Abstract
Precision medicine is a concept which is recently gaining momentum in all branches of medicine. In particular in psychiatry it is greatly needed given the huge societal costs of psychiatric disorders and given the long time needed to observe benefit from treatments and the response variability. The future will be based on biological determinants, however until such an interesting but still futuristic aim will be reached, at present we may only rely on clinical features to guide our individualized prescription which is currently still frequently based on personal opinion and subjective previous experiences. The aim of this review is to offer an overview of the main aspects to take into consideration when prescribing an antidepressant treatment to reach the best precision medicine using clinical information. More than 40 compounds are available for treating depression and a similar amount of compounds for other psychiatric disorders. The process of matching the profile of the patient with all different profiles of available compounds is therefore quite complex. Our everyday prescribing procedure should take into consideration a number of factors such as the knowledge of the profile of available compounds versus the symptomatology profile of the subject, previous efficacy, medical comorbidities, tolerability profile, individual preferences, and family history. While we are waiting more complex algorithms including biological or genetic measures, it is possible to optimize our current prescription practice by using all available information in order to obtain as much as possible an evidence based precision medicine prescription.
Keywords: Antidepressive agents, Depression, Precision medicine, Psychiatry, Psychopharmacology
INTRODUCTION
In the office of the psychiatrist, a patient is sitting in front of the doctor. This is the first time they meet and, after 30 minutes of interview, the doctor formulates a diagnosis of major depression after detecting many depressive symptoms including insomnia. At this point the doctor starts the discussion about the prescription of an antidepressant:
Doctor: “I understand that you are feeling a lot of suffering from your depressive condition and I believe that an appropriate pharmacologic treatment would help you”.
Patient: “Thanks doctor, please prescribe me the most effective antidepressant. However consider that I heard that many drugs may induce weight gain, and I’m very worried about it. Further, given the needs of my daily work as a driver, I would prefer drugs not causing somnolence”.
Doctor: “I see”. At this point the doctor thinks about prescribing bupropion, which profile matches perfectly the patient needs in terms of tolerability, with no weight gain1) and no somnolence.2) However it has been demonstrated that bupropion may have a detrimental effect on sleep,2) and the patient previously reported that he is suffering from insomnia. Therefore other drugs which have a favorable profile on weight gain, no somnolence but not possibly increasing insomnia are citalopram and escitalopram, which the doctor is about to choose.
Patient: “Also I forgot to tell you that 2 years ago I suffered from a myocardial infarction, and my cardiologist told me that there are still some electrocardiographic abnormalities, since then I am taking Plavix 75 mg” (clopidogrel).
Doctor: “I see”. Then the doctor remembers that a warning has been issued for both citalopram and escitalopram regarding patients with cardiac diseases. Therefore he must think about another compound. Both fluoxetine and sertraline seem to have the profile needed in this specific clinical case, however, fluoxetine is known to reduce the efficacy of the maintenance treatment with clopidogrel (by interference with the CYP2C19 metabolism) which the patient is taking after the myocardial infarction.3)
Doctor: “Taking into consideration the clinical profile of your depression, your preferences and your medical comorbidities, I think that the best option is to start sertraline”.
The choice of the best compound for each patient is always a very difficult procedure. In a very short period of time during the consultation with the patient we have to choose among many compounds taking into consideration a large number of factors such as symptomatology profile, previous efficacy, medical comorbidities, subject preferences, family history and so on.
The difficulty of such process is exemplified above. At present we may use more than 40 compounds licensed for treating depression and a similar amount of compounds for other psychiatric disorders. The process of matching the profile of the patient with all different profiles of available compounds is therefore quite complex. Moreover not all is known about the factors which make some drugs work for some patients and not for others also considering all possible clinical and pharmacological factors. Therefore it is well known that in psychiatry pharmacologic treatments follow a trial and error procedure, however this methods leads frequently to start treatment with the first compound we have in mind or the one we use more frequently not considering all the available information which may lead to a more individualized, more effective and more tolerated treatment. In other words what is called precision medicine. Guidelines are usually of small help in selecting the most appropriate compound, also given the many factors which have to be considered that are not usually covered by guidelines. Guidelines in fact are mainly suggesting the use of classes and differences across single compounds are not usually reported.
