Abstract
Major advances in mononuclear phagocyte biology have been realized, yet key questions pertinent to health and disease remain, including in the visual system. One problem concerns how dendritic cells can trigger immune responses from certain tightly regulated immune privileged sites of the eye. Another, albeit separate, problem involves whether functional specializations exist for microglia versus monocytes in neurodegenerating retinas. We examine novel insights in eye immune privilege and, separately, review recent inroads concerning retinal degeneration. Both themes have been extensively studied in the visual system, and exhibit parallels highlighted here with recent findings in CNS mononuclear phagocytes and in the periphery.
The last decade has seen big advancements in our understanding of cells of the mononuclear phagocyte system (MPS), which include monocytes, macrophages, and dendritic cells (DCs)1. While their central role in triggering and/or shaping immune responses has been appreciated, key advances regarding their respective developmental origins have only recently been established 2. From these studies, it is now understood that the ontogeny of tissue-resident macrophages is largely distinct from that of ‘classical’ (or conventional) DCs, signifying that these respective MPS populations are indeed unique3 (notwithstanding monocytes, which can take on functionalities of either 4). Other landmark studies have recently discovered the embryonic basis of adult microglia (CNS-resident macrophages5, 6, 7, 8, 9), addressing the long-standing debate regarding the origins of these cells10.
Key questions regarding MPS functionality still remain. For one, the tissue-specific cues that enable MPS cells to effectively trigger adaptive immune responses in tightly regulated, immune-privileged sites are poorly understood, which we focus on here in the context of certain compartments of the visual system. Wayne Streilein described immune privilege as “nature’s way” 11 of providing protection from deleterious immune responses to highly delicate and vital organ systems. These include the visual, central nervous, and reproductive (testis and pregnant uterus) 12, 13 systems. Certain mechanisms of immune privilege vary between these systems, and, as seen in the eye (Fig 1), even vary across different compartments within a given system14, 15. Some facets of immune privilege have also been recognized in the pathogenesis of certain diseases, including the immune evasion in tumor settings or by pathogens 16, 17. Thus, this area of research has broad implications in health and disease, as previously reviewed by Niederkorn 18.
Figure 1. Immune privilege sites of the eye.
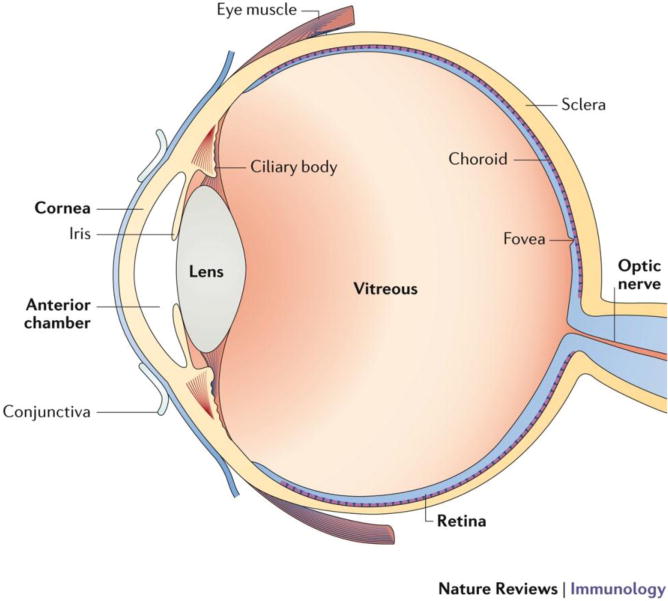
The eye is divided into an anterior and a posterior segment. The anterior segment includes the cornea, anterior chamber, iris, and lens. The posterior segment includes the vitreous, retina, and choroid. Immune privileged sites and tissues of the eye include the cornea, anterior chamber, lens, vitreous, retina, and subretinal space. MPS cell types in the eye include: Cornea (DCs and macrophages 29); iris (DCs and macrophages 30); cilliary body (macrophages 30); choroid (macrophages 30); retina (microglia 119).
A separate, but also important, problem involves potential specialized functions for distinct MPS populations in certain neurodegenerative diseases of the CNS, including in the retina (Fig 1). Although immune privilege (or loss thereof) has been related to the pathogenesis of neurodegeneration, and has been addressed elsewhere 19, 20, 21, 22, 23, 24, we do not focus on this area here. Instead, we examine recent findings establishing that monocyte-derived cells are recruited to the CNS parenchyma in disease and are derived from the bone marrow 25, 26, whereas adult microglia are largely derived from erythro-myeloid progenitors formed during primitive hematopoiesis 5, 6, 7, 8, 10, 27. More specifically, we will evaluate the question of whether these cells are intrinsically different at a functional level given their distinct ontogenies 9.
In the visual system, which we concentrate on here, both the themes of immune privilege and neurodegeneration have been extensively studied. In this Review, we first explore newly appreciated pathways that may explain how MPS cells activate adaptive immune networks with respect to the immune privileged cornea and retina. We then shift topics, to examine recent findings regarding MPS cell function in neurodegenerative diseases of the retina, such as glaucoma, retinitis pigmentosa, and age-related macular degeneration (AMD). Both parts of the Review also draw parallels to new findings concerning MPS biology in the CNS and periphery.
Eye immune privilege breach and the MPS
The concept of immune privilege has been revised in recent years, as highlighted in Box 1. New questions have arisen regarding how MPS cells function in immune privileged sites. On the one hand, subverting immune privilege is permissive to autoimmunity, as recently reviewed in Ref 28; on the other hand, subversion promotes immune defenses that are needed in the context of infectious diseases. Recent findings in the eye suggest several ways in which DCs may accomplish this feat in certain compartments of the eye. The types of DCs and macrophages that are harbored in the cornea have been reviewed elsewhere 29, 30. Additionally, note, that not all insults to the eye result in a permanent loss to immune privilege14, 31, but we do not directly address this area here. Instead, we concentrate on novel concepts that include lymphatic vessel outgrowth (that is, lymphangiogenesis) into the immune privileged cornea (which is not part of the CNS), and peripheral nerve signals that are capable of causing a breach in this immune privilege. Finally, we also review recent data implicating gut commensal microorganisms in T cell activation and consequent autoimmune attack of the retina (which is part of the CNS). We discuss the role of DCs in these contexts and the homeostatic and pathological implications in humans.
Box 1. Evolution of immune privilege.
Sir Peter Medawar coined the term ‘immune privilege’ in the 1940s, using it in the context of transplant immunology. In contrast to allogeneic grafts placed within conventional sites like the skin, those placed in the brain parenchyma or the eye anterior chamber were protected from the fate of immune rejection for extraordinarily long intervals or even indefinitely, in the absence of neovascularization 138. He also made the observation that privileged sites lacked the presence of patent lymphatic vessels, which at the time explained his theory for how these sites were afforded “privilege” from certain adaptive immune responses. New findings, however, suggest that non-conventional pathways of drainage permit antigen from these sites to access lymphatic networks or the spleen 34, 36, 78, 139, 140. Yet still, this level of access is limited 38 and thought to be preferential to activation of regulatory immune networks 141, such as anterior chamber associated immune deviation (ACAID) described by Wayne Streilein and colleagues 11. Furthermore, innate immune responses are suppressed in immune privileged sites 142, and breach of the blood brain barrier by T cells is relatively limited in the steady state 15.
