Abstract
Objective
In 2002, the Korean Society for Affective Disorders developed the guidelines for the treatment of major depressive disorder (MDD), and revised it in 2006 and 2012. The third revision of these guidelines was undertaken to reflect advances in the field.
Methods
Using a 44-item questionnaire, an expert consensus was obtained on pharmacological treatment strategies for MDD 1) without or 2) with psychotic features, 3) depression subtypes, 4) maintenance, 5) special populations, 6) the choice of an antidepressant (AD) regarding safety and adverse effects, and 7) non-pharmacological biological therapies. Recommended first, second, and third-line strategies were derived statistically.
Results
AD monotherapy is recommended as the first-line strategy for non-psychotic depression in adults, children/adolescents, elderly adults, patient with persistent depressive disorder, and pregnant women or patients with postpartum depression or premenstrual dysphoric disorder. The combination of AD and atypical antipsychotics (AAP) was recommended for psychotic depression in adult, child/adolescent, postpartum depression, and mixed features or anxious distress. Most experts recommended stopping the ongoing initial AD and AAP after a certain period in patients with one or two depressive episodes. As an MDD treatment modality, 92% of experts are considering electroconvulsive therapy and 46.8% are applying it clinically, while 86% of experts are considering repetitive transcranial magnetic stimulation but only 31.6% are applying it clinically.
Conclusion
The pharmacological treatment strategy in 2017 is similar to that of Korean Medication Algorithm for Depressive Disorder 2012. The preference of AAPs was more increased.
Keywords: Algorithms, Depressive disorder, Drug therapy, Guideline
INTRODUCTION
The purpose of the clinical guideline is to assist clinicians’ decisions on proper treatment options and to improve the quality of medication treatments in danger of bias from overwhelming research informations.1)
Depressive disorder is a heterogeneous and complex disorder that has various symptoms, clinical courses and outcome including treatment response to pharmacotherapy, or to non-pharmacological somatic therapy,2) and that is related with cognitive and occupational function, quality of life, suicide and socioeconomic burden.3)
For the purpose of clinical guideline, therefore, the Korean Medication Algorithm Project for Depressive Disorder that is a task force within the Korean Society for Affective Disorders (KSAD), one of the 23 nonprofit scientific and educational psychiatrists’ societies under the Korean Neuropsychiatric Association, developed the Korean Medication Algorithm for Major Depressive Disorder in 2002 (KMAP-MD 2002),4) and conducted first revision in 2006 (The Korean Medication Algorithm for Depressive Disorder, KMAP-DD 2006),5) second revision in 2012 (KMAP-DD 2012),2) and this third revision of KMAP-DD in 2017.
The KMAP-DD series contain seven sections giving pharmacological treatment strategies for 1) major depressive disorder (MDD) without psychotic features, 2) MDD with psychotic features, 3) dysthymia and other depressive disorder subtypes, 4) maintenance treatment, 5) treatment strategies for special populations, 6) the choice of an AD in the context of safety, adverse effects and comorbid physical illnesses, and 7) non-pharmacological biological therapies. An exception is KMAP-MD 2002, which contains few newer antidepressants (AD) and atypical antipsychotics (AAP) and has a different methodology compared with later KMAP-DDs. The KMAP-DD 2006, 2012, and 2017 series is the expert’s consensus guideline, with current evidence on treatment of depressive disorder evaluated by a KMAP executive committee, consisting of 12 well-trained psychiatrists with extensive clinical experience in the field of mood disorders in Korea. In this revision, there is a few modifications to the questionnaire (Table 1). For example, because of the introduction of the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) in 2013, the specifiers “mixed features” and “anxious distress” were included in “subtype” section C in this revision to enable comparisons of clinical pharmacological treatment before and after 2013.
Table 1.
Comparison among first (2006), second (2012), and third (2017) revisions of the Korean Medication Algorithm for Depressive Disorder
| First revision in 2006 | Second revision in 2012 | Third revision in 2017 | |
|---|---|---|---|
| Depressive episode | Mild | Mild to moderate | Same as 2012 |
| Moderate | Non-psychotic severe | ||
| Non-psychotic severe | Psychotic severe | ||
| Psychotic severe | |||
| AD dosage and duration of treatment | Present | Deletion | Change: duration of initial treatment and number of choosing AD as initial treatment |
| Subtype | Dysthymia | Dysthymia | Dysthymia |
| Minor depressive disorder | Minor depressive disorder | Minor depressive disorder | |
| Atypical features | Atypical features | Atypical features | |
| Melancholic features | Melancholic features | Melancholic features | |
| Seasonal pattern | Seasonal pattern | ||
| Mixed features | |||
| Anxious distress | |||
| Comorbid physical illness | Absent | Newly added | Same as 2012 |
| Special population | Child only | Child and adolescent | Same as 2012 |
| Elderly | |||
| Women | |||
| Non-pharmacological biological therapy | ECT only | Including TMS, phototherapy, nutritional therapy, sleep deprivation, VNS, DBS as well as ECT | Same as 2012 |
| Response rate of review committee | 66.3% (67/101) | 54.5% (67/123) | 54.9% (79/144) |
AD, antidepressant; ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; VNS, vagal nerve stimulation; DBS, deep brain stimulation.
We summarized the results of third revision of Korean experts’ opinions on the pharmacological treatment of patients with depressive disorder and compared the results between the KMAP series.
METHODS
The overall study design and method of previous revisions were retained in this revision. To obtain the experts’ consensus, we composed a review committee and the review committee completed the modified questionnaire. The data were statistically analyzed.
Review Committee
The composition criteria for the review committee were the same as those of KMAP-DD 2012. We recruited 144 Korean psychiatrists who were life-long members of KSAD, had more than 15 years of clinical experience in the field of mood disorders, and who had each published at least one paper related to mood disorders during the previous year. Members worked in a wide variety of clinical settings (university hospitals, n=97; general and mental hospitals, n=34; private psychiatric clinics, n=13). All members of the review committee provided written informed consent for their participation in this survey. Of the 144 psychiatrists, 79 (54.9%) responded to our survey. Respondents received a predetermined fee for their participation.
Questionnaire
The KMAP-DD third-revision questionnaire was a modification of the instrument used for the KMAP-DD 2012 guidelines.2) The questionnaire included 7 sections and 44 general categories organized into 117 sub-items that offered 876 options. These were organized into the following sections: 1) MDD without psychotic features; 2) MDD with psychotic features; 3) persistent depressive disorder (dysthymia) and treatment for other clinical subtypes (melancholic features, atypical features, seasonal pattern, mixed features, anxious distress, and minor depressive disorder); 4) strategies for maintenance treatment; 5) special populations (children and adolescents, elderly persons, and women); 6) AD selection according to safety, tolerability, or comorbidity; and 7) non-pharmacological biological therapy (electroconvulsive therapy [ECT], repetitive transcranial magnetic stimulation [rTMS], etc.).
The executive committee decided to include the newer AD, such as desvenlafaxine, and vortioxetine. However, some drugs introduced in psychiatric congress but not yet available in Korea, such as levomilnacipran and vilazodone, were not included in this revision (Table 2). In this revision, AAP, such as amisulpride, aripiprazole, blonanserin, clozapine, paliperidone, quetiapine, risperidone, and ziprasidone; and typical antipsychotics were included.
Table 2.
Lists of drugs used in the Korean Medication Algorithm for Depressive Disorder 2017
| Antidepressant | Escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline |
| Dsvenlafaxine, duloxetine, milnacipran, venlafaxine | |
| Bupropion | |
| Mirtazapine | |
| Moclobemide | |
| Tianeptine | |
| Agomelatine* | |
| TCA (amitriptyline, clomipramine, imipramine, etc) | |
| Antipsychotics | Amisulpride, aripiprazole, blonanserin, clozapine, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone, typical antipsychotics |
| Mood stabilizer | Carbamazepine, lamotrigine, lithium, valproate |
| Augmentation drugs | Buspirone, gabapentin, ketamine, pindolol, psychostimulant, thyroid hormone, topiramate |
TCA, tricyclic antidepressant.
Agomelatine is temporarily withdrawn in Korea, owing to an issue with the management system for insurance issue.
Rating Scale
Each treatment option was scored on a nine-point scale. Nine indicates extremely appropriate, 7 to 8 indicates usually appropriate, 4 to 6 indicates ambivalence about its appropriateness, 2 to 3 indicates usually inappropriate (a treatment the clinician would rarely use), and 1 indicates extremely inappropriate (a treatment the clinician would never use). The remaining 12 questions, which related to the interval before switching AD, the duration of AD and antipsychotic treatment, and other relevant issues, were open-ended.
When answering, reviewers were asked to consider real practical treatment options rather than ideal practices, and to choose “q” if they had insufficient experience or information to answer a question.