Precision medicine intends to offer to clinicians the possibility to tailor the treatment according to the best possible evidence of effectiveness and tolerability for each subject. This aim is to be reached through a number of tools ranging from biologic measures to observable clinical features.
The task is challenging because of the huge complexity of psychiatric disorders which biological determinants are only partially known. Similarly, the large number of psychotropic medications available may often ingenerate confusion because of their different pharmacodynamic profile, not always completely known, such as the example of lithium, which reflects on their variable effect on each subjects’ different biologic background. In the present paper the focus will be on antidepressants but similar issues may be applied also to all psychiatric treatments.
MAIN TEXTS
The Need of Precision Medicine in Psychiatry
Precision medicine is a concept which is recently gaining momentum in all branches of medicine. In particular in psychiatry it is greatly needed given the huge societal costs of psychiatric disorders and, mainly, given the long time needed to observe benefit from treatments and the response variability.
The Future Will Be Based on Biological Determinants
It is known that over 50% of the variance of antidepressant response and tolerability is genetically controlled. Therefore in a not too distant future we will be able to identify the most suitable treatments for each subject on the basis of the genetic profile, as it is already happening in other fields of medicine. However at present only few genetic and non genetic biomarkers are known and their predictive power is still to be fully understood for a routine use in clinical practice.4) A combination of biological markers and clinical variables is the most promising strategy that at present has been suggested to reach a valid individualization of treatment.5) To reach this valuable aim a large study will be promoted in the United States under the name Precision Medicine Initiative.6) This study will include one million subjects followed for a number of years with the collection of clinical and biologic variables to the aim of identifying the signature of each treatment by disease outcome including psychiatric disorders and depression. The process to identify and validate biologic markers is indeed quite complex and the commercial products available at present are still to be considered preliminary. Once they will be fully developed we will use them in our everyday clinical practice.
However, until such an interesting but still futuristic aim will be reached, at present we may only rely on clinical features to guide our individualized prescription. What is particularly interesting is that clinical variables alone indeed constitute a very powerful tool which is not always correctly implemented in clinical practice.
The aim of the following sections will be to offer an overview of the main aspects to take into consideration when prescribing an antidepressant treatment to reach the best precision medicine at present using clinical information.
Are Guidelines Useful?
Guidelines and expert opinions are of great help for our everyday clinical practice. However the detail of information is not completely satisfying. Guidelines are very useful to have information about what not to do, how to avoid errors, but they lack of specificity when coming to the detail of the complexity described above. The large majority of guidelines for example suggest to use serotonin reuptake inhibitors or similar recent compounds licensed for treating depression; however, we may use more than 40 compounds and specific indication how to choose among them is missing, with some exceptions which offers a certain degree of detail such as the Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline.7) Therefore the clinician is basically left alone in the decision, and must rely on the vast knowledge that is available from reviews and meta-analysis in literature but that may result difficult to summarize. The aim of the following sections is to provide an overview of the decisional process referring to the original documents for an in depth coverage. The process would be much easier if there are some compounds which are more effective than others.
Which is the Most Effective Antidepressant?
All patients, such as the one described at the beginning of the article, ask for the most effective antidepressant and, similarly, all clinicians would like to offer to their patients the most effective compound. This would solve the precision medicine difficulty: we could prescribe the most effective compound to all subjects. Unfortunately no such compound exist. In the recent years many studies aimed to identify the most effective antidepressants. One of the most influential is a network meta-analysis published few years ago.8) In this study all antidepressants were ranked based on their efficacy and tolerability, however results raised some concerns. In fact it is common clinical experience that no overall best antidepressant exists, given that each compound has a unique and specific efficacy and tolerability profile based on unique pharmacodynamic profile. As an example, in the study of Cipriani et al.8) mirtazapine resulted to be among the best antidepressants, but we all know that mirtazapine specific pharmacodynamic profile is causing sedation and weight gain in most subjects. Therefore mirtazapine it is not generally indicated, for example, in subjects with atypical depression which is characterized by hypersomnia and increased appetite. So, no most effective antidepressant exists, and we should use further criteria for choosing the compound.
