ABSTRACT
Objective: Staphylococcus aureus is a particularly difficult pathogen to eradicate from the respiratory tract. Previous studies have highlighted the intracellular capacity of S.aureus in several phagocytic and non-phagocytic cells. The aim of this study was to define S.aureus interaction within a murine alveolar macrophage cell line.
Methods: Cell line MH-S was infected with Newman strain. Molecular mechanisms involved in phagocytosis were explored. To assess whether S.aureus survives intracellularly quantitative (gentamicin protection assays and bacterial plating) and qualitative analysis (immunofluorescence microscopy) were performed. Bacterial colocalization with different markers of the endocytic pathway was examined to characterize its intracellular trafficking.
Results: We found that S.aureus uptake requires host actin polymerization, microtubule assembly and activation of phosphatidylinositol 3-kinase signaling. Time course experiments showed that Newman strain was able to persist within macrophages at least until 28.5 h post infection. We observed that intracellular bacteria are located inside an acidic subcellular compartment, which co-localizes with the late endosome/lysosome markers Lamp-1, Rab7 and RILP. Colocalization counts with TMR-dextran might reflect a balance between bacterial killing and intracellular survival.
Conclusions: This study indicates that S.aureus persists and replicates inside murine alveolar macrophages, representing a privileged niche that can potentially offer protection from antimicrobial activity and immunological host defense mechanisms.
KEYWORDS: invasion, macrophage, persistence, phagocytosis, Staphylococcus aureus
Introduction
Staphylococcus aureus is an important human pathogen in both community and hospital settings. Infections can involve any organ system and can range from asymptomatic colonization to virulent forms of septicemia.1 The ability to cause such a wide range of infections is the result of its wide repertoire of virulence factors2 and strategies that evade recognition by the innate immune system.3 Resistance of S. aureus to methicillin and other current available treatments is increasing, becoming an important clinical problem.4
Although skin and soft tissue infections represent the major burden of staphylococcal disease, infections of the bloodstream and lower respiratory tract are of major interest because of the associated high morbidity and mortality and prolonged treatment requirements.5 Respiratory tract is a major reservoir of both methicillin susceptible and methicillin resistant S aureus (MRSA).6,7 S. aureus is a major cause of pneumonia following Influenza and is one of the most frequent etiological agents of ventilator associated pneumonia.8,9
Resident alveolar macrophages play a critical role in the clearance of bacteria from the lung by their capacity for phagocytosis and killing. This process can be conceptually divided into the phagosome formation and subsequent evolution into a degradative compartment, through the phagosome maturation process whereby the phagosome gains microbicidal activity. Macrophage maturation aids clearing infection, and at the same time it generates route antigens for presentation on MHC molecules to activate the adaptive immune system.10 Phagosome maturation is a process that involves sequential fusions and interactions with sub-compartments of the endocytic pathway.11 This maturation process is characterized by the acquisition of different proteins, such as antigens, GTPases, proteases, and ATPase in a choreographed sequence of events that culminates with the formation of the phagolysosome.12
Some pathogens have developed strategies to counteract the microbicidal effect of macrophages. These mechanisms include: inhibition of phagocytosis by preventing opsonophagocytosis or blocking specific signaling pathways,13 avoiding delivery to the lysosome, and release in the cytoplasm, as is the case for Listeria monocytogenes14 or arresting phagosome maturation, creating an optimal niche for replication, an essential feature of Mycobacterium tuberculosis survival.15
There is accumulating evidence that S. aureus is able to survive within eukaryotic host cells, both professional phagocytes, and non-professional phagocytes.16 This intracellular stage may be crucial for persistence, dissemination and infection of distant anatomic sites using phagocytes as a ‘Trojan horse’ delivery system.17 The role of neutrophils in innate host defense against S.aureus infections, and strategies to circumvent their function are being extensively investigated.18,19 In the same direction, there is interest in exploring the interplay between S.aureus and macrophages.20-23 Regarding staphylococcal lower respiratory tract infections, the murine model of pneumonia is the most explored one,24 and the evaluation of specific antibodies targeting virulence factors secreted during pneumonia is currently under investigation.25 So, exploring an in-vitro model using alveolar macrophage cell line can also be a valuable approach, as shown for Legionella pneumophila.26 A focus on exploring MRSA impact and importance during carriage and lung infections is justifiable, although the role of methicillin susceptible strains need also to be considered. Indeed persistent isolation of S.aureus in respiratory clinical samples has been reported regardless of cloxacillin resistance.27 Thus, to have a better understanding of the host-pathogen interaction specifically in the lower respiratory tract, one important aspect is to focus on S.aureus interaction with alveolar macrophage.
Thus, the objective of our study was to define the intracellular life style of S.aureus in an experimental infection model with a murine alveolar cell line.
Results
S.aureus survives inside murine macrophages
To investigate the molecular mechanisms used by MH-S to engulf S.aureus, infections were performed in the presence of drugs that inhibit specific host cell functions. Cytochalasin D (Cyt D) (prevents actin polymerization) and nocodazol (affects microtubule polymerization) significantly reduced bacterial engulfment, indicating that phagocytosis process requires the correct assembly of host F-actin and microtubule network. Depletion of cellular cholesterol with cholesterol-binding reagents, such as methyl-β-cyclodextrin (MβCD), nystatin, or filipin, slightly decreased bacterial phagocytosis but only nystatin pre-incubation reached statistical significance, as shown in Fig. 1(a). Since the generation of phosphoinositides is linked to the phagosome formation,28 we evaluated the contribution of phosphatidylinositol 3-kinase contribution (PI3K) signaling pathway on S.aureus phagocytosis. Pre-treatment of MH-S with LY294002, a specific inhibitor of PI3K activity, resulted in a decreased bacterial count, indicating phagocytosis blockage.
Figure 1.

