Abstract
Aims
We tested the hypothesis that CB1/CB2 receptor double knockout would produce significant increases in infarct size and volume and significant worsening in clinical score, using two mouse models, one of permanent ischemia and one of ischemia/reperfusion.
Main methods
Focal cerebral infarcts were created using either photo induced permanent injury or transient middle cerebral artery occlusion. Infarct volume and motor function were evaluated in cannabinoid receptor 1/ cannabinoid receptor 2 double knockout mice.
Key Findings
The results surprisingly revealed that CB1/CB2 double knockout mice showed improved outcomes, with the most improvements in the mouse model of permanent ischemia.
Significance
Although the number of individuals suffering from stroke in the United States and worldwide will continue to grow, therapeutic intervention for treatment following stroke remains frustratingly limited. Both the cannabinoid 1 receptor (CB1R) and the cannabinoid 2 receptor (CB2R) have been studied in relationship to stroke. Deletion of the CB2R has been shown to worsen outcome, while selective CB2R agonists have been demonstrated to be neuroprotective following stroke. Although initial studies of CB1R knockout mice demonstrated increased injury following stroke, indicating that activation of the CB1R was neuroprotective, later studies of selective antagonists of the CB1R also demonstrated a protective effect. Surprisingly the double knockout animals had improved outcome. Since the phenotype of the double knockout is not dramatically changed, significant changes in the contribution of other homeostatic pathways in compensation for the loss of these two important receptors may explain these apparently contradictory results.
Keywords: Stroke, cannabinoid1 receptor, cannabinoid 2 receptor, double knockouts, photo injury, transient middle artery occlusion
INTRODUCTION
Although the number of individuals suffering from stroke in the United States and worldwide will continue to grow, therapeutic intervention for treatment following stroke remains frustratingly limited. Stroke is the third leading cause of death and the primary cause of adult disability in the industrial world1,2. The development of novel therapies to treat the secondary expansion of damage following stroke are desperately needed. It has been clearly demonstrated that inflammation following stroke is both a component of secondary injury and of healing3,4. Therefore modulation of inflammatory changes resulting from stroke represents an attractive target for treatment.
Treatment with cannabinoids has been proposed as a potential way to beneficially modify inflammatory changes following stroke5–7. The endocannabinoid system, comprised of the enzymes responsible for the production of endogenous cannabinoids, cannabinoid receptors, and the enzymes responsible for the degradation of the endogenous cannabinoids have all been viewed as potential therapeutic targets for the treatment of stroke8,9. Both the cannabinoid 1 receptor (CB1R) and the cannabinoid 2 receptor (CB2R) have been studied in relationship to stroke. The CB1R, primarily located on neurons, inhibits synaptic transmission. Endogenous cannabinoids released from the post-synaptic terminal serve as retrograde neurotransmitters for this receptor. The CB2 receptor is primarily located on cells involved in inflammatory responses10–12. While it is clear that there are alterations of the endocannabinoid system following stroke, and that selective agonists and antagonists of cannabinoid receptors can influence outcome following stroke, our understanding of these changes and possibilities is far from complete. Deletion of the CB2R has been shown to worsen outcome, while selective CB2R agonists have been demonstrated to be neuroprotective following stroke5,7.
Although initial studies of CB1R knockout mice demonstrated increased injury following stroke, indicating that activation of the CB1R was neuroprotective, later studies of selective antagonists of the CB1R also demonstrated a protective effect13–16. As a result of conflicting reports, a better understanding of the effects of deletion of the cannabinoid receptors is needed. A complication to interpretation of results from knockout studies is the ever present problem that permanent deletion of a receptor almost invariably results in other compensatory changes, especially when involving receptors that are as important for homeostasis as the cannabinoid receptors. However, based on the current literature, it would be predicted that the effect of double CB1/CB2 receptor knockout would be as or more deleterious than either knockout alone.