A Series of Steps Is Needed for a Good Clinically Based Precision Medicine
Given the lack of clear indications coming from guidelines and the lack of most effective compounds, unfortunately some clinicians prescribe according to personal opinion and subjective previous experiences. This is exactly the contrary of an evidence-based precision medicine and should be avoided. Therefore we need to choose according to other evidence-based criteria.
The first criterion is past response: if a subject already received benefit from a specific compound, this is the strongest criteria for prescribing the same compound again. If this information is missing, we may rely on the information about response in the same family. Given that first degree relatives share 50% of the genetic background, a positive response to a specific compound in a first-degree relative of the patient is also a very strong indication for the use the same compound, unless contraindicated for other reasons.9) A third very relevant set of criteria to take into consideration are the possible pharmacokinetic and pharmacodynamic interactions.10) Virtually all compounds have a variable degree of modulation of CYP enzymes leading to possible variations of plasma level of concomitant medications or the compound itself, which may lead to toxicity or to consequences deriving from an artificially lowered or increased plasma level. But also pharmacodynamic interactions may be relevant, as an example for many antidepressants, bleeding may happen in combination with some compounds. It is therefore advisable to check for both possible interactions using available web tools (e.g., https://www.drugs.com/drug_interactions.html).
Pharmacodynamic Profile as a Guide for Specific Symptomatology Profiles?
The about 40 compounds that we have for treating depression differs considerably about their pharmacodynamic profile. It would then seem very rational to choose on the basis of the specific profile of the compound vs. the specific symptomatology of the patient.11) As an example, a patient suffering from depression characterized by a predominant anhedonia and lethargy may benefit more from compounds which block the noradrenaline and the dopamine transporters, given that these two neurotransmitters have been associated with a more activating effect. Conversely, a patient with severe anxiety and insomnia may benefit more from compounds with anticholinergic or HT2c blocking effects. Even if this sounds very reasonable and many clinicians adopt similar algorithms in their clinical practice, specific evidence is still lacking and trials aimed at demonstrating the superiority of such algorithms vs. standard care would be very interesting.
However, one relevant pharmacodynamic feature which may be used clinically is the detachment induced by some compounds.12) Even if it not completely known which are the exact mechanisms involved, it is clear from a series of studies that some compounds cause a variable degree of detachment in the subject. This phenomenon has been observed both in human and animal studies. In technical terms the term “detachment” stand for the decrease of negative affects, i.e. the subject shows a reduced involvement and emotional response when negative events happen. This may be observed as early as few hours after administration and persists for the time coming. For some authors this is part of the mechanism of action of antidepressants.13) In any case it is very useful to use compounds which cause a relevant detachment, such as paroxetine, in subjects which look very reactive to negative and stressful environments. Interestingly this effect has been observed also in healthy subjects. However, for some subjects, this feeling may be perceived as negative, in particular when also positive affects are reduced (“Doctor, I feel better from my depression but I feel like living in a numb, nothing matters to me anymore”), in this case a dose reduction or change of the compound is indicated.
Medical Comorbidities Have to Be Considered
Considering the impact of the ageing population another important criterion to be considered is the presence of medical comorbidities. A number of compounds may be contraindicated in case of specific medical disorders. Detailed information about individualized treatment in patients with specific medical disorders may be found elsewhere,14) just as an example we should consider to avoid compounds with long half life in patients with hepatic impairment or compounds that are decreasing respiratory function in patients with chronic respiratory diseases.
But the tolerability profile of a specific compound is not only relevant in cases of concomitant medical disorder it should be considered also for patients without medical comorbidities in order to increase compliance. In fact it has been reported that compliance in outpatient settings can be as low as 50%, and this is mainly due to the fact that patients hardly tolerate the presence of mild but bothering side effects such as weight gain, sexual dysfunction, gastrointestinal symptoms or sleepiness. The range of side effects induced by the different compounds is in fact highly variable and it constitutes probably the most relevant criteria for choosing the best compound for each subject.
Tolerability as a Guide for Individualized Treatment?