Phagocytosis and dynamics of Newman S.aureus survival in MH-S. (a) Molecular mechanisms involved on S.aureus phagocytosis by MH-S cells. Eukaryotic cells were left untreated or pre-treated for 1 h with nocodazole, MβCD, nystatin, filipin or LY294002 and for 30 min with Cyt D. Cells were then infected for 30 min, and after 1 hour treatment with gentamicin (200 µg/ml), bacterial uptake was quantified by cell lysis, serial dilution and viable counting on LB agar plates. Results are expressed in c.f.u. per well. Data are representative of 3 independent experiments. *, p < 0.05 results are significantly different from the ones for untreated cells (control). (b) Time course analysis of intracellular S. aureus Newman in infected MH-S. Cells were infected for 30 min (MOI 25:1). Wells were later washed and incubated with medium containing gentamicin (200 µg/ml) for 1 h to eliminate extracellular bacteria, and then with medium containing gentamicin (25 µg/ml) (white) or media without gentamicin (black) for up to 28.5 h. Intracellular bacteria were quantified by lysis, serial dilution and viable counting on LB agar plate. Log 10 c.f.u./well are the average of at 6 independent experiments. *p < 0.05 results are significantly different between time points, for both conditions. (c) MH-S cells were infected with S.aureus as described above and the percentage of macrophages containing intracellular bacteria was assessed over time. At least 300 cells belonging to 3 independent experiments were counted per time point considered. (d) Percentage of infected macrophages containing one to 2, 3 to 5 or more than 5 intracellular bacteria (determined by extra and intracellular differential staining) over time. At least 300 cells belonging to 3 independent experiments were counted per time point considered. (e). Representative immunofluorescence analysis of infected MH-S. Image was taken at 3.5 h post infection and shows nuclei stained with Hoeschst 33342 (blue), actin cytoskeleton stained with rhodamine-phaloidin (red) and S.aureus stained with secondary antibody conjugated to Cy2 (green). (f) Cytotoxicity in S.aureus infected MH-S. Cells were infected with a MOI of 25:1. After 30 min of contact, wells were washed and later incubated with gentamicin (200 µg/ml) (white) or media without gentamicin (black) until 24 h. At specific time points, cell viability was determined by means of neutral red uptake assay. Experiments were performed by triplicate in 3 independent occasions, p < 0.05. All comparisons were statistically significant when comparing values obtained in the presence of antibiotic. Statistical significant differences were observed between 6 h and 18 h for those wells without antibiotic.
To determine the fate of S.aureus once inside MH-S macrophages, intracellular bacteria were assessed by the gentamicin protection assay following differing incubation periods and microscopically using differential staining. Results indicate that macrophages were able to engulf S.aureus, and that once inside the macrophage, S.aureus was able to survive intracellularly [Fig. 1(b)]. The increase for the earliest time point to the last one was statistically significant (p < 0.05). Although the number of intracellular bacteria in MH-S macrophages decreased during the first hour post infection, counts remained constant till 16.5 h post infection. From that time point, there was an increase in the number of intracellular bacteria up to 28.5 h post infection that was the last time point considered.
Given that antibiotics can be internalized and delivered to terminal lysosomes as phagocytosed bacteria,22 the experiment was also performed in the absence of gentamycin (25 µg/ml) for the long-term incubation periods, to ensure that the presence of antibiotic was not affecting intracellular bacteria (Fig 1b). Intracellular bacterial counts showed a similar trend between conditions, thus the observations presented are due to S. aureus biology, and not to experimental design.
Microscopy analysis revealed that the percentage of infected macrophages was similar during the time course, suggesting the absence of re-infections [Fig. 1(c)]. The number of macrophages containing 1 to 2 bacteria decreased along time [Fig. 1(d)]. The number of macrophages containing between 3 and 5 bacteria tend to decrease slightly along time. In contrast, the number of macrophages containing more than 5 bacteria increased, suggesting the microorganism was not only able to survive, but also to replicate inside macrophages [Fig. 1(e)]. We also analyzed whether S.aureus exerts a cytotoxic effect on macrophages. For that, we estimated the viability of infected MH-S cells by the neutral red uptake assay.29 S.aureus infection was associated with a slight increased cell death from 18 h post-infection that reached 20% cell death after 24 h post infection [Fig. 1(f)]. The experiment was also performed in the absence of antibiotic for long-term incubation. Results show, that in the absence of gentamycin, the increase in cytotoxicity begins at 6 h post-infection, reaching similar values at 24 h.
Collectively, these results show that Newman strain is able to survive and replicate within MH-S and that the phagocytosis process is an event dependent on host cytoskeleton and PI3K/Akt signaling pathway.
S.aureus subcellular localization
Since S.aureus is able to survive within macrophages, we hypothesized that it could inhibit or divert the normal process of phagosome maturation or withstand in the hostile environment of a mature phagolysosome. We examined the presence of markers specific of the consecutive sub-compartments of the endocytic pathway, on the S.aureus containing compartment. Early endosome antigen 1 (EEA-1) is an endosome-specific peripheral membrane protein found in early phagosomes.11 As shown in Fig. 2(a), we detected the presence of EEA-1 on 40% of phagosomes at 30 min post infection. The overlap of the phagosome containing S. aureus with this marker dropped to 20% from 45 min post infection. We then sought to determine whether the phagosome acquires late endosomal markers such as lysosome-associated membrane protein 1 (Lamp-1) and Rab7. At 0.5 h post infection, Lamp-1 was detected on almost 50% of S.aureus containing phagosomes. At 1.5h post infection, the percentage increased up to 80%. From that moment and until the last time point considered, the percentage of colocalization remained constant at 100%. [Fig. 2(b)]. Rab7 is another characteristic marker of late phagosome. It is a small G-protein that controls vesicular transport between phagosomes and late endosomes or lysosomes in the endocytic pathway.11 Almost 100% of S.aureus containing phagosomes were positive for GFP-Rab7 since 1.5 h post-infection [Fig. 2(c)]. To determine the activation status of Rab 7, cells were transfected with a plasmid containing GFP fused to the C-terminal Rab7-binding domain of Rab-interacting lysosomal protein (RILP), named RILP-C33. RILP is a Rab7 effector protein that exclusively recognizes the active conformation of Rab7 (bound to GTP). As shown in Fig. 2(d), RILP-C33-GFP co-localized with the majority of S.aureus containing phagosomes at all time points analyzed.
