hypoxia induces de-differentiation and the emergence of immature phenotypes among cells, as well as maintaining stem-cell-like phenotypes to create a protective effect in both hematopoietic stem cells and solid tumor stem cells [3]. Secondly, abnormal tumor vasculature makes the delivery and infiltration of chemotherapeutic agent difficult in a hypoxic microenvironment. Finally, hypoxia promotes tumor cells at a low proliferation rate and becomes insensitive to the pressure of apoptosis [4]. Accordingly, clinical data have demonstrated that hypoxia is correlated with increased tumor aggressiveness and poor prognoses [5]. Particularly in chronic myeloid leukemia (CML), hypoxia was correlated with CML tumor burden [6].
Hypoxia-inducible factor (HIF) is considered to be a central regulator of hypoxia [7]. Of the 3 HIF-α isotypes (1α, 2α, and 3α), HIF-1α is particularly important in hypoxia response [8]. Under normal oxygen conditions, prolyl-hydroxylase (PHD) introduces a hydroxyl group to Hif-1α and functions as a recognition motif for the breakdown of essential proteins involved in the process. Consequently, under low-oxygen conditions, PHD proteins are polyubiquinated and broken down by ubiquitin ligase Siah2. This inhibits the introduction of a hydroxyl group to Hif-1a. Siah2 mediates the ubiquitination and degradation of substrates that are important in hypoxia-activated signaling pathways and contributes to the degradation of multiple targets, including cellular biological function and tumorigenesis [9–11]. Hence, we hypothesized that Siah2 may serve as a target inhibitor in CML resistance.
Thus, our study aimed to evaluate the expression and function of Siah2 in CML patients, as well as in cell lines, and to examine whether vitamin K3 (a Siah2 inhibitor) can improve the chemo-sensitivity of CML cells in a hypoxic microenvironment. These findings could provide a foundation for clinical protocols in which Siah2 inhibitor overcomes drug resistance and provides a therapeutic benefit in cases of CML pathogenesis.
Material and Methods
Patients
The protocol was approved by the Ethics Committee of Nanfang Hospital and by Southern Medical University (Guangzhou, Guangdong, China). Patients were admitted at Nanfang Hospital according to the guidelines of its Ethics Committee and the Helsinki Declaration. All patients and healthy donors signed the consent form. CML has an evolutionary course comprising 3 clinical phases based on both clinical and pathological features [12]. The chronic phase (CP) is characterized by an increase in immature and mature myeloid elements, and retention of hematopoietic differentiation. The disease may then progress through an accelerated phase (AP), or directly to an acute or blast phase (BP) when there are ≥30% blasts in the bone marrow or extramedullary blastic disease, and presents a very poor prognosis [13].
Clinical samples were taken from the bone marrow of CML-CP patients (n=13), CML-BC (n=9) patients, and healthy volunteers (n=4) to isolate bone marrow mononuclear cells (BMMCs). Diagnoses were based on hematologic, cytogenetic, and molecular assays. We selected 4 healthy donors (mean age=34, range=25~40, male: female ratio=2: 2), 13 CML-CP patients (mean age=32, range=21~45, male: female ratio=2: 3), and 9 CML-BP patients (mean age=40, range=33~49, male: female ratio=3: 2).
Cells
PBMCs were isolated by Ficoll separation from the bone marrow samples of the CML patients and volunteers. The cells were suspended in RPMI-1640 medium with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin-streptomycin. K562cells and K562imatinib-resistant cells (K562-R cells) were cultured in the above-mentioned media. The cells were maintained in 5% CO2 at 37°C. K562 cells and K562-R cells were further supplemented with imatinib (IM).
Hypoxic conditions
We analyzed cells by treating them in low-oxygen state (1%O2) for 12 h, 24 h, or 48 h using a hypoxia chamber (In vivo 400: Ruskin Technologies Ltd, Bridgend, UK). For our analysis, we used cell culture media, trypsin/PBS, and PBS, which were equilibrated previously with 1% O2 overnight before any changes were made, in order to prevent the exposure of the cells to atmospheric air (20% O2) under conditions such as medium change, unless mentioned otherwise.