In the present set of experiments, we tested the hypothesis that CB1/CB2 receptor double knockout would produce significant increases in infarct size and volume and significant decrease in clinical score. We tested this hypothesis using two mouse models of stroke, the middle cerebral artery occlusion model (MCAO) of transient ischemia/reperfusion, and the photothrombosis model of permanent ischemia.
METHODS
Animals
Male wild type and CB1 receptor/CB2 double knockout mice (CB1 -/- CB2 -/-) generated on a full C57Bl/6 background were used. The mice used in the photothrombotic studies were 11 weeks old and weighed 27–30 grams. The cerebral ischemia studies were conducted on 7 to 8-week-old mice weighing 18–23 grams. Wild type and CB1/CB2 receptor knockout mice were age matched littermates obtained from the Center for Substance Abuse Research (CSAR) Breeding Core at Temple University. Studies were conducted in accordance with the guidelines approved by the Institute for Animal Care and Use Committee at Temple University as well as the National Institute of Health Guidelines. Animals were maintained under a 12-h light/dark cycle on a regular chow diet and had access to food and water ad libitum before and after the procedure. The experimenters were blinded to the treatment groups throughout the experiments.
Prior to inducing stroke, the animals were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg) (1:1) at a volume of 1 mL/kg. Prior to any intervention, an appropriate anesthetic plane was confirmed by regular respiration, lack of response to toe pinch, and lack of response to corneal touch, and anesthetized body temperature was maintained at 37°C ± 0.5°C using heating lamps and a heating pad.
Photothrombotic cerebral ischemia
The technique was carried out as described by Kleinshnitz et al 17. Briefly, 0.1 mL of Rose Bengal (Sigma Aldrich) 10 mg/mL dissolved in 0.9% saline was injected intraperitoneally. The fur on the head was clipped and the skin and periosteum covering the right parietal bone were removed and the head secured in position. A cold light source was positioned over the exposed area, and 7 minutes after the injection of dye, the light was activated. After 20 minutes of illumination at approximately 28,000 lux, the mouse was returned to its home cage.
Middle Cerebral Artery Occlusion and Reperfusion (MCAO/R) and Cerebral Blood Flow Monitoring (CBF)
After ensuring each animal was brought to an appropriate anesthetic plane, the skin on the dorsal aspect of the head was retracted and the periosteum removed. An ink mark 2 mm poster and 4 mm lateral to Bregma was placed over the right parietal bone. Body temperature was maintained at 37°C ± 0.5°C with heating lamps and a heating pad during the period of occlusion. Middle cerebral artery occlusion (MCAO) was induced with the intraluminal filament method and slight modifications7,14,18. Briefly, after securing the animal in place, a midline incision was made on the ventral aspect of the neck. Using an operating microscope, the submaxillary glands were located and retracted to allow a clear visualization of the trachea and surrounding anatomy. The right external carotid artery (ECA) was identified, ligated with 6-0 silk suture, and cauterized distal to the bifurcation of the common carotid artery (CCA) into the ECA and internal carotid artery (ICA). Another 6-0 silk suture was tied around the right ICA loosely. A third 6-0 silk suture was tied around the right ECA proximal to the point of cauterization. The vagus nerve was separated from the CCA with care taken to not damage it.
A laserPro Blood Perfusion Monitor (TSI, Inc., Shoreview, MN, USA) was employed to monitor regional cerebral blood flow (rCBF) before ischemia and during MCAO. A 1-mm diameter microfiber laser-Doppler probe was aligned so that it covered the mark previously indicated on the parietal bone. Baseline rCBF readings were collected. The second suture placed around the ICA was tightened. With a microvascular clamp, the CCA was clamped. A 30-gauge needle made a small incision in the ECA. A blunted 5-0 monofilament nylon suture coated with poly-L-lysine (0.1% in deionized water, Sigma Inc., St. Louis, MO USA) was inserted into the ECA, advanced into the Circle of Willis, and finally, to the origin of the middle cerebral artery (MCA) 19. Slight resistance upon advancing indicated it was in the proper position. The third suture was secured around the ECA to prevent the suture from dislodging and preclude backflow. The MCAO was considered adequate if rCBF showed a sharp decrease to 25% of baseline levels 20. After 50 minutes, the nylon suture was withdrawn and the ECA permanently tied and cauterized. Reperfusion was confirmed when pulsations were again observed in the ICA.