Figure 2.
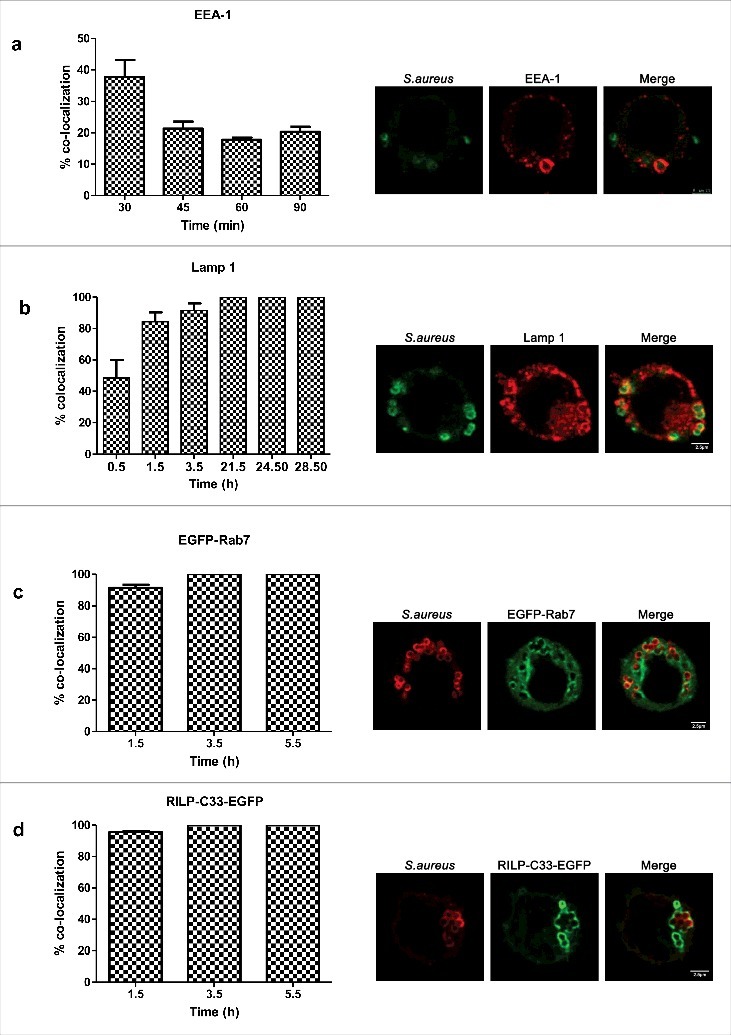
Phagosome maturation during S.aureus infection. (a) Percentage of colocalization of S.aureus and the early endosomal marker EEA-1 over a time course (images were taken 0.5 h post infection). S.aureus was stained with rabbit anti-S.aureus and Cy2-conjugated donkey anti-rabbit (green) antibodies. EEA1 was stained with goat anti-EEA1 and rhodamine-conjugated donkey anti-goat (red) antibodies. (b) Percentage of colocalization of S.aureus and Lamp-1 over a time course (images were taken 24.5 h post infection). S.aureus was stained with rabbit anti-S.aureus and Cy2-conjugated donkey anti-rabbit (green) antibodies. Lamp-1 was stained with mouse anti-lamp-1 and rhodamine-conjugated donkey anti-rat antibodies. (c) Percentage of colocalization of S.aureus and GFP-Rab7 over a time course (images were taken 1.5h post infection). Cells were transfected 24 h before the experiment with GFP-Rab7. S.aureus was stained with rabbit anti-S.aureus and texas red-conjugated donkey anti-rabbit (red) antibodies. (d) Percentage of colocalization of S.aureus and RILP-C33-EGFP over a time course (images were taken 3.5 h post infection). Cells were transfected 24 h before the experiment with RILP-C33-EGFP. S.aureus was stained with rabbit anti-S.aureus and texas red-conjugated donkey anti-rabbit (red) antibodies. Images are representative of triplicate coverslips in 3 independent experiments. At least 300 infected cells belonging to 3 independent experiments were counted per marker and time point considered.
Since the interaction of Rab7 with RILP drives fusion with lysosomes,30,31 we sought to determine whether S.aureus containing compartment co-localizes with lysosomal markers. Although there are no markers that can unambiguously distinguish late endosomes from lysosomes, mounting/existing evidence indicates that an acidic luminal pH is characteristic of the phagolysosomal fusion.11,32 We used the fixable acidotropic probe lysotracker to monitor acidic organelles. We found a major overlap between the dye and the S.aureus containing phagosome. At 1.5 h post infection the percentage of colocalization was 75% and from 21.5h post infection till last time analyzed it was 100%. These results hence indicate that the compartment is acidic [Fig. 3(a)]. To further evaluate whether compartments of the endocytic pathway, including lysosomes were involved in the phagosome maturation, we assessed the colocalization of bacteria containing compartment with tetramethylrhodamine-labeled dextran (TMR-dextran). Prior to bacterial infection, macrophages were pulsed with TMR-dextran for 2 h followed by 1h chase in dye-free medium to ensure that the probe was delivered from early and recycling endosomes to lysosomes. Pulse-chase protocols with TMR-dextran are extensively used in the literature to label lysosomes.33 We observed that at 1.5 h and 3.5 h post infection, 26% and 37% of compartments co-localized with TMR-dextran [Fig. 3(b)]. At 5.5h and 24.5h post infection, 47% of the intracellular S.aureus co-localized with TMR-dextran, suggesting that phagosomes containing S.aureus either partially prevent phagolysosome delivery, or rather delay it. When incubating macrophages with UV-killed S.aureus, almost 65% of S.aureus containing phagosome already co-localized with TMR-dextran after 15 and 30 min post infection.
Figure 3.
