Abstract
Purpose of review
This review explores recent research investigating the contribution of satellite cells (skeletal muscle stem cells) during muscle fiber atrophy as seen in periods of disuse, illness and aging.
Recent findings
Studies indicate reduced satellite cell activity and density in a variety of acute and chronic conditions characterized by robust muscle wasting. The direct contribution of satellite cells to unloading/denervation and chronic illness-induced atrophy remains controversial. Inflammation that accompanies acute trauma and illness likely impedes proper satellite cell differentiation and myogenesis, promoting the rapid onset of muscle wasting in these conditions. Transgenic mouse studies provide surprising evidence that age-related declines in satellite cell function and abundance are not causally related to the onset of sarcopenia in sedentary animals.
Summary
Recent clinical and pre-clinical studies indicate reduced abundance and dysregulated satellite cell activity that accompany muscle atrophy during periods of disuse, illness and aging, providing evidence for their therapeutic potential.
Keywords: muscle stem cell, Pax7, burn, aging, cachexia
Introduction
Loss of skeletal muscle mass (atrophy) occurs following an absence of muscle loading and recruitment (unloading/disuse, neural damage), as a consequence of disease (cancer, burn injury, heart failure, sepsis, AIDS, etc.), or in conjunction with aging (sarcopenia). The decrement in muscle mass impairs recovery, increases morbidity and reduces quality of life. Satellite cells, skeletal muscle-resident stem cells, are key mediators of muscle plasticity through contributions to myofiber hypertrophy. Additionally, they are indispensable for myofiber regeneration/repair following injury. Recent clinical and pre-clinical studies have shown perturbations in satellite cell activity and abundance that accompany muscle atrophy. However, controversy surrounds the role of satellite cells in mediating muscle atrophy resulting from disuse and during illness, as well as their role in promoting restoration of atrophied muscle. In this review, we will explore recent findings concerning the role of satellite cells during atrophy with an emphasis on unloading, disease and aging in both animal and clinical studies.
Satellite cell interactions with unloading/disuse and denervation-mediated atrophy
Unloading/disuse
Mechanical unloading induces a myriad of detrimental changes in skeletal muscle, including rapid atrophy and decreased strength and functional capacity (1). Rodent models often achieve mechanical unloading through hind limb suspension (HLS), yielding rapid atrophy over 1–2 weeks. Nakanishi et al. exposed adult female rats to 14 days of bilateral HLS (2). Myosin heavy chain (MHC) type 1 and 2 fibers of the soleus muscle exhibited atrophy in concert with reduced satellite cell content. Pax7+ and MyoD+ satellite cell abundance was reduced in HLS animals, as was the frequency of myogenin+ differentiating satellite cells (2). These findings suggest muscle disuse impairs satellite cell proliferation and differentiation, and reduces general abundance. A similar study employed 10 days of unloading in adult male rats, studying atrophy in the tibialis anterior (TA) muscle (3). Atrophy was present only in MHC type 2x and 2b fibers in the TA, with reduced satellite cell content only associated with these fiber types (3). This evidence suggests that reduced satellite cell content and function may hinder the regenerative and regrowth responses that ordinarily follow periods of disuse. Similar decrements in oxidative (soleus) (2) and glycolytic (TA) (3) muscle satellite cell abundance during mechanical unloading offer evidence for coordinated regulation of satellite cell activity in metabolically-divergent skeletal muscles.
A recent clinical study reports that 14 days of bed rest in middle-aged adults (51 ± 1 years) not only induces robust skeletal muscle atrophy, but also decreases in satellite cell content (4*). Intriguingly, the diminution in Pax7+ satellite cell abundance was significantly correlated with myofiber atrophy (cross-sectional area [CSA]), offering evidence that a relationship between atrophy and satellite cell alterations may exist in humans (4*). Short-term bed rest (5 days) in older adults (69 ± 2 years) induced a similar degree of myofiber atrophy (~25%) in an accelerated time frame (5**). Reidy et al. also show a 35–40% decrement in MHC type 1 satellite cell quantity following 5 days of bed rest, in addition to a positive correlation between myofiber CSA and satellite cell content (5**). However, 14-day unilateral leg immobilization in young males (24 ± 1 years) yielded conflicting results (6). Their myofiber atrophy was less severe compared to subjects undergoing bed rest (4*, 5**), suggesting that a critical atrophic threshold and/or age progression may be necessary to deleteriously impact satellite cell abundance and activity. It is important to note that a total absence of gravitational loading (bed rest, HLS) may also contribute to satellite cell maladaptation.
