Pentadecanoic acid (15:0) and heptadecanoic acid (17:0), the dairy-specific saturated fatty acids have been inversely, while inflammation and oxidative stress have been positively related to the risk of cardiovascular disease (CVD). Both fatty acid metabolism and inflammation and oxidative stress may be influenced by adiposity. In the current cross-sectional analyses among adolescents (mean age 15 years), we determined whether overweight status modified the associations between dairy fatty acids (pentadecanoic acid (15:0) and heptadecanoic acid (17:0)) represented in serum phospholipids (PL) and markers of inflammation and oxidative stress. Six biomarkers for inflammation and oxidative stress were analyzed, including circulating adiponectin, C-reactive protein (CRP), cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and urinary 15-keto-dihydro-PGF2α (15-keto) and 8-iso-PGF2α (F2-iso). Generalized linear regression analyses, adjusted for age, gender, race, tanner score, total energy intake and physical activity, revealed that PL dairy fatty acids were inversely associated with CRP, F2-iso and 15-keto in overweight, but not in normal weight adolescents (all Pinteraction < 0.05). However, higher level of PL dairy fatty acids was associated with lower IL-6 among all adolescents. Further adjustment for dietary intake of calcium, vitamin D, protein, total flavonoids, and ω-3 fatty acids did not materially change the findings. Dairy-specific saturated fats, i.e., 15:0 and 17:0 fatty acids, may contribute to the potential health benefits of dairy products, especially for overweight adolescents.
IntroductIon
The obesity epidemic has been increasing over the past 30 years among adults, adolescents, and children (1). Childhood obesity may track into adulthood, and predict adult disease (2). Inflammation and oxidative stress are common factors linking obesity and cardiovascular disease (CVD) risk (3). Although, low levels of inflammation and oxidative stress are normal in everyday tissue repair and acute injury in healthy people, chronic elevated levels of inflammatory and oxidative stress markers, such as C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) (4), 15-keto-dihydro-PGF2α (15-keto) (5), and 8-iso-PGF2α (F2-iso) (6), have been observed with excess adiposity in both adults and children and with CVD in adults (3,7). Further, levels of adiponectin, a protein hormone that is exclusively secreted from adipose tissue, have been inversely related to CVD risk (8,9).
Another contributor to the development of CVD is dietary fat intake (10), and saturated fatty acids (SFA) have been widely shown to be positively related to levels of inflammation and other CVD risk factors, such as high blood pressure, elevated total and low-density lipoprotein cholesterol, insulin resistance and metabolic syndrome (10–14). However, not all individual SFA have the same physiologic effects (11). In this regard, 15:0 (pentadecanoic acid) and 17:0 (heptadecanoic acid) fatty acids, which come primarily from dairy products, are of particular interest (11). Elevated levels of 15:0 and 17:0 fatty acids in blood, as objective biomarkers for dairy intake (15,16), have been linked to lower insulin resistance syndrome, triglycerides and risk of developing myocardial infarction among adults (17,18).
Evidence is limited in youth regarding the associations of 15:0 and 17:0 fatty acids with CVD risk, other than a study showing that the proportion of 15:0 fatty acid in serum cholesterol esters was inversely correlated with total cholesterol level in healthy adolescents (19). It is worth noting that adiposity, in addition to dietary fat intake, may influence the endogenous fatty acid profile and metabolism. For example, obese adolescents had lower co-3 serum polyunsaturated fats and higher serum SFA, compared to their lean counterparts (20), and in an earlier report, we observed that adolescent overweight was associated with an adverse serum fatty acid profile and specific patterns of desaturase activity (21).
In the present study among adolescents mean age 15 years, we aimed to determine whether 15:0 and 17:0 fatty acids in serum phospholipids (PL) were related to inflammation and oxidative stress, and whether these associations differed by the weight status of adolescents. We hypothesized that PL 15:0 and 17:0 fatty acids would be inversely associated with IL-6, TNF-α, CRP, 15-keto and F2-iso, but positively associated with adiponectin, and these relations would be enhanced in the overweight adolescents.