Cell viability assay
During the logarithmic growth phase, the cells were exposed to various concentration of IM and/or Vitamin K3 under 1% O2 conditions and then incubated with CCK-8 solution. The optical density (OD) was calculated based on 450 nm absorption values using an auto-179-mated enzyme-linked immunosorbent assay (ELISA) reader (Perk-180, Elmer, USA).
Cell cycle analysis
The cell cycle was assessed via propidium iodide (PI) staining and measured with a FACSCaliburTM flow cytometer. Samples were processed as follows: 1) Harvested the cells in the appropriate manner and washed them in PBS. 2) Fixed the cells in cold 70% ethanol, added drop-wise to the pellet while vortexing. 3) Fixed them for 30 min at 4°C. 4) Washed them in PBS and centrifuged them. 5) Treated the cells with ribonuclease and added 50 μl of a 100 μg/ml sock of RNase. 6) Added 200 μl PI (from 50 μg/ml stock solution). The cell distribution of each phase of the cell cycle was evaluated with ModFit LT software (BD Biosciences). For analysis, the first gate on the single-cell population was used as pulse width vs. pulse area. Then, we applied this gate to the scatter plot and gated out obvious debris. We combined the gates and applied it to the PI histogram plot. Apoptosis of cells was measured using an Annexin V-FITC/PI apoptosis detection kit according to the protocol. The samples were analyzed in flow cytometry.
Quantitative real-time polymerase chain reaction (QRT-PCR)
Total RNA was extracted from cells using TRIzol reagent (Takara Bio Inc., Otsu, Shiga, Japan) and then reverse-transcribed into cDNA. The sequence of primers used was as follows: Siah2: forward 5′-TTGCTCATCAGTTGCCACTTCC-3′ and reverse 5′-CAGGAGTAGGGACGGTATTCACA-3′; for HIF-1α: forward 5′-TTGCTCATCAGTTGCCACTTCC-3′and reverse5′-AGCAATTC ATCTGTGCTTTCATGTC-3′; and for GAPDH: forward 5′-ACCACA GTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′. Reactions were carried out in a Roche Light Cycler 480 system (Roche Diagnostics GmbH, Mannheim, Germany) with a SYBR Premix ExTaq kit (Takara Bio Inc., Otsu, Shiga, Japan). Using the 2−ΔΔCT method, data were normalized to GAPDH expression.
Western blotting analysis
Cells were washed with PBS and lysed in buffer (50 mM Hepes, 150 mM NaCl, 1% TritonX-100, 0.1% SDS, 50 mM NaF, 10 mM NaPPi, 2 mM NaVO3, 10 mM EDTA, 2 mM EGTA, 1 mM PMSF, and 10 μg/ml leupeptin) on ice for 10 min. Protein lysate from the cells was mixed with loading buffer, denatured for 10 min at 65°C, separated on SDS-PAGE, and later transferred to a PVDF membrane. After blocking (5% dry milk in Tris-buffered saline-T), membranes were incubated at 4°C overnight with primary antibodies. After incubation with secondary antibodies, the signals were visualized by chemiluminescence using a chemiluminescence (ECL) detection device (Millipore, Billerica, MA, USA). Optical densities were visualized and measured as arbitrary units via an LAS3000 Imager (Fugifilm, Greenwood, SC, USA). The results were normalized to GAPDH using the Multi-gauge software program (Version 22.0, Fujifilm, Greenwood, SC, USA).
ELISA
To measure vascular endothelial growth factor (VEGF) secretion from the K562 and K562-R cell lines, we randomly selected cells for 24-h incubation under normoxia (20% O2) or hypoxia (1% O2) conditions. We collected supernatants and measured the levels of VEGFA by using an ELISA kit (R&D Systems).
Statistical analysis
Data are presented as means ± standard deviation (SDs). The statistical analysis of the data was carried out with GraphPad Prism 5 software (Version 5 for Windows; GraphPad, San Diego, CA). Our study utilized a paired Student’s t-test to observe the findings regarding the cohorts chosen for this experiment. Statistical significance was set at P<0.05. We carried out the procedures 4 times.