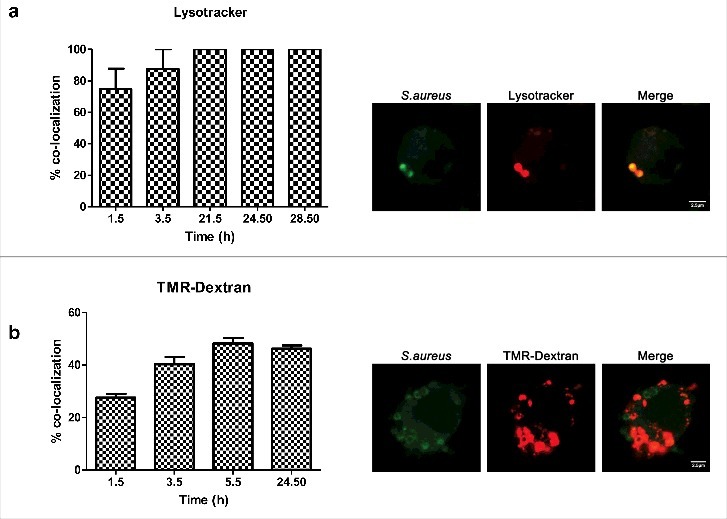
Colocalization of S.aureus with phagolysosomal markers. (a) Percentage of colocalization of S.aureus inside acidic compartments over a time course (images were taken 1.5 h post infection). S.aureus was stained with rabbit anti-S.aureus and Cy2-conjugated donkey anti-rabbit (green) antibodies. Acidic compartments were loaded with lysotracker (red). (b) Percentage of colocalization of S.aureus with TMR-dextran (red) over a time course (images were taken 3.5 h post infection). Cells were pulse–chased with TMR-dextran, then infected and fixed at the indicated times. S.aureus was stained with rabbit anti-S.aureus and Cy2-conjugated donkey anti-rabbit (green) antibodies.
Collectively, these findings suggest that S.aureus trafficked across classical endocytic pathway, but delay its phagolysosomal fusion.
Inhibition of compartment acidification does not affect S.aureus intracellular survival
Phagosome acidification has been shown to be essential for the intracellular survival of some pathogens.34,35 Therefore, since we have localized S.aureus inside acidic compartment, we investigated the effect of inhibiting compartment acidification on S.aureus survival. Bafilomycin A1 is a specific inhibitor of the vacuolar type H+-ATPase (V-ATPase) that inhibits the acidification of organelles containing this enzyme, such as lysosomes and endosomes. To assess the effect of vacuolar acidification on intracellular S.aureus survival, cells were treated with bafilomycin A1 (100 nM) at the onset of gentamicin treatment and bacteria were enumerated by plating at different time points [Fig. 4(a)]. Results show that although there is a slight decrease of bacterial number when comparing bafilomycin treated vs DMSO (regardless of the time point selected), S.aureus is able to persist in bafilomycin treated cells. Compartment acidification was sensitive to bafilomycin A1, thereby lysotracker staining was removed confirming dependence on the cellular vacuolar H+-ATPase [Fig. 4(b)]. Altogether, these observations suggest that compartment acidification is not critical for survival.
Figure 4.
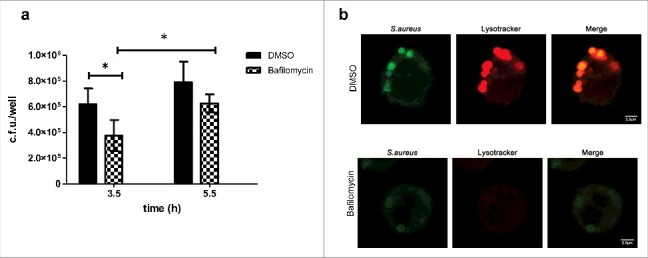
Importance of vacuolar acidification on S.aureus survival (a) Effect of bafilomycin on intracellular bacterial counts. MH-S were treated with bafilomycin A1 (100 nM) at the onset of gentamicin treatment and bacteria were quantified by lysis, serial dilution and viable counting on LB agar plates. Data, shown as c.f.u./well are the average of 3 independent experiments; *, p < 0.05. (b) Microscopy analysis showing that acidification was sensitive to bafilomycin A1. Upper panel shows cells treated with DMSO (control): lysotracker staining overlaps with S.aureus containing compartment whereas lower panel shows cells treated with bafilomycin A1, where overlap between S.aureus and lysotracker is absent (images taken at 3.5 h post infection).
Rab 14 and PI3K/Akt axis
At least 18 Rab GTPases have been implicated in phagosomal maturation. Interestingly, some microorganisms such as Salmonella, M.tuberculosis, and Klebsiella pneumoniae target Rab14 to prevent phagosomal maturation.36-38 To explore whether S.aureus may also target Rab14 to control the phagosome maturation, we monitor Rab14 by infecting MH-S with GFP-Newman and later staining coverslips with anti-Rab14 antibody. However, no colocalization was detected from 0.5 h to 5.5 h post-infection [Fig. 5(a)]. Given that Rab recruitment can be transient and thus difficult to assess, and that phagosomes containing S. aureus partially prevented or delayed phagolysosome delivery to lysosomes, we also explored the potential implication of Rab14 using transfection experiments. Cells were transfected with a Rab14 dominant-negative construct (DN-Rab14) or control vector (pcDNA3) and later infected with Newman strain. As shown in Fig. 5(b), bacterial counts were statistically lower in cells transfected with DN-Rab14 plasmid in comparison to pcDNA3 transfected cells at all time points considered, suggesting that the overexpression of inactive Rab14 enabled almost a complete phagolysosome maturation and therefore elimination of intracellular S.aureus. To confirm these results microscopically, we assessed colocalization of TMR-dextran in transfected cells with both plasmids. As expected the percentage of colocalization was higher in DN-Rab14 transfected cells (62.9% at 1.5 h post infection and 61.7% at 3.5 h post infection) in comparison to control conditions (36.8% at 1.5 h and 53.9% at 3.5 h) [Fig. 5 (c)].
Figure 5.