Lymphangiogenesis overcomes immune privilege in the cornea
While there is a paucity of lymphatics within immune privileged sites (except for the testis 32) under normal physiological conditions, access to blood vessels or drainage pathways in lymph vessels of adjacent structures has been recognized, as recently reviewed 14. Examples include drainage from: cerebrospinal fluid through meningeal lymphatics 33, 34; eye anterior chamber into extraorbital lymphatic vessels 35; and the maternal–fetal interface into uterine lymphatics 36, 37. Hence, we use the term ‘pauci-lymphatic’ as a designation for such sites. The cornea is one such example of a pauci-lymphatic 38, immune privileged site 39. Specifically, the cornea proper is completely avascular, whereas the limbus (the very periphery of the cornea) is amply vascularized (Fig 2).
Figure 2. Pauci-lymphatic state and corneal immune privilege.
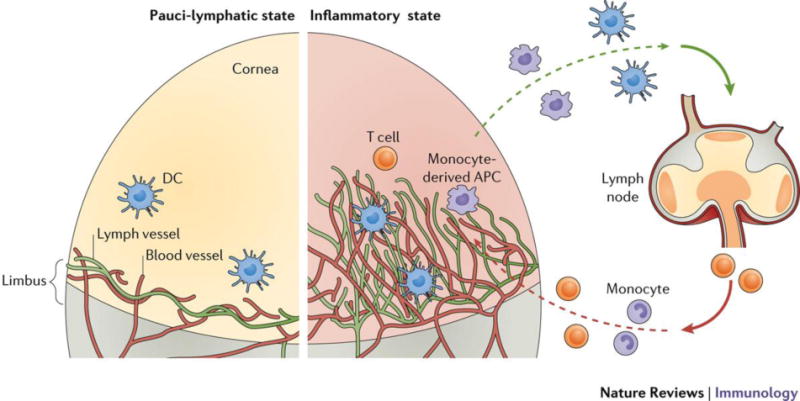
Under normal physiological conditions, drainage is limited, as lymphatic (and blood) vessels are restricted to the limbus. Several key factors have been identified that retain the cornea in this pauci-lymphatic state (see text). These mechanisms are subverted in certain inflammatory settings, and lymphangiogenesis results in lymphatic vessel invasion of the cornea. The latter is associated with amplification of DC egress and consequent T cell activation in the draining LN.
Numerous mechanisms that maintain this ‘angiogenic privilege’ 40 have been defined, such as the repression of certain pro-lymphangiogenic factors. One example is via suppression of vascular endothelial growth factor C (VEGF-C) expression, mediated in part by membrane type 1-matrix metalloproteinase signaling that suppresses macrophage production of VEGF-C 41. Likewise, the matricellular protein thrombospondin 1 (TSP1) also suppresses macrophage expression of VEGF-C, as described by Masli and colleagues 42. Another mechanism involves inhibiting the expression of the VEGF-C receptor, VEGFR2, which is mediated in part through forkhead box protein C1 (FOXC1) – a member of the large Forkhead box transcription factor family involved in vascular development 43. Finally, VEGF-C decoy receptors also inhibit lymphangiogenesis by sequestering VEGF-C away from lymphatic endothelial cells 44, 45.
Subversion of these inhibitory processes occurs most notably under certain inflammatory settings, as we have shown in the allergic eye disease (AED) model 46, 47 and others have shown in models of corneal transplantation 48, HSV-1 keratitis 49, and dry eye disease 50. In such settings, an outgrowth of lymphatics from the limbus invades the cornea (Fig 2). In some settings, there is also a concurrent invasion of blood vessels (Fig 2). For these reasons the cornea has been a widely utilized model site to study the mechanisms underpinning pathologic neovascularization – a noteworthy example is the work in tumor angiogenesis by Judah Folkman 51. To overcome the aforementioned inhibitory activities in the cornea, certain factors are switched on 52. Such factors, which are expressed during inflammation, include the carbohydrate-binding protein galectin-8 53 and interleukin-17 (IL-17) 50, and contribute to corneal lymphangiogenesis through lymphatic endothelial cell sprouting and proliferation, respectively. Additional factors involved in overcoming immune privilege in the cornea have been reviewed elsewhere 54.
Evidence of limited drainage in the cornea was shown by Collin.38 With respect to restricted migration of antigen-laden DCs to the lymph node, these ideas are largely derived from observations in the inflammatory lymphangiogenic setting 55, where their ability to migrate and consequently activate T cells is augmented. The latter was demonstrated in the context of abrogated immune privilege in the corneal transplant model 56. However, the isolated role of lymphangiogenesis might not be identified in this system because inflammation also results in increased frequencies of DC maturation and egress into the lymphatics 57, 58, as well as recruitment of monocyte-derived cells 59. One study demonstrated that lymphangiogenesis can be experimentally inhibited (using an integrin inhibitor) in the presence of corneal inflammation 60. In so doing, the authors observed that consequent T cell activation is reduced despite the abrogation of corneal immune privilege 60. However, the authors did not quantify DC migration to the lymph node. Other evidence that corneal lymphangiogenesis, and possible migration of DCs, may lead to a breach in immune privilege can be garnered from reports showing the presence of corneal autoimmune T cell responses associated with lymphangiogenesis 61. Examples were demonstrated in a Sjogren’s syndrome-like model of corneal disease, and in models of dry eye disease 42, 46, 62, 63, 64.
In short, significant advances have been made in understanding how the immune privileged cornea maintains its pauci-lymphatic state, and how its subversion is associated with augmented DC migration and T cell responses. However, current methods used to stimulate lymphangiogenesis require an inflammatory insult, which in and of itself may trigger augmented DC migration. Work from our group and others helped to reveal the role of CC-chemokine receptor 7 (CCR7) in migration of corneal DCs to the lymph nodes 29, 46, 65, 66, 67, 68, which could be another way that DC migration may be regulated independently of lymphangiogenesis 66. Corneal abrasion was also demonstrated to increase antigen drainage to the lymph nodes, signifying a key role for the epithelium in maintaining immune privilege 69. Finally, the relative contribution of other factors, such as neuropeptides 70, and corneal nerves in immune privilege is also important, as we discuss in the next section.
Peripheral nerve regulation in breaching corneal immune privilege
Understanding how immune responses are altered through the hardwiring of the nervous system’s reflex arcs is an emerging field,71, 72, 73 and recent evidence suggests a role for this process in breaching immune privilege. This hard-wired activity can facilitate rapid adaptation to environmental perturbations for maintenance of tissue homeostasis. It was recently shown how this neuro-immune reflex in the gut lumen regulates macrophage function distally in the muscularis externa, as pathogens activate extrinsic sympathetic neurons that innervate this site 72. With respect to the eye, another fascinating study revealed the possible existence of a putative neuro-immune reflex capable of breaching corneal immune privilege 74. The authors showed that a certain nerve injury in one cornea causes the loss of immune privilege in the fellow cornea, and showed further data possibly suggesting that breach of immune privilege in both eyes is coupled through such a reflex response (Fig 3).