Results
The Siah2 expression of CML patients and cell lines under normoxic conditions
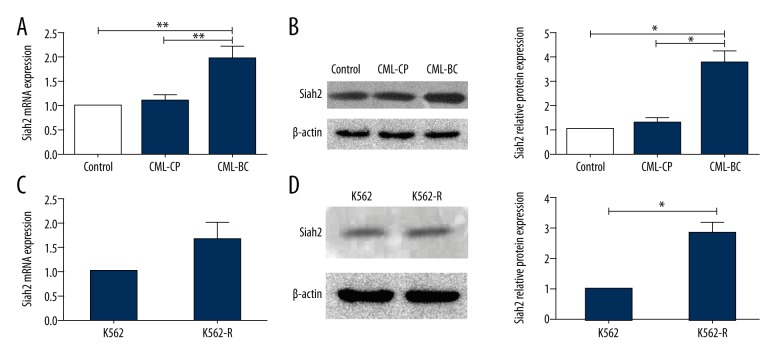
The expression of Siah2 from CML patients (CML-CP and CML-BC), healthy donors, and cell lines was detected via Q-PCR and Western blotting, as shown in Figure 1. The results show that Siah2 mRNA levels in CML-BC patients were significantly higher than those of the CML-CP patients and healthy donors. Furthermore, our study found no variations between CML-CP patients and healthy donors (Figure 1A, 1B). In the cell lines, the analysis of Siah2 mRNA levels showed insignificant changes between K562 cells and K562-R cells (Figure 1C). Western blotting analysis revealed similar findings (Figure 1D).
Figure 1.
Siah2 was expressed in CML patients and cell lines. (A) Siah2 mRNA expressions, on QPCR, of CML-CP patients, CML-BC patients, and healthy controls. (B) Siah2 protein immunoreactivity, on western blots, of CML-CP patients, CML-BC patients and healthy controls. β-actin served as a loading control. (C) Siah2 mRNA expressions of K562 and K562-R cells were analyzed via QPCR. (D) Siah2 protein expressions of cell lines were analyzed via western blots. Kruskal-Wallis test was employed, whereby statistical significance was obtained for continuous variables using a non-parametric analysis of variance. For statistical significance, patient groups were compared by employing Dunn’s multiple-comparison test. ** P<0.01.
The Siah2 expression of K562 cells and K562-R cells under hypoxia
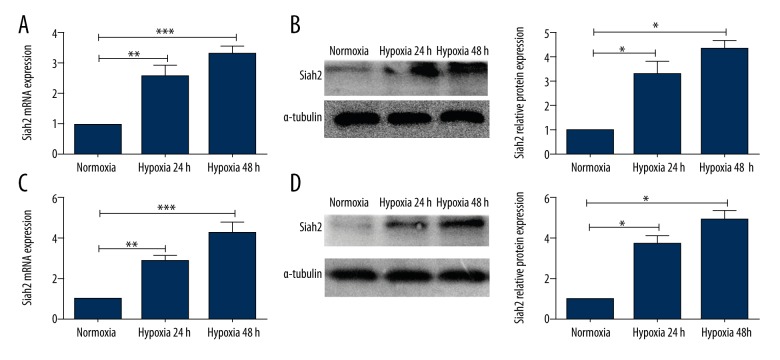
To confirm the impact of hypoxia on Siah2, we monitored changes in Siah2 expression in K562 cells and K562-R cells. We utilized a modular incubation chamber containing humidified hypoxic air to create hypoxic conditions for the analyzed cells. K562 cells and K562-R cells were cultured under hypoxic conditions (1% O2) for 24 h and 48 h. QPCR and Western blotting analyses showed that compared to normoxia, both K562 cells and K562-R cells showed increased expression of Siah2 mRNA and protein levels in hypoxic conditions from 24 h to 48 h, and that the change in K562-R cells was more apparent (Figure 2). However, the Siah2 mRNA and protein levels did not change in normoxia conditions at 24 h and 48 h.
Figure 2.