Data Analysis
Mean of each question or option were calculated. And the presence or absence of consensus on each option/question was determined using a chi-square test to identify differences between groups. No significant difference between groups indicated lack of consensus. We then calculated the means and 95% confidence intervals (CI) of the experts’ scores and divided them into three categories according to the lowest 95% CI: first-line/preferred treatment, ≥6.5; second-line/reasonable treatment, <6.5 and ≥3.5; and third-line/inappropriate treatment, <3.5. Treatment of choice (TOC) was defined as an option that was rated at 9 points by 50% or more of the experts. The SPSS ver. 15.0 software package (SPSS Inc., Chicago, IL, USA) was used for the analyses of preference rankings and multiple responses.
Development of Treatment Guidelines and Algorithms
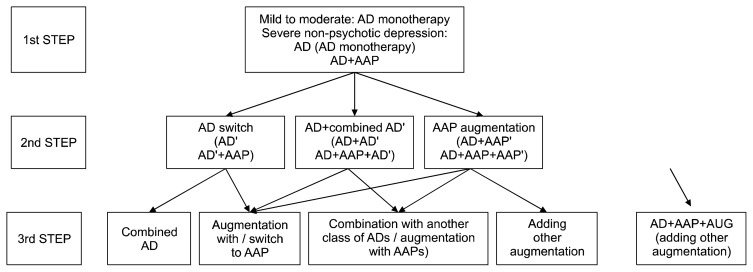
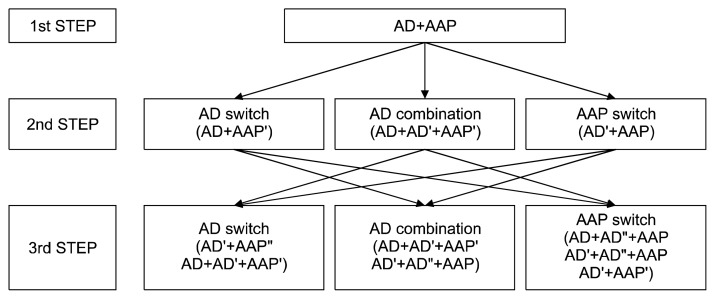
After discussing these results and reviewing the current evidences, considering Korean clinical situations, the executive committee drew up the third revised KMAP-DD algorithms (Figs. 1, 2), and will distribute them to the Korean psychiatrists and related experts.
Fig. 1.
Korean Medication Algorithm for Depressive Disorder 2017: Non-psychotic depression.
AD, antidepressant; AAP, atypical antipsychotics; AD′, another antidepressant; AD″, other antidepressant; AAP′, another atypical antipsychotics; AAP″, other atypical antipsychotics.
Fig. 2.
Korean Medication Algorithm for Depressive Disorder 2017: Psychotic depression.
AD, antidepressant; AAP, atypical antipsychotics; AD′, another antidepressant; AD″, other antidepressant; AAP′, another atypical antipsychotics; AAP″, other atypical antipsychotics.
Ethics
The present study was conducted according to the Declaration of Helsinki. The study protocol was approved by the Institutional Review or Ethics Committee at each study site.
The revision process was funded entirely by KSAD without external financial support.
RESULTS
Treatment Strategy for Acute Depression with or without Psychotic Features (Table 3)
Table 3.
Initial and next treatment strategies for depressive disorder between the Korean Medication Algorithm for Depressive Disorder 2017, 2012, and 2006
| Depressive episode | Third revision (2017) | Second revision (2012) | First revision (2006) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | |
| Initial treatment strategy | ||||||
| Mild to moderate episode | AD monotherapy* | AD+AD | AD monotherapy* | AD+AD | AD monotherapy* | AD+AD |
| AD+AAP | AD+AAP | AD+AUG | ||||
| AD+MS | ||||||
| Severe episode | AD monotherapy | AAP monotherapy | AD monotherapy | AD+AAP | AD monotherapy | AD+AD |
| AAP monotherapy | AD+AAP | |||||
| AD+AAP | AD+AD | AD+AUG | ||||
| AD+MS | AD+AD | |||||
| ECT | ECT | |||||
| Psychotic depression | AD+AAP* | AD+AAP* | AD+TAP | AD+AAP* | AD+TAP | |
| AAP monotherapy | ECT | |||||
| AD+AD | ||||||
| ECT | AD+AUG | |||||
| AD+AD | AD monotherapy | |||||
| AD monotherapy | AAP monotherapy | |||||
| 2nd treatment strategy | ||||||
| Mild to moderate episode (No response) | Switching AD | AUG | Switching AD | Adding AAP | Switching AD | AUG |
| Adding AD | Adding other AD | AUG | Adding other AD | Adding AAP | ||
| Adding AAP | ||||||
| Severe episode (Partial response) | Adding AD | Switching AD | Adding other AD | AUG | Adding other AD | Switching AD |
| Adding AAP | AUG | Adding AAP | Switching AD | AUG | Adding AAP | |
| Psychotic depression (Inadequate response) | Switching AAP | Adding AAP | Adding other AD | Adding other AAP | Adding AAP | AUG |
| Adding AD | AUG | Switching AAP | Switching AD | |||
| Switching AD | Adding TAP | Switching AD | AUG | Adding other AD | ||
| Adding TAP | ||||||
AD, antidepressant; AAP, atypical antipsychotics; MS, mood stabilizer; AUG, augmenting drugs (buspirone, gabapentinm, ketamine, pindolol, psychostimulant, thyroid hormone, topiramate); ECT, electroconvulsive therapy; TAP, typical antipsychotics.
Treatment of choice, defined as an option that was rated at 9 points by 50% or more of the experts.
Initial strategies for depressive episode
For non-psychotic MDD, mild-to-moderate depressive episodes, AD monotherapy (95% CI, 8.5–8.8 in third revision) was recommended as the TOC in the third revision, as same as in previous revisions. For non-psychotic severe episode, AD monotherapy and AD+AAP were the preferred (first-line) strategy, which indicates that preference for AAP was increased over that of the previous KMAP series (Table 4). For psychotic severe episode, AD+AAP was the TOC in all three KMAP-DD revisions. Second strategies when initial strategies have no or partial response
Table 4.
Comparison of preference of antipsychotics in the Korean Medication Algorithm for Depressive Disorder
| Preference of atypical antipsychotics | Third revision (2017) | Second revision (2012) | First revision (2006) (when using AP) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Non-psychotic | Psychotic | Non-psychotic | Psychotic | ||
| Amisulpride | 5.0 (4.6–5.5) | 6.0 (5.6–6.3) | 5.5 (5.0–5.9) | 6.6 (6.1–7.0) | 5.8 (5.3–6.2) |
| Aripiprazole | 8.3 (8.2–8.5)*,† | 8.3 (8.1–8.5)* | 7.9 (7.6–8.2)* | 7.9 (7.6–8.2)* | 6.3 (5.8–6.7) |
| Blonanserin | 4.6 (4.2–5.0) | 6.1 (5.7–6.5) | 4.4 (3.7–5.1) | 5.8 (5.1–6.4) | - |
| Clozapine | 2.7 (2.3–3.1) | 3.9 (3.4–4.3) | 2.9 (2.4–3.4) | 4.1 (3.6–4.6) | 3.5 (3.0–4.0) |
| Olanzapine | 6.0 (5.6–6.4) | 7.3 (7.0–7.7)* | 6.6 (6.2–7.0) | 7.6 (7.3–7.9)* | 7.1 (6.7–7.5)* |
| Paliperidone | 4.5 (4.1–5.0) | 6.9 (5.6–6.5) | - | - | - |
| Quetiapine | 7.8 (7.6–8.0)* | 7.9 (7.7–8.1)* | 7.7 (7.4–8.0)* | 8.1 (7.8–8.3)* | 7.3 (6.9–7.7)* |
| Risperidone | 5.3 (4.8–5.7) | 6.7 (6.3–7.1) | 6.0 (5.5–6.4) | 7.3 (6.9–7.6)* | 7.3 (6.9–7.7)* |
| Ziprasidone | 5.1 (4.6–5.6) | 5.9 (5.6–6.3) | 5.7 (5.2–6.3) | 6.5 (6.1–6.9) | 6.5 (6.0–6.9) |
| Typical antipsychotics | 2.9 (2.5–3.3) | 4.0 (3.4–4.3) | 3.2 (2.8–3.6) | 4.5 (4.0–5.0) | 4.8 (4.3–5.3) |
Values are presented as mean (95% confidence interval). AP, antipsychotics.
First-line drug maximum score of preference is 9 points.
Treatment of choice, defined as an option that was rated at 9 points by 50% or more of the experts.
When the patient is unresponsive to initial strategies, switching and adding AD or AAP were preferred, while with a partial response, switching was preferred to add other drugs.
AD Choices
Preferred AD for initial treatment
For mild-to-moderate depressive episodes, escitalopram (95% CI, 8.4–8.7) and sertraline (95% CI, 7.8–8.2) were the TOCs and fluoxetine, paroxetine, serotonin-norepinephrine reuptake inhibitors (SNRIs, duloxetine, milnacipran, venlafaxine, desvenlafaxine), and mirtazapine were recommended as first-line AD treatments. For nonpsychotic severe episodes, escitalopram, venlafaxine, and mirtazapine were the TOC, and fluoxetine, paroxetine, sertraline, duloxetine, and desvenlafaxine were the first-line drugs. For psychotic depression, escitalopram was the TOC, and other selective serotonin reuptake inhibitors (SSRIs) except fluvoxamine, SNRIs, and mirtazapine were the first-line drugs.