Methods and Procedures
Study Population
Data for the present study were collected in a prospective cohort study of obesity, insulin resistance and CVD risk factors in adolescents that was approved by the human subjects committee of the University of Minnesota. Briefly, over 12,000 children in grades 5-8 enrolled in Minneapolis Public Schools were screened for blood pressure, height and weight in 1995. Participants for the prospective study were randomly selected after stratification by sex, race (black and white), and systolic blood pressure (22). Informed consent for participation was obtained from 401 children and their parents. Clinic visits were conducted at mean ages 13, 15, 19, and 23 years, with the number of the participants varying across visits. The present study includes 305 adolescents seen at mean age 1 5 years (year 3 of the study) with complete data, including serum fatty acid measurements, biomarkers of inflammation and oxidative stress, anthropometric measurements, and laboratory studies.
Measurements
Clinical measurements
A wall-mounted stadiometer and a balance scale were used to measure height (cm) and weight (kg), respectively. BMI was calculated as weight in kg/height in m2. Overweight is defined as gender-age specific BMI≥85 percentile according to the Centers for Disease Control and Prevention growth chart (23). Blood pressure was measured twice using a random-zero sphygmomanometer, while participants were seated and the two values averaged in analyses. Tanner stage of children was assessed by a pediatrician, according to pubic hair in boys, and pubic hair and breast development in girls. The skinfold formula method of Slaughter (24) was used to calculate the %body fat, lean body mass and fat-free mass. A modified Paffenbarger physical activity questionnaire was used to estimate energy expenditure (25).
Diet assessment
Dietary intake was assessed by a 1 27-item Willett food frequency questionnaire (26), from which intakes of total energy and nutrients for each participant were estimated.
Laboratory measurement
Fasting blood specimens were collected and stored at –70 °C until analyses (22). The serum PL fatty acid profile was analyzed by gas-liquid chromatography as described previously (27). Individual PL fatty acids were expressed as %total PL fatty acids. Six biomarkers for inflammation and oxidative stress were analyzed, including circulating adiponectin, CRP, cytokines (IL-6 and TNF-α), and urinary F2-iso and 15-keto. Adiponectin, IL-6 and TNF-α were measured using a noncommercial enzyme-linked immunosorbent assay developed by the cytokine laboratory at the University of Minnesota and comparable to the commercial Quantikine ELISA kit (R&D Systems, Minneapolis, MN) (28). An ultrasentitive colorimetric competitive enzyme-linked immunosorbent assay was used to measure serum CRP level (29). Both 15-keto and F2-iso were analyzed from overnight urine samples by radioimmunoassay, and divided by urine creatinine concentration (5,6).
Statistical Analysis
Descriptive data are presented as mean values stratified by normal weight and overweight. Data with skewed distributions were log-transformed prior to analysis; results were back-transformed and presented as geometric means. PL 15:0 and 17:0 fatty acids were analyzed both individually and together (the sum of 15:0 + 17:0 represented “dairy fatty acids”). Generalized linear regression models were used to evaluate the relations between levels of biomarkers of inflammation/oxidative stress, serum dairy fatty acids and weight status. Effect mo dification of weight status was tested by including an interaction term between weight status and fatty acids in the model. When effect modification was statistically significant, the relations between PL dairy fatty acids and biomarkers of inflammation and oxidative stress were stratified by weight status. Otherwise, levels of biomarkers of inflammation and oxidative stress were examined across quintiles of fatty acids among all participants. Potential confounders were controlled in the analysis by adjusting for age, sex, race, tanner score, total energy intake, and physical activity; and further for BMI when appropriate. Statistical models were also adjusted for dietary intake of calcium, potassium, phosphorus, vitamins A and D, protein, and co-3 fatty acids, which have been shown to be potential bioactive components in dairy; as well as the dietary intakes of total favonoids, representing intake of fruit and vegetables associated with the dairy intake (30). Statistical significance was considered at P < 0.05. All analyses were conducted using SAS (version 9.2; SAS Institute, Cary, NC).