The Siah2 expression of K562 cells and K562-R cells under hypoxia. (A, B) Siah2 expression at mRNA and protein levels of K562 cells under hypoxia. (C, D) Siah2 mRNA expression and protein immunoreactivity levels of K562-R cells were analyzed under hypoxia. *** P<0.001; ** P<0.01.
VitaminK3 reduces K562 cells’ and K562-R cells’ resistance to IM under hypoxic conditions
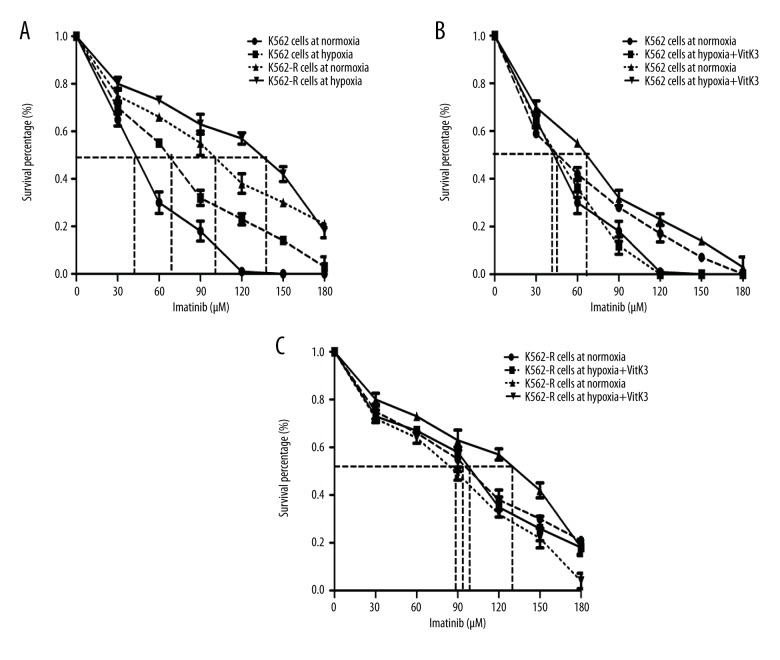
Our study aimed to determine whether leukemic cells become sensitive to IM, the frontline therapy for CML, in response to hypoxia. K562 cells and K562-R cells were maintained in complete medium at 21% O2 (normoxia) or 1% O2 (hypoxia) for 24 h in the presence or absence of IM. A cell viability assay via CCK-8 showed that hypoxia significantly increased the ability of cells to survive upon treatment with IM in comparison with cells under normoxic conditions (Figure 3A). This increase was significantly attenuated by pre-treating cells with Vitamin K3 (15 mM) under hypoxia conditions (Figure 3B, 3C). Interestingly, Vitamin K3 did not influence cell viability in either cell line under normoxia. Additionally, the IC50 of IM for K562 cells and K562-R cells under hypoxia was 1.62-fold and 1.38-fold higher, respectively, as compared with cells under normoxia, as shown in Figure 3A. After Vitamin K3 treatment, the IC50 of IM for K562 cells and K562-R cells under hypoxia was 1.51-fold and 1.59-fold, respectively, lower than that seen under hypoxia without VitaminK3 treatment (Figure 3B, 3C). These results indicate that hypoxia seems to protect leukemic cells from chemotherapy damage and that Vitamin K3 (an inhibitor of Siah2) counteracts this hypoxic protection.
Figure 3.
The imatinib sensitivities of K562 cells and K562-R cells with or without VitaminK3 treatment under normoxia or hypoxia. (A) The cell activities of CML cells with imatinib treatment under normoxic or hypoxic conditions. The chemosensitivity of K562 (B) and K562-R (C) cells was improved by VitaminK3 stimulation in hypoxia. *** P<0.001; ** P<0.01; * P<0.05.