AD choice in light of adverse effects, safety, and comorbid physical illness
We asked the experts to choose three ADs when considering adverse effect, drug safety, and comorbid physical illness, respectively. Considering adverse effect, bupropion, mirtazapine, and tianeptine were preferred in terms of sexual dysfunction. Bupropion, fluoxetine, and escitalopram were preferred for sedation and somnolence. For weight gain, fluoxetine, bupropion, and tianeptine were preferred. For insomnia, mirtazapine, paroxetine, and tricyclic antidepressants (TCAs) were preferred. For gastrointestinal (GI) trouble, mirtazapine, tianeptine and bupropion were recommended. For anticholinergic side effect, escitalopram, sertraline, and bupropion were selected.
In matters of safety, for hypo- or hypertension, escitalopram, sertraline, and tianeptine; for serotonin syndrome, bupropion, tianeptine, and agomelatine; for seizure, escitalopram, sertraline, and tianeptine; for arrhythmia, escitalopram, sertraline, and tianeptine; for suicidality, mirtazapine, bupropion, and tianeptine were recommended.
In matters of comorbid physical illness, escitalopram and sertraline were recommended as first-line AD considering diabetes mellitus, thyroid disease, liver disease, and renal disease.
Organizing these findings by drug, bupropion was recommended by the Korean expert group based on considerations of sexual dysfunction, sedation, and weight gain. Furthermore, mirtazapine was a preferred AD based on considerations of insomnia, GI problems, and suicidality. Escitalopram was preferred based on considerations of anticholinergic side effects, hypo- or hypertension, arrhythmia, seizures, diabetes mellitus, and diseases of thyroid, liver, or kidney.
Treatment Duration with Initial AD before Next Strategy (Switching to or Adding Other AD, etc.) and Maintenance Treatment
Treatment duration with initial AD until switching to another AD
The experts were asked “How long do you keep using the initial drug until the next strategic change, such as switching or adding, due to lack of efficacy?”
With AD monotherapy for non-psychotic mild-to-moderate depressive episode, their answer was a minimum, 2.92 (±1.39) to maximum, 6.41 (±3.64) weeks. With AD monotherapy for severe episode, the answer was 2.82 (±2.35) to 6.05 (±5.34) weeks. When there is no response to the initial AD for psychotic depression, they wait for 2.34 (±1.95) to 4.71 (±3.77) weeks, while with partial response they wait for 3.37 (±1.86) to 6.49 (±3.69) weeks. When there is no response to AAP for psychotic depression, their answer was 2.26 (±1.91) to 4.61 (±3.77) weeks, while with partial response the answer was 3.28 (±1.76) to 6.26 (±3.09) weeks.
Duration of maintenance treatment of psychotic depression after remission (Table 5)
Table 5.
Duration of maintenance treatment
| Ongoing drug | Number of depressive episode | Taper and discontinue | After using some duration, taper and discontinue | Maintain continuously | |
|---|---|---|---|---|---|
|
| |||||
| Number (%) | Duration (wk) | ||||
| AD | 1 | 0 | 68 (86.0) | 19.8–46.8 | 11 (14.0) |
| 2 | 0 | 54 (68.4) | 34.8–78.4 | 25 (31.6) | |
| 3 or more | 0 | 13 (16.5) | 41.8–88.9 | 66 (83.5) | |
| AAP | 1 | 12 (15.2) | 61 (77.2) | 13.1–31.3 | 6 (7.6) |
| 2 | 3 (3.8) | 61 (77.2) | 21.6–49.8 | 15 (19.0) | |
| 3 or more | 1 (1.3) | 29 (36.7) | 28.8–59.6 | 49 (62.0) | |
The duration of AD+AAP treatment for psychotic depression after remission depends on the number of depressive episodes experienced by the patient. The majority of experts (86% for first episode; 54% for second episode) recommended that the ongoing AD treatment be stopped 19.8 to 46.8 weeks after the first episode and 34.8 to 78.4 weeks after a second episode. Experts recommended that the initial AAP therapy be maintained for 13.1 to 31.3 weeks for a first episode and 21.6 to 49.8 weeks for a second episode. However, following three or more episodes, 66% of the respondents recommended, “maintaining the ongoing AD as long as possible,” and 62.0% recommended, “maintaining the ongoing AAP as long as possible.”
Maintenance dose of ongoing AD and AAP after remission
The experts were asked, “How long do you maintain the dosage of ongoing drugs after remission, if there are no safety issues?” Most experts recommended maintaining 75% of the AD dose and 50% of the AAP dose used in the acute stage.
Treatment Strategies for Persistent Depressive Disorder (Dysthymia) and Strategies according Subtype or with Specifiers Mixed or Anxious distress
Treatment strategies for persistent depressive disorder
AD monotherapy with escitalopram was the TOC for persistent depressive disorder. AD choice according subtype of depressive episode
For the patients with melancholic features, escitalopram and venlafaxine were the TOC and fluoxetine, paroxetine, sertraline, duloxetine, milnacipran, desvenlafaxine, and mirtazapine were the first-line ADs. With regard to atypical and seasonal depression, escitalopram, fluoxetine, sertraline, SNRIs, bupropion, and mirtazapine were commonly recommended as first-line treatments. Paroxetine was the first-line treatment for seasonal pattern, but not for atypical features.
Treatment strategies and AD choice according specifiers, mixed features and anxious distress in depressive episode (Table 6)
Table 6.
Initial treatment strategies and drugs of choice for anxious distress or mixed features
| Subtype of depressive disorder | Initial treatment strategies | AD | AAP, MS | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | |
| Anxious distress | AD+AAP | MS monotherapy | Escitalopram | Fluvoxamine | Quetiapine | Lithium |
| AD monotherapy | AD+AD | Fluoxetine | Milnacipran | Valproate | ||
| AD+MS | Paroxetine | Bupropion | Carbamazepine | |||
| AAP monotherapy | Sertraline | Moclobemide | Lamotrigine | |||
| AD+TAP | Duloxetine | Tianeptine | Aripiprazole | |||
| Venlafaxine | Agomelatine | Olanzapine | ||||
| Desvenlafaxine | TCA | Risperidone | ||||
| Mirtazapine | Ziprasidone | |||||
| Other AAP* | ||||||
| Mixed features | AD+AAP | AAP monotherapy | Escitalopram | Fluvoxamine | Aripiprazole | Carbamazepine |
| AD+MS | MS monotherapy | Fluoxetine | Paroxetine | Quetiapine | Lamotrigine | |
| AD monotherapy | Sertraline | Duloxetine | Valproate | Risperidone | ||
| AD+TAP | Venlafaxine | Milnacipran | Olanzapine | Ziprasidone | ||
| AD+AD | Bupropion | Desvenlafaxine | Lithium | Other AAP* | ||
| ECT | Mirtazapine | Moclobemide | ||||
| Tianeptine | ||||||
| Agomelatine | ||||||
| TCA | ||||||
AD, antidepressant; AAP, atypical antipsychotics; MS, mood stabilizer; TAP, typical antipsychotics; ECT, electroconvulsive therapy; TCA, tricyclic antidepressant.
Amisupride, blonanserin, clozapine, paliperidone.
For mixed features, AD+AAP and AD+mood stabilizer (MS) were the first-line strategies and AAP, MS, or AD monotherapy were recommended not as first-line, but as second-line strategies. As preferred ADs, escitalopram, fluoxetine, sertraline, venlafaxine, bupropion, and mirtazapine were recommended, and as MSs, lithium, valproate, aripiprazole, olanzapine, and quetiapine were recommended. These strategies indicate that when treating MDD with mixed features, the experts would be cautious or concerned about manic switching or bipolarity.
With regard to anxious distress, AD monotherapy or AD+AAP were the initial treatment strategies. MS monotherapy, AD+AD, AD+MS, AAP monotherapy, AD+ TAP and ECT were recommended as the second strategies. As an initial AD, escitalopram, fluoxetine, paroxetine, sertraline, duloxetine, venlafaxine, desvenlafaxine, and mirtazapine were preferred. And quetiapine was the first AAP for anixious distress.
Treatment Strategies for Special Populations (Table 7)
Table 7.