Neurological Evaluation
The severity of neurological deficits was evaluated 24 hours after the ischemic insult using a five point deficit score. The scale utilized the following criteria adapted from Hata: 0 = normal motor function, 1 = flexion of torso and of contralateral forelimb on lifting of the animal by the tail, 2 = circling but normal posture at rest, 3 = leaning while at rest, 4 = no spontaneous motor activity or lateral rolling18.
Infarct Volume Assessment
Animals were euthanized with an overdose of pentobarbital (200 mg/kg intraperitoneal) 24hr after cerebral ischemia. Brains were submerged in cold PBS briefly and then cut into 6 2 mm coronal sections using a mouse brain matrix (Zivic Lab, Pittsburgh, PA, USA). The brain sections were placed in 2% triphenyltetrazolium chloride (Sigma, Inc) dissolved in saline and stained for 5 minutes at 37°C in the dark. The brain sections were fixed in 4% paraformaldehyde at 4°C for 24hr. Next, the anterior and posterior face of each section was scanned by a flatbed color scanner (Microtek Inc., Carson, CA USA). Images were saved as JPEG files and analyzed with Image-J Software (NIH). The infarct volumes were expressed as mm3as well as the percentage of overall brain tissue after correcting for edema using the following formulas
Statistical analyses
Data were analyzed using either Student’s unpaired t-tests or two-way ANOVA using Graph Pad Prism version 6.
Results
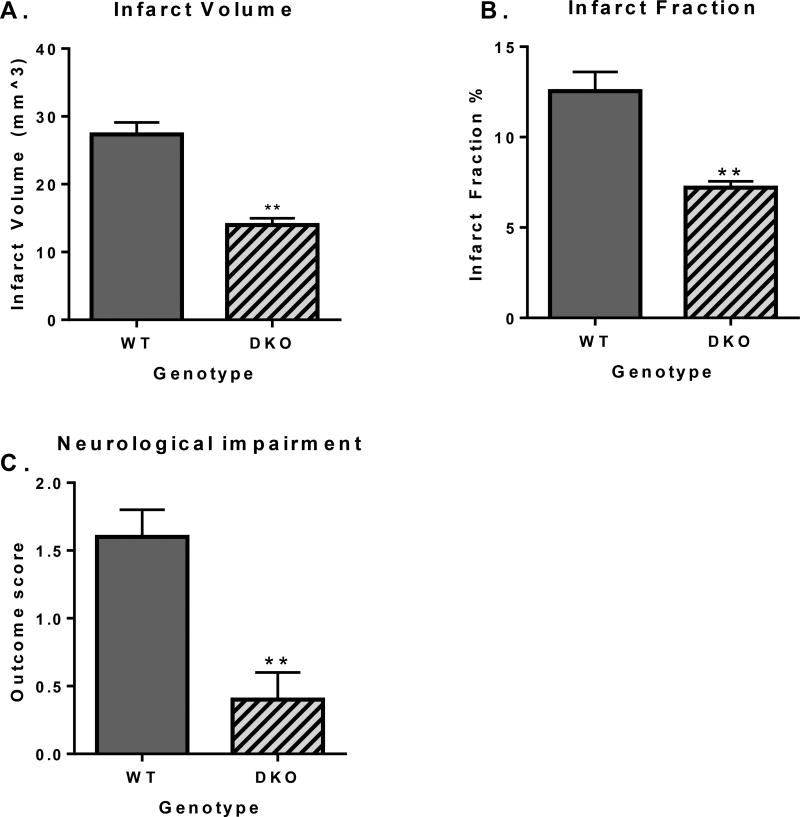
Student’s unpaired t-test showed a significant decrease in infarct volume (p = 0.0002) and infarct fraction (p = 0.001), as well as a lower clinical score (p = 0.003) in CB1/CB2 DKOs as compared with wild type controls, indicative of improved outcome in the DKOs following photoinjury (Figure 1).