Denervation
Discordantly, denervation-induced muscle atrophy has been associated with satellite cell activation (commonly assessed by increased MyoD expression) (7). Electrically stimulated denervated muscle elevates MyoD+ nuclear abundance and embryonic MHC+ fibers (regeneration indicators), enhancing satellite cell differentiation potential (7). According to Xing et al., this enhanced differentiation partially restored muscle mass (7). Additional work in rodent models supports the therapeutic potential of exogenously administered miR-206 (microRNA responsible for skeletal muscle differentiation) to attenuate denervation-induced muscle atrophy through satellite cell differentiation (8**). Huang et al. show that miR-206 inhibits both transforming growth factor-β and muscle fibrosis (8**) – factors that impede satellite cell differentiation and regeneration (9). Similarly, satellite cell-derived miR-206 (expressed during a growth stimulus) was recently shown to attenuate fibrotic tissue expansion, improving muscle plasticity (10). Therefore, both satellite cell-intrinsic and –extrinsic miR-206 may promote positive muscle adaptation.
Neuromuscular dysfunction and muscle fibrosis have also been studied through an inducible transgenic mouse model that conditionally depletes satellite cells (SC-dep). Nerve transection of these mice showed that SC-dep exacerbated myofiber atrophy and promoted fibrotic tissue accumulation in skeletal muscle (11**). Unsurprisingly, neuromuscular junction disruption stimulated local satellite cell differentiation and fusion in wild-type mice (11**). In SC-dep however, neuromuscular junction regeneration was impaired, suggesting that satellite cells are integral for re-innervation. Liu et al. suggest that fewer post-synaptic myonuclei (derived from satellite cells) observed in SC-dep mice likely interfere with the initiation of the gene expression program required for neuromuscular junction regeneration (11**).
Satellite cell alterations during chronic and acute illness
Chronic illness-induced atrophy
The prevalence of chronic conditions such as cancer, diabetes and congestive heart failure continues to rise globally, and recent evidence highlights dysregulated satellite cell activity contributing to muscle atrophy in the presence of these conditions. Brzeszczyńska et al. showed reduced myogenin (terminal satellite cell differentiation marker) expression in cancer patients experiencing weight loss compared to healthy controls (12). Pro-inflammatory cytokines were also elevated in cancer patients (12). Specifically, IL-6 impedes satellite cell differentiation; however, transient inhibition of Stat3, which is downstream of IL-6 signaling, promoted satellite cell expansion and skeletal muscle repair post-injury (13).
Impaired satellite cell differentiation is also observed in children with type 1 diabetes, and a mouse model of the condition (14*). D’Souza et al. reported a similar decline in satellite cell activity in both rodent and clinical samples, with experiments on isolated single fibers suggesting that the decline in satellite cell function was intrinsic to satellite cells (14*). The Notch signaling pathway was also explored because it regulates satellite cell quiescence, expansion, and differentiation. However, Notch ligand expression differed between rodent and clinical samples, clouding interpretation (14*). Future research is needed to determine the influence of Notch signaling on satellite cell dysregulation in diabetic muscle.
Alternatively, over-activation of the renin-angiotensin system contributes to cachectic muscle-wasting in chronic conditions such as congestive heart failure (CHF) and chronic kidney disease. Recently, Yoshida et al. showed that angiotensin type 1 and 2 receptors (AT1R, AT2R) regulate different stages of satellite cell proliferation and activation (15**). In a mouse model of CHF, there was a marked reduction in muscle regeneration that was associated with attenuated AT2R expression (15**). Eloquent promoter reporter analysis experiments identified intron 2 of AT2R as a transcriptional enhancer element that promotes satellite cell differentiation. CHF however, suppressed the AT2R intron 2 enhancer activity, leading to diminished AT2R expression that likely contributes to impaired satellite cell differentiation and muscle regeneration (15**). Altered AT2R expression may also occur in other chronic conditions (chronic obstructive pulmonary disease, chronic kidney disease, etc.) characterized by perturbations in the renin-angiotensin system, and offers a new target to restore proper satellite cell activity levels to combat pervasive muscle wasting.