Treatment strategies for major depressive disorder in special populations
| Special population and disorder | Severity of episode | Initial treatment strategies | AD | AAP, MS | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | ||
| Child and adolescent | |||||||
| Disruptive mood dysregulation disorder | AAP monotherapy | Escitalopram | Fluoxetine | Aripiprazole | Risperidone | ||
| MS monotherapy | Sertraline | Quetiapine† | |||||
| AD monotherapy | Desvenlafaxine | Valproate | |||||
| AD+AAP | Bupropion | Lamotrigine | |||||
| MS+AAP† | Mirtazapine | Lithium† | |||||
| MS+AD† | Fluvoxamine† | ||||||
| Venlafaxine† | |||||||
| MDD | Mild to moderate episode | AD monotherapy* | AD+AD† | Escitalopram | Sertraline | ||
| AD+AAP† | Fluoxetine | Bupropion | |||||
| AD+MS† | Paroxetine† | ||||||
| Fluvoxamine† | |||||||
| Duloxetine† | |||||||
| Venlafaxine† | |||||||
| Mirtazapine† | |||||||
| Severe episode | AD monotherapy | AD+AD | Escitalopram | Venlafaxine | |||
| AD+AAP | AAP monotherapy† | Fluoxetine | Duloxetine | ||||
| AD+MS† | Paroxetine | ||||||
| Bupropion | |||||||
| Mirtazapine† | |||||||
| Sertraline† | |||||||
| Fluvoxamine† | |||||||
| Desvenlafaxine† | |||||||
| Psychotic severe episode | AD+AAP* | AD monotherapy† | Escitalopram | Sertraline | Aripiprazole | Quetiapine | |
| AAP monotherapy† | Fluoxetine | Venlafaxine | Risperidone | Amisulpride† | |||
| AD+AD† | Duloxetine | Olanzapine† | |||||
| AD+TAP† | Bupropion | Paliperidone† | |||||
| AD+MS† | Paroxetine† | ||||||
| Mirtazapine† | |||||||
| Fluvoxamine† | |||||||
| Desvenlafaxine† | |||||||
| Elderly | |||||||
| MDD | Mild to moderate episode | AD monotherapy* | AD+AAP | Escitalopram* | Paroxetine | ||
| AD+AD | Sertraline | Fluvoxamine | |||||
| AD+MS | Duloxetine | Bupropion | |||||
| AAP monotherapy | Milnacipran | Tianeptine | |||||
| Venlafaxine | Moclobemide | ||||||
| Desvenlafaxine | TCA | ||||||
| Fluoxetine | |||||||
| Mirtazapine | |||||||
| Severe episode | AD monotherapy | AD+AD | Escitalopram* | Fluoxetine | |||
| AD+AAP | AD+MS | Sertraline | Fluvoxamine | ||||
| AAP monotherapy | Duloxetine | Paroxetine | |||||
| ECT | Milnacipran Venlafaxine | Bupropion | |||||
| Tianeptine | |||||||
| Desvenlafaxine | Moclobemide | ||||||
| Mirtazapine | TCA | ||||||
| Psychotic severe episode | AD+AAP* | AD monotherapy | Escitalopram* | Fluvoxamine | Aripiprazole* | Olanzapine | |
| AAP monotherapy | Fluoxetine | Paroxetine | Quetiapine | Risperidone | |||
| ECT | Sertraline | Bupropion | Amisulpride | ||||
| AD+AD | Duloxetine | Tianeptine | Blonanserin | ||||
| AD+TAP | Milnacipran Venlafaxine | Moclobemide | Paliperidone | ||||
| AD+MS | TCA | Ziprasidone | |||||
| Desvenlafaxine | |||||||
| Mirtazapine | |||||||
| Women | |||||||
| Premenstrual dysphoric disorder | AD monotherapy* | Anxiolytics | Escitalopram* | Fluvoxamine | |||
| MS | Fluoxetine | Milnacipran | |||||
| Others | Sertraline | Venlafaxine | |||||
| Paroxetine | Bupropion | ||||||
| Duloxetine | Mirtazapine | ||||||
| Desvenlafaxine | Moclobemide | ||||||
| Tianeptine | |||||||
| TCA | |||||||
| MDD in pregnancy | Mild to moderate episode | AD monotherapy | AAP monotherapy | ||||
| AD+AAP | |||||||
| ECT | |||||||
| Severe episode | AD monotherapy | AD+AAP | |||||
| AAP monotherapy | |||||||
| ECT | |||||||
| Psychotic severe episode | AD+AAP | AD monotherapy | |||||
| ECT | AAP monotherapy | ||||||
| Postpartum depression | Mild to moderate episode | AD monotherapy* | AD+AAP | ||||
| MS monotherapy | |||||||
| MS+AAP | |||||||
| AD+MS | |||||||
| AAP monotherapy | |||||||
| ECT | |||||||
| Severe episode | AD monotherapy | MS+AAP | |||||
| AD+AAP | MS monotherapy | ||||||
| AAP monotherapy | |||||||
| AD+MS | |||||||
| ECT | |||||||
| Psychotic severe episode | AD+AAP* | AAP monotherapy | |||||
| MS+AAP | |||||||
| AD monotherapy | |||||||
| ECT | |||||||
| MS monotherapy | |||||||
| AD+MS | |||||||
AD, antidepressant; AAP, atypical antipsychotics; MS, mood stabilizer; MDD, major depressive disorder; TAP, typical antipsychotics; ECT, electroconvulsive therapy; TCA, tricyclic antidepressant.
Treatment of choice,
no consensus.
Depressive disorder in child or adolescent
In contrast to MDD in adults, the results on children and adolescents with MDD contained more “no consensus” values. There is no first-line treatment for disruptive mood dysregulation disorder (DMDD). AAP, MS, or AD monotherapy were recommended as second-line treatment. Only escitalopram and aripiprazole were first-line ADs and AAPs, respectively.
AD monotherapy for non-psychotic severe episodes was the recommended first-line treatment for children and adolescents with mild-to-moderate and severe depressive episodes without psychotic features. The combination of AD+AAP was recommended as the first-line treatment for severe episodes with psychotic features. Escitalopram and fluoxetine were the first-line ADs. AD+ AAP for psychotic depression was the recommended first-line strategy, escitalopram and fluoxetine the recommended ADs, and aripiprazole and risperidone the recommended AAPs.
Elderly patients with MDD
AD monotherapy was the TOC for geriatric patients with mild-to-moderate depressive episodes. AD monotherapy and AD+AAP were the first-line strategies for severe episodes without psychotic features, whereas combination therapy with AD+AAP was the TOC for severe episodes with psychotic features. Moreover, escitalopram was a TOC for all three types of episode.
Women with depressive disorder
AD monotherapy was the first-line treatment option for premenstrual dysphoric disorder (PMDD). Escitalopram was a TOC for PMDD.
For MDD in pregnancy, AD monotherapy was recommended as a first-line treatment for mild-to-moderate and non-psychotic, severe depression. However, AD+AAP and ECT were recommended for psychotic severe depression. For postpartum depression, AD monotherapy was the TOC for mild-to-moderate episodes, and both AD monotherapy and combination therapy with AD and AAP were recommended as the first-line treatment for severe episodes without psychotic features. For severe episodes with psychotic features, AD+AAP were the recommended TOC.
Non-pharmacological Biological Treatment
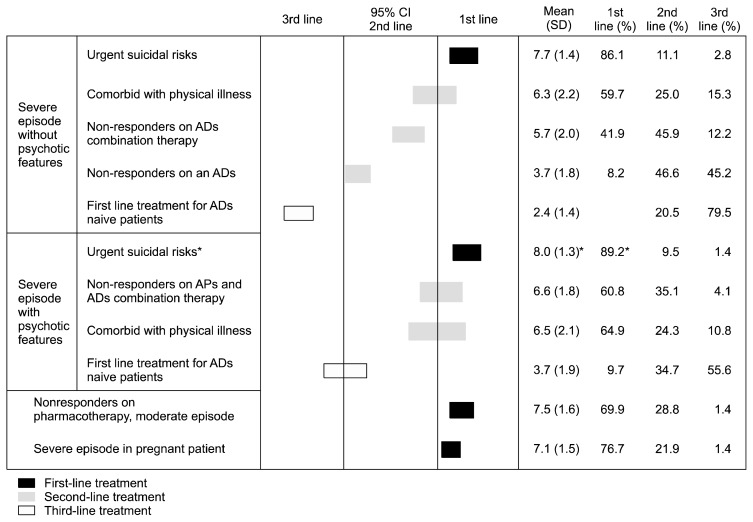
ECT (Fig. 3)
Fig. 3.
Indications of electro-convulsive therapy.
95% CI, 95% confidential interval; SD, standard deviation; ADs, antidepressants; APs, antipsychotics.
*Treatment of choice.
Ninety-two percent of experts considered ECT a MDD treatment modality and 46.8% of experts were applying it for MDD in clinical practice. On average, one expert conducts ECT with 5.6 persons per year, with 2.9 sessions per patient per week, totaling 9.6 sessions per patient during one treatment plan. The first-line indications for ECT were urgent suicidal risks in patients with non- or psychotic severe episode, non-responder on pharmacotherapy with moderate episode, or severe episode in pregnant patient.