Results
Descriptive Characteristics
Characteristics of the participants are listed in Table 1. The majority of study participants were white (80.3%) and male (57.1%). Normal weight adolescents were older than those who were overweight. Compared to their normal weight counterparts, the overweight adolescents had higher BMI (28.4 kg/ m2 vs. 20.6 kg/m2), greater waist circumference (91.0 cm vs. 73.7 cm) and %body fat (45.7% vs. 26.6%) (all P < 0.001). However, the two groups did not differ in physical activity or dietary intakes of total energy and selected nutrients.
Table 1. Unadjusted mean (±s.d.) values of characteristics among normal and overweight adolescents (n = 305).
| Characteristics | Normal weight (n = 192) | Overweight (n = 113) | P value |
|---|---|---|---|
| Boys, n (%) | 112 (58.3) | 62 (54.9) | |
| Whites, n (%) | 154 (80.2) | 92 (80.5) | |
|
| |||
| Mean ± s.d. | |||
|
|
|||
| Age, years | 15.2 ± 1.2 | 14.7 ± 1.2 | <0.001 |
| Tanner score | 4.6 ± 0.7 | 4.5 ± 0.7 | 0.48 |
| BMI (kg/m2) | 20.6 ± 1.9 | 28.4 ± 5.2 | <0.001 |
| Waist (cm) | 73.7 ± 7.1 | 91.0 ± 13.3 | <0.001 |
| Lean body mass (kg) | 44.3 ± 9.5 | 42.8 ± 9.2 | 0.18 |
| %Body fat | 26.6 ± 9.9 | 45.7 ± 13.8 | <0.001 |
| Physical activity scores | 5,676 ± 6,370 | 5,238 ± 6,251 | 0.56 |
| Total energy intake (kcal) | 2,700 ± 1,377 | 2,609 ± 1,371 | 0.57 |
| Dietary intakes of nutrientsa | |||
| Potassium (mg) | 3,223 ± 119 | 3,153 ± 151 | 0.72 |
| Phosphorus (mg) | 1,534 ± 57 | 1,559 ± 75 | 0.79 |
| Calcium (mg) | 1,106 ± 49 | 1,196 ± 68 | 0.28 |
| Vitamin A (IU) | 9,951 ± 472 | 8,843 ± 548 | 0.13 |
| Vitamin D (IU) | 299 ± 17 | 323 ± 24 | 0.42 |
| ω-3 fatty acids (%kcal) | 0.05 ± 0.05 | 0.04 ± 0.06 | 0.11 |
| Total flavonoids (mg) | 11 ± 1 | 10 ± 1 | 0.32 |
| Phospholipids dairy fatty acids (%total phospholipids fatty acids) | |||
| 15:0 (pentadecanoic acid) | 0.22 ± 0.10 | 0.21 ± 0.04 | 0.14 |
| 17:0 (heptadecanoic acid) | 0.47 ± 0.05 | 0.46 ± 0.06 | 0.01 |
| Dairy fatty acids (15:0 + 17:0) | 0.70 ± 0.12 | 0.67 ± 0.08 | 0.01 |
| Biomarkers of inflammation and oxidative stress | |||
| Adiponectin (mg/l) | 15.0 ± 0.4 | 11.9 ± 0.5 | <0.001 |
| IL-6 (pg/ml)a | 2.2 ± 0.1 | 2.5 ± 0.2 | 0.11 |
| TNF-α (pg/ml)a | 3.9 ± 0.2 | 4.6 ± 0.3 | 0.04 |
| CRP (ng/ml)a | 0.74 ± 0.03 | 0.95 ± 0.06 | <0.001 |
| F2-isoprostanes (nmol/mmol creatinine)a | 0.31 ± 0.01 | 0.36 ± 0.02 | 0.01 |
| 15-keto-PGF2α (nmol/mmol creatinine) | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.12 |
Overweight is defined as gender-age specific BMI≥85 percentile according to the CDC growth chart (23).
CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Geometric means.