Rab 14, PI3K-Akt axis and intracellular survival of S.aureus. (a) Absence of colocalization of S.aureus and Rab14 over a time course (images were taken 1.5 h post infection). Cells were infected with GFP-S.aureus. Rab14 was stained with rabbit anti-Rab14 and Rhodamine Red-conjugated donkey anti-rabbit (red) antibodies. Images are representative of triplicate coverslips in 3 independent experiments. (b) Quantification of intracellular bacteria in transfected MH-S with plasmid pcDNA3 or with Rab14 dominant negative construct (DN Rab 14) over a time course. Cells were transfected 24 h before the experiment, and cells were infected as described above. Data shown as c.f.u./well are the average of 3 independent experiments; *, p < 0.05. (c) Percentage of colocalization of S.aureus with TMR-dextran (red) over a time course (images were taken 3.5h post infection). Cells were transfected 24 h before the experiment with DN Rab 14 and pcDNA3 constructs. Cells were pulse–chased with TMR-dextran, then infected and fixed at the indicated times. S.aureus was stained with rabbit anti-S.aureus and Cy2-conjugated donkey anti-rabbit (green) antibodies. (d) Immunoblot analysis of total Akt, and Akt and AS160 phosphorylation (P-Akt and P-AS160) in lysates of MH-S infected with S.aureus for the indicated times. Detection of Akt-Ser473 phosphorylation by Western blotting with rabbit anti-phosphoSer473 Akt and goat anti-rabbit conjugated to horseradish peroxidase antibodies (first panel). Detection of AS160 (Thr642) phosphorylation with rabbit anti-phosphoThr642 AS160 and goat anti-rabbit conjugated to horseradish peroxidase antibodies (second panel). Detection of total Akt by Western blotting with rabbit anti- Akt and goat anti-rabbit conjugated to horseradish peroxidase antibodies (third panel). Detection of tubulin was used as loading control (fourth panel). LY294002 (75 μM) was added to the cells during gentamicin treatment and kept until the end of the experiment. Data are representative of 3 independent experiments. (e) Quantification of intracellular bacteria in MH-S infected with S.aureus, which were mock-treated (black bar) or treated with Akt inhibitor X (10µM) or LY294002 (75 µM). Treatments were added after the time of contact, during the gentamicin treatment and kept until cells were lysed for bacterial enumeration. Data, shown as c.f.u./well are the average of 3 independent experiments.
Several pathogens target the PI3K-Akt axis to manipulate intracellular trafficking on their own benefit.39 This is because Akt inactivates the Rab14 GTPase activator, named AS160 thereby preventing AS160 recruitment to the phagosomal membrane. This fact extends Rab14 activation, avoiding phagosome maturation.39-41 We have previously shown that PI3K is necessary for S.aureus internalization. As PI3K has been also related with phagosome maturation,28 we further explored whether PI3K had a role during intracellular trafficking of S. aureus containing phagosome. Akt is a downstream effector of PI3K, which becomes phosphorylated upon activation of the PI3K signaling cascade. Western blot analysis revealed that S.aureus infection process induced Akt activation (P-Akt) in a PI3K-dependent manner [Fig. 5(d)], and LY294002 administration during gentamicin treatment inhibited Akt phosphorylation. Additionally, Western blot analysis also showed that infection with S.aureus triggered the phosphorylation of AS-160 in a PI3K-Akt dependant manner, since the presence of LY294002 inhibitor prevented S.aureus-induced AS-160 phosphorylation [Fig. 5(d)].
We next sought to determine the particular contribution of PI3K-Akt axis to the intracellular survival of S.aureus. Inhibition of PI3K-Akt axis did not affect intracellular survival counts. Treatment of cells with PI3K inhibitor, LY294002 after the time of contact did not affect the number of intracellular bacteria recovered at the 4 time points considered, as compared with non treated cells as similar bacterial counts were obtained. Similarly, treatment of cells with the Akt inhibitor (AKT X) did not reduce the intracellular bacterial counts, as shown in Fig. 5 (e). Collectively, these results suggest that PI3K-Akt signaling pathway is not relevant for the survival of S.aureus once inside the macrophage.
Discussion
In the present study we provide evidences showing that Newman S.aureus survives inside within murine alveolar macrophages in a time course experiment and have characterized its intracellular trafficking.
The infection rate was relatively high and constant during the time course. S.aureus is indeed a prototype of pyogenic pathogen with a great ability to adhere and invade tissues. The phagocytosis of S.aureus within murine macrophages involved the reorganization of actin cytoskeleton, as well as the activation of PI3K signaling pathway.32 The same signaling pathway was also involved in internalization of S.aureus in bovine endothelial cells42; suggesting that the mechanism is somehow common for phagocytic and non-phagocytic cells. Once inside the macrophage, Newman S.aureus strain was found to be viable at least until 28 h post infection that corresponds to the latest time point analyzed. During this time course, Newman was able to replicate to a degree that bactericidal activity of macrophages was exceeded, as shown by plating and microscopy counts after gentamicin protection assays. During the first hour post infection, there is a decrease in the number of bacteria recovered and this can be explained by the fact that bacteria adhered, and not yet phagocyted are eliminated by the action of gentamicin. In addition, we cannot exclude the possibility that some macrophages might be able to counteract the infection process in this early period of time. In fact, during the second phase that goes till 16.5h post infection, bacterial counts remain constant along time, suggesting that there is a balance between macrophage bactericidal activity and S.aureus survival. Finally, and till the last time point considered, intracellular bacterial counts gradually increase indicating that S.aureus not only remains viable, but that it is also able to replicate efficiently inside the cell. In the same direction, the number of bacteria per infected cell also increased along time. It is important to mention that although a certain degree of cytotoxicity was detected, the percentage of infected cell along time remained quite similar. In a recent paper, authors demonstrated in a murine model persistence inside alveolar macrophages.21 Similarly, Kubica M et al, found that S.aureus was able to persist intracellularly inside human monocyte-derived macrophages in vacuoles, before escaping into the cytoplasm.20
In our study, once S.aureus has been internalized by macrophages, it resides in a vacuolar compartment. Intracellular trafficking dynamics characterization has covered the same sampling time points as the plating experiment. The pathogen transits temporarily through a compartment with early endosome features, being positive for EEA-1 during the first 30 minutes post infection, but colocalization rapidly decreased. Later, and till the last time point analyzed, it acquires late endosome characteristics,36 including the markers Lamp-1 and Rab7, as well as its effector protein RILP. Jubrail J et al also found that the majority of S.aureus containing compartments were positive for Lamp-1 in macrophages infected with Newman strain.21 Tranchemontagne ZR et al also reported in differentiated THP-1 challenged with strain USA300, Lamp-1 positive containing phagosomes.23 And finally, in RAW 264.7 also using strain USA 300, authors also reported a high percentage of Lamp-1 colocalization after 30 min of infection.22 In contrast, Seto S et al, found that S.aureus viability decreased quickly in RAW 264.7, showing no signs of intracellular survival or proliferation.43 Authors reported a high proportion of Rab7-positive S.aureus containing phagosomes, from the early hours post-infection till 6h post infection.