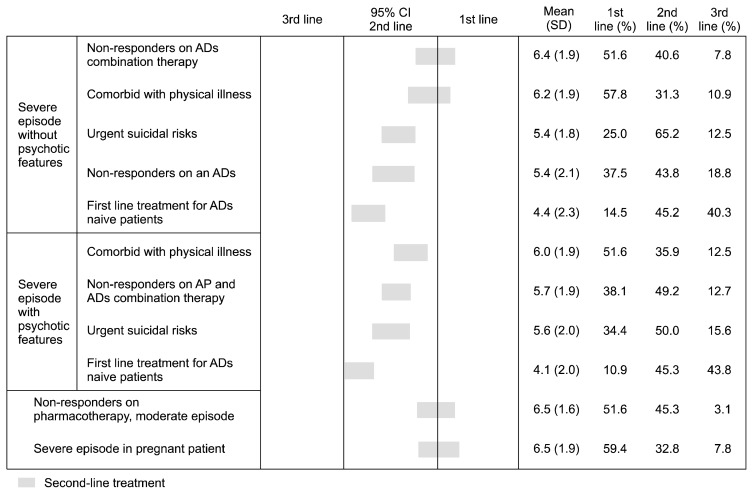
Indications for rTMS (Fig. 4)
Fig. 4.
Indications of repetitive transcranial magnetic stimulation.
95% CI, 95% confidential interval; SD, standard deviation; ADs, antidepressants; APs, antipsychotics.
Eighty-six percent of experts considered rTMS an MDD treatment option, but only 31.6% apply it in clinical practice for MDD. On average, one expert conducts rTMS with 12.7 persons per year, with 4.1 sessions per patient per week, totaling 12.6 sessions per patient during one treatment plan. In Korea, experts recommended rTMS as a second-line treatment option for MDD without urgent risks.
Choice of complementary or novel agents for treatment-resistant depressive disorder
Light therapy, nutritional therapy (omega-3, megavitamin), vagus nerve stimulation, S-adenosylmethionine, deep brain stimulation, and sleep deprivation were considered as second-line treatment options for MDD.
DISCUSSION
Are Expert Consensus and Evidence-based Guidelines Contradictory?
There are two types of guidelines, experts’ consensus and evidence-based. Most evidences are derived from randomized controlled trials (RCTs) with strict inclusion and exclusion criteria that do not reflect the complexity of various real clinical situations, and from meta-analyses of RCTs. Thus, there can be a gap between real-world practice and evidence from RCTs. Moreover, common problems of meta-analyses include small sample size, inadequate power, study heterogeneity, lack of extractable data, lack of interchangeable measurement instruments and definitions of outcomes, and other differences in the design of the studies whose data are utilized. On the other hand, clinical-consensus guidelines have a common problem that overall reliability and validity is questionable.6)
Our process for the present revision had two phases. First, we focused on the consensus emerging from various clinical situations, which RCTs cannot assess. We thereafter proceeded to an open discussion that addressed and evaluated the evidence. The recent guidelines of the Canadian Network for Mood and Anxiety Treatment has introduced the concept that the basis of guidelines should be balanced between systematic reviews and consensus expert opinion obtained from experienced clinicians, rather than depending exclusively on formalized evidence summaries.7) We agree with Möller,8) the first speaker on the International College of Neuropsychopharmacology (CINP) treatment guidelines for bipolar disorder at the 2016 CINP world seminar in Korea, who said that evidence-based and clinical-experience-based medicine are not contradictory, but complementary. For example, treatment recommendations for MDD with mixed features or with anxious distress or DMDD can be based on expert experience in the current absence of RCT-based evidence, with the proviso that the recommendations be validated by such evidence in the near future.
Treatment Strategy for Non- or Psychotic Depression
The preferred initial treatment strategy for non-psychotic MDD was AD monotherapy regardless of the severity of the depressive episode, as recommended in KMAP-DD 2012 and 2006. Compared with previous revisions, the notable finding in this revision is that preference for AAP has increased (Table 4); In KMAP-DD 2012 and 2006, the combination of AD+AAP was the second-line treatment, but in this revision, AD+AAP were recommended as a first-line strategy for non-psychotic severe episode. It was also recommended as a second strategy when the initial strategies give no- or partial responses. In the third revision, adding AAP was the preferred next strategy, while adding AAP was second-line among next strategies (Table 3). Although the recommendation grade for AAP as an initial treatment in nonpsychotic depression is weak,9) adjunctive AAP treatment in treatment-resistant depression (TRD) or failure of initial AD treatment has consistent supporting evidence,10–13) and is recommended in various guidelines, such as the Texas Medication Algorithm Project, Major Depressive Disorder Algorithms14) and the World Federation of Societies of Biological Psychiatry Guidelines for Biological Treatment of Unipolar Depressive Disorders (WFSBP 2013).15)
Increased preference for AAP reflects the efficacy of AAP in the treatment of non-psychotic MDD as well as TRD. Nelson and Papakostas16) reported that in their meta-analysis of 16 trials (n=3,480), adjunctive AAP significantly achieved more responses than AD monotherapy (odds ratio [OR], 1.69; 95% CI, 1.46–1.95; p<0.00001), as well as higher remission rates (OR, 2.00; 95% CI, 1.69–2.37; p<0.00001). Moreover, Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 recommended aripiprazole, quetiapine, and risperidone as first-line adjunctive drugs for nonresponse or partial response to an initial AD.17)
AAP treatment is associated with an increased risk of discontinuation due to adverse events16) and is less well tolerated than are SSRIs18); thus, AAP augmentation, rather than AAP monotherapy, may be more appropriate for patients with nonpsychotic MDD.
In the comparison between clinical guidelines of MDD by Wang et al.,19) such as APA 2010,20) WFSBP 2013,15) CANMAT 2016,17) and National Institute for Clinical Excellence (NICE) 2009,21) these guidelines recommended AD monotherapy as a first-line treatment for MDD without psychotic features while AD+AAP as well as AD monotherapy were recommended for the treatment of severe depressive episode without psychotic features in KMAP-DD 2017, which suggested the increased preference of AAP in Korea.
For psychotic depression, AD+AAP is the TOC in this revision, as in previous revisions. WFSBP 2013 presented AD+AAP as recommendation grade 3 (defined as limited positive evidence from controlled trials).15) It is not clear which is more effective, adding or switching within the same class of AD or using a different class. Connolly and Thase22) concluded that the strength of the evidence supporting augmentation and that supporting switching to a new agent after failure of a first-line SSRI were similar, with remission rates between 25% and 50%; thus, the data did not provide unequivocal support for either switching within or switching between AD classes. However, CANMAT 200918) summarized evidence that switching to another AD in non-responders results in good response and remission rates, and CANMAT 201617) recommended switching to another AD in cases of no response to the initial treatment, or adding another AD in cases of partial response to initial treatment, which is consistent with KMAP-DD third revision.
AD Choice
Preferred AD and as initial treatment
The results that escitalopram and sertraline were the TOC for non-psychotic mild-to-moderate and severe episode, and escitalopram was the TOC for psychotic depression are in contrast to those of KMAP-DD 2012, in which there was no TOC among the ADs. These results are similar to those of Cipriani et al.,23) who published a network meta-analysis showing that among 12 second-generation ADs, escitalopram, mirtazapine, sertraline, and venlafaxine had superior response relative to other ADs. The results that SSRIs except fluvoxamine, SNRIs except milnacipran, and mirtazapine were recommended for first-line AD treatment of psychotic depression show a trend of AD choice similar to that of KMAP-DD 2012. CANMAT 2016 recommended vortioxetine as well as SSRIs, SNRIs, agomelatine, bupropion, and mirtazapine as first-line.17) Interestingly, quetiapine was recommended as a second-line AD with TCAs, trazodone, moclobemide, selegiline, levomilnacipran, and vilazodone. Although agomelatine was withdrawn in Korea and it is not available now due to issues with the system for management of insurance price, agomelatine was recommended as a first-line AD by the Korean experts in agreement with CANMAT 200918) and CANMAT 2016,17) whereas there had been no consensus regarding agomelatine in 2012. Although no clear difference in efficacy among ADs was apparent,24,25) other factors including safety issues, clinical experience, and the rapidity of onset of AD action may underlie differences in preference among psychiatrists.26)
Influences on AD choice due to adverse effect, safety, and comorbid physical illness
Adverse effects
Comparisons of recommendations considering the side effects and safety of AD and the impact of comorbid diabetes mellitus by American (APA, 2000),27) Canadian 2007,28) and English (NICE, 2009)21) guidelines are fully subscribed to in KMAP-DD second revision.5) Generally, the recommendations of the KMAP-DD third revision were similar to those of the KMAP-DD 2012. With the exception of concerns about their sexual side effects, sedation, GI trouble, insomnia, and suicidality, the experts preferred SSRIs when considering issues related to safety, whereas they preferred bupropion when considering issues related to sexual dysfunction or sedation. Mirtazapine was preferred considering insomnia, GI problems, or suicidality. Considering weight gain, fluoxetine was recommended as the first-line AD instead of bupropion (50.8% in 2012, 22.1% in this survey). Because bupropion may be related less with weight gain and more frequently with headache and dry mouth, the preference for bupropion to avoid weight gain has decreased. CANMAT 201617) reviewed patients taking bupropion-XL, and found that they had more headache and dry mouth, as often as 28% and 34%, respectively, compared to other ADs.