The mean level of PL dairy fatty acids in normal weight adolescents was 0.70% of total PL fatty acids, which was significantly higher (P = 0.01) than that of overweight adolescents (i.e., 0.67% of total PL fatty acids). Overweight adolescents had higher levels of TNF-α (3.9 pg/ml vs. 3.6 pg/ml, P = 0.04), CRP (0.95 ng/ml vs. 0.74 ng/ml, P < 0.001) and F2-iso (0.36 vs. 0.31 nmol/mmol creatinine, P = 0.01) than normal weight adolescents, while the level of adiponectin was significantly higher in normal weight adolescents (15.0 mg/l vs. 11.9 mg/l, P < 0.001).
Effect Modification
As shown in Figure 1a-c after adjusting for age, gender, ethnicity, tanner score, total energy intake and physical activity, weight status significantly modified the relations between PL dairy fatty acids and biomarkers of inflammation and oxidative stress. Among overweight, but not normal weight adolescents, higher levels of PL dairy fatty acids were associated with lower CRP, F2-iso and 15-keto (all P trend ≤ 0.01). Similar patterns were also observed when 15:0 and 17:0 fatty acids were examined individually (data not shown), although there was no association between PL 15:0 fatty acid and 15-keto in either the normal or overweight group (Pinteraction = 0.39). No effect modification of weight status was observed for the relation between PL dairy fatty acids and either TNF-α or IL-6. Further controlling for the dietary intakes of calcium, potassium, phosphorus, vitamins A and D, proteins, total flavonoids, and ω-3 fatty acids did not materially change these results.
Figure 1.
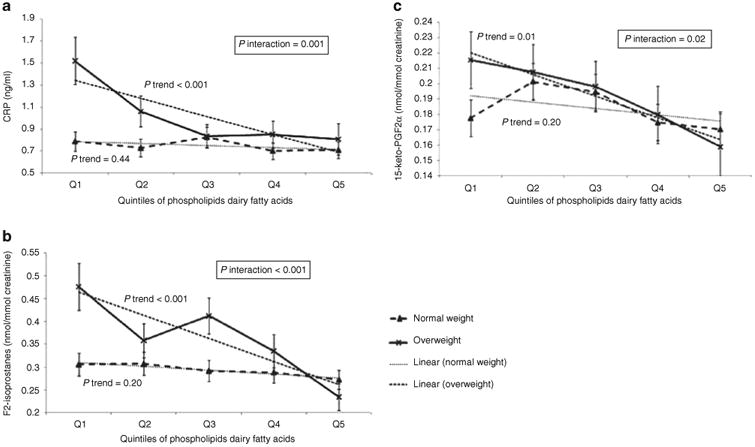
The interactions between weight status and biomarkers of dairy fats in relation to inflammation and oxidative stress, adjusted for gender, age, ethnicity, tanner score, total calorie intake and physical activity (n = 305). (a) Association of phospholipids dairy fatty acids with C-reactive protein (CRP) among normal weight and overweight adolescents. (b) Association of phospholipids dairy fatty acids with 8-iso-PGF2α among normal weight and overweight adolescents. (c) Association of phospholipids dairy fatty acids with 15-keto-PGF2α among normal weight and overweight adolescents. Geometric mean levels for CRP and F2-isoprostanes.
PL 17:0 fatty acid was associated with adiponectin in opposite directions in the two weight groups, after adjusting for age, gender, ethnicity, tanner score, total energy intake and physical activity (Pinteraction = 0.03, Table 2). Levels of PL 17:0 fatty acid were positively related to adiponectin in overweight adolescents (P trend = 0.08). In contrast, the inverse association was observed between PL 17:0 fatty acid and adiponectin in normal weight group (P trend = 0.06). Further controlling for the dietary intakes of calcium, potassium, phosphorus, vitamins A and D, proteins, total favonoids, and ω-3 fatty acids strengthened these relations (P trend = 0.01 and 0.04 for overweight and normal weight groups, respectively).