S.aureus co-localized with lysotracker, indicator of acidic organelles during all time course, indicating that intracellular survival was indeed possible within an acidic niche. This has also been reported in previous studies.22,44,45 Indeed, Leimer N et al found in A549 cells that acidic environment facilitates the formation of non stable small colony variants,46,47 that were able to persist within lysosomes.48 In contrast, Jubrail J et al found that in differentiated THP-1, Newman S.aureus was contained in an endosome which failed to acidify correctly, despite high intracellular bacterial burden.21 In our experience S.aureus was able to survive inside the vacuole regardless of the pH, as treatment with bafilomycin, which inhibits vacuolar acidification did not substantially decreased intracellular counts. Therefore, the alteration of the niche pH did not result bactericidal. Instead, Tranchemontage ZR et al showed that the inhibition of phagosome acidification had a critical impact on USA300 strain survival, and altered the expression of agr regulatory systems.23
The percentage of dextran colocalization reported at 24.5 h post infection (∼50%) is lower than previously reported.22 In that study, authors found percentages of dextran colocalization around 70% at an early time point (30 min post infection), both in RAW 264.7 murine macrophages and primary human M-CSF derived macrophages infected with USA300. Some differences between both studies could explain these differences: cell line, considering the origin (human/murine), as well as the cell sub-type (alveolar/peritoneal/ peripheral blood), strain (USA300/ Newman) and the time point selected (30 min/ time course until 24.h post-infection).
In our experience, Rab 14 did not colocalize with S.aureus containing phagosome at any time point considered. Seto et al found that colocalization with Rab14 had a peak at 30 min and only lasted till 1h post infection.43 These data suggest that Rab14 recruitment can be transient, and thus difficult to assess. In addition, Rab proteins do not necessarily accumulate on vesicles to which they contribute trafficking, for example they can be localized on vesicles delivered to phagosomes, without being accumulating on the phagosome itself.49,50 Kyei GB et al identified a critical role for the small GTPase Rab14 in maintaining mycobacterial phagosome maturation block, by inhibiting phagosome-late endosome fusion.41 Similarly, the role of Rab14 in phagosome maturation has also been demonstrated in a Drosophila model with S.aureus infection.51 In our experience, transfection with a DNRab14 plasmid, overexpressing inactive Rab14 enabled an almost complete intracellular bacterial elimination. However, percentages of colocalization with TMR-dextran of 50% suggest that phagolysosomal fusion might be somehow delayed, suggesting a slower kinetic. Overall, these results might reflect a balance between intracellular bacteria killing and intracellular bacteria growth, and do not allow us to drawn firm conclusions.
PI3K-Akt axis is involved in bacterial intracellular trafficking. Kuijl et al demonstrated that Salmonella typhimurium activates Akt to prevent phagosome-lysosome fusion, acting as a key regulator of at least 3 GTPases and 2 cell host pathways involved in intracellular survival.39 It has been described that Akt-1 phosphorylates AS160, which main role is to activate in turn GTPase Rab14.40 By western-blot analysis we showed that S.aureus infection was able to activate both Akt and AS160, and that total Akt expression was constant at all time points.
Akt is also implicated in the intracellular survival of others pathogens, such as M.tuberculosis, suggesting that this kinase might be a central host factor targeted by pathogens to take control over specific cellular functions.40 Since S.aureus infection activated Akt in vitro, we speculated that activated Akt may also promote intracellular survival. However, Akt inhibition did not result in a significant decrease in bacterial intracellular counts, suggesting that although the microorganism activates PI3K-Akt pathway during phagocytosis, bacteria do not aim it for intracellular survival. Further experiments are needed to define and determine the mechanism by which S. aureus modulates, and alters phagolysosomal maturation process. This analysis, which cannot easily be addressed, becomes very complex and it was not the scope of the current study.
Different experimental studies have thus demonstrated that S.aureus is able to persist within different types of host cells holding a remarkable capacity of adaptation.16 There is a special emphasis placed on characterizing phenol soluble modulins,44 α-toxin,52,53 leukocidins,54,55 and regulatory systems26 role during S.aureus adaptation and intracellular survival. However, survival mechanisms involved (whether in a vacuole or in the cytoplasm, for example), as well as its intracellular trafficking (colocalization with endosomal/ fluid markers, phagosomal escape and pH conditions) are highly dependent on cell origin (human or murine) and sub-type (phagocytic and non phagocytic), bacterial strain used and MOI considered, as well as growth phase used for infection.56-58 So, all these factors need to be carefully considered when comparing studies because discrepancies might be reflecting important biologic differences depending on the cell line and bacteria tested.
Long-term survival, especially inside professional phagocytes may be a mechanism of dissemination with important implications in treatment options; whereas survival inside specific non-phagocytic cells, such as epithelials, osteoblasts may contribute to chronic infections, nasal carriage and/or transient colonization of skin and mucous membranes. For example, some chronic and therapy refractory staphylococcal infections, have been associated with the presence of small colony variants, which display an altered bacterial phenotype, adapted to the intracellular environment revealing the evidence of intracellular adaptation and persistence during human infections.46,47,59
Further research exploring bacterial factors that contribute to intracellular survival, and host factors leading to S.aureus clearance or survival during lung infections are poorly documented and are a rich area for future research. The ability to survive and/or replicate intracellularly together with its capacity of adaptation can contribute significantly to the global progression of the staphylococcal infection. To establish and define which mechanisms enable S.aureus to remain in an intracellular stage in an experimental model is of crucial importance as it opens new therapeutical approaches and at the same time provides insights into the pathogenesis of staphylococcal infections.