Safety
Cardiovascular effects: cardiovascular side effects are rare with SSRI use, and the expert group strongly preferred SSRIs rather than other ADs if cardiovascular effects are an issue. However, there have been reports of mild bradycardia in patients treated with fluoxetine, fluvoxamine, and paroxetine29) and case reports of arrhythmia and syncope in response to treatment using SSRIs.30,31) This suggests that clinical cardiac monitoring is necessary when using SSRIs, despite the fact that SSRIs are less associated with adverse cardiovascular events than are TCAs.
Suicidality: SSRIs are related to nearly twice the risk (OR, 1.92) of suicide and suicidal attempts among adolescents in observational studies32) and in the US FDA direction that manufacturers of all AD revise their labeling as the result of an increase in suicidality among children and adolescents. The US FDA warning changed the target period from childhood and adolescence to young adulthood (18–24 years) during initial treatment.33) Given these results and issues, mirtazapine rather than an SSRI was preferred by the Korean experts considering suicidality. Although the relation between AD and suicidality is not clear,28) careful monitoring and assessment for suicidality should be undertaken at the beginning of AD treatment, particularly in adolescent and young adults.
Comorbid physical illness: Epidemiological studies have shown that the prevalence of depression ranges from 9% to 43% among patients with physical illnesses, including diabetes mellitus,34) cardiac disease,35) cancer,36) pain,37) and stroke.38) Although fluoxetine did not cause clinically significant changes in blood glucose levels in patients with diabetes mellitus or in thyroid hormone levels in patients with thyroid disease,39) regular monitoring of the blood glucose and thyroid hormone levels in the depressed patient with diabetes mellitus or thyroid disease is recommended.40) As SSRIs are metabolized in the liver, depressed patients with renal disease do not need to reduce AD dosage41) but depressed patients with liver disease do.42)
Treatment Duration with the Initial AD until Activation of Next Strategy (Switching to or Adding Other AD, etc.) due to Lack of Efficacy; Maintenance Treatment
Treatment duration with initial AD until switching to another AD
Recommended treatment duration (2.92–6.41 weeks for mild-to-moderate, 2.82–6.05 weeks for severe episode) with the initial AD for non-psychotic depression was relatively shorter than in KMAP-DD 2012 (3.20–7.49 weeks). However, the initial AD treatment duration for psychotic depression was similar to that of KMAP-DD 2012 (No response, 2.3–4.7 weeks vs. 2.4–4.7 weeks in 2012; Partial response, 3.4–6.5 weeks vs. 3.4–6.9 weeks in 2012).
Recently, the clinical implications of early improvement, defined as >20% to 30% reduction from baseline on a depression rating scale after 2 to 4 weeks of depression, have been emphasized. Evidence-based guidelines offer no recommendations on how long to maintain treatment with the initial AD in the expectation of seeing a response. Moreover, early improvement is correlated with later prognosis at 6 to 12 weeks and 2 to 4 weeks is considered the best duration for waiting for a response to the initial AD, based on low quality evidence.43) Thus, failure to see an early improvement should cause the expert to apply a shorter waiting duration at the initial AD treatment.
Duration of maintenance treatment after remission: psychotic depression (Table 6)
The notion that the duration of the initial AD treatment depends on the number of recurring episodes of psychotic depression did not change over 2016, 2012, and 2006. For a first and second episode, experts recommended at least 5 to 20 months of treatment. For three or more episodes, 83.5% and 62.0% of experts recommended not to discontinue the AD or the AAP, respectively. CANMAT 200918) recommended 6 to 9 months as maintenance therapy, and 2 years or more for those with a risk factor for recurrence. Recent meta-analyses of 72 trials (1–12 months, n=14,450) and 34 trials (more than 12 months, n=7,253) found significant benefit of AD over placebo,44) and results from 16 maintenance RCTs showed that the AD was superior to placebo in terms of recurrence (18% vs. 37%, respectively).45)
In summary, the duration of maintenance therapy for depressed patients depended on risk factors for recurrence, such as number of episodes, severity, psychiatric or physical comorbidity, and family history.
Treatment Strategies for Persistent Depressive Disorder (Dysthymia), and Strategies Specific to Subtype or Specifiers Such as Mixed or Anxious Distress
Treatment strategies for persistent depressive disorder
The initial strategy, AD monotherapy, was the same as that of KMAP-DD 2012. The difference was that the preference for bupropion was increased, moving it from a second-to a first-line drug. Among first-line drugs, the preference for SSRIs was higher than for SNRIs.
Treatment strategies specific to subtype
Melancholia
Little information about the most effective agents for the melancholic and atypical subtypes is available.18) Compared with KMAP-DD 2012, the preferences for escitalopram and venlafaxine were increased, which became the TOCs. Contrary to results of KMAP-DD 2012, the preference for bupropion for melancholia was decreased, to second-line. The APA 2010 describes ECT or pharmacotherapy as effective for the treatment of melancholia, and TCAs and SNRIs as more effective than SSRIs. These AD choices may reflect the core symptoms of melancholia, such as insomnia, anxiety, and psychomotor retardation.20)
Atypical features and seasonal pattern
Among first-line ADs, less sedative ADs were selected. Paroxetine was the only SSRI recommended as a second-line AD, which may reflect concerns about the atypical symptoms, such as hypersomnia and psychomotor retardation. Because of the negative impact of drug-drug interactions, moclobemide was not selected as a first-line treatment in considering any subtype or any severity. Level-1 evidence was found for the use of bupropion to prevent winter depressive disorder.46) APA 2010 recommended pharmacotherapy and adjunctive phototherapy, and described bupropion SR as approved by the US FDA for MDD with seasonal pattern.20)
Treatment strategies for specifiers mixed feature and anxious distress (Table 6)
The survey on specifiers is a newly added set of questions in this revision. Initial strategies for “with anxious distress” were AD+AAP and AD monotherapy. However, among AAP and MS, quetiapine was only first-line, which indicated the cause treatment with AAP and combined AD. WFSBP 2013 recommended SSRI, venlafaxine or TCA for MDD patients with prominent anxiety symptoms due to potential benefits from those drugs.15)
Regarding MDD with mixed features, the first-line strategies were AD monotherapy and AD+AAP. In the survey for the Korean Medication Algorithm for Bipolar Disorder 2014, MS+AAP was the TOC for bipolar I disorder, with mixed features indicating the initial treatment strategy.47) However, in this survey, MS+AAP were not included as options in the questionnaire, which need to be added in the next revision. CANMAT 2016 recommended monotherapy with lurasidone or ziprasidone owing to their efficacy compared with placebo.48,49)
Treatment Strategies for Special Populations (Table 7)
Treatment strategy for children and adolescents
DMDD is a new disorder introduced in DSM-5. The US National Institute of Mental Health offers severe mood dysregulation, and DSM-5 newly recognizes a disorder including two key symptoms, severe recurrent temper outbursts, and persistent irritability observable by others. DMDD symptoms are common in the child and adolescent, and the prevalence range is 2% to 5%.50,51) Psychostimulant was excluded in this survey because the experts were asked to answer concerning DMDD in the absence of comorbid attention deficit/hyperactivity disorder (ADHD). There was no first-line strategy for DMDD and a lack of evidence, but some positive results have been reported with psychostimulants, with an ongoing divalproex sodium trial for ADHD and an adjunctive risperidone trial for tic disorder. The Korean experts cautiously recommended AAP, MS, or AD monotherapy, with AD+ AAP as a second-line treatment plan. In treating tic disorder, aripiprazole is more favorable than risperidone in terms of side effects.52) In this survey, aripiprazole was preferred.