Table 2. Overweight modified the relation between adiponectin and quintiles of phospholipids 17:0 fatty acid among normal and overweight adolescents (n = 305).
| Weight group | Quintiles of phospholipids 17: fatty acid | P trend | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Model 1a | Normal | 16.1 ± 1.1 | 17.0 ± 1.0 | 14.3 ± 1.0 | 14.5 ± 1.0 | 14.5 ± 0.9 | 0.06 |
| Overweight | 10.5 ± 1.0 | 11.6 ± 1.0 | 12.9 ± 1.0 | 12.8 ± 1.2 | 12.5 ± 1.3 | 0.08 | |
| Model 2b | Normal | 15.8 ± 1.2 | 16.7 ± 1.0 | 13.9 ± 1.0 | 14.4 ± 1.1 | 14.2 ± 1.0 | 0.04 |
| Overweight | 9.6 ± 1.1 | 11.0 ± 1.0 | 12.5 ± 1.1 | 12.8 ± 1.2 | 12.7 ± 1.3 | 0.01 | |
Pinteraction = 0.03.
Adjusted for age, gender, race, tanner score, total energy intake and physical activity.
Further adjusted for dietary intakes of calcium, vitamin D, protein, total favonoids, and ω-3 fatty acids.
Other Associations of Fatty Acids with Inflammation and Oxidative Stress
In main effects analysis and adjusting for gender, age, ethnicity, tanner score, total calorie intake and physical activity (Figure 2), adolescents with the highest quintile level of PL dairy fatty acid had the lowest IL-6 (P trend < 0.001) compared to those whose PL dairy fatty acid level was in the lowest quintile. Individual 15:0 and 17:0 fatty acids followed the same pattern. Notably, these inverse associations were not significantly influenced after further adjusting for BMI or dietary intakes of nutrients (data not shown). Null results were observed for TNF-α in all analyses (data not shown).
Figure 2.
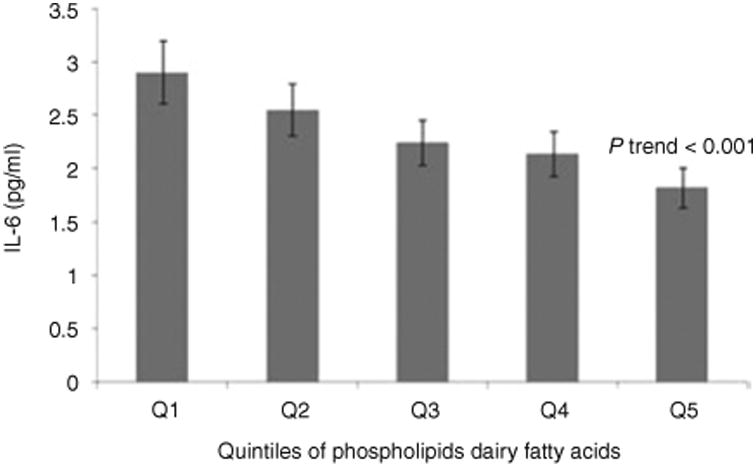
The associations between biomarkers of dairy fats in serum phospholipids and inflammation, adjusted for gender, age, ethnicity, tanner score, total calorie intake and physical activity (n = 305). Geometric mean level for IL-6.
Discussion
In this cross-sectional study, dairy fatty acids (i.e., 15:0 and 17:0 fatty acids) in serum PL were found to be inversely related to biomarkers of CRP, F2-iso and 15-keto only among overweight, but not among normal adolescents. However, IL-6 was inversely related to PL dairy fatty acids independent of participants' weight status.
Few studies have directly linked dairy fatty acids to inflammation and oxidative stress among adolescents. Our findings are in agreement, however, with previous studies of adults showing that higher levels of dairy fatty acids in blood or adipose tissues were associated with decreased risk of CVD, e.g., lower triglycerides and small dense low-density lipoprotein particles, and reduced risk of stroke, insulin resistance syndrome and myocardial infarction (17,18,31,32). In contrast, a positive association between plasma 15:0 fatty acid and risk of ischemic heart disease was found in a study of 32,826 adult women (33). A study in adolescents found that 15:0 fatty acid measured in cholesterol esters was inversely related to total cholesterol (19).