Figure 1.
Effect of CB1/CB2 double knockout on infarct volume, infarct fraction, and clinical score in a mouse model of permanent ischemia (photoinjury). A: X axis: genotype, Y axis, infarct volume in mm3. Double knockout (DKO) mice show significantly smaller infarct volume as compared to wild type controls (p = 0.0002). B: X axis: genotype, Y axis, infarct fraction as a percent of the whole ipsilateral hemisphere. DKO mice show significantly smaller infarct fraction as compared to wild type controls (p = 0.0014). C: X axis: genotype, Y axis, outcome of the neurological assessment (a higher score indicates more severe impairment). DKO mice show significantly improved neurological performance compared to wild type controls (p = 0.003).
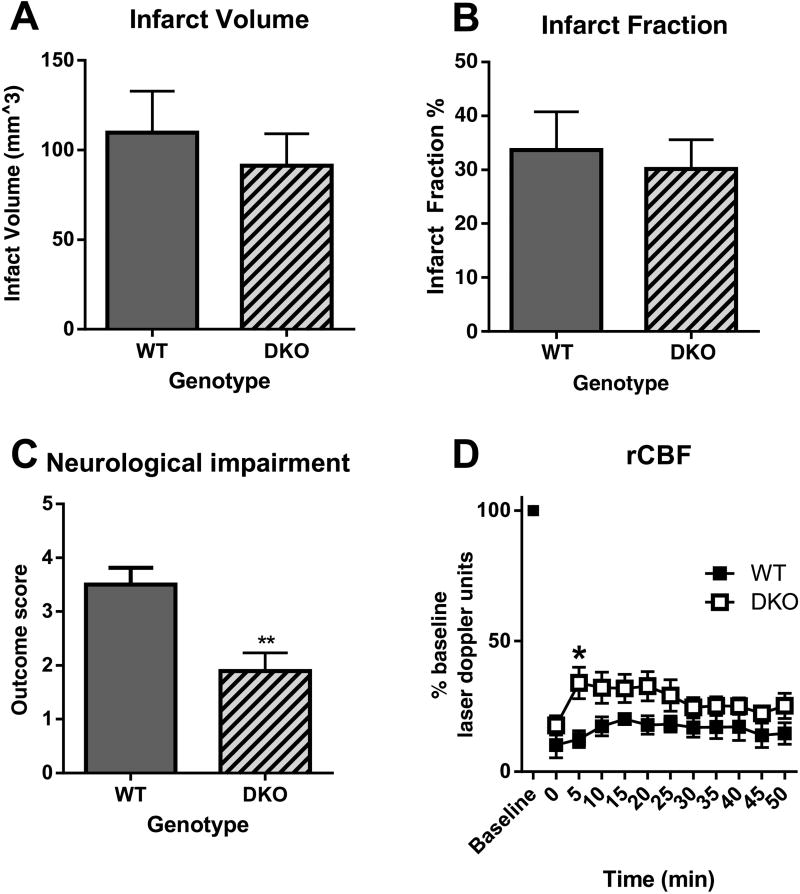
Student’s unpaired t-test showed no significant decrease in infarct volume or infarct fraction, but a significant improvement of clinical score (p = 0.008) in CB1/CB2 DKOs as compared with wild type controls (Figures 2A-C). Furthermore, two-way ANOVA with repeated measures showed there was a significant main effect of genotype on rCBF, as flow was significantly improved in the DKOs as compared to the wild type controls [F (10, 88) = 36.25, p < 0.0001]. Sidak’s multiple comparisons test showed a significant different within the second 5 minutes of occlusion between wildtype and CB1/CB2 DKO mice. There was no significant main effect of time and no significant interaction (Figure 2D).