Figure 3. Putative neural response in bilateral breach of corneal immune privilege.
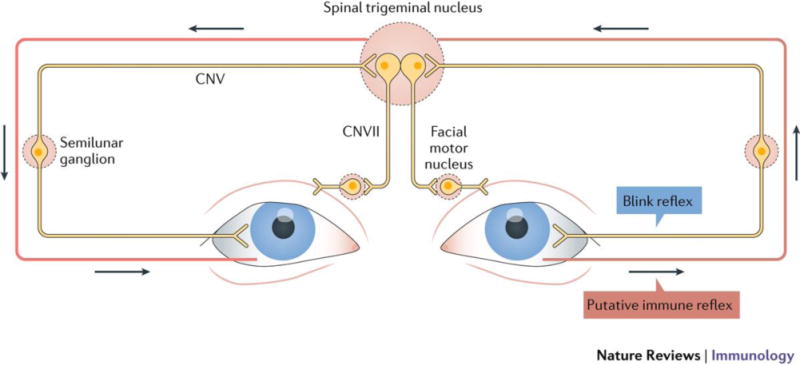
The somatosensory nerves of the blink reflex arc (outlined in grey) may serve as the conduit for bilateral breach in corneal immune privilege (outlined in red). The blink reflex arc is initiated from corneal stimulation such as touch, which signals through cranial nerves (CN), V to VII via the trigeminal nucleus, resulting in blinking in both eyes. Severing corneal nerves in a certain manner in one eye results in a breach of corneal immune privilege in the fellow eye, which is mediated by substance P. Breach in immune privilege through this pathway may be beneficial in the setting of infection, where infection in one eye may prepare the bilateral eye by allowing it to mount an adaptive immune response.
Authors of this cornea study took advantage of the unique acceptance rates in the mouse corneal transplant model 75 to examine the loss of immune privilege. Certain fully MHC mismatched donor cornea to host mouse combinations enjoy a spontaneous 50% acceptance rate, which is attributed to immune privilege. By contrast, the same donor-to-host combination placed in conventional sites, like the skin, yields 0% acceptance. Corneal immune privilege also exists in humans, as corneal transplantation is the only type of solid organ/tissue graft where HLA tissue typing is not standardly performed 76. When the authors severed the nerves in a certain manner in one cornea, the expected spontaneous graft acceptance in the fellow eye was abolished in mice, which was associated with increased allogeneic T cell activity. Hence, severing corneal nerves in one eye breaches immune privilege of the unmanipulated contralateral cornea. A similar finding was corroborated by a study involving laser burn injury to the retina in mice 77. Intriguingly, there is an analogous human condition called sympathetic ophthalmia, in that a penetrating injury to one eye results in a uveitic inflammation in both eyes 78, although a neurogenic etiology for sympathetic ophthalmia has not been established.
Multiple lines of evidence suggest that this bilateral immune privilege breach of the cornea is a neuro-immune reflex. First, the factor required to cause this response was shown to be the neuropeptide, substance P74. Second, elevated levels of substance P were detected in the contralateral eye more than twelve days following the severing procedure, whereas increased neuropeptide in the serum was only detectable for several days. Thus, rather than a possible role for systemic or circulating substance P, the data suggest that the nerves in the unmanipulated contralateral cornea are the source of this neuropeptide. If true, it may implicate a putative reflex that may travel via afferent axons, perhaps by way of the trigeminal ganglia (Fig 3). Intriguingly, this response does not follow normal circuitry, a previously recognized feature reviewed by Tracey 79. However, this specific pathway in the cornea has yet to be definitively proven.
The exact mechanism by which substance P-producing nerves in the contralateral cornea cause augmented T cell responses during immune privilege breach remains an open question. One possibility is that substance P causes qualitative pathogenic changes in corneal mononuclear phagocytes, as we have previously hypothesized 80. For example, it was demonstrated that skin sensory nerves drive dermal DCs to trigger a psoriasiform disease 81. Similarly, another group showed that expression of calcitonin gene-related peptide by nociceptive fibers activated dermal DCs to promote cutaneous candidiasis resistance 82. In the gut, one study identified a microbiota-driven crosstalk between muscularis macrophages and enteric neurons that regulate peristalsis 83. In the eye, a close proximity between nerves and myeloid cells in the cornea has also been documented 80, 84, 85, and recent findings suggest that DC production of ciliary neurotrophic factor contributes to corneal nerve regeneration 84. Hence, it is conceivable that substance P-expressing corneal nerves that cause a breach in immune privilege may do so by altering mononuclear phagocytes in a manner that promotes activation of T cells upon certain provocation.
The possible evolutionary advantage for this putative neuro-immune reflex may be in the augmentation of host immune defense against blinding bilateral corneal infection. Acanthamoeba keratitis causes corneal nerve damage 86, and thus a unilateral infection may trigger an immune privilege breach in the contralateral eye. Such a result would potentially prevent a host from succumbing to bilateral corneal blindness, and there is some evidence in human Acanthamoeba keratitis to support this concept 87. Future work is needed to address such a possibility. Also needing to be resolved is if commensal microorganisms may differentially modulate this response, which we will review in the next section.
The gut microbiota enables evasion of immune privilege in the retina
The underpinning mechanisms for why the gut microbiome has such a profound effect on host physiology is another fascinating and timely area of investigation, and may be relevant to how DCs activate T cell responses that affect the immune privileged retina 88. However, for reasons not understood, the surface of the normal eye has an extraordinarily low density and diversity of commensal bacteria, which is dissimilar to any other barrier site (reviewed in Ref 89). For example, whereas human saliva yields 106−108 CFUs/mL 90, human tear fluid yields as low as 102−103 CFUs/mL 91. Furthermore, Gadjeva pointed out that other work using 16S rRNA sequencing does not indicate whether these data represent live bacterial colonization or instead transiently existing live or dead bacteria 89.
By contrast, the normal gut microflora has been shown to influence retinal health and the CNS at large. Recent evidence suggested that gut microbiota-dependent signals activate Th17 cells specific for the retinal antigen interphotoreceptor retinoid-binding protein (IRBP) that cause experimental autoimmune uveitis (EAU) 92. This particular model results in spontaneous development of uveitis by two months. A similar phenomenon was shown with encephalomyelitic T cells in a model of experimental autoimmune encephalomyelitis (EAE) 93, which is induced by active immunization with the myelin-related antigen myelin oligodendrocyte glycoprotein (MOG). Likewise, it was reported that normal gut flora is associated with increased Th17 cell responses and clinical disease in EAE 94. Interestingly, the latter report showed that the same augmented disease response occurred when the gut flora was monocolonized with the specific commensal, segmented filamentous bacteria (SFB), and thus implicated this bacterium in EAE immunopathogenesis. Similarly, the mouse colony in the EAU study mentioned above harbored SFB 92. Furthermore, R161 T cell receptor transgenic T cells, which are specific for IRBP, were activated independently of their cognate antigen, which could suggest a similar role for SFB. However, the authors found that SFB depletion with the antibiotic vancomycin did not abrogate EAU. Hence, the gut commensal species that produces the signals that lead to the activation of uveitogenic T cells in this model remains unknown.