PL fatty acids are involved in cell membrane activities such as cell signaling transduction, molecular transport, and maintenance of membrane fluidity (34). It has been shown that PL fatty acid profiles represent short-term (weeks) fatty acid consumption (35) and may be a more objective measure of fatty acids that cannot be synthesized by humans (36). 15:0 and 17:0 ft this category of fatty acids, reflecting dietary intake of dairy products (15,16). It is known that overweight individuals under-report dietary intake, especially dietary fat (37). Thus, measures of PL fatty acids are likely to provide a more accurate assessment of dietary fat.
Dietary consumption of dairy products, despite being rich in SFA, do not appear to be associated with CVD risk (38–40). Limited evidence has shown that higher dairy product intake substantially suppresses oxidative stress and inflammation among overweight and obese adults (41). However, neither the underlying mechanisms nor the specific dairy product components related to these effects, in terms of 15:0 or 17:0 fatty acids, have been defined. In the present study, the inverse associations of PL dairy fatty acids with CRP, F2-iso, 15-keto and IL-6 remained stable, even after the adjustment for dietary intake of nutrients (e.g., calcium, ω-3 fatty acids, etc (30)) that may be related to the beneficial effects of dairy products. However, residual confounding cannot be ruled out, including other nutritional components and behavior factors associated with dairy intake.
In examining the role of adiposity in the relation between SFA metabolism and inflammation and oxidative stress (12), we found that PL dairy fatty acids were inversely related to CRP, F2-iso and 15-keto only in overweight adolescents, but not in their normal weight counterparts. Despite nonsignificant trends of linear associations which seemed to be due to a threshold effect, higher adiponectin was related to an elevated proportion of PL 17:0 fatty acids among overweight adolescents. Further adjustment for other dietary nutrient intakes tended to enhance this positive association. These results support our hypothesis that adiposity may play a critical role in the cross-talk between endogenous fatty acids and biomarkers of inflammation and oxidative stress. The lower CVD risk factors in normal weight adolescents, compared to their overweight counterparts, may be one explanation for the difference between the two weight status groups (42). It is known that increased macrophage recruitment to the adipose tissues which is mediated by obesity results in low-grade inflamma-tion (12). Consistently, we found that normal weight adolescents had lower percent body fat and subsequently, lower levels of CRP and F2-iso, but higher adiponectin than overweight adolescents. In previous studies, similar effect modification of weight status has shown higher plasma ω-3 polyunsaturated fats associated with lower risk of metabolic syndrome and inflammation only in overweight, but not normal weight adolescents (42). In addition, among 330 adults aged 18+ years with BMI of about 20–40 kg/m2, Makhoul et al. showed that eicosapentaenoic acid and docosahexaenoic acid in red blood cells was inversely related to levels of triglycerides and CRP among overweight and obese people (43).
Our study, for the first time, reported the interaction between PL dairy fatty acids and adiposity in relation to inflammation and oxidative stress. Nonetheless, compared to other fatty acids, such as eicosapentaenoic acid, docosahexaenoic acid, and other SFA, the absorption, distribution and metabolism of odd-numbered SFA (i.e., dairy fatty acids in this study) in human body have been less investigated. A study in the mouse demonstrated that compared to even-numbered fatty acids, odd-numbered fatty acids were more likely to accumulate in epididymal fat rather than being β-oxidized in liver (44). Meanwhile, overweight and obese individuals are well known to have greater production of free fatty acids in their adipocytes, but lower β-oxidation and a higher rate of fatty acid uptake in other tissues (45). Terefore, one may speculate that the preferred accumulation of dairy fatty acids may augment their potential beneficial effects. However, there is no evidence so far showing whether and how dairy fatty acids are involved in anti-inflammatory/anti-oxidative cell signaling network from a molecular mechanism perspective.