Figure 2.
Effect of CB1/CB2 double knockout on infarct volume, infarct fraction, clinical score, and regional cerebral blood flow in a mouse model of transient ischemia/reperfusion (MCAO). A: X axis: genotype, Y axis, infarct volume in mm3. Double knockout (DKO) are not significantly different compared to wild type controls. B: X axis: genotype, Y axis, infarct fraction as a percent of the whole ipsilateral hemisphere. DKO mice are not significantly different as compared to wild type controls (p = 0.0014). C: X axis: genotype, Y axis, outcome of the neurological assessment (a higher score indicates more severe impairment). DKO mice show significantly improved neurological performance compared to wild type controls (p = 0.008). D: X axis, time following middle cerebral artery occlusion, Y axis, % baseline flow measured in laser Doppler units. Double knockout (DKO) mice show significantly improved neurological performance compared to wild type controls (two way ANOVA significant main effect of genotype F= 36.25, p < 0.0001; Sidak’s multiple comparison test shows significance within the second 5 minutes of occlusion * p<0.05)).
DISCUSSION
Previous reports have indicated that deletion of either the CB1R or the CB2R increased damage following stroke7,13 The surprising finding from the current investigation was that combined deletion of both the CB1R and the CB2R in a single animal resulted in a decrease in infarct size in a model of permanent ischemia and improved recovery following stroke following both permanent and transient ischemia.
The CB1R provides for a negative feedback mechanism to inhibit synaptic transmission. Depolarization of the postsynaptic terminal results in the formation of the endogenous cannabinoid 2-AG, which then diffuses through the membrane to serve as a retrograde neurotransmitter, inhibiting the anterograde release of either excitatory or inhibitory neurotransmitters from the presynaptic terminal8,9. This provides an explanation for the consistent finding that antagonism or deletion of the CB1R increases brain excitotoxic injury25–28. However, early studies demonstrated that deletion of the CB1R in mice exacerbated injury following stroke. As the CB1R has been found to play an important role in normal neuronal development, it is therefore possible that chronic deletion of the CB1R causes neurons to be much more susceptible to injury, and that acute or conditional deletion would not have the same effect. This hypothesis is supported by the finding that administration of the selective CB1R antagonist SR141716 (rimonabant) was neuroprotective following ischemia reperfusion injury. Interpretation of these results is complicated by the fact that SR141716 may interact with other non- CB1Rs, including 5-HT1A and Vanilloid VR1 receptor6,17,29–31. The contribution of the CB1R to secondary injury following stroke has therefore remained unclear.
The results obtained from modulation of activity of the CB2R have been more consistent than the CB1R results. The CB2R is present on numerous cells that are involved in inflammatory responses following stroke, including microglia, monocytes/macrophages, dendritic cells, neutrophils, lymphocytes and endothelial cells32–35. The CB2R contributes to downregulation of proinflammatory responses by all of these cells. Numerous studies have provided evidence that activation of the CB2R on endothelial cells decreases expression of adhesion molecules, reduces leukocyte rolling and adhesion, and causes tightening of the blood brain barrier5,36,37. These actions contribute to decreasing inflammatory cell invasion of the brain and reduce their contribution of secondary injury following stroke. We have also demonstrated that activation of the CB2R on both microglia and dendritic cells, resident inflammatory cells within the central nervous system, attenuates their production of pro-inflammatory cytokines and their contribution to the promotion of inflammation. In addition, there is significant evidence that direct activation of CB2R on peripheral inflammatory cells also decreases their proinflammatory phenotype34. Reports of CB2R on neurons also indicate the possibility that the CB2R is present in very restricted areas38,39and therefore could also play a role in neuroprotection. Interestingly, a group of investigators have reported that administration of what they describe as a CB2 inverse agonist is protective following traumatic brain injury40,41. While these studies demonstrated that the agent investigated (SMM-189) functions as a CB2 inverse agonist in HEK-CNG cells, the possibility remains that this agent could actually function as a selective CB2 agonist for microglial cells activated following traumatic brain injury. This, if true, would provide an explanation for the apparent conflicting results. Clearly, additional work is required to elucidate the precise mechanism(s) through which selective modulators of CB2 receptor activation provide neuroprotection.
