Abstract
Objective
Schizophrenia is a severe, debilitating mental disorder characterized by behavioral abnormalities. Although several studies have investigated the role of oxidative stress and the effects of antipsychotic drugs on oxidative markers in schizophrenia, adequate information is not available on these issues. The aim of this study is to determine the changes in oxidative status and thiol disulfide homeostasis in schizophrenic patients using atypical antipsychotic drugs.
Methods
Thirteen schizophrenic patients using atypical antipsychotic drugs and 30 healthy controls were included this study. The concentrations of total oxidant status (TOS), total antioxidant status (TAS), native thiol, total thiol, and disulfide levels were determined in the study population.
Results
The TAS (p=0.001), total thiol, and native thiol levels (p<0.001) were higher in the patients compared to the controls, whereas the TOS and disulfide levels were lower in the patients than in the controls (p<0.001).
Conclusion
These results may suggest that atypical antipsychotic drugs have a useful therapeutic effect by reducing oxidative stress via the inhibition of the formation of disulfide bonds. The study population number was one of the limitations of this study. Therefore, further studies are needed to establish the association between thiol disulfide homeostasis in schizophrenic patients using atypical antipsychotic drugs.
Keywords: Schizophrenia, Thiols, Disulfides, Antipsychotics
INTRODUCTION
Schizophrenia, a severe debilitating mental disorder, is characterized by behavioral abnormalities. The onset of schizophrenia illness typically occurs in late adolescence or early adulthood. Schizophrenia is characterized by delusions, hallucinations, disorganized behavior and progressive cognitive deficits with postulated neurodevelopmental origin. The point prevalence of schizophrenia has been reported as five per thousand in the population.1–4) Differences in age, gender, duration of illness, exposure to antipsychotic drug could contribute towards the variability of morphometric findings.5) Many genetic and environmental factors have been indicated for the etiopathogenesis of schizophrenia.6) Oxidant-antioxidant balance disorders lie under several disease of central nervous system. Increasing evidence indicates that disturbances of antioxidant defense system and presence of oxidative stress may play a role in the biochemical mechanism underlying the schizophrenia.7) Oxidative stress is defined as a disturbance in the oxidant/antioxidant balance in favor of the former. The oxidant-antioxidant balance is an important mechanism for homeostasis in reactive oxygen metabolites and free radicals are parts of the normal human metabolism.8) Excess reactive oxygen species can cause oxidative damage in vulnerable targets such as polyunsaturated fatty acids, thiol groups and DNA.9) Several biomarkers were evaluated to demonstrate the biochemical alterations related to oxidative stress in patients with schizophrenia.10) Total antioxidant status (TAS), total oxidant status (TOS) and oxidative stress index (OSI), which are novel biomarkers of oxidative stress, are defined by Erel.10,11) Several studies were performed to investigate TAS and TOS levels in patients with schizophrenia. However, the reports are controversial.9,12–17)
Thiols are found in albumin and cysteine derived molecules such as glutathione, homocysteine, and γ-glutamylcysteine. The most abundant thiol in plasma is serum albumin. Thiols are good reductants. Thiols (RSH) can undergo oxidation reactions, which form a wide range of products, such as disulfide bonds.18) A disulfide bond is a covalent bond called an SS-bond or disulfide bridge. Under the conditions of oxidative stress, the oxidation of cysteine residues can lead to the reversible formation of mixed disulfides between protein thiol groups and low-molecular-mass thiols. The formed disulfide bonds can again be reduced to thiol groups; thus, dynamic thiol–disulfide homeostasis is maintained.19) Thiol disulfide homeostasis has critical roles in antioxidant protection, detoxification, signal transduction, apoptosis, the regulation of enzymatic activity and transcription factors, and cellular signaling mechanisms.20)
In this study, we evaluated the changes in OSI, TAS, TOS, native thiol, total thiol, and disulfide concentrations in schizophrenic patients using atypical antipsychotic drugs. The aim of this study was to determine the effects of atypical antipsychotic drugs on disulfide stress. To the best of our knowledge, this is the first study that evaluates the thiols and disulfide bound formation in schizophrenic patients using atypical antipsychotic drugs. This study provides an important opportunity to advance the understanding of the relationship between oxidative stress and atypical antipsychotics use in patients with schizophrenia.