The prevalence rates of depression in children and adolescents are 2% and 4 to 8%, respectively.53) Similar to the recommendations of KMAP-DD 2012, AD monotherapy was recommended as the TOC for mild-to-moderate, and AD monotherapy and AD+AAP were the TOC for psychotic severe episodes. Aripiprazole and risperidone were recommended as first-line AAPs for psychotic severe depression. However, it is not clear whether AD therapy is as effective in children and adolescents as it is in adults; furthermore, ADs may increase the risk of suicide or self-harm in adolescents18) and may adversely affect young patients with bipolar disorder, particularly those who experienced the onset of depression before 24 years of age.54) Thus, clinical guidelines for children and adolescents recommend that psychological approaches, including cognitive-behavioral therapy (CBT), interpersonal therapy (IPT), psychoeducation, emotional support, and personal psychotherapy need be considered before pharmacotherapy for uncomplicated mild depression,55,56) and suggest that pharmacotherapy be reserved for patients with moderate or severe depressive episodes.53,57–59)
In this revision, the first-line ADs for children and adolescents with MDD were escitalopram and fluoxetine. A recent Cochrane review of 19 trials with subjects aged 6 to 18 years (n=3,335) reported that fluoxetine was significantly more effective than placebo, that sertraline was significantly effective with a small effect size, and that paroxetine did not prove efficacious in this population. The authors recommended fluoxetine as the TOC for the child/adolescent with MDD.60,61)
Treatment strategy for elderly adults
AD+AAP as well as AD monotherapy were newly recommended as first-line for non-psychotic severe depression. Aripiprazole and quetiapine were first-line AAP for elderly psychotic depression. Adjunctive aripiprazole with various ADs was found effective for elderly depression.62) CANMAT 2016 recommended switching to quetiapine and aripiprazole or their combination for inadequate response to initial treatment in elderly depression.63) SSRIs except paroxetine, recommended as second-line, became first-line ADs for the elderly. These results may reflect paroxetine’s anticholinergic effect.64,65)
Despite lack of evidence in treating elderly depression, it is clear that certain factors should be considered. Aging has an effect on the incidence and treatment outcomes of depression; the drug-drug interactions resulting from polypharmacy and the various comorbid physical illnesses should be taken into account.66) Because the vegetative symptoms of physical illnesses and impaired cognitive functioning may be misdiagnosed as symptoms of depression, 67) readjusting the dosage schedule or titration should be undertaken with caution.
Treatment strategy for women with PMDD or postpartum depression
As in the KMAP-DD 2012, the KMAP-DD 2017 recommends AD monotherapy as the TOC for PMDD and escitalopram is the TOC for PMDD; the other SSRIs, duloxetine, and desvenlafaxine were the recommended first-line drugs, consistent with previous studies.68)
The survey of MDD in pregnancy is a new section in this revision. MDD treatment should be chosen in light of a clear benefit-risk evaluation, taking into account possible harmful effects of the drugs on the fetus, potential malnutrition without MDD treatment, and risk of substance abuse including tobacco.69,70) CANMAT 2016 recommends escitalopram and sertraline as second-line while CBT and IPT are recommended as first-line, with recommendation to be cautious of paroxetine and clomipramine, which may be related to cardiac malformations.71) For postpartum depression, initial treatment strategies here are similar to those of KKAP-DD 2012, except that MS+AAP was recommended as first-line in 2012 for mild-to-moderate and psychotic severe episode, but are second-line in this revision.
ADs are the mainstays of the treatment of women with PMDD or postpartum depression.71) However, preference for AAP has been increasing over revisions because the efficacy of monotherapy and adjunction with AAP has been proven via various clinical trials.72–74)
The ADs with the least influence on postpartum and breast-feeding, such as escitalopram and sertraline75) were also recommended by the Korean experts.
Non-pharmacological Biological Therapy
Consistent with KMAP-DD 2012, ECT was recommended as a first-line strategy for non-psychotic severe MDD with urgent suicidal risk, and as a second-line strategy for non-responders to AD monotherapy or combination therapy and combined with physical illness (Fig. 3). TMS was also a second strategy for non-responder on AD combination therapy in severe episodes without psychotic features, and for non-responders to pharmacotherapy in moderate episodes. CANMAT 2016 recommended ECT as a second-line treatment for TRD with MDD, and rTMS as a first-line treatment based on the efficacy, tolerability, and safety. Most Korean experts consider ECT (92.4%) and rTMS (86.0%) good treatment strategies, but only 44.3% of experts have executed ECT, and only 31.6% have used rTMS.76) These results show that real practice in Korea is less accepting of ECT and rTMS. However, the executive committee recommended that ECT could be applied when depressed patients have potential suicidality or attempt. The frequencies of use of adjunctive complementary agents such as phototherapy, omega-3 nutritional therapy, and megavitamin with initial treatment drugs were 27.8%, 22.8%, and 12.7%, respectively. When used as adjunctives for TRD, the frequencies were 29.1%, 19.0%, and 8.9%, respectively.
Although the frequency of use of phototherapy was very low, CANMAT 2016 recommended monotherapy with phototherapy as a first-line treatment for seasonal MDD and mono- or adjunctive phototherapy as a second-line treatment for non-seasonal, mild-to-moderate MDD.77) Its combination with complementary therapy is recommended as a second strategy for treatment-refractory patients in this revision and in CANMAT 2016.77)
Advantages and Limitations of KMAP-DD Third Revision
A major limitation of the present study is that it was based on the consensus of Korean experts rather than on evidence. As stated earlier, we believe that the expert consensus and the evidence-based guidelines are complimentary, not contradictory. Second, the review committee may have been too small (n=144) to reach a valid consensus and to select a TOC. However, given that there are only 3,750 psychiatrists in Korea and given that the total membership of the KSAD is only 258, a sample of 144 psychiatrists may be sufficient. Finally, we did not explore psychosocial approaches, which should be addressed in a future study.
In summary, the pharmacological treatment strategy in KMAP-DD third revision was similar to that of KMAP-DD 2012; however, preference for the first-line use of AAPs was greater in 2012 than in 2006. Moreover, recommendations for specific ADs according to population, side effects, and safety issues reflect recent evidence.
To our knowledge, KMAP-DD third revision is the only expert’s consensus guideline in the world that has been updated and revised in almost every 4-year period since 2002. We expect it to provide clinicians with useful information about the specific strategies and medications appropriate for treating patients with MDD.
Acknowledgments
The present manuscript is a secondary publication of our group’s papers which were already published in the Korean language. Though we have already published the papers in Korea, we decided to present and share the results with the experts who speak English according to conditions for acceptable secondary publications as stated in Uniform Requirements for Manuscripts Submitted to Biomedical Journals by International Committee of Medical Journal Editors.
This study was supported by the Korean Society for Affective Disorders and the Korean College of Neuropsychopharmacology. This research did not receive any specific grant from funding agencies in the commercial sector. The authors report no conflicts of interest in this work.
REFERENCES
- 1.Field M, Lohr KN. Guidelines for clinical practice: from development to use. Washington, D.C: National Academy Press; 1992. pp. 32–35. [PubMed] [Google Scholar]
- 2.Seo JS, Song HR, Lee HB, Park YM, Hong JW, Kim W, et al. The Korean Medication Algorithm for Depressive Disorder: second revision. J Affect Disord. 2014;167:312–321. doi: 10.1016/j.jad.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clin Psychopharmacol Neurosci. 2016;14:1–16. doi: 10.9758/cpn.2016.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MS, Lim SW, Cha JH, Chung SK, Kim KS, Kasper S, et al. The Korean Medication Algorithm for Major Depressive Disorder (KMA-MDD): report of the Korean society of depressive and bipolar disorders. Int J Psychiatry Clin Pract. 2006;10:186–194. doi: 10.1080/13651500600633584. [DOI] [PubMed] [Google Scholar]
- 5.Seo JS, Min KJ, Kim W, Seok JH, Bahk WM, Song HC, et al. Korean medication algorithm for depressive disorder 2006 (I) J Korean Neuropsychiatr Assoc. 2007;46:453–460. [Google Scholar]
- 6.Fountoulakis KN, Young A, Yatham L, Grunze H, Vieta E, Blier P, et al. The International College of Neuropsychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), Part 1: background and methods of the development of guidelines. Int J Neuropsychopharmacol. 2017;20:98–120. doi: 10.1093/ijnp/pyw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam RW, Kennedy SH, Parikh SV, MacQueen GM, Milev RV, Ravindran AV CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: introduction and methods. Can J Psychiatry. 2016;61:506–509. doi: 10.1177/0706743716659061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Möller HJ. The CINP bipolar algorithm project: Are everything clinical practice and guideline supported clinical decision making in contrast?. 30th CINP World Congress of Neuropsychopharmacology; Jul 3–5, 2016; Seoul, Korea. p. S25-01. [Google Scholar]
- 9.American Psychiatric Association (APA) Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Washington, D.C.: APA; 2010. [Google Scholar]
- 10.Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010;(12):CD008121. doi: 10.1002/14651858.CD008121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10:e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen XJ, Wang LM, Liu ZL, Huang A, Liu YY, Hu JY. Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res. 2014;47:605–616. doi: 10.1590/1414-431X20143672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Keitner GI, Qin B, Ravindran AV, Bauer M, Del Giovane C, et al. Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol. 2015;18:pyv060. doi: 10.1093/ijnp/pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suehs B, Argo TR, Bendele SD, Crismon ML, Trivedi MH, Kurian B, et al. Texas medication algorithm project procedural manual: major depressive disorder algorithms. Austin, TX: Texas Department of State Health Services; 2008. [Google Scholar]