As mentioned in the introduction, reports of single knockouts for either CB1 or CB2 receptors would lead one to predict an increase in infarct size and poorer motor performance in the double knockout animals. Surprisingly, we found that following transient ischemia, damage was not greater in the double knockout as compared with wild type controls. Furthermore, neurological function was significantly improved based on the clinical score. This may be the result of the finding that the magnitude of blood flow reduction was less during the occlusion period in the double knockout animals as compared with the wild type controls, demonstrating improved autoregulatory ability in the double KO mice following occlusion. One possible explanation of this improved flow would be vascular remodeling in the double receptor knockout mice. Unfortunately it is not possible to evaluate this using the laser Doppler method because it does not measure absolte values of flow but instead a change in flow from baseline.
Interestingly, the results of this investigation demonstrated that the double knockout mice are less susceptible to damage in the model of permanent ischemia. The photothrombosis model of permanent ischemia was chosen because of its high reproducibility of lesion size42. Damage is thought to be the result of the generation of free radicals following exposure of the Rose Bengal in the cerebral vasculature to light. Although platelet aggregation and coagulation may contribute to the injury with this model, it has been shown that the injury can occur even without the presence of functionally active platelets17. While it was not possible to use the laser Doppler technique to evaluate flow with the photothrombosis model, flow has been found to be almost completely eliminated in the area of illumination. Therefore the smaller reduction in blood flow in the area of illumination is unlikely to be an explanation for the greater protective effect of the CB2 agonist observed with this model. However, it is possible that flow was improved in the ischemic penumbra, contributing to less expansion of the infarct. It is also possible that the smaller magnitude of injury in the permanent ischemia model afforded the possibility for greater recovery in the double knockout animals.
One concern we had with using the photothrombosis model in these experiments was the possibility that changes in bone density could interfere with light transmission to the brain43,44. Cannabinoids have been shown to influence bone growth in a number of studies. We therefore examined whether there was a decrease in light transmission in a small number of knockout animals (data not shown). In fact, light transmission was actually enhanced in the double knockout animals and therefore an increase in bone density cannot explain the results obtained.
Conclusion
The reason the double knockouts do not show a similar susceptibility to injury following stroke to the single knockouts of these receptors remains unclear. Both the CB1 and CB2 receptor are important contributors to homeostasis. Since the phenotype of the double knockout is not dramatically changed, there must be significant changes in the contribution of other homeostatic pathways in compensation for the loss of these two important receptors. One pathway intimately related to the endocannabinoid system is the eicosanoid system. Future investigations will explore alterations in the eicosanoid system following deletion of the cannabinoid receptors.