METHODS
Subjects
The study subjects were composed of 30 patients (18 males and 12 females; 19–70 years old [mean age, 42.13±14.45 years]) and 30 controls (16 males and 14 females; 20–66 years old [mean age, 40.59±9.08]). No significant difference was observed between the groups in terms of age (p=0.609) and gender (p=0.247). Current mental status and personal or family history of any mental disorder was assessed by a clinical psychiatrist. Patients with impaired renal and thyroid function; diabetes mellitus; rheumatic disease; liver disease; malignancy; and pregnancy were excluded from the study. For the healthy controls, the exclusion criteria included a clinical suspicion of infections (body temperature out of the range of 36–38°C, heart rate >90 beats/minute, respiratory rate >20 times/minute, and white blood count >12,000/mm3 or <4,000/mm3); the presence of liver disease, kidney disease, rheumatic disease, or malignancy; pregnancy, and smoking. Neither patients nor control subjects suffered from drug or alcohol abuse/ dependence. All patients met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) diagnosis of schizophrenia. Patients had a mean duration of illness of 10.77±8.65 years. All patients had been receiving stable doses of oral atypical antipsychotic drugs (risperidone, olanzapine, aripiprazole, amisulpride, and quetiapine) for at least 12 months before entry into this study. The protocol was approved by the ethical committee of Cumhuriyet University Medical Faculty (No. 2016–02/02). All the procedures performed in the study involving the human participants were done in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all the individual participants included in the study.
Blood Sampling
Overnight fasting blood samples were collected from all participants in a red top tube (Becton Dickinson, Oxford, UK). The serum was separated, aliquoted, and stored at −20°C before use. The samples of the patients and the controls were assayed in the same assay batches.
Determination of OSI, TAS, TOS, and Thiol/Disulfide Levels
TAS levels were measured using commercially available colorimetric kits (Rel Assay Diagnostic, Gaziantep, Turkey). The novel automated method is based on the bleaching of characteristic color of a more stable ABTS [2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation by antioxidants. The assay precision were <3%. The results were expressed as mmol Trolox equivalent/L. TOS levels were measured using commercially available colorimetric kits (Rel Assay Diagnostic). In this method; oxidants present in the sample oxidized the ferrous ion-o-dianisidine complex to ferric ions. The oxidation reaction was enhanced by glycerol molecules abundantly present in the reaction medium. The ferric ion produced a colored complex with xylenol orange in an acidic medium. The color intensity, which could be measured spectrophotometrically, was related to the total amount of oxidant molecules present in the sample. Results were expressed as micromolar hydrogen peroxide equivalent per liter (μmol H2O2equivalent/L). OSI, which is the indicator of the oxidative stress degree, was used as a parameter to assess redox status. Calculation of OSI were done according to the formula: OSI=TOS/TAS.21) Thiol/disulphide levels were measured with a newly developed method by Erel and Neselioglu.20) The essential principle of the Erel and Neselioglu method is the reduction of disulfide bonds (S=S) to reactive thiol groups in the presence of NaBH4. In this test dynamic disulphide bonds (−S–S−) in the sample are reduced to functional thiol groups (−SH) by NaBH4. The unused NaBH4 remnants are completely removed by formaldehyde. Thus, this prevents the extra reduction of the DTNB and further reduction of the formed disulphide bond, which are produced after the DTNB reaction. The total thiol content of the sample is measured using modified Ellman reagent. When the results obtained by subtracting native thiol from total thiol were divided into two, the disulfide level was obtained. When disulfide, native thiol, and total thiol levels were divided to each other, a disulfide/native thiol, disulfide/total thiol, and native thiol/total thiol ratio was obtained as a result.