Unfortunately no antidepressant is completely free of side effects. We have seen that the criterion of efficacy is not offering relevant information for the choice of the compound. Therefore the careful choice of the best tailored tolerability profile is probably the largest part of precision medicine we may use at present in the clinical practice. Commonly used antidepressants are in fact generally well tolerated but they present a range of side effects from common ones, which are not severe but may impair compliance in our patients, to less common side effects which are sometimes severe.
Sleep disturbances are very common in many psychiatric disorders including major depression. Regarding antidepressants, almost all of them have some impact on sleep. This feature is of great help in the individualization of the treatment. Unfortunately drug labels and current guidelines are of little help for choosing the compound on the basis of its effects on sleep because for many drugs they report both insomnia and somnolence. Also the clinical experience is quite variable. An interesting, though unpublished, research questioned 1,000 psychiatrists on a list of antidepressants asking their opinion for each of the compounds if it is mainly sedating or activating. Results showed a surprising variety of opinions, with the very interesting case of paroxetine which was sedating in the opinion of half of the clinicians and activating for the other half. This is probably the clearest example about the need of evidence based data for our clinical activity.
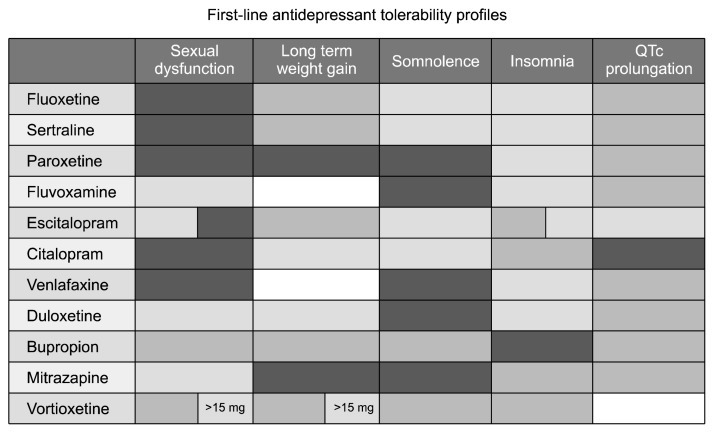
In a recent meta-analysis we ranked available compounds on the basis of their feature of inducing diurnal somnolence or insomnia,2) a summary is reported in Figure 1. Obviously there is an interindividual difference, but an overall guidance may be of use in clinical practice.
Fig. 1.
Tolerability profile of most common antidepressants based on published meta analyses. Darkness indicates more side effects, while brightness for less side effects.
Sexual dysfunction is another important issue both during the disease and as a side effect of many medications, therefore it is particularly troublesome for subjects and may lead to spontaneous treatment discontinuation. Sexual dysfunction is in fact extremely common during the depressive episodes to the point that it has been included into rating scales as a symptom of overall depressive severity. But sexual dysfunction is also a common side effect of many psychiatric treatments including antidepressants. Therefore in clinical practice it is particularly difficult to deal with this dysfunction: when recovering, patients are supposed to improve in their sexual functioning, however treatment may counterbalance this improvement by causing itself treatment induced sexual dysfunction. This time course has been observed many times in clinical practice and may lead to lack of compliance or to the false belief that depression is still present. Therefore a detailed explanation to patients of this phenomenon is needed but it is also very useful to have evidence based knowledge about the degree of sexual dysfunction induced by each available compound. In another meta analysis, we observed a large degree of variability, with some compounds (venlafaxine, fluoxetine, paroxetine, sertraline and citalopram) showing a treatment induced sexual dysfunction in more than 80% of the subjects, while others, such as escitalopram, duloxetine and fluvoxamine much less and bupropion resulting as mildly stimulating, probably due to its dopaminergic pharmacodynamic profile.15–17)
Weight gain is a further important and common side effect of antidepressants and of the large majority of psychiatric medications. Patients are very frequently concerned about weight gain and it is a common reason for lack of compliance, not to mention metabolic consequences. Also for this common side effect we performed a ranking of available compounds observing a large variability, particularly in the long term, with mirtazapine and paroxetine leading to an increase in weight of about 2 to 3 kg while others are more neutral and bupropion is on average leading to weight loss, again probably because if its dopaminergic profile.1)
CONCLUSION
Is a Precision Medicine Approach Possible on Clinical Data?