Acknowledgments
This work was supported by NIDA grants P30DA013429 (Ellen M Unterwald) and T32DA007237 (Ellen M Unterwald).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics-- 2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–89. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–96. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Mahadevan A, Amere M, Li H, Ganea D, Tuma RF. Unique effects of compounds active at both cannabinoid and serotonin receptors during stroke. Transl Stroke Res. 2012;3:348–56. doi: 10.1007/s12975-012-0197-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward S, Tuma RF. Endocannabinoids. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the Neurological Sciences. 2. Oxford: Academic Press: Elsevier Inc; 2014. pp. 42–7. [Google Scholar]
- 9.Rajesh M, Mukhopadhyay P, Godlewski G, et al. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem Biophys Res Commun. 2006;350:1056–62. doi: 10.1016/j.bbrc.2006.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turcotte C, Blanchet MR, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73:4449–70. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–85. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–5. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–60. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–50. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 16.Amantea D, Spagnuolo P, Bari M, et al. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17beta-estradiol. FEBS J. 2007;274:4464–775. doi: 10.1111/j.1742-4658.2007.05975.x. [DOI] [PubMed] [Google Scholar]
- 17.Kleinschnitz C, Braeuninger S, Pham M, et al. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008;39:1262–8. doi: 10.1161/STROKEAHA.107.496448. [DOI] [PubMed] [Google Scholar]
- 18.Hata R, Mies G, Wiessner C, et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–75. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya D, Hong S, Kayama T, Panter SS, Weinstein PR. Effect of suture size and carotid clip application upon blood flow and infarct volume after permanent and temporary middle cerebral artery occlusion in mice. Brain Res. 2003;970:131–9. doi: 10.1016/s0006-8993(03)02300-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Martin BR, Adler MW, et al. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009;4:249–59. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 23.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Vannucci SJ, Willing LB, Goto S, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol Pharmacol. 1998;54:459–62. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- 26.Abood ME, Rizvi G, Sallapudi N, McAllister SD. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci Lett. 2001;309:197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- 27.van der Stelt M, Veldhuis WB, Maccarrone M, et al. Acute neuronal injury, excitotoxicity, and the endocannabinoid system. Mol Neurobiol. 2002;26:317–46. doi: 10.1385/MN:26:2-3:317. [DOI] [PubMed] [Google Scholar]
- 28.Schlicker EK. M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sciences. 2001;22:565–72. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- 29.Pegorini S, Zani A, Braida D, Guerini-Rocco C, Sala M. Vanilloid VR1 receptor is involved in rimonabant-induced neuroprotection. Br J Pharmacol. 2006;147:552–9. doi: 10.1038/sj.bjp.0706656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichenbach ZW, Li H, Ward SJ, Tuma RF. The CB1 antagonist, SR141716A, is protective in permanent photothrombotic cerebral ischemia. Neurosci Lett. 2016;630:9–15. doi: 10.1016/j.neulet.2016.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JK, Park MS, Kim YS, et al. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007;67:620–5. doi: 10.1016/j.surneu.2006.08.077. discussion 5. [DOI] [PubMed] [Google Scholar]
- 32.Adhikary S, Kocieda VP, Yen JH, Tuma RF, Ganea D. Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood. 2012;120:3741–9. doi: 10.1182/blood-2012-06-435362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhikary S, Li H, Heller J, et al. Modulation of inflammatory responses by a cannabinoid-2- selective agonist after spinal cord injury. J Neurotrauma. 2011;28:2417–27. doi: 10.1089/neu.2011.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong W, Li H, Tuma RF, Ganea D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol. 2014;287:1–17. doi: 10.1016/j.cellimm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arevalo-Martin A, Garcia-Ovejero D, Gomez O, et al. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol. 2008;153:216–25. doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez SH, Hasko J, Skuba A, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–16. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persidsky Y, Ho W, Ramirez SH, et al. HIV-1 infection and alcohol abuse: neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav Immun. 2011;25(Suppl 1):S61–70. doi: 10.1016/j.bbi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang HY, Gao M, Liu QR, et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111:E5007–15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onaivi ES. Commentary: Functional Neuronal CB2 Cannabinoid Receptors in the CNS. Curr Neuropharmacol. 2011;9:205–8. doi: 10.2174/157015911795017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiner A, Heldt SA, Presley CS, et al. Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int J Mol Sci. 2014;16:758–87. doi: 10.3390/ijms16010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bu W, Ren H, Deng Y, et al. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front Neurosci. 2016;10:449. doi: 10.3389/fnins.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med. 2009;1:8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bab I, Zimmer A, Melamed E. Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann Med. 2009;41:560–7. doi: 10.1080/07853890903121025. [DOI] [PubMed] [Google Scholar]
- 44.Bab I, Zimmer A. Cannabinoid receptors and the regulation of bone mass. Br J Pharmacol. 2008;153:182–8. doi: 10.1038/sj.bjp.0707593. [DOI] [PMC free article] [PubMed] [Google Scholar]