Statistical Analysis
Sample size was determined as 30 observations for each group, based on α=0.05 and β=0.20. Power of the actual performed test was obtained as 80.26%. Analyses were conducted using Power Analysis Statistical System (PASS) ver. 11.0 (NCSS Statistical Software, Kaysville, UT, USA). A Shapiro-Wilk test was used to determine the distribution characteristics of the variables. Student t tests and Mann-Whitney U tests were used to compare the differences of the parametric and nonparametric variables between the groups, respectively. A chi-square tests were used to compare differences in terms of gender. The results were expressed as mean±standard deviation. A p value less than 0.05 level was considered as statistically significant.
RESULTS
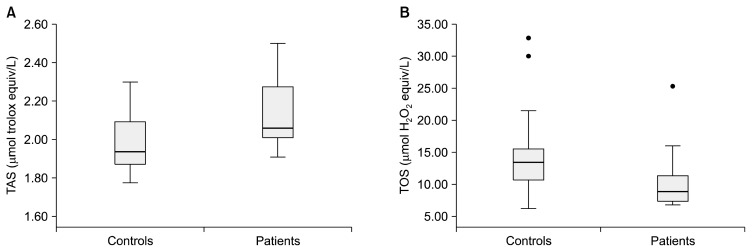
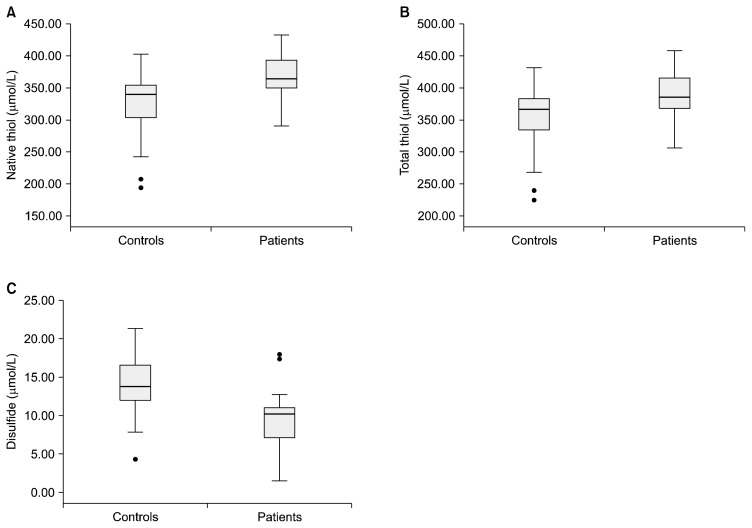
The median TAS levels were 1.93 (1.87–2.09) and 2.05 (2.00–2.27) μmol trolox equiv/L in the controls and patients, respectively. The median TOS levels were 13.40 (10.65–15.53) in the controls and 8.85 (7.25–11.42) μmol H2O2 equiv/L in the patients. Statistically significant differences were observed between the patients and controls in terms of TAS (p=0.001) and TOS (p<0.001). The TAS and TOS levels in the controls and patients are shown in a boxplot in Figure 1. The median OSI values were determined as 4.00 (3.42–5.75) and 6.75 (5.65–7.70) in patients and controls, respectively. The mean native thiol, total thiol, disulfide, disulfide/native thiol%, disulfide/total thiol, native thiol/total thiol%, albumin, and total protein values are provided in Table 1. The native thiol, total thiol, and disulfide levels in the controls and patients are illustrated in a boxplot in Figure 2. As seen in Table 1, the total and native thiol values are higher in the patients than in the controls, whereas the disulfide value is higher in the controls. These observed differences in native thiol, total thiol, and disulfide concentrations were statistically significant.
Fig. 1.