Acute trauma and illness-induced atrophy
Critically ill individuals and acute trauma survivors often experience weakness and functional disability that can persist for years – elucidating the underlying cellular mechanisms that contribute to the rapid loss of muscle mass and function is critical to developing evidence-based therapeutic strategies. Dos Santos et al. showed rapid and robust atrophy in patients 7 days following intensive care unit (ICU) discharge, and ~75% of patients demonstrated persistent atrophy at 6 months following ICU discharge (16). Notably, critically ill patients showed elevated protein degradation through the ubiquitin–proteasome system, but not the autophagosomal-lysosomal system at 7 days post-discharge, but neither the ubiquitin–proteasome nor the autophagosomal-lysosomal system was related to atrophy at 6 months post-discharge (16). However, decreased satellite cell abundance was observed at both 7 days and 6 months post-discharge, and was associated with persistent muscle atrophy (16). Specifically, in the 75% of patients who failed to regain quadriceps muscle size, reduced satellite cell density was observed compared to the 25% of patients who restored their atrophied muscle mass (16). While previous work has shown no deleterious effect on regrowth following atrophy in SC-dep healthy mice (17), the diminished satellite cell content in critically ill patients may play a causative role in poor muscle regrowth and sustained atrophy.
Similarly, in pediatric patients who have suffered a severe burn injury (>30% total body surface area), we observed reductions in satellite cell abundance (18*). Indices of muscle regeneration and myonuclear apoptosis were elevated in burn subjects (18*), both of which require satellite cell activity/fusion for repair. While a subset of satellite cells showed evidence of proliferation (Ki67+ satellite cells undergoing mitosis) in burn patients, there was also evidence of satellite cell apoptosis that was significantly correlated with burn severity (18*). Burn injury-induced myounclear turnover and myofiber regeneration require adequate and appropriate satellite cell activity and suggest a critical role for satellite cells in skeletal muscle atrophy and recovery following burn trauma. Similar findings reported by Song et al. show activation of satellite cells alongside myonuclear apoptosis in mouse muscle following a scald burn (19**). However, the observed myogenesis post-scald likely does not counterbalance the increased cell death after burn, contributing to burn-induced cachexia (19**). Additionally, inflammation through tumor necrosis factor-α may attenuate the myogenic response to a thermal injury, mitigating the recovery of atrophied muscle (19**). Findings from Corrick et al. further support that satellite cell terminal differentiation is impaired post-burn (20). When primary human skeletal muscle satellite cells were differentiated to myotubes in vitro and exposed to serum from burn patients, there was a reduction in myogenic fusion signaling and impairment of myogenesis (fewer nuclei per myotube) (20). Diminished myonuclear accrual during differentiation was also associated with reduced myotube size, emphasizing the integral role of satellite cells in the recovery of lean muscle following a burn injury (20). Results from these recent studies highlight the need for a greater understanding of burn-induced dysregulation of satellite cell activity to identify targeted therapies to promote muscle recovery.
Acute orthopedic injuries, such as those involving the rotator cuff (RC) or anterior cruciate ligament (ACL), can include prolonged muscle atrophy that results in pain, weakness, loss of function, and biomechanical instability. Following ACL injury, Noehren and colleagues reported reduced abundance of satellite cells in the injured limb compared to participants’ non-injured limb – which was not restored following surgical reconstruction and rehabilitation (21**, 22). Reported satellite cell deficits were also associated with quadriceps muscle atrophy and additional morphological maladaptations following ACL injury (21**). Reductions in satellite cell density have also been reported in atrophied/functionally impaired supraspinatus muscle following RC injury (23). Recently, Gigliotti et al. showed that satellite cells exhibited low basal levels of proliferative activity (Pax7+ cells in S-phase) in supraspinatus muscles following RC injury versus control deltoid muscles (23). Another study from the same group exposed isolated fibers of post-RC injury supraspinatus biopsies to isosorbide dinitrate (nitric oxide donor drug) (23). Drug exposure induced greater satellite cell proliferation in vitro (24). Enhancement of satellite cell proliferative capacity post-RC injury may enable regeneration and improve successful reversal of muscle atrophy following RC surgical repair (24). While these studies demonstrate reduced satellite cell density following an orthopedic injury, further work is needed to define the underlying mechanisms behind these reductions.