- 15.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14:334–385. doi: 10.3109/15622975.2013.804195. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry. 2016;61:540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117( Suppl 1):S26–S43. doi: 10.1016/j.jad.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 19.Wang HR, Bahk WM, Seo JS, Woo YS, Park YM, Jeong JH, et al. Korean Medication Algorithm for Depressive Disorder: comparisons with other treatment guidelines. Clin Psychopharmacol Neurosci. 2017;15:199–209. doi: 10.9758/cpn.2017.15.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Treatment of patients with major depression and practical guideline [Internet] American Psychiatric Association; 2010. [cited at 2016 Nov 10]. Available from: http://psychiatryonline.org/guidelines.aspx. [Google Scholar]
- 21.National Institute for Health and Clinical Excellence (NICE) Quick reference guide. Depression: management of depression in primary and secondary care. London: NICE; 2009. [Google Scholar]
- 22.Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. doi: 10.2165/11587620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 24.Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143:415–426. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas GI, Fava M. A metaanalysis of clinical trials comparing moclobemide with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. Can J Psychiatry. 2006;51:783–790. doi: 10.1177/070674370605101208. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, Churchill R, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. doi: 10.1002/14651858.CD006528.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 2nd ed. Washington, D.C.: American Psychiatric Association; 2000. [PubMed] [Google Scholar]
- 28.Bauer M, Bschor T, Pfennig A, Whybrow PC, Angst J, Versiani M, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World J Biol Psychiatry. 2007;8:67–104. doi: 10.1080/15622970701227829. [DOI] [PubMed] [Google Scholar]
- 29.Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem. 1999;6:469–480. [PubMed] [Google Scholar]
- 30.Buff DD, Brenner R, Kirtane SS, Gilboa R. Dysrhythmia associated with fluoxetine treatment in an elderly patient with cardiac disease. J Clin Psychiatry. 1991;52:174–176. [PubMed] [Google Scholar]
- 31.Friedman EH. Fluoxetine-induced bradycardia. J Clin Psychiatry. 1991;52:477. [PubMed] [Google Scholar]
- 32.Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009;180:291–297. doi: 10.1503/cmaj.081514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration (FDA) Antidepressant use in children, adolescents, and adults [Internet] Silver Spring, MD: US FDA; 2007. May 2, [updated 2016 Apr 13; cited at 2013 Nov 20]. Available from: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm096273.htm. [Google Scholar]
- 34.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 35.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/S0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 36.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 37.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52:253–264. doi: 10.1016/S0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- 39.Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Shelton RC, Winn S, Ekhatore N, Loosen PT. The effects of antidepressants on the thyroid axis in depression. Biol Psychiatry. 1993;33:120–126. doi: 10.1016/0006-3223(93)90311-Z. [DOI] [PubMed] [Google Scholar]
- 41.de Carvalho GA, Bahls SC, Boeving A, Graf H. Effects of selective serotonin reuptake inhibitors on thyroid function in depressed patients with primary hypothyroidism or normal thyroid function. Thyroid. 2009;19:691–697. doi: 10.1089/thy.2008.0261. [DOI] [PubMed] [Google Scholar]
- 42.Mandrioli R, Mercolini L, Saracino MA, Raggi MA. Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem. 2012;19:1846–1863. doi: 10.2174/092986712800099749. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima S, Uchida H, Suzuki T, Watanabe K, Hirano J, Yagihashi T, et al. Is switching antidepressants following early nonresponse more beneficial in acute-phase treatment of depression?: a randomized open-label trial. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1983–1989. doi: 10.1016/j.pnpbp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19:pyv076. doi: 10.1093/ijnp/pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldessarini RJ, Lau WK, Sim J, Sum MY, Sim K. Duration of initial antidepressant treatment and subsequent relapse of major depression. J Clin Psychopharmacol. 2015;35:75–76. doi: 10.1097/JCP.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 46.Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry. 2005;58:658–667. doi: 10.1016/j.biopsych.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Seo JS, Bahk WM, Lee JG, Woo YS, Jeong JH, Wang HR, et al. Korean Medication Algorithm for Bipolar Disorder 2014: depressive episode. Korean J Psychopharmacol. 2014;25:68–78. [Google Scholar]
- 48.Patkar A, Gilmer W, Pae CU, Vöhringer PA, Ziffra M, Pirok E, et al. A 6 week randomized double-blind placebo-controlled trial of ziprasidone for the acute depressive mixed state. PLoS One. 2012;7:e34757. doi: 10.1371/journal.pone.0034757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suppes T, Silva R, Cucchiaro J, Mao Y, Targum S, Streicher C, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173:400–407. doi: 10.1176/appi.ajp.2015.15060770. [DOI] [PubMed] [Google Scholar]
- 50.American Psychiatric Association. Diagnostic and statistical manual of mental disorders fifth edition (DSM 5) Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- 51.Baweja R, Mayes SD, Hameed U, Waxmonsky JG. Disruptive mood dysregulation disorder: current insights. Neuropsychiatr Dis Treat. 2016;12:2115–2124. doi: 10.2147/NDT.S100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo HK, Lee JS, Paik KW, Choi SH, Yoon SJ, Kim JE, et al. Open-label study comparing the efficacy and tolerability of aripiprazole and haloperidol in the treatment of pediatric tic disorders. Eur Child Adolesc Psychiatry. 2011;20:127–135. doi: 10.1007/s00787-010-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birmaher B, Brent D AACAP Work Group on Quality Issues. Bernet W, Bukstein O, Walter H, Benson RS, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 54.Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: misdiagnosis, antidepressant use, and a proposed definition for bipolar spectrum disorder”. Can J Psychiatry. 2002;47:125–134. doi: 10.1177/070674370204700202. [DOI] [PubMed] [Google Scholar]
- 55.Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein JB, Jacobs RH, Reinecke MA. Cognitive-behavioral therapy for adolescent depression: a meta-analytic investigation of changes in effect-size estimates. J Am Acad Child Adolesc Psychiatry. 2007;46:1403–1413. doi: 10.1097/chi.0b013e3180592aaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein RE GLAD-PC Steering Group. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007;120:e1313–e1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 58.Hughes CW, Emslie GJ, Crismon ML, Posner K, Birmaher B, Ryan N, et al. Texas children’s medication algorithm project: update from Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 59.Zuckerbrot RA, Cheung AH, Jensen PS, Stein RE, Laraque D GLAD-PC Steering Group. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): I. Identification, assessment, and initial management. Pediatrics. 2007;120:e1299–e1312. doi: 10.1542/peds.2007-1144. [DOI] [PubMed] [Google Scholar]
- 60.Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11:CD004851. doi: 10.1002/14651858.CD004851.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388:881–890. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 62.Steffens DC, Nelson JC, Eudicone JM, Andersson C, Yang H, Tran QV, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26:564–572. doi: 10.1002/gps.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacQueen GM, Frey BN, Ismail Z, Jaworska N, Steiner M, Lieshout RJ, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 6. special populations: youth, women, and the elderly. Can J Psychiatry. 2016;61:588–603. doi: 10.1177/0706743716659276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- 65.Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int Clin Psychopharmacol. 2014;29:185–196. doi: 10.1097/YIC.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexopoulos GS, Katz IR, Reynolds CF, 3rd, Carpenter D, Docherty JP. Expert Consensus Panel for Pharmacotherapy of Depressive Disorders in Older Patients. The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001:1–86. Spec No Pharmacotherapy. [PubMed] [Google Scholar]
- 67.Kim JM, Hong JP, Kim SD, Kang HJ, Lee YS. Development of a Korean version of the Perceived Deficits Questionnaire-Depression for patients with major depressive disorder. Clin Psychopharmacol Neurosci. 2016;14:26–32. doi: 10.9758/cpn.2016.14.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biggs WS, Demuth RH. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2011;84:918–924. [PubMed] [Google Scholar]
- 69.Le Strat Y, Dubertret C, Le Foll B. Child marriage in the United States and its association with mental health in women. Pediatrics. 2011;128:524–530. doi: 10.1542/peds.2011-0961. [DOI] [PubMed] [Google Scholar]
- 70.Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107–1111. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]
- 71.Ng RC, Hirata CK, Yeung W, Haller E, Finley PR. Pharmacologic treatment for postpartum depression: a systematic review. Pharmacotherapy. 2010;30:928–941. doi: 10.1592/phco.30.9.928. [DOI] [PubMed] [Google Scholar]
- 72.Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68:224–236. doi: 10.4088/JCP.v68n0207. [DOI] [PubMed] [Google Scholar]
- 73.Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/S1092852900020216. [DOI] [PubMed] [Google Scholar]
- 74.El-Khalili N, Joyce M, Atkinson S, Buynak RJ, Datto C, Lindgren P, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2010;13:917–932. doi: 10.1017/S1461145710000015. [DOI] [PubMed] [Google Scholar]
- 75.Molyneaux E, Howard LM, McGeown HR, Karia AM, Trevillion K. Antidepressant treatment for postnatal depression. Issues Ment Health Nurs. 2017;38:188–190. doi: 10.1080/01612840.2016.1182409. [DOI] [PubMed] [Google Scholar]
- 76.Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. neurostimulation treatments. Can J Psychiatry. 2016;61:561–575. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. complementary and alternative medicine treatments. Can J Psychiatry. 2016;61:576–587. doi: 10.1177/0706743716660290. [DOI] [PMC free article] [PubMed] [Google Scholar]