Box plots for TAS (A) and TOS (B) levels. The image of each group shows the box with median (horizontal line within the box); the interquartile range (IQR), corresponding to the 25th to 75th percentiles (lower and upper limit of the box); nearest observations within 1.5 IQRs (the whiskers) and outliers (circles within 3 IQR).
TAS, total antioxidant status; TOS, total oxidant status.
Table 1.
Comparison of the serum levels native thiol, total thiol, disulfide, albumin and total protein concentrations
| Patient (n=30) | Control (n=30) | p value* | |
|---|---|---|---|
| Age (yr) | 42.13±14.45 | 40.59±9.08 | 0.609 |
| Native thiol (μmol/L) | 370.03±34.48 | 324.47±47.65 | <0.001 |
| Total thiol (μmol/L) | 388.71±36.13 | 352.56±47.99 | 0.001 |
| Disulfide (μmol/L) | 9.3±3.73 | 14.04±3.54 | <0.001 |
| SS/SH % | 2.53±1.05 | 4.44±1.37 | <0.001 |
| SS/total thiol % | 2.39±0.95 | 4.05±1.15 | <0.001 |
| SH/total thiol % | 95.21±1.90 | 91.88±2.30 | <0.001 |
| Albumin (g/dl) | 4.25±0.49 | 4.27±0.43 | 0.856 |
| Total protein (g/dl) | 6.50±0.66 | 6.58±0.84 | 0.704 |
Values are presented as mean±standard deviation. SS, disulphide; SH, native thiol.
The significance between control and patient groups.
Fig. 2.
Box plots for native thiol levels (A), total thiol levels (B) and disulphide levels (C). The image of each group shows the box with median (horizontal line within the box); the interquartile range (IQR), corresponding to the 25th to 75th percentiles (lower and upper limit of the box); nearest observations within 1.5 IQRs (the whiskers) and outliers (circles within 3 IQR).
DISCUSSION
The major findings of the study were that (i) TAS, total thiol, and native thiol levels were higher in the patients than controls and (ii) TOS and disulfide levels were lower in the patients than controls. Numerous studies investigated the relationships between schizophrenia and TAS and TOS levels.9,13–17) In these studies, inconsistent results were found. In 2009, Virit and co-workers9) demonstrated that, lower TAS concentrations in patients with schizophrenia and also in the same study no significant difference was observed between the patients and controls in terms of TOS levels. Sertan Copoglu et al.15) showed that decreased TOS levels in patients compared to healthy controls. Bahceci et al.16) reported decreased concentrations of TAS but increased concentrations of TOS levels in schizophrenic patients with respect to controls. Al-Chalabi and co-workers22) demonstrated that the increased concentrations of TAS in patients taking olanzapine compared to drug-free patients. Inconsistent results between studies might arise from differences in the patients’ selection criteria, differences in disease etiology, exposure to antipsychotic treatment, sampling of patients at different stages of the disease, and study population number. Our findings were in accordance with the studies done by Sertan Copoglu et al.15) and Al-Chalabi et al.22) in terms of TOS and TAS concentrations, respectively. It was also suggested in the literature that atypical antipsychotic drugs might affect oxidative status by increasing the antioxidant levels and decreasing the oxidative stress.22–25) In our study, all the patients were using only atypical antipsychotic drugs. Therefore, we thought that the reason for the increased concentrations of TAS and decreased concentrations of TOS might be related to atypical antipsychotic drug use in patients with schizophrenia.