Interestingly, among the six biomarkers of inflammation and oxidative stress examined, the effect modification of weight status observed between dairy fatty acids and CRP, F2-iso and 15-keto was absent for IL-6 and null results were found for TNF-α in all the analyses. Further, our findings were also slightly different for individual 15:0 and 17:0 fatty acids, such as in relation to 15-keto and adiponectin. This may be due to the complicated production network of inflammatory and oxidative stress signals in response to their activators. Future studies are warranted, in order to elucidate the cross-talk between dairy fatty acids, adiposity and inflammation/oxidative stress.
The current analysis was cross-sectional and therefore limited the ability to draw any conclusion about temporality or causality of the relations. The sample size was relatively small; nevertheless, there was sufficient power to detect significant associations between serum PL dairy fatty acids and biomarkers of inflammation and oxidative stress. Although, statistically significant effect modification and fatty acid-biomarker relations may be due to chance, the majority of results after controlling for multiple comparisons were consistent with our proposed priori hypotheses. Assessing serum PL dairy fatty acids, instead of self-report dietary dairy fat intake, provided an objective evaluation of nutrient-CVD risk factor associations. Although residual confounding may still exist, we accounted for factors that may influence adolescents' fatty acid metabolism, as well as the possible bioactive components that may contribute to the beneficial effects of dairy foods. The examination of the modification effect of body weight status, a particularly relevant factor, considered the strong association between overweight and markers of inflammation and oxidative stress.
In summary, we observed that dairy fatty acids were beneficially associated with reduced CVD risk factors prior to adulthood in overweight, as opposed to normal weight adolescents. These results support a goal of increasing dairy fatty acid intake early in life, to set a dietary pattern consistent with preventing or delaying the development of CVD in later life (46).
Acknowledgments
H.W., L.M.S., A.R.S. designed research; H.W., L.M.S., B.V., S.B., J.S., A.M., A.R.S. conducted research; B.V., S.B., d.R.J., c.P.H. provided essential materials; H.W., L.M.S., c.P.H. analyzed data; H.W., L.M.S., B.V., S.B., J.S., A.M., d.R.J., A.R.S. wrote the paper. L.M.S., A.R.S. had primary responsibility for final content. All authors read and approved the final manuscript. This study was funded by the National Institutes of Health by grants #HL52851 and #MO1RR00400.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- 3.Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 4.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 5.Basu S. Radioimmunoassay of 15-keto-13,14-dihydro-prostaglandin F2alpha: an index for inflammation via cyclooxygenase catalysed lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids. 1998;58:347–352. doi: 10.1016/s0952-3278(98)90070-9. [DOI] [PubMed] [Google Scholar]
- 6.Basu S. Radioimmunoassay of 8-iso-prostaglandin F2alpha: an index for oxidative injury via free radical catalysed lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids. 1998;58:319–325. doi: 10.1016/s0952-3278(98)90042-4. [DOI] [PubMed] [Google Scholar]
- 7.Lazarou C, Panagiotakos DB, Chrysohoou C, Andronikou C, Matalas AL. C-reactive protein levels are associated with adiposity and a high inflammatory foods index in mountainous Cypriot children. Clin Nutr. 2010;29:779–783. doi: 10.1016/j.clnu.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 9.Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2009;10:269–277. doi: 10.1111/j.1399-5448.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 11.Astrup A, Dyerberg J, Elwood P, et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr. 2011;93:684–688. doi: 10.3945/ajcn.110.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 13.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 14.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 16.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr. 1998;68:291–295. doi: 10.1093/ajcn/68.2.291. [DOI] [PubMed] [Google Scholar]
- 17.Warensjö E, Jansson JH, Cederholm T, et al. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr. 2010;92:194–202. doi: 10.3945/ajcn.2009.29054. [DOI] [PubMed] [Google Scholar]
- 18.Warensjö E, Jansson JH, Berglund L, et al. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br J Nutr. 2004;91:635–642. doi: 10.1079/BJN20041080. [DOI] [PubMed] [Google Scholar]
- 19.Samuelson G, Bratteby LE, Mohsen R, Vessby B. Dietary fat intake in healthy adolescents: inverse relationships between the estimated intake of saturated fatty acids and serum cholesterol. Br J Nutr. 2001;85:333–341. doi: 10.1079/bjn2000279. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson M, Mårild S, Brandberg J, et al. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity (Silver Spring) 2006;14:1931–1939. doi: 10.1038/oby.2006.225. [DOI] [PubMed] [Google Scholar]
- 21.Steffen LM, Vessby B, Jacobs DR, Jr, et al. Serum phospholipid and cholesteryl ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk factors in adolescents. Int J Obes (Lond) 2008;32:1297–1304. doi: 10.1038/ijo.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–707. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. CDC Growth Charts: United States. [Accessed on Dec 15, 2009];2000 http://www.cdc.gov/growthcharts/cdc_charts.htm.