To summarize, the MPS has various ways in which to activate adaptive immune responses in eye immune privilege. In the cornea, evidence suggests that the presence of lymphangiogenesis amplifies the migration of antigen-laden DCs to the lymph node for consequent activation of T cell responses. Alternatively, (somatosensory) nerves in the cornea can release neuropeptides that breach corneal immune privilege and permit activation of T cells. Potentially, this pathway enables corneal DCs to activate T cells in the lymph node, but the latter needs to be confirmed. Finally, studies mentioned above suggest that commensal-derived antigens from non-privileged distal sites lead to the activation of T cells that can, in turn, attack host cells in immune privileged tissues. The DCs in the gut lamina propria are possibly stimulating these T cells in EAU, but this point also needs to be tested. Additionally, pathogens can stimulate immune responses via innate lymphoid cells or via γδ T cells95. Finally, gut commensals can affect the CNS, via altering microglial activity, which we also do not address here 96,97. However, the focus of the next section is on the role of microglia (as well as monocyte-derived cells) in the context of retinal degenerative diseases.
The MPS and neurodegeneration in the retina
The recent paradigm shift that adult microglia are derived from fetal origins as opposed to adult definitive hematopoiesis is reshaping our knowledge about the isolated role of these cells in neurodegenerative diseases. Within the CNS parenchyma, including the neural retina, microglia comprise the majority of the MPS (Fig 4). Efforts have shifted to better understand the relationship between positive and negative impacts of microglia and monocyte-derived cells in neurodegeneration, including in the retina. Such work may lead to novel therapies to preserve neuronal function through individually targeting these populations 98.
Figure 4. Visual circuit and cell types in the retina.
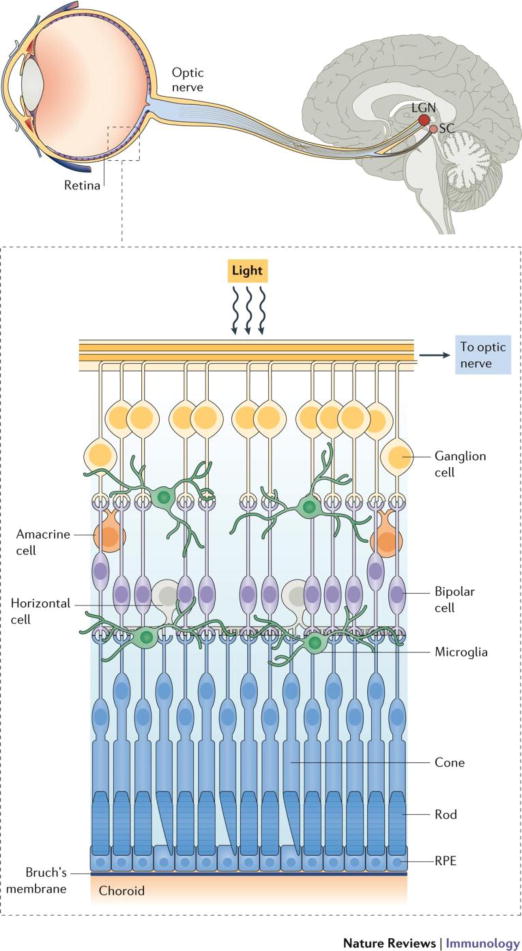
Light travels through the cornea and lens to the retina, where photoreceptor cells (PR) translate light into signals that are relayed from bipolar cells to the retinal ganglion cells (RGC). Axons extend outside of the retina through the optic nerve to the lateral geniculate nucleus (LGN), where the signal is passed to neurons that carry it to the visual cortex. Microglia reside in and extend their processes throughout the synaptic regions in the neural retina. Retinal pigmented epithelium (RPE) is very important for the visual cycle function, and has tight junctions and other factors that aid in the maintenance of retinal immune privilege. The area between the RPE and PRs is designated the subretinal space. Beneath the RPE are the choroid and sclera which comprise the larger outer structure of the eye. In AMD, pathogenesis begins at the level of the choroid, whereas photoreceptors is the primary site of degeneration in retinitis pigmentosa. In contrast, the RGCs and their axons degenerate in glaucoma.
This section examines the role of microglia and monocyte-derived cells in retinal neurodegenerative disease, although other immune and non-immune cells also play a role. We specifically address blinding diseases not widely considered to have an immune etiology. One such disease is glaucoma, which involves axonal degeneration and dropout of retinal ganglion cells (RGCs) (Fig 4), leading to blindness. Also emphasized here is animal work on retinitis pigmentosa, which is an umbrella term for numerous inherited conditions that are characterized by degeneration of photoreceptor cells (Fig 4). We also examine AMD – a condition where clinical disease begins at the level of the choroid, which is just below the neural retina (Fig 4). For these diseases, we review new reports indicating a central role for mononuclear phagocytes in disease pathobiology, and examine the possible distinct roles of microglia and monocyte-derived cells in such settings.
Microglia and the classical complement cascade in RGC degeneration
Our understanding of microglia has evolved tremendously, and in addition to appreciating their roles as immune sentinels of the CNS, we are now aware of their vital role in synaptic maturation and maintenance 99, 100, 101, 102. In health, the involvement of microglia and the classical complement cascade has emerged as a central factor in synaptic plasticity, as investigated in the visual system103. An important study by Barres and colleagues helped initiate this line of research through their demonstration that complement component 1q (C1q), which is the initiating protein in the classical complement cascade, is needed for eliminating inappropriate synaptic connections during postnatal development 104. Stevens and colleagues then went on to show that microglia carry out this elimination process, as demonstrated by examining the anatomical refinement of connections between the retina and geniculate ganglion 105. Specifically, they demonstrated that neuronal derived C1q tags excess synapses and consequently selects them for elimination by microglia. These studies proved that microglia are crucial to maintaining healthy synapse function and identified the mechanism by which this activity occurs at the level of RGCs and retinogeniculate connections (Fig 4).
The classical complement cascade also plays a pathologic role, as recently discovered in certain neurodegenerative diseases. In mice, the healthy adult CNS has a low presence of C1q, but in the adult retina, C1q becomes upregulated and synaptically relocalized in RGCs in DBA/2J mice 104. This strain spontaneously develops a glaucoma-like disease, which includes degeneration of RGCs106, and genetic deletion of C1q helps protect them from this fate 107. A remarkably similar finding in Alzheimer’s disease models was recently discovered108, possibly suggesting parallel mechanisms in glaucoma. Authors showed that C1q associated with synapses before overt plaque deposition and that abrogating the classical complement cascade reduces the extent by which microglia eliminate early synapse loss in the hippocampus 108. A similar mechanism was demonstrated in a neuroinvasive disease mouse model of West Nile virus infection109.