In the present study we found higher concentrations of the native and total thiol and lower concentrations of disulfide in the patients compared to controls. Homocysteine, which has thiols, is becoming increasingly recognized as an important substance in the pathogenesis of schizophrenia. There is a positive correlation between homocysteine concentration and schizophrenia.26–30) One of the most relevant mechanisms explaining the association between homocysteine and schizophrenia is the effect of oxidative stress caused by homocysteine associated molecules on N-methyl-D-aspartate receptors.26) Glutathione is the major intracellular non-protein thiol.18) Unlike homocysteine, glutathione is considered to be one of the most important endogenous antioxidants in the body.31) Recent studies have shown a correlation between the decreased concentrations of glutathione and the pathophysiology of schizophrenia.31–33) It has been also demonstrated that a negative correlation between brain glutathione level and negative symptom in patients with schizophrenia.32) Gysin et al.31) reported that under oxidative conditions, impaired glutathione synthesis is a vulnerability factor for schizophrenia. Additionally, decreased concentrations of glutathione peroxidase and glutathione reductase have been seen in patients with schizophrenia.34–38) Although, it has been reported that the impaired metabolisms of thiol containing substances such as homocysteine and glutathione in schizophrenic patients.26–33) There are no studies on the effect of antipsychotic drugs on thiol disulfide homeostasis in schizophrenic patients. In our study, native thiol and total thiol levels are found to be higher in patients taking atypical antipsychotic drugs. It has been demonstrated that the relationship between atypical antipsychotic drug use, decreased levels of lipid peroxidation and increased antioxidant enzyme activities were reported.24,39–41) Taken together, these results suggest that atypical antipsychotic drugs may have a protective effect on thiol disulfide homeostasis against to oxidative stress. The present findings seem to be consistent with our earlier observations which found that the increased concentrations of TAS and decreased concentrations of TOS may be related to atypical antipsychotic drug use.
The main limitation of this study was that the concentrations of homocysteine, reduced glutathione, and oxidized glutathione were not assessed. Very small sample size, the inclusion of several antipsychotics and cross sectional design were other limitations of this study.
In conclusion, the evidence from this study suggests that atypical antipsychotic drugs have also a useful therapeutic effect by reducing oxidative stress via inhibiting the formation of disulfide bounds. We think that these findings enhance our understanding of the relationship between oxidative stress and atypical antipsychotics. More broadly research on the effects of atypical antipsychotics on thiol disulfide homeostasis would help us to establish a greater degree of accuracy on this matter.
REFERENCES
- 1.Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30:323–338. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatasubramanian G. Understanding schizophrenia as a disorder of consciousness: biological correlates and translational implications from quantum theory perspectives. Clin Psychopharmacol Neurosci. 2015;13:36–47. doi: 10.9758/cpn.2015.13.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roh D, Chang JG, Yoon S, Kim CH. Antipsychotic prescribing patterns in first-episode schizophrenia: a five-year comparison. Clin Psychopharmacol Neurosci. 2015;13:275–282. doi: 10.9758/cpn.2015.13.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SJ, Shim JC, Kong BG, Kang JW, Moon JJ, Jeon DW, et al. The relationship between language ability and cognitive function in patients with schizophrenia. Clin Psychopharmacol Neurosci. 2015;13:288–295. doi: 10.9758/cpn.2015.13.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumari AA, John JP, Halahalli HN, Paul P, Thirunavukkarasu P, Purushottam M, et al. Effect of polymorphisms of three genes mediating monoamine signalling on brain morphometry in schizophrenia and healthy subjects. Clin Psychopharmacol Neurosci. 2015;13:68–82. doi: 10.9758/cpn.2015.13.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 7.Bošković M, Vovk T, Kores Plesničar B, Grabnar I. Oxidative stress in schizophrenia. Curr Neuropharmacol. 2011;9:301–312. doi: 10.2174/157015911795596595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 9.Virit O, Altindag A, Yumru M, Dalkilic A, Savas HA, Selek S, et al. A defect in the antioxidant defense system in schizophrenia. Neuropsychobiology. 2009;60:87–93. doi: 10.1159/000239684. [DOI] [PubMed] [Google Scholar]