Vitamin K3 sensitizes leukemic cells to IM in a cell-cycle-arrest-dependent manner
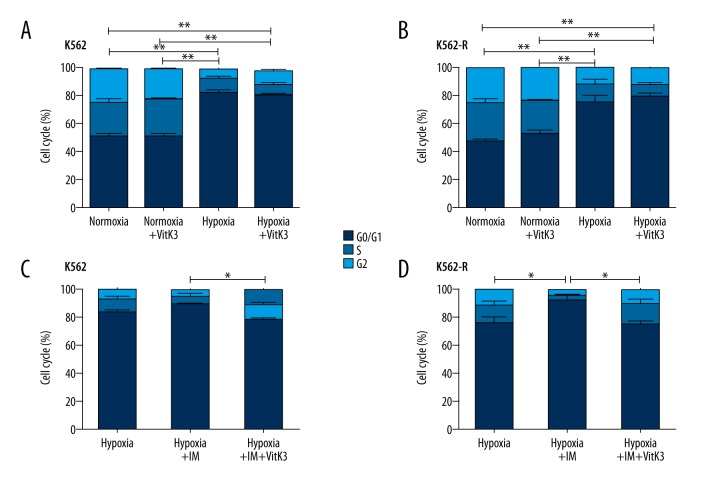
As the above results have demonstrated, hypoxia can inhibit the proliferation of CD34-positive cells derived from patients with CML [14], and hypoxia clearly reduced the proliferation of K562 and K562-R cells by maintaining the cells in a quiescent state. In the cell cycle analysis, hypoxia increased the proportions of cells in the G0/G1 phase in both cell lines (Figure 4A, 4B). Additionally, IM can also significantly increase the proportion of cells in the G0/G1 phase among K562 and K562-R cells (Figure 4C, 4D). Nevertheless, when IM was combined with Vitamin K3 treatment, the proportion of cells in the G0/G1 phase in both cell lines was significantly decreased under hypoxic conditions, while Vitamin K3 alone did not induce such an effect (Figure 4A–4D).
Figure 4.
Apoptotic cells were measured via flow cytometry in K562 cells and K562-R cells under imatinib, hypoxia, and/or VitaminK3 treatment. (A, C). The cell cycle of K562 cells was evaluated via flow cytometry after these cells were subjected to hypoxia, imatinib, and/or VitaminK3 stimulation (B, D). The cell cycle of K562-R cells was analyzed after hypoxia, imatinib and/or VitaminK3 treatment. IM, imatinib. VitK3, VitaminK3. ** P<0.01; * P<0.05.
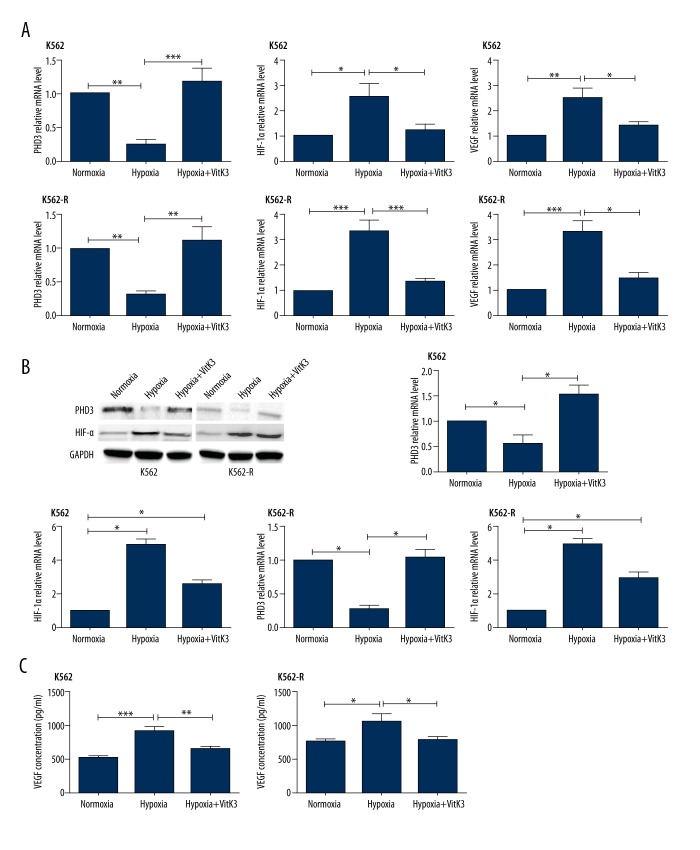
Vitamin K3 inhibits leukemic cells’ VEGF expression through the Siah2-PHD3-HIF pathway
In order to analyze the effect of Vitamin K3 on Siah2 activity, we observed the variations in the stability of Siah2 substrates. Our study sought to determine whether preventing Siah2 ubiquination has any effect on the targeted ubiquination of the substrates associated with this process, such as PHD3. Therefore, we assessed the expression of PHD3, HIF-1α, and VEGF in K562 and K562-R cells at the mRNA and protein levels. As expected, HIF-1α was not observed under normoxic conditions in either of the 2 cell lines. Under hypoxia, leukemic cells showed lower PHD3 and higher HIF-1α and VEGF levels in comparison with those observed under normoxia. As an inhibitor of Siah2, Vitamin K3 was found to have significant effects (Figure 5).