Of note, dysregulation of complement, albeit the alternative cascade, is implicated in AMD - a different neurodegenerative condition110, 111. However pathogenesis in AMD is thought to involve upstream accumulation of pathologic lipid deposits under the retina, as opposed to synapse elimination. This topic has been reviewed elsewhere 112.
These studies underscore a dichotomous nature of microglia. On the one hand, C1q enables microglia to shape healthy neuronal connections in the visual system, while on the other hand, the inappropriate reactivation of C1q in adulthood is suspected to make microglia pathogenic in glaucoma-like neurodegeneration. It is undefined as to whether this mechanism is involved in photoreceptor degeneration, which we will focus on in the next section. In addition, infiltrated monocytes are also involved in retinal degeneration, including in glaucoma models113. Hence, discriminating such recruits versus microglia, and parsing out their respective unique involvements in retinal degeneration, is another important area of research.
Phenotypical differences of microglia vs. monocyte-derived cells in the retina
While it is now understood that microglia have distinct origins from monocyte-derived macrophages that are recruited into the inflamed CNS4, distinguishing between the two populations is technically challenging. Their different origins were demonstrated in a seminal study that used parabiosis to demonstrate that microglia are not replaced by circulating monocytes, but are instead a long-lived, self-renewing population 25, 26. Subsequent findings by another group showed that adult microglia are seeded in utero by yolk sac-derived progenitors5. Of note, this ontogeny is in contrast to the origins of macrophages that reside in the periphery, as macrophage populations from peripheral tissues show mixed ontogenies (that is, they can be comprised of fetal liver-derived monocytes and/or adult bone marrow-derived monocytes)27, 114, 115, 116, 117.
The retina in degenerative conditions is permissive to the recruitment of monocytes, which differentiate into macrophages113, 118, 119, 120, 121. These recruits share phenotypical markers with microglia. Numerous approaches have been devised to study these two populations independently, but many have notable drawbacks (Table 1). These approaches include mice bearing a green fluorescent protein (GFP) reporter gene knockin under the promoter control of the fractalkine receptor CX3CR1 (Cx3cr1GFP/WT), and a red fluorescent protein (RFP) under the chemokine receptor CCR2 promoter (Ccr2RFP/WT). However, microglia and monocyte-derived macrophages are both CX3CR1(+) and CCR2(-)119, and thus this approach cannot faithfully discriminate between the two populations. Another approach utilizes bone marrow chimeras to distinguish between microglia (host-derived) and recruited monocytes (donor-derived). However, the combination of irradiation and bone marrow transplantation (BMT) results in low-grade pathology and the recruitment of monocyte-derived cells (or their precursors) into the retina26, 119, 121, 122. Parabiosis is another approach that can be used to circumvent this problem 26, but drawbacks include partial blood chimerism and technical challenges.
Table 1.
Advantages and disadvantages of numerous mouse models used to study microglia
| Common techniques for studying microglia | Utilization and Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Chimeras | |||
|
| |||
| Bone marrow (BM) chimera |
|
|
26, 119, 121, 122 |
| BM chimera with lead shielding of head |
|
|
119, 122 |
| Parabiosis* |
|
|
25, 26 |
|
| |||
| Null Reporter mice | |||
|
| |||
| CX3CR1eGFP/eGFP or CX3CR1eYFP/eYFP |
|
|
19, 119, 120 |
| CCR2RFP/RFP |
|
|
119, 120, 122 |
|
| |||
| Cre-Lox combinations | |||
|
| |||
| CX3CR1CreER or CX3CR1eYFP-CreER |
|
|
100, 116, 119, 126 |
| CX3CR1CreER; DTA flox |
|
|
128 |
| CX3CR1CreER; DTR flox |
|
|
100 |
Never been done in the retina; only brain and spinal cord
Abbreviations: CCR2, CC-chemokine receptor 2; CNS, central nervous system; CreER, Cre recombinase – estrogen receptor; CX3CR1, CX3C-chemokine receptor 1; DTx, diphtheria toxin; eYP, enhanced yellow fluorescent protein GFP, green fluorescent protein; KO, knockout; MPS, mononuclear phagocyte system; RFP, red fluorescent protein;
Only a Cre-Lox approach has been shown to faithfully identify the two compartments simultaneously, as devised independently by two groups 100, 116, and we subsequently established in the retina119. The strategy utilizes Cx3cr1CreER transgenic mice for conditional Cre-LoxP-mediated modifications of CX3CR1-expressing cells, including microglia, monocytes and macrophages100, 116, 119. The long-lived microglia retain those modifications while short-lived monocytes are replaced by non-modified monocytes within a few weeks (Fig 5). Our group has mated the Cx3cr1YFP-CreER mouse to a R26RFPCre reporter119. After tamoxifen activation of Cre and a short ‘wash out’ period, we were able to faithfully discriminate RFP-labeled microglia from non-labeled monocyte-derived cells in the light-induced model of retinal degeneration. Furthermore, we found that retinal microglia have a unique surface marker phenotype (that is, CD45lo, F4/80lo, CD11clo, MHC class IIlo), which is preserved in the degeneration setting. In contrast, recruited monocyte-derived macrophages are CD45hi, F4/80hi, CD11chi, MHC class IIhi 119, which is consistent with respect to CD45 expression shown in earlier work123. We also identified a small but significant population of MHC class IIhi macrophages in the retina, similar to what was previously shown124. Intriguingly, we showed that these MHC class IIhi cells are long-lived, albeit radiosensitive, macrophages119. The respective roles of microglia versus monocyte-derived macrophages in retinal degenerative are further reviewed in the next section.
Figure 5. Definitive discrimination of microglia vs blood monocytes.
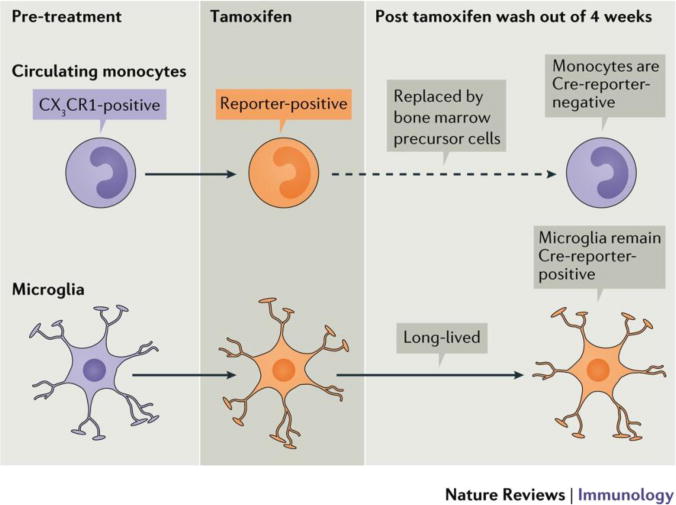
Use of Cx3cr1CreER;Reporterflox (e.g. RFP, DTR, etc.) mice allows faithful visualization of microglia, which requires the following time considerations depicted in this figure. After tamoxifen (Tam) administration, microglia retain reporter expression indefinitely, whereas circulating monocytes retain expression for 4 weeks. We refer to this time-frame as the “wash out“ period, which correlates with monocyte turnover rates from the bone marrow. Hence, faithful reporter visualization in microglia alone requires inclusion of the wash out period.