Contribution of satellite cells to sarcopenia
Several recent review articles provide in-depth assessment of cell-intrinsic and -extrinsic modifications to satellite cells that contribute to their functional decline with advancing age (25–27). Age-related declines in satellite cell abundance have also been well-documented in rodents (28*) and humans (29); as such, satellite cells remain a popular therapeutic target in the management of sarcopenia. Novel mouse models allowing for the specific and conditional depletion of satellite cells (SC-dep) offer a direct assessment of their contribution to age-related muscle atrophy. Multiple labs have independently utilized such models, depleting satellite cells in young mice (4–6 months), and then allowing them to age (20–24 months) (28*, 30**). Both groups (SC-dep and wild-type) showed age-related declines in myofiber size consistent with a sarcopenic model; surprisingly however, there were largely no exacerbations nor earlier onset of age-related atrophy in SC-dep mice (28*, 30**). Keefe et al. also tracked satellite cell fusion across the lifespan through eloquent genetic lineage tracing experiments. Satellite cell fusion was observed in ~30% of hind limb myofibers and ~70% of diaphragm myofibers in 20 month old mice (30**). With substantial satellite cell fusion occurring in sedentary mice during the lifespan, the absence of an effect of satellite cell depletion on sarcopenia development becomes even more surprising (28*, 30**). Conflicting results on absolute levels of satellite cell fusion in sedentary mice have recently been reported: some evidence suggests pervasive fusion in uninjured myofibers (31*), while other results show minimal fusion events through 6 months of age (32**). While clear evidence exists for the development of age-related deficits in functional capacity and self-renewal within satellite cells, recent evidence in sedentary mice (28*, 30**) would not support a causal relationship to the onset of sarcopenia.
Conclusion
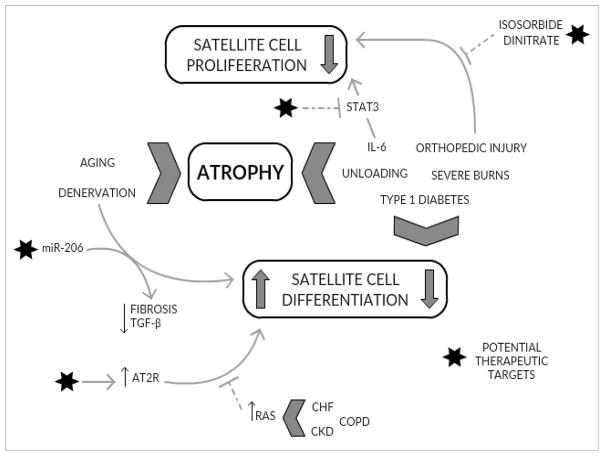
Significant recent attention has been paid to the regulatory role of satellite cells in mediating skeletal muscle atrophy and recovery (Figure 1). Clear deficits in functional activity and abundance have emerged in both acute and chronic clinical models that present with significant muscle wasting. However, a causal role for satellite cells in mediating the various atrophic phenotypes remains unsettled.
Figure 1. Perturbations in satellite cell activity accompany acute and chronic muscle wasting.
Various pathologies that promote muscle atrophy also affect satellite cell function. Enhancement of intrinsic factors (miR-206, AT2R) within satellite cells may address impaired differentiation and improve skeletal muscles’ regenerative response. Additionally, targeting satellite cell proliferative capacity through exogenous inputs (isosorbide dinitrate) may restore reduced abundance.
Key points.
Recent studies have shown perturbations in skeletal muscle satellite cell activity and abundance that accompany atrophy and functional decline.
Conflicting evidence exists as to the contribution of satellite cells to unloading and denervation-induced muscle atrophy.
Traumatic injury and illness induces robust systemic inflammation that likely contributes to impaired myogenesis and promotes the onset of muscle wasting.
Age-related muscle atrophy is associated with diminished activity and decreased abundance of satellite cells; however, it is unlikely that reduced satellite cell density and fusion contribute directly to the onset of sarcopenia under sedentary conditions.
Acknowledgments
Financial support and sponsorship
C Fry is supported by the by the UTMB Claude D. Pepper Older Americans Independence Center NIH/NIA grant P30 AG024832.