- 24.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 25.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87:43–47. [PubMed] [Google Scholar]
- 27.Boberg M, Croon LB, Gustafsson IB, Vessby B. Platelet fatty acid composition in relation to fatty acid composition in plasma and to serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin Sci. 1985;68:581–587. doi: 10.1042/cs0680581. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Jr, et al. Influence of waist on adiponectin and insulin sensitivity in adolescence. Obesity (Silver Spring) 2009;17:156–161. doi: 10.1038/oby.2008.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 30.Lock AL, Destaillats F, Kraft J, German JB. Introduction to the proceedings of the symposium “Scientific Update on Dairy Fats and Cardiovascular Diseases”. J Am Coll Nutr. 2008;27:720S–722S. doi: 10.1080/07315724.2008.10719749. [DOI] [PubMed] [Google Scholar]
- 31.Sjogren P, Rosell M, Skoglund-Andersson C, et al. Milk-derived fatty acids are associated with a more favorable LDL particle size distribution in healthy men. J Nutr. 2004;134:1729–1735. doi: 10.1093/jn/134.7.1729. [DOI] [PubMed] [Google Scholar]
- 32.Warensjö E, Smedman A, Stegmayr B, et al. Stroke and plasma markers of milk fat intake–a prospective nested case-control study. Nutr J. 2009;8:21. doi: 10.1186/1475-2891-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86:929–937. doi: 10.1093/ajcn/86.4.929. [DOI] [PubMed] [Google Scholar]
- 34.Torkhovskaya TI, Ipatova OM, Zakharova TS, Kochetova MM, Khalilov EM. Lysophospholipid receptors in cell signaling. Biochemistry Mosc. 2007;72:125–131. doi: 10.1134/s0006297907020010. [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 36.Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133(Suppl 3):925S–932S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 37.Heitmann BL, Lissner L, Osler M. Do we eat less fat, or just report so? Int J Obes Relat Metab Disord. 2000;24:435–442. doi: 10.1038/sj.ijo.0801176. [DOI] [PubMed] [Google Scholar]
- 38.Crichton GE, Bryan J, Buckley J, Murphy KJ. Dairy consumption and metabolic syndrome: a systematic review of findings and methodological issues. Obes Rev. 2011;12:e190–e201. doi: 10.1111/j.1467-789X.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 39.Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens. 2011 doi: 10.1038/jhh.2011.3. e-pub ahead of print 10 February 2011. [DOI] [PubMed] [Google Scholar]
- 40.Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;93:158–171. doi: 10.3945/ajcn.2010.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zemel MB, Sun X, Sobhani T, Wilson B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr. 2010;91:16–22. doi: 10.3945/ajcn.2009.28468. [DOI] [PubMed] [Google Scholar]
- 42.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 43.Makhoul Z, Kristal AR, Gulati R, et al. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.39. e-pub ahead of print 23 March 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotoh N, Moroda K, Watanabe H, et al. Metabolism of odd-numbered fatty acids and even-numbered fatty acids in mouse. J Oleo Sci. 2008;57:293–299. doi: 10.5650/jos.57.293. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta. 2010;1801:1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Ness AR, Maynard M, Frankel S, et al. Diet in childhood and adult cardiovascular and all cause mortality: the Boyd Orr cohort. Heart. 2005;91:894–898. doi: 10.1136/hrt.2004.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]