Material and methods
Bacterial strain and culture conditions
S.aureus Newman strain has been used extensively in animal models of staphylococcal disease due to its robust virulence phenotype.60 When indicated, a GFP-Newman strain was used instead. Bacteria were grown in Luria-Bertani (LB) at 37°C on an orbital shaker (180 rpm). To UV kill bacteria, samples were UV irradiated (1 J for 10 min) in a Bio-link Blx crosslinker (Vilber Lourmat).
Eukaryotic cell culture
Murine alveolar macrophages MH-S (ATTC, CRL-2019) were grown on RPMI-1640 tissue culture medium supplemented with 10% heat-inactivated calf serum (FCS), Hepes 10mM and antibiotics/antifungals at 37°C in an humidified atmosphere and 5% CO2.
Infection of macrophages
Cells were seeded in 24-well tissue culture plates at 5 × 105 cells per well 24h before the experiment. Bacteria were grown in 5 ml of LB and harvested in the exponential phase (2500 x g, 20 min, 22°C), washed once with PBS, and resuspended using vortex and pipetting. Absence of bacterial aggregates was checked visually. A suspension containing approximately 1 × 109 cfu/ml was prepared in 10 mM PBS (pH 6.5). Cells were infected with 25 μl of this suspension to get a MOI of 25:1 in a final volume of 500 μl RPMI 1640 tissue culture medium supplemented with 10% FCS and Hepes 10 mM. To synchronize infection, plates were centrifuged at 200 × g during 5 min. Plates were incubated at 37°C in a humidified 5% CO2 atmosphere. After 30 min of contact, cells were washed 5 times with PBS and incubated for 1 hour with 500 μl RPMI 1640 containing 10% FCS, Hepes 10 mM, gentamicin (200 μg/ml), followed by an additional incubation of 1 hour (or more, depending on interested time point) with 500 μl RPMI 1640 containing 10% FCS, Hepes 10 mM, gentamicin (25 μg/ml). The same protocol was performed in the absence of gentamycin (25 μg/ml) for the long-term incubation periods.To determine intracellular bacterial load, cells were then washed 3 times with PBS and lysed with 300 μl of 0.025% saponin in PBS for 10 min at room temperature. Serial dilutions were plated on LB to quantify the number of intracellular bacteria. Data are represented as c.f.u. per well. All experiments were done with triplicate samples at least on 3 independent occasions.
When exploring phagocytosis mechanisms, MH-S were pre-incubated for 1 hour with nocodazole (50μg/ml), nystatin (25 μg/ml), filipin (5 μg/ml), LY294002 (75 μM) or for 30 min with Cyt D (5 μg/ml) before carrying out infections as described above. Cells were also pre-incubated with MβCD (1mM) for 1 hour, washed twice with PBS to remove cholesterol and later infected as described. When indicated, LY294002 (75 μM), Akt X (10 μM) or bafilomycin A1 (100 nM) were added to the cells at the onset of the gentamicin treatment and kept until the end of the experiments. Exposure to these drugs had no effect on cell and bacterial viability under the conditions tested. All drugs were purchased from Sigma.
Neutral red assay and cell viability
Cell viability was determined by assessing the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes.29 MH-S were seeded on 96-well tissue culture plates at 5 × 105 cells/well 24 h before the experiment. Cells were then infected with a MOI of 25:1 in a final volume of 200 μl of RPMI-1640 culture medium supplemented with 10% heat-inactivated FCS and 10 mM Hepes. To synchronize infection, plates were centrifuged at 200 x g during 5 min. Plates were incubated at 37°C in a humidified 5% CO2 atmosphere. After 30 min of contact, cells were washed twice with PBS and incubated with 200 μl RPMI 1640 containing 10% FCS, 10 mM Hepes, gentamicin (200 μg/ml) during 6 h, 18 h and 24 h. Alternatively, cells were incubated with gentamicin (200 μg/ml) for 1 h, and later cells were incubated with media without antibiotic during the selected time points. After that, medium was aspirated and cells were washed twice with PBS and later incubated with 100 μl of freshly prepared neutral red medium at a final concentration of 40 μg/ml for 2 h. Wells were then washed once with PBS and the remaining biomass-adsorbed neutral red was dissolved with 150 μl neutral red destain solution (50% ethanol 96%, 49% deionised water, 1% glacial acetic acid). Staining was quantified by determining the OD at 540 nm in a microplate reader, and used to compare the relative neutral red staining of uninfected cells, infected cells and cells lysed completely with 1% Triton X-100. Experiments were performed by triplicate in 3 independent occasions.
Generation of a rabbit polyclonal anti-S.aureus serum
Rabbit anti-S.aureus serum was produced by Charles River Laboratories through repeated immunization of one rabbit with a mixture of acetone killed bacteria. This mixture was obtained from an overnight-grown culture of Newman strain, which was centrifuged, resuspended in acetone to half of the original volume and agitated for 24 h. Acetone killed bacteria were centrifuged, resuspended in one-tenth of the original acetone volume and agitated for 1 h. Finally, the suspension of acetone-killed bacteria was dried with a vacuum desiccator.
Immunofluorescence microscopy
Cells were seeded on 12 mm circular coverslips in 24-well tissue culture plates. Infections were performed as described before for intracellular bacterial counts. When indicated, cells were washed 3 times with PBS, and fixed with 3.7% paraformaldehyde (PFA) in PBS (pH 7.4) for 15 min. For early endosome antigen 1 (EEA-1) staining, cells were fixed with 2.5% PFA for 10 min at room temperature, followed by 5% PFA with 80% methanol at −20°C for 5 min.