Microglia and monocyte-derived cells in photoreceptor degeneration
Exploration of the non-redundant contributions of microglia and monocyte-derived cells in neurodegenerative diseases has only recently begun, but early studies suggest important differences125 . In a model of light-induced retinal degeneration, our lab used the Cx3cr1CreER-YFP:R26RFP mouse described above to definitively discriminate RFP-labeled microglia from non-labeled monocyte-derived macrophages119 (Fig 5, Table 1). Interestingly, these two cell types localized to different regions of the photoreceptor cell. Whereas the majority of microglia moved to below the photoreceptor outer segment, monocyte-derived macrophages appeared throughout the depth of the degenerating retina, including next to the photoreceptor soma. Hence, it may be possible that microglia are removing distal debris of the outer segment, whereas monocyte-derived cells may be phagocytosing around the photoreceptor soma; but this remains to be proven. Evidence of differential localization of MPS cells in the spinal cord was also recently demonstrated in EAE. Authors showed that monocyte-derived macrophages made axoglial contact, whereas microglia processed debris at a more distal location125.
The functions of specific MPS populations in retinochoroidal disease was also recently reported126. The authors used a mouse model that mimics certain features of AMD pathogenesis. In this model, a laser burn is administered to rupture Bruch’s membrane, which separates the retinal pigment epithelium (RPE) and the choroid. This perturbation leads to the outgrowth of new blood vessels and vascular leakage into the retina, similar to what is seen in neovascular AMD. The authors used Cx3cr1CreER mice to delete the floxed gene for the interferon-α/β receptor (Ifnar1) prior to laser injury in retinochoroidal macrophages, which include retinal microglia and putative CX3CR1+ macrophages in the choroid. Strikingly, the severity of choroidal neovascular lesions and vascular leakage was worsened by conditional deletion of IFNAR1 in retinochoroidal macrophages, suggesting a central protective role for IFNAR1 signaling. While these findings are important, it was not determined whether the effect was due to conditional loss of IFNAR1 in microglia, in choroidal macrophages or from both populations (Table 1). This question is significant because the injury originates at the choroid and consequent vascular leakage is due to choroidal neovessels. Regarding human disease, while IFNα2 therapy has been tried in patients with AMD with little success 127, not much is known about IFNβ therapy in this regard.
With respect to retinitis pigmentosa, it was shown that MPS cells actively phagocytose injured photoreceptors128. The authors generated a Cx3cr1CreER;R26DTA (diphtheria toxin A) mouse for tamoxifen-induced selective depletion of all CX3CR1+ cells, which includes microglia and monocyte-derived cells (Table 1). This transgene combination was bred onto mice homozygous for the retinal degeneration 10 (rd10) mutation of phosphodiesterase 6B (Pde6b), which develop photoreceptor degeneration within the first three weeks of life. With continuous depletion of CX3CR1+ phagocytes, mice showed delayed and reduced degeneration out to post-natal day 50. However, methods used could not discriminate whether microglia and/or monocyte-derived cells were the effector phagocytes because both populations would be depleted in this system (Table 1). Furthermore, depletion was not started until post-natal day 21, a time when monocyte recruitment presumably had already begun since retinal levels of CCL2 (which signals via CCR2 to promote monocyte recruitment) are significant at this time-point129. Nonetheless, these findings suggest that the mononuclear phagocytes have important pathogenic roles in certain forms of inherited degeneration.
Other reports suggest an isolated contribution of monocyte-derived cells to the pathobiology of photoreceptor degeneration in mouse models, although the effector mechanisms are not completely understood. It was demonstrated that monocyte-derived cells, recruited through CCR2, contribute to the spontaneous age-related photoreceptor degeneration that occurs in CX3CR1-deficient mice 120, and a similar pathway was also found to be relevant in human AMD and photoreceptor degeneration 130. Likewise, another study showed that CCR2-deficient mice bred onto Pde6brd10 mice reduced photoreceptor degeneration 129, although the effect was less dramatic than what was seen in the aforementioned report using Cx3cr1CreER;R26DTA mice 128.
Finally, the type 2 cytokine, IL-33, has recently emerged in the literature as a player in retinal degenerative disease. New findings suggest that pathogenic monocyte recruitment in photoreceptor degeneration is mediated by IL-33, which is produced by Müller glia 131. For reasons not fully understood, another group found that IL-33 was not relevant in recruitment activity, albeit using a different model system in laser induced choroidal neovascularization model. In this system, IL-33 played a central role in regulating pathologic neovascularization at the level of the choroid132. In contrast, IL-33 does have a role in recruitment of monocytes following optic nerve crush in mice 133 (which models aspects of glaucoma), as well as in experimental spinal cord injury. Intriguingly, this monocyte-recruitment activity played a reparative role in the disease setting, as previously shown in a model of glutamate intoxication of RGCs113. Hence, monocyte-derived cells are not universally pathogenic in all forms of retinal degeneration.
To summarize, in the steady state, microglia are important for maintaining healthy synapses. In degenerative diseases, microglia are not always protective and monocyte-derived cells are not always pathogenic. Furthermore, recent findings show that both the local environment and epigenetics shape macrophage identity and function in the steady state134, 135, 136, 137, which may suggest that monocyte-derived cells can take on microglial functionality in neurodegeneration, or vice versa. Hence, future work is required to continue to parse out these different compartments, and to help determine if and how therapies can be designed to specifically target either cell types 98.
Concluding remarks
The advent of new techniques for assessing the ontogeny of MPS cells has increased our understanding of the roles of these cells in breaching immune privilege and in the pathobiology of neurodegenerative disease. Ontogeny research has helped show that DCs are indeed a unique branch of the MPS, and in turn will facilitate the discovery of DC-specific mechanisms in the formation of adaptive immunity relevant to privileged sites, including the cornea and retina. Ontogeny studies have also helped distinguish microglia from monocyte-derived cells and the non-redundant functions of these cell types in neurodegeneration (including in that of the retina) are now being addressed. Though complexities and open questions still remain, the reciprocal relationship between research advances in ontogeny and function, as outlined here, is augmenting our understanding of the MPS. In so doing, the vast implications of these cells in health and disease promises to become fully realized, for the visual system and throughout the human body.
Key points.
Pathological lymphangiogenesis of the adult cornea is associated with breach of corneal immune privilege, and is permissive for MPS cells in triggering T cell responses.
Severing corneal nerves in one eye causes a breach in corneal immune privilege in both eyes, suggesting the presence of a neuro-immune reflex that may involve MPS cells.
Commensals in the gut, and perhaps gut MPS cells, are capable of triggering T cells that cause autoimmune of the immune privileged retina.