Figure 5.
PHD3, HIF-1α, and VEGF expression levels of both cell lines were evaluated via QPCR, western blot, and ELISA after hypoxia and VitaminK3 stimulation. (A) PHD3, HIF-1α, and VEGF mRNA expression levels of K562 and K562-R cells with or without VitaminK3 stimulation under normoxic or hypoxic conditions. (B) PHD3 and HIF-1α protein immunoactivity of both cell lines on western blots under normoxia, hypoxia, and hypoxia + VitaminK3 treatment. (C) VEGF secretion of both cell lines after hypoxia and/or VitaminK3 stimulation. *** P<0.001; ** P<0.01; *P<0.05.
Discussion
We assessed whether Siah2 and hypoxia affected chemo-resistance in chronic myeloid leukemia patients. Our study demonstrated that preventing the signaling pathways of Siah2 and hypoxia can be used for therapeutic purposes in cases of CML pathogenesis. Protein ubiquitination plays a vital role in hypoxia response. Ubiquitination is performed via the successive reaction of ubiquitin-activating enzyme E1, ubiquitin-transferring enzyme E2, and a substrate-specific ubiquitin ligase E3, which allows recognition by targeting certain substrates for ubiquination [15]. Siah2 belongs to the RING finger ubiquitin ligase E3 and is important in the regulation of tumor development under hypoxic conditions [10]. Accordingly, considerable research has been devoted to understanding the roles of Siah2 in tumor development and the effects its inhibition may have. In solid tumors, Siah2 has been reported to contribute to tumor aggressiveness and chemo-resistance in breast, prostate, ovarian, lung, and oral tumors [16–19]. For the first time, our study found that Siah2 was overexpressed in CML-BC patients as compared to CML-CP patients. Consistent with these findings, K562-R cells showed a significant increase in Siah2 expression as compared to K562 cells in a hypoxic microenvironment.
Similar to solid tumors, CML cells survive and grow in a hypoxic bone marrow microenvironment. Under hypoxic conditions, tumor cells adapt through the up-regulation of HIF-1α protein, which in turn promotes the expression of various genes, including VEGF. VEGF serves a quintessential role in the process of angiogenesis and promotes blood vessel preservation. Active blood vessels aid in tumor cell growth via providing oxygen and nutrient supplies, along with the expulsion of metabolic products that have been broken down into simpler constituents. In solid tumors, it is well-established that angiogenesis plays an important role in tumor development and metastasis. Furthermore, angiogenesis prevention could be used as a pathway via which to effectively treat cancer patients [20]. In contrast to solid tumors, the leukemic tumor microenvironment mainly exists in the bone marrow and can be divided into an “osteoblastic niche” and a “vascular niche.” The former is responsible for providing the environment for long-term quiescent hematopoietic stem cells, and the latter fosters the proliferation and differentiation of short-term hematopoietic progenitors [21]. Both niches are important for the growth and development of leukemic stem cells. Several studies have suggested that increased vascular density within the bone marrow is found in many blood diseases, such as leukemia, myelodysplastic syndromes, and myelomas. Enhanced VEGF markers cannot provide effective prognoses for blood-related pathologies. Hence, enhanced vascular density could serve as an important prognostic marker for the overall survival of patients affected by hematological diseases [22–24]. Moreover, in CML, the expression of VEGF is consistent with Bcr-Abl1 fusion kinase, and imatinib may suppress the expression of VEGF [25]. Therefore, we speculate that a higher level of VEGF expression may be closely related to leukemic cells’ chemo-resistance in CML, and the inhibition of VEGF could be used as a novel therapy for increasing the sensitivity of leukemic cells to chemotherapy. However, the molecular mechanism behind the angiogenic reaction in the leukemic microenvironment has been poorly characterized.