Footnotes
Conflicts of interest
The authors have no conflicts of interest
References cited
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):91–6. doi: 10.1093/gerona/glu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakanishi R, Hirayama Y, Tanaka M, Maeshige N, Kondo H, Ishihara A, et al. Nucleoprotein supplementation enhances the recovery of rat soleus mass with reloading after hindlimb unloading-induced atrophy via myonuclei accretion and increased protein synthesis. Nutr Res. 2016;36(12):1335–44. doi: 10.1016/j.nutres.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Babcock LW, Knoblauch M, Clarke MS. The role of myostatin and activin receptor IIB in the regulation of unloading-induced myofiber type-specific skeletal muscle atrophy. J Appl Physiol (1985) 2015;119(6):633–42. doi: 10.1152/japplphysiol.00762.2014. [DOI] [PubMed] [Google Scholar]
- 4*.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 2016;120(8):965–75. doi: 10.1152/japplphysiol.00799.2015. The decline in satellite cell content was significantly correlated to myofiber atrophy, suggesting a potential relationship between degree of atrophy and satellite cell maladaptations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, et al. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5-days of bed rest. Rejuvenation Research. 2017 doi: 10.1089/rej.2017.1942. Daily protein supplementation and neuromuscular electrical stimulation did not mitigate disuse-induced atrophy of SC decline, suggesting more intense interventions may be needed to offset even relatively short periods of unloading in older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijders T, Wall BT, Dirks ML, Senden JM, Hartgens F, Dolmans J, et al. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci (Lond) 2014;126(8):557–66. doi: 10.1042/CS20130295. [DOI] [PubMed] [Google Scholar]
- 7.Xing H, Zhou M, Assinck P, Liu N. Electrical stimulation influences satellite cell differentiation after sciatic nerve crush injury in rats. Muscle Nerve. 2015;51(3):400–11. doi: 10.1002/mus.24322. [DOI] [PubMed] [Google Scholar]
- 8**.Huang QK, Qiao HY, Fu MH, Li G, Li WB, Chen Z, et al. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats Through Regulation of Satellite Cell Differentiation via TGF-beta1, Smad3, and HDAC4 Signaling. Med Sci Monit. 2016;22:1161–70. doi: 10.12659/MSM.897909. Exogenous miR-206 improved satellite cell differentiation and helped mitigate denervation-induced atrophy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell. 2017;20(1):56–69. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife. 2015:4. doi: 10.7554/eLife.09221. Denervation promotes activation of satellite cells during recovery, but Liu et al. show a reciprocal relationship, that satellite cell activity is needed to regenerate neuromuscular junctions after injury. Induction of a specific gene expression program within satellite cell-derived myonuclei is likely integral to re-establish neuromuscular junctions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzeszczynska J, Johns N, Schilb A, Degen S, Degen M, Langen R, et al. Loss of oxidative defense and potential blockade of satellite cell maturation in the skeletal muscle of patients with cancer but not in the healthy elderly. Aging (Albany NY) 2016;8(8):1690–702. doi: 10.18632/aging.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20(10):1182–6. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.D’Souza DM, Zhou S, Rebalka IA, MacDonald B, Moradi J, Krause MP, et al. Decreased Satellite Cell Number and Function in Humans and Mice With Type 1 Diabetes Is the Result of Altered Notch Signaling. Diabetes. 2016;65(10):3053–61. doi: 10.2337/db15-1577. Both patients with and a mouse model of type 1 diabetes demonstrate impaired satellite cell dynamics through alterations in Notch signaling; however, differences in rodent and human satellite cell Notch signaling highlights species-specific adaptations following the onset of T1D. [DOI] [PubMed] [Google Scholar]
- 15**.Yoshida T, Delafontaine P. An Intronic Enhancer Element Regulates Angiotensin II Type 2 Receptor Expression during Satellite Cell Differentiation, and Its Activity Is Suppressed in Congestive Heart Failure. J Biol Chem. 2016;291(49):25578–90. doi: 10.1074/jbc.M116.752501. CHF attenuates satellite cell differentiation through repression of an intron enhancer for AT2R, contributing to CHF-induced muscle atrophy and impaired muscle regeneration following injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos C, Hussain SN, Mathur S, Picard M, Herridge M, Correa J, et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am J Respir Crit Care Med. 2016;194(7):821–30. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 17.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, et al. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012;303(8):C854–61. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Fry CS, Porter C, Sidossis LS, Nieten C, Reidy PT, Hundeshagen G, et al. Satellite cell activation and apoptosis in skeletal muscle from severely burned children. J Physiol. 2016 doi: 10.1113/JP272520. Significant cachexia following a severe burn injury promotes long-term functional deficits in children, and is associated with concurrent activation and death of satellite cells. The reduced abundance of satellite cells following a burn injury likely promotes rapid muscle wasting due to impaired regeneration and replacement of myonuclei that have undergone apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Song J, Saeman MR, De Libero J, Wolf SE. Skeletal Muscle Loss is Associated With TNF Mediated Insufficient Skeletal Myogenic Activation After Burn. Shock. 2015 doi: 10.1097/SHK.0000000000000444. A scald burn injury in rodents activates myogenesis, but is insufficient to rescue skeletal muscle atrophy and myonuclear apoptosis. The systemic hyper-inflammatory response to burn injury likely attenuates satellite cell-induced muscle repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrick KL, Stec MJ, Merritt EK, Windham ST, Thomas SJ, Cross JM, et al. Serum from human burn victims impairs myogenesis and protein synthesis in primary myoblasts. Front Physiol. 2015;6:184. doi: 10.3389/fphys.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Noehren B, Andersen A, Hardy P, Johnson DL, Ireland ML, Thompson KL, et al. Cellular and Morphological Alterations in the Vastus Lateralis Muscle as the Result of ACL Injury and Reconstruction. J Bone Joint Surg Am. 2016;98(18):1541–7. doi: 10.2106/JBJS.16.00035. Protracted muscle weakness following an ACL injury has long been attributed to arthrogenic muscle inhibition and poor neural input. Noehren et al. show that distinct maladaptations occur within the quadriceps muscle itself following an ACL tear, including reduced abundance of Pax7+ satellite cells, which are not restored following surgical reconstruction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res. 2016 doi: 10.1002/jor.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gigliotti D, Leiter JR, MacDonald PB, Peeler J, Anderson JE. Altered Satellite Cell Responsiveness and Denervation Implicated in Progression of Rotator-Cuff Injury. PLoS One. 2016;11(9):e0162494. doi: 10.1371/journal.pone.0162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gigliotti D, Leiter JR, Macek B, Davidson MJ, MacDonald PB, Anderson JE. Atrophy, inducible satellite cell activation, and possible denervation of supraspinatus muscle in injured human rotator-cuff muscle. Am J Physiol Cell Physiol. 2015;309(6):C383–91. doi: 10.1152/ajpcell.00143.2015. [DOI] [PubMed] [Google Scholar]
- 25.Brack AS, Munoz-Canoves P. The ins and outs of muscle stem cell aging. Skelet Muscle. 2016;6:1. doi: 10.1186/s13395-016-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa-Victor P, Munoz-Canoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med. 2016;50:109–17. doi: 10.1016/j.mam.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Clin Nutr Metab Care. 2017;20(3):186–90. doi: 10.1097/MCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 28*.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21(1):76–80. doi: 10.1038/nm.3710. The lifelong genetic depletion of satellite cells in mice did not accelerate or exacerbate the onset or severity of sarcopenia, questioning the therapeutic potential of satellite cells to address age-related muscle atrophy and functional decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014;36(2):545–7. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, et al. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun. 2015;6:7087. doi: 10.1038/ncomms8087. While lineage tracing studies demonstrate satellite cell fusion into myofibers in sedentary mice throughout the lifespan, the depletion of satellite cells and their fusion did not contribute to the development of sarcopenia in aged mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin BB. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle. 2015;5:42. doi: 10.1186/s13395-015-0067-1. Significant fusion of satellite cells into uninjured myofibers provide intriguing data for greater levels of basal myonuclear turnover in adult mice. The direct role or necessity of these fusion events remains in question. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Rozo M, Li L, Fan CM. Targeting beta1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med. 2016;22(8):889–96. doi: 10.1038/nm.4116. Lineage tracing studies in mice through 6 months of age do not show significant fusion of satellite cells in uninjured myofibers, providing conflicting evidence to results from Pawlikowski (31), suggesting mouse strain or lineage tracing methodologies may contribute to differences in basal satellite cell fusion rates. [DOI] [PMC free article] [PubMed] [Google Scholar]