While microglia play a key role in shaping healthy synapses in the visual system, these cells may also contribute to of retinal ganglion cell dysfunction in glaucoma.
Microglia and monocyte derived cells are both present in the retina in a model of photoreceptor degeneration, and these distinct MPS lineages possess phenotypical differences, as confirmed in Cx3cr1Cre systems.
Early studies using such Cx3cr1Cre systems implicate the possibility for functional specializations of microglia versus monocyte-derived cells in photoreceptor degenerative diseases.
Acknowledgments
Grant Funding: R01 (Saban), RPB (Saban), F32 (Reyes)
Footnotes
Competing interest statement
The authors declare no competing interest.
References
- 1.Ginhoux F, Guilliams M, Naik SH. Editorial: Dendritic Cell and Macrophage Nomenclature and Classification. Frontiers in immunology. 2016;7:168. doi: 10.3389/fimmu.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature reviews Immunology. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nature reviews Immunology. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mildner A, Yona S, Jung S. A close encounter of the third kind: monocyte-derived cells. Adv Immunol. 2013;120:69–103. doi: 10.1016/B978-0-12-417028-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 5.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. This study showed that yolk sac macrophages in utero give rise to microglia in the adult setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeffel G, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nature neuroscience. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 9.Shemer A, Jung S. Differential roles of resident microglia and infiltrating monocytes in murine CNS autoimmunity. Seminars in immunopathology. 2015;37:613–623. doi: 10.1007/s00281-015-0519-z. [DOI] [PubMed] [Google Scholar]
- 10.Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harbor perspectives in biology. 2015;7:a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nature reviews Immunology. 2003;3:879–889. doi: 10.1038/nri1224. This review covers tolerogenic immune networks that play a central role in ocular immune privilege and anteriro associated immmune deveation. [DOI] [PubMed] [Google Scholar]
- 12.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 13.Fijak M, Meinhardt A. The testis in immune privilege. Immunological reviews. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 14.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal immunology. 2008;1:372–381. doi: 10.1038/mi.2008.27. This review covers our recent understanding of immune privilege and the different mechanisms that support this setting in various organ systems. [DOI] [PubMed] [Google Scholar]
- 15.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends in immunology. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nature reviews Immunology. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature immunology. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 19.Combadiere C, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. The Journal of clinical investigation. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo JS, et al. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. The Journal of experimental medicine. 2003;197:1335–1344. doi: 10.1084/jem.20022041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Frontiers in immunology. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Housset M, Sennlaub F. Thrombospondin-1 and Pathogenesis of Age-Related Macular Degeneration. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2015;31:406–412. doi: 10.1089/jop.2015.0023. [DOI] [PubMed] [Google Scholar]
- 23.Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends in immunology. 2015;36:354–363. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamiri P, Sugita S, Streilein JW. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem Immunol Allergy. 2007;92:86–93. doi: 10.1159/000099259. [DOI] [PubMed] [Google Scholar]
- 25.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 26.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nature neuroscience. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 27.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 28.Caspi RR. Ocular autoimmunity: the price of privilege? Immunological reviews. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 29.Saban DR. The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. The ocular surface. 2014;12:87–99. doi: 10.1016/j.jtos.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunological reviews. 2010;234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 31.Lucas K, Karamichos D, Mathew R, Zieske JD, Stein-Streilein J. Retinal laser burn-induced neuropathy leads to substance P-dependent loss of ocular immune privilege. J Immunol. 2012;189:1237–1242. doi: 10.4049/jimmunol.1103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–383. [PMC free article] [PubMed] [Google Scholar]
- 33.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louveau A, et al. Corrigendum: Structural and functional features of central nervous system lymphatic vessels. Nature. 2016;533:278. doi: 10.1038/nature16999. [DOI] [PubMed] [Google Scholar]
- 35.Camelo S, Kezic J, Shanley A, Rigby P, McMenamin PG. Antigen from the anterior chamber of the eye travels in a soluble form to secondary lymphoid organs via lymphatic and vascular routes. Investigative ophthalmology & visual science. 2006;47:1039–1046. doi: 10.1167/iovs.05-1041. [DOI] [PubMed] [Google Scholar]
- 36.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. The Journal of clinical investigation. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collin HB. Lymphatic drainage of 131-I-albumin from the vascularized cornea. Investigative ophthalmology. 1970;9:146–155. This original study characterizes the kinetics of antigen drainage to the lymphatics from the pauci-lymphatic cornea. [PubMed] [Google Scholar]
- 39.Billingham RE, Boswell T. Studies on the problem of corneal homografts. Proceedings of the Royal Society of London Series B, Biological sciences. 1953;141:392–406. doi: 10.1098/rspb.1953.0049. [DOI] [PubMed] [Google Scholar]
- 40.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Current opinion in ophthalmology. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Wong HL, et al. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nature communications. 2016;7:10824. doi: 10.1038/ncomms10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cursiefen C, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. The Journal of experimental medicine. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo S, et al. Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2015–2020. doi: 10.1073/pnas.1109540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albuquerque RJ, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nature medicine. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh N, et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood. 2013;121:4242–4249. doi: 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HS, et al. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Investigative ophthalmology & visual science. 2015;56:3140–3148. doi: 10.1167/iovs.14-16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahadome SD, et al. Classical dendritic cells mediate fibrosis directly via the retinoic acid pathway in severe eye allergy. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cursiefen C, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Investigative ophthalmology & visual science. 2002;43:2127–2135. [PubMed] [Google Scholar]
- 49.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. The Journal of experimental medicine. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chauhan SK, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 52.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. The Journal of clinical investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen WS, et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nature communications. 2016;7:11302. doi: 10.1038/ncomms11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bock F, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Progress in retinal and eye research. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Maruyama K, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. The Journal of clinical investigation. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nature medicine. 2004;10:813–815. doi: 10.1038/nm1078. This study showed that antigen presenting cells from the cornea migrate to the draining lymph node. [DOI] [PubMed] [Google Scholar]
- 57.Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121:1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 58.Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol. 2010;185:4691–4697. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pajoohesh-Ganji A, et al. Partial denervation of sub-basal axons persists following debridement wounds to the mouse cornea. Laboratory investigation; a journal of technical methods and pathology. 2015;95:1305–1318. doi: 10.1038/labinvest.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietrich T, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Investigative ophthalmology & visual science. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 62.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal immunology. 2014;7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gandhi NB, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. Journal of leukocyte biology. 2013;94:1293–1301. doi: 10.1189/jlb.1012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, et al. CD8(+) cells regulate the T helper-17 response in an experimental murine model of Sjogren syndrome. Mucosal immunology. 2014;7:417–427. doi: 10.1038/mi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlereth S, Lee HS, Khandelwal P, Saban DR. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. The American journal of pathology. 2012;180:2351–2360. doi: 10.1016/j.ajpath.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hos D, et al. Blockade of CCR7 leads to decreased dendritic cell migration to draining lymph nodes and promotes graft survival in low-risk corneal transplantation. Experimental eye research. 2016;146:1–6. doi: 10.1016/j.exer.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Hua J, et al. Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis. Investigative ophthalmology & visual science. 2016;57:1457–1467. doi: 10.1167/iovs.15-17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kodati S, et al. CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Investigative ophthalmology & visual science. 2014;55:5871–5877. doi: 10.1167/iovs.14-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang Z, Kuffova L, Liu L, Forrester JV. Soluble antigen traffics rapidly and selectively from the corneal surface to the eye draining lymph node and activates T cells when codelivered with CpG oligonucleotides. Journal of leukocyte biology. 2014;95:431–440. doi: 10.1189/jlb.0612294. [DOI] [PubMed] [Google Scholar]