While we are waiting for more complex algorithms including biological or genetic measures, it is possible to optimize our current prescription practice by using all available evidence based information. Although guidelines are of some help, in the present review I summarized all the steps which should be undertaken to reach a treatment which is individualized on the clinical features of our patient taking into consideration all what is known about the over 40 compounds we may use to treat depression.
A small summary of the profile of the most common compounds is displayed in Figure 1. Obviously there are no ‘good’ and ‘bad’ drugs, each effect may be considered troublesome in some subjects and useful in others. As an example weight gain may be also beneficial in subjects which present a relevant weight loss due to the disorder.
Despite the fact that time during consultations is always little, that available information about compounds is large and constantly in development, we should strive to optimize our prescribing procedure to obtain as much as possible an evidence based precision medicine prescription.
Acknowledgments
Dr. Serretti is or has been consultant/speaker for Abbott, Abbvie, Angelini, Astra Zeneca, Clinical Data, Boheringer, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier.
REFERENCES
- 1.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 2.Alberti S, Chiesa A, Andrisano C, Serretti A. Insomnia and somnolence associated with second-generation antidepressants during the treatment of major depression: a metaanalysis. J Clin Psychopharmacol. 2015;35:296–303. doi: 10.1097/JCP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 3.Delavenne X, Magnin M, Basset T, Piot M, Mallouk N, Ressnikoff D, et al. Investigation of drug-drug interactions between clopidogrel and fluoxetine. Fundam Clin Pharmacol. 2013;27:683–689. doi: 10.1111/fcp.12021. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri C, Crisafulli C, Calabrò M, Spina E, Serretti A. Progress and prospects in pharmacogenetics of antidepressant drugs. Expert Opin Drug Metab Toxicol. 2016;12:1157–1168. doi: 10.1080/17425255.2016.1202237. [DOI] [PubMed] [Google Scholar]
- 5.Perlis RH. Abandoning personalization to get to precision in the pharmacotherapy of depression. World Psychiatry. 2016;15:228–235. doi: 10.1002/wps.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankar PL, Parker LS. The Precision Medicine Initiative’s All of Us Research Program: an agenda for research on its ethical, legal, and social issues. Genet Med. 2017;19:743–750. doi: 10.1038/gim.2016.183. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 9.Franchini L, Serretti A, Gasperini M, Smeraldi E. Familial concordance of fluvoxamine response as a tool for differentiating mood disorder pedigrees. J Psychiatr Res. 1998;32:255–259. doi: 10.1016/S0022-3956(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 10.Porcelli S, Fabbri C, Spina E, Serretti A, De Ronchi D. Genetic polymorphisms of cytochrome P450 enzymes and antidepressant metabolism. Expert Opin Drug Metab Toxicol. 2011;7:1101–1115. doi: 10.1517/17425255.2011.597740. [DOI] [PubMed] [Google Scholar]
- 11.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical application. New York: Cambridge University Press; 2013. [Google Scholar]
- 12.Serretti A, Calati R, Goracci A, Di Simplicio M, Castrogiovanni P, De Ronchi D. Antidepressants in healthy subjects: what are the psychotropic/psychological effects? Eur Neuropsychopharmacol. 2010;20:433–453. doi: 10.1016/j.euroneuro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. London: John Wiley & Sons; 2015. [Google Scholar]
- 15.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 16.Jeon SW, Han C, Ko YH, Yoon SY, Pae CU, Choi J, et al. Measurement-based treatment of residual symptoms using clinically useful depression outcome scale: Korean validation study. Clin Psychopharmacol Neurosci. 2017;15:28–34. doi: 10.9758/cpn.2017.15.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh KS, Shin E, Ha J, Shin D, Shin Y, Lim SW. Early improvement in one week predicts the treatment response to escitalopram in patients with social anxiety disorder: a preliminary study. Clin Psychopharmacol Neurosci. 2016;14:161–167. doi: 10.9758/cpn.2016.14.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]