HIF-1α protein plays a key role in hypoxia because its expression is regulated in an oxygen-dependent manner and it is involved in many activities related to cellular adaptation to oxygen-poor conditions. The Siah2-PHD3 pathway may play an important role in tumor development, which is considered to be the most prominent PHD regulating HIF-1α under normoxia. PHD3 actively hydroxylates and negatively regulates HIF when oxygen is abundant and is inhibited in hypoxia, enabling HIF-1α to become active. In embryonic fibroblasts isolated from Siah2-knockout mice, HIF-1α expression was significantly decreased and accompanied by the reduced expression of VEGF when cells were under hypoxic conditions. Nevertheless, PHD3 expression was obviously increased when compared with cells taken from wild-type mice [26]. In accordance with the previous research, our study demonstrated that both K562 and K562-R cells had lower levels of PHD3 and higher levels Siah2, HIF-1α, and VEGF expression as compared with those seen under normoxia. Moreover, Vitamin K3 had significant effects, increasing leukemic cells’ sensitivity to imatinib treatment. Although many preclinical studies have indicated that the direct blockage of VEGF could slow down the progression of hematologic malignancies, this suppression was limited in a tumor-type-specific and context-dependent manner [27,28]. Compared with VEGF, Siah2 regulates a wide range of physiological and pathological responses to a hypoxic microenvironment. The inhibition of Siah2 could prevent the bypassing of the anti-angiogenesis mechanism and allow residual leukemic cells to survive. Therefore, an inhibitor of Siah2, combined with TKIs, may be a promising approach to relapsing and refractory CML.
Conclusions
For the first time, our study indicated that Siah2 was overexpressed in CML-BP patients as compared to CML-CP patients. In accordance with this, K562-R cells showed a significant increase in Siah2 expression as compared to K562 cells in a hypoxic microenvironment. In vitro, both cell lines had a lower PHD3 and higher Siah2, HIF-1α, and VEGF expression levels under hypoxia. As an inhibitor of Siah2, Vitamin K3 restrains the expression of Siah2, HIF-1α, and VEGF and may increase leukemic cells’ sensitivity to imatinib. The inhibition of Siah2, combined with TKIs, might provide a therapeutic opportunity for CML, especially for imatinib-resistant patients. Nonetheless, further studies are required to elucidate the extensive molecular mechanisms at work in the leukemia-supportive microenvironment and leukemic chemo-resistance.
Footnotes
Source of support: This study was supported by the Science and Technology Planning Project of Guangdong Province, China (2014A020211019), the Medical research foundation of Guangdong province (No. 2015124162336427), Science Foundation of Nan fang Hospital (No. 2016A002), Medical Scientific Research Foundation of Guangdong Province, China (No. A2017543),Science and Technology Planning Project of Shaoguan City, China (No. 2017cx/008), and Health and Family Planning Research Project of Shaoguan City, China (No. Y17019)
References
- 1.Brown JM, Giaccia AJ. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998;58(7):1408–16. [PubMed] [Google Scholar]
- 2.Hammond EM, Asselin MC, Forster D, et al. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol) 2014;26(5):277–88. doi: 10.1016/j.clon.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Muz B, de la Puente P, Azab F, Luderer M, Azab AK. The role of hypoxia and exploitation of the hypoxic environment in hematologic malignancies. Mol Cancer Res. 2014;12(10):1347–54. doi: 10.1158/1541-7786.MCR-14-0028. [DOI] [PubMed] [Google Scholar]