- 10.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Camkurt MA, Fındıklı E, Tolun Fİ, Bakacak M, Bal NG, Sakallı H, et al. Probable preventive effects of placenta from oxidative stress; Evaluation of total antioxidant status, total oxidant status and oxidative stress index in fetal cord blood during the delivery. Psychiatry Res. 2016;240:222–225. doi: 10.1016/j.psychres.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Pazvantoglu O, Selek S, Okay IT, Sengul C, Karabekiroglu K, Dilbaz N, et al. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63:693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 14.Ustundag B, Atmaca M, Kirtas O, Selek S, Metin K, Tezcan E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin Neurosci. 2006;60:458–464. doi: 10.1111/j.1440-1819.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 15.Sertan Copoglu U, Virit O, Hanifi Kokacya M, Orkmez M, Bulbul F, Binnur Erbagci A, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015;229:200–205. doi: 10.1016/j.psychres.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Bahceci B, Bagcioglu E, Kokacya MH, Dilek AR, Bahceci I, Selek S. Prolidase activity and oxidative stress in patients with schizophrenia: a preliminary study. J Pak Med Assoc. 2015;65:131–135. [PubMed] [Google Scholar]
- 17.Devanarayanan S, Nandeesha H, Kattimani S, Sarkar S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: a case control study. Clin Chem Lab Med. 2016;54:447–452. doi: 10.1515/cclm-2015-0212. [DOI] [PubMed] [Google Scholar]
- 18.Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Motor S, Ozturk S, Ozcan O, Gurpinar AB, Can Y, Yuksel R, et al. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int J Clin Exp Med. 2014;7:1089–1093. [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Chalabi BM, Thanoon IA, Ahmed FA. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology. 2009;59:8–11. doi: 10.1159/000202823. [DOI] [PubMed] [Google Scholar]
- 23.Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Wei Z, Bai O, Richardson JS, Mousseau DD, Li XM. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2003;73:364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Phuong VT, Lee CH, Lee JG, Seo MK, Cho HY, et al. Effects of antipsychotic drugs on BDNF, GSK-3β, and β-catenin expression in rats subjected to immobilization stress. Neurosci Res. 2011;71:335–340. doi: 10.1016/j.neures.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front Behav Neurosci. 2014;8:343. doi: 10.3389/fnbeh.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine J, Sela BA, Osher Y, Belmaker RH. High homocysteine serum levels in young male schizophrenia and bipolar patients and in an animal model. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1181–1191. doi: 10.1016/j.pnpbp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Kevere L, Purvina S, Bauze D, Zeibarts M, Andrezina R, Rizevs A, et al. Elevated serum levels of homocysteine as an early prognostic factor of psychiatric disorders in children and adolescents. Schizophr Res Treatment. 2012;2012:373261. doi: 10.1155/2012/373261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouaziz N, Ayedi I, Sidhom O, Kallel A, Rafrafi R, Jomaa R, et al. Plasma homocysteine in schizophrenia: determinants and clinical correlations in Tunisian patients free from antipsychotics. Psychiatry Res. 2010;179:24–29. doi: 10.1016/j.psychres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Adler Nevo G, Meged S, Sela BA, Hanoch-Levi A, Hershko R, Weizman A. Homocysteine levels in adolescent schizophrenia patients. Eur Neuropsychopharmacol. 2006;16:588–591. doi: 10.1016/j.euroneuro.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altuntas I, Aksoy H, Coskun I, Cayköylü A, Akçay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med. 2000;38:1277–1281. doi: 10.1515/CCLM.2000.201. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich-Muszalska A, Olas B, Głowacki R, Bald E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology. 2009;59:1–7. doi: 10.1159/000202822. [DOI] [PubMed] [Google Scholar]
- 36.Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. 20081. [DOI] [PubMed] [Google Scholar]
- 37.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. 2007;40:1026–1031. doi: 10.1016/j.clinbiochem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37:43–51. doi: 10.1016/S0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- 40.Pillai A, Parikh V, Terry AV, Jr, Mahadik SP. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2007;41:372–386. doi: 10.1016/j.jpsychires.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]