- 70.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 71.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 72.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiu IM, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paunicka KJ, et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:1490–1501. doi: 10.1111/ajt.13240. This study showed that severing corneal nerves in a certain manner in one eye leads to a bilateral breach in corneal immune privilege. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niederkorn JY. Cornea: Window to Ocular Immunology. Curr Immunol Rev. 2011;7:328–335. doi: 10.2174/157339511796196593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18:162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez Rueda MA, Mathew R, Cho KS, Chen D, Stein-Streilein J. Bilateral Loss of the Ocular Immune Privilege After Optic Nerve Crush in One Eye. IOVS. 2009;50 [Google Scholar]
- 78.Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Frontiers in immunology. 2012;3:338. doi: 10.3389/fimmu.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nature neuroscience. 2017;20:156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 80.Blanco T, Saban DR. The cornea has “the nerve” to encourage immune rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:1453–1454. doi: 10.1111/ajt.13238. This reviews covers the neuro-immune reflex responses in health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riol-Blanco L, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kashem SW, et al. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao N, Yan C, Lee P, Sun H, Yu FS. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. The Journal of clinical investigation. 2016;126:1998–2011. doi: 10.1172/JCI85097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seyed-Razavi Y, Chinnery HR, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investigative ophthalmology & visual science. 2014;55:1313–1320. doi: 10.1167/iovs.13-12995. [DOI] [PubMed] [Google Scholar]
- 86.Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends in parasitology. 2006;22:175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Cruzat A, et al. Contralateral Clinically Unaffected Eyes of Patients With Unilateral Infectious Keratitis Demonstrate a Sympathetic Immune Response. Investigative ophthalmology & visual science. 2015;56:6612–6620. doi: 10.1167/iovs.15-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kugadas A, Gadjeva M. Impact of Microbiome on Ocular Health. The ocular surface. 2016;14:342–349. doi: 10.1016/j.jtos.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Evaldson G, Heimdahl A, Kager L, Nord CE. The normal human anaerobic microflora. Scandinavian journal of infectious diseases Supplementum. 1982;35:9–15. [PubMed] [Google Scholar]
- 91.Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye (Lond) 1991;5(Pt 1):70–74. doi: 10.1038/eye.1991.14. [DOI] [PubMed] [Google Scholar]
- 92.Horai R, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. This study demonstrated that gut commensals result in T cell autoreactivty that cause exeprimental autoimmune uveitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 94.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moser B, Brandes M. Gammadelta T cells: an alternative type of professional APC. Trends in immunology. 2006;27:112–118. doi: 10.1016/j.it.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature neuroscience. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erny D, Hrabe de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2016 doi: 10.1111/imm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biber K, Moller T, Boddeke E, Prinz M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov. 2016;15:110–124. doi: 10.1038/nrd.2015.14. [DOI] [PubMed] [Google Scholar]
- 99.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 100.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 103.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. This study demonstrated the role of C1q in synaptic pruning in development and axonal neurodegeration in the visual system of DBA2/j mice. [DOI] [PubMed] [Google Scholar]
- 105.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. This study demonstrated the role of microglia in C1q mediated pruning of synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anderson MG, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 107.Howell GR, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. The Journal of clinical investigation. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. This study showed that microglia mediated elimination of synapses is involved in the pathogeneisis of Alzheimers in mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vasek MJ, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hageman GS, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E3040–3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding JD, et al. The role of complement dysregulation in AMD mouse models. Adv Exp Med Biol. 2014;801:213–219. doi: 10.1007/978-1-4614-3209-8_28. [DOI] [PubMed] [Google Scholar]
- 113.London A, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. The Journal of experimental medicine. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 115.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. The Journal of experimental medicine. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scott CL, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nature communications. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caicedo A, Espinosa-Heidmann DG, Pina Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Experimental eye research. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 119.O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Scientific reports. 2016;6:20636. doi: 10.1038/srep20636. This study showed that microglia and monocyte derived macrophages are both definitively present in a retinal degeneration model, and characterized their phenotypical differences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sennlaub F, et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5:1775–1793. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- 122.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nature neuroscience. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 123.Sedgwick JD, et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dick AD, Ford AL, Forrester JV, Sedgwick JD. Flow cytometric identification of a minority population of MHC class II positive cells in the normal rat retina distinct from CD45lowCD11b/c+CD4low parenchymal microglia. The British journal of ophthalmology. 1995;79:834–840. doi: 10.1136/bjo.79.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. The Journal of experimental medicine. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luckoff A, et al. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol Med. 2016;8:670–678. doi: 10.15252/emmm.201505994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Interferon alfa-2a is ineffective for patients with choroidal neovascularization secondary to age-related macular degeneration. Results of a prospective randomized placebo-controlled clinical trial. Pharmacological Therapy for Macular Degeneration Study Group. Arch Ophthalmol. 1997;115:865–872. doi: 10.1001/archopht.1997.01100160035005. [DOI] [PubMed] [Google Scholar]
- 128.Zhao L, et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015;7:1179–1197. doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo C, et al. Knockout of ccr2 alleviates photoreceptor cell death in a model of retinitis pigmentosa. Experimental eye research. 2012;104:39–47. doi: 10.1016/j.exer.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 130.Eandi CM, et al. Subretinal mononuclear phagocytes induce cone segment loss via IL-1beta. Elife. 2016;5 doi: 10.7554/eLife.16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xi H, et al. IL-33 amplifies an innate immune response in the degenerating retina. The Journal of experimental medicine. 2016;213:189–207. doi: 10.1084/jem.20150894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Theodoropoulou S, et al. Interleukin-33 regulates tissue remodelling and inhibits angiogenesis in the eye. J Pathol. 2017;241:45–56. doi: 10.1002/path.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 134.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nature immunology. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 135.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van de Laar L, et al. Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity. 2016;44:755–768. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 137.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British journal of experimental pathology. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 139.Kaur G, Mital P, Dufour JM. Testisimmune privilege - Assumptions versus facts. Animal reproduction/Colegio Brasileiro de Reproducao Animal. 2013;10:3–15. [PMC free article] [PubMed] [Google Scholar]
- 140.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. The Journal of experimental medicine. 1981;153:1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin HH, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. The Journal of experimental medicine. 2005;201:1615–1625. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Streilein JW, Stein-Streilein J. Does innate immune privilege exist? Journal of leukocyte biology. 2000;67:479–487. doi: 10.1002/jlb.67.4.479. [DOI] [PubMed] [Google Scholar]