- 4.Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8(7):790–97. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 6.Azab AK, Weisberg E, Sahin I, et al. The influence of hypoxia on CML trafficking through modulation of CXCR4 and E-cadherin expression. Leukemia. 2013;27(4):961–64. doi: 10.1038/leu.2012.353. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama K, Qi J, Ronai Z. The ubiquitin ligase Siah2 and the hypoxia response. Mol Cancer Res. 2009;7(4):443–51. doi: 10.1158/1541-7786.MCR-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Li H, Xi HS, Li S. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119(11):2595–607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House CM, Moller A, Bowtell DD. Siah proteins: Novel drug targets in the Ras and hypoxia pathways. Cancer Res. 2009;69(23):8835–38. doi: 10.1158/0008-5472.CAN-09-1676. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K, Frew IJ, Hagensen M, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117(7):941–52. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.House CM, Hancock NC, Moller A, et al. Elucidation of the substrate binding site of Siah ubiquitin ligase. Structure. 2006;14(4):695–701. doi: 10.1016/j.str.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: Update on biology and treatment. Oncology (Williston Park, NY) 1999;13:169–80. [PubMed] [Google Scholar]
- 13.Rumjanek VM, Vidal RS, Maia RC. Multidrug resistance in chronic myeloid leukaemia: how much can we learn from MDR-CML cell lines? Biosci Rep. 2013;33(6) doi: 10.1042/BSR20130067. pii: e00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desplat V, Faucher JL, Mahon FX, et al. Hypoxia modifies proliferation and differentiation of CD34(+) CML cells. Stem Cells. 2002;20(4):347–54. doi: 10.1634/stemcells.20-4-347. [DOI] [PubMed] [Google Scholar]
- 15.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Liu Y, Meng F, et al. Overexpression of Siah2 is associated with poor prognosis in patients with epithelial ovarian carcinoma. Int J Gynecol Cancer. 2016;26(1):114–19. doi: 10.1097/IGC.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh SC, Kuo SN, Zheng YH, et al. The E3 ubiquitin ligase SIAH2 is a prosurvival factor overexpressed in oral cancer. Anticancer Res. 2013;33(11):4965–73. [PubMed] [Google Scholar]
- 18.Knauer SK, Mahendrarajah N, Roos WP, Kramer OH. The inducible E3 ubiquitin ligases SIAH1 and SIAH2 perform critical roles in breast and prostate cancers. Cytokine Growth Factor Rev. 2015;26(4):405–13. doi: 10.1016/j.cytogfr.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Muller S, Chen Y, Ginter T, et al. SIAH2 antagonizes TYK2-STAT3 signaling in lung carcinoma cells. Oncotarget. 2014;5(10):3184–96. doi: 10.18632/oncotarget.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Konopleva M, Tabe Y, Zeng Z, Andreeff M. Therapeutic targeting of microenvironmental interactions in leukemia: Mechanisms and approaches. Drug Resist Updat. 2009;12(4–5):103–13. doi: 10.1016/j.drup.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrabovszky E, Liposits Z. Novel aspects of glutamatergic signalling in the neuroendocrine system. J Neuroendocrinol. 2008;20(6):743–51. doi: 10.1111/j.1365-2826.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 23.Li WW, Hutnik M, Gehr G. Antiangiogenesis in haematological malignancies. Br J Haematol. 2008;143(5):622–31. doi: 10.1111/j.1365-2141.2008.07372.x. [DOI] [PubMed] [Google Scholar]
- 24.Korkolopoulou P, Viniou N, Kavantzas N, et al. Clinicopathologic correlations of bone marrow angiogenesis in chronic myeloid leukemia: A morphometric study. Leukemia. 2003;17(1):89–97. doi: 10.1038/sj.leu.2402769. [DOI] [PubMed] [Google Scholar]
- 25.Mayerhofer M, Valent P, Sperr WR, et al. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100(10):3767–75. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 26.Frew IJ, Hammond VE, Dickins RA, et al. Generation and analysis of Siah2 mutant mice. Mol Cell Biol. 2003;23(24):9150–61. doi: 10.1128/MCB.23.24.9150-9161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z, Hattori K, Zhang H, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17(3):604–11. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]
- 28.Dias S, Hattori K, Heissig B, et al. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA. 2001;98(19):10857–62. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]