S.aureus was stained with rabbit polyclonal anti-S.aureus serum diluted 1:500. Actin cytoskeleton was stained with texas red-X-phalloidin (T 7471, Invitrogen) diluted 1:200. Host cell nuclei was stained with Hoechst 33342 (Hoechst 33342, Invitrogen) diluted 1:2500. Early endosomes were stained with goat anti-EEA1 antibody (N-19; Santa Cruz Biotechnology) diluted 1:50. Late endosomes were stained with rat anti-Lamp-1 (1D4B; Developmental Studies Hybridoma Bank) diluted 1:150. For the detection of Rab14, cells were infected with GFP-Newman, using the same protocol as with Newman strain. Anti-Rab14 (C-terminal) rabbit antibody was used (Sigma, R0656) diluted 1:800. Donkey anti-rabbit, donkey anti-rat and donkey anti-goat secondary antibodies conjugated to rhodamine, Cy5 or Cy2 were purchased from Jackson Immunological and diluted 1:200.
Fixable dextran 70 000 MW labeled with Texas red (TMR-dextran, D1864, Life technologies) was used to label lysosomes in a pulse - chase assay, given that endocytosed TMR-dextran is delivered and retained by endocytic compartments. Briefly, alveolar macrophages seeded on glass coverslips were labeled by pulsing with 250 μg/ml TMR-dextran for 2 h at 37°C in 5% CO2 in culture medium. To allow the TMR-dextran to accumulate in lysosomes, medium was removed, and cells were washed 3 times with PBS and incubated for 1h in dye-free medium (chase). After the chase period, cells were infected with live or UV-killed S.aureus as described above. Acidic compartments were loaded with 0.5 μM lysotracker (Red DND-99,Invitrogen), 45 min before PFA fixation. At the end of the infection period, the residual fluid marker was removed by washing the cells 3 times with PBS, followed by fixation.
Staining was performed in 10% horse serum and 0.1% saponin in PBS. Coverslips were washed twice in PBS containing 0.1% saponin, once in PBS, and incubated for 30 min with primary antibodies. Staining protocol for Rab14 included a permeabilization (PBS-saponin 0.4%, 10 min) and blocking (PBS-saponin 0.05%, 10% horse serum, 30 min) step. Coverslips were then washed twice in 0.1% saponin in PBS and once in PBS and incubated for 30 min with secondary antibodies. Finally, coverslips were washed twice in 0.1% saponin in PBS, once in PBS and once in H2O, mounted on Aqua Poly/Mount (18606, Polysciences).
Depending on the marker, S.aureus containing compartment was considered positive when it fulfilled these criteria: (i) the marker was detected throughout the area occupied by the bacterium and (ii) the marker was detected around/enclosing the bacterium or (iii) the marker was concentrated in this area, in comparison to the immediate surroundings. For each individual infected cell, intracellular bacteria located inside the compartment (positive colocalization) and outside the compartment (negative) were recorded. The percentage of positive colocalization was calculated as: total number of bacteria located in the compartment/ total number of intracellular bacteria (regardless of being in the compartment or not). To determine the percentage of bacteria that co-localized with each marker, all bacteria located inside a minimum of 100 infected cells were analyzed in each experiment. Experiments were performed by triplicate in 3 independent occasions. Specimens were observed either on a Leica DM6000B epifluorescence microscope for quantitative analysis and a Leica TCS SP5 confocal microscope for image analysis.
Plasmids and transient transfection
For transient transfections with GFP-Rab 7,61 RILP-C33-EGFP,30 pcDNA343 or DN-Rab14,43 MH-S cells were seeded in 24-well plates at a density of 3 × 105 cells per well 24 h before the experiment. Cells were transfected with 750 ng of DNA using Xfect Transfection Reagent (Clontech) following the manufacturer's instructions. Twenty-four hours post-transfection cells were infected as indicated. In all cases, cells were fixed, stained and later analyzed by immunofluorescence microscopy. After 24 h, cells were washed twice with PBS, infected and intracellular bacterial load was determined as described previously.
Detection of total Akt, P-Akt and P-AS160 by Western blotting
MH-S were seeded on 6-well tissue culture plates at 2 × 106 cells/well. Cells were infected as described previously. At indicated time points, cells were washed 3 times with cold PBS, scraped and lysed with 100 μl lysis buffer (1 × SDS Sample Buffer, 62.5 mM Tris-HCl pH 6.8, 2% w/v SDS, 10% glycerol, 50 mM DTT, 0.01% w/v bromophenol blue) on ice. Samples were sonicated, boiled at 100°C for 10 min and cooled on ice before polyacrylamide gel electrophoresis and Western Blotting.
Total Akt and Akt and AS160 phosphorylation (P-Akt, P-AS160) were detected with primary rabbit anti Akt (Akt1, Akt2 and Akt3), primary rabbit anti-phospho Ser473 Akt and primary rabbit anti-phospho Thr642 AS160 antibodies (Cell Signaling Technology) diluted 1:1.000 and with secondary goat anti-rabbit antibody conjugated to horseradish peroxidase (Thermo Scientific) diluted 1:10,000. Tubulin was detected with primary mouse anti-tubulin antibody (Sigma) diluted 1:3000 and secondary goat anti-mouse antibody (Pierce) conjugated to horseradish peroxidase diluted 1:1,000. To detect tubulin, membranes were reprobed after stripping of previously used antibodies using Western Blot Stripping Buffer (Thermo Scientific). Images were recorded with a GeneGnome HR imaging system (Syngene).
Statistical analysis
Statistical analyses were performed using the 2-tailed unpaired t-test (2 groups) or ANOVA (multiple groups). P < 0.05 was considered statistically significant. The analyses were performed using GraphPad Prism 5 (Graph Pad Software).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Kok van Kessel (UMC, Utrecht) and I.Lasa (Universidad Pública de Navarra/ CSIC) for providing S.aureus Newman and GFP-Newman strains, respectively. We are indebted to S. Grinstein, J. Neefjes and Y. Koide for sending us plasmids. We are grateful to members of the Laboratory “Infection and Immunity” for helpful discussions. We thank the IGTP Microscopy Core Facility and staff (MP. Armengol and G. Requena) for their contribution to this publication.We also thank Oriol Martos for his kind technical assistance.
Funding
This work has been funded by the project PI13/01418 which is part of “Plan Nacional de I+D +I” and co-funded by ISCIII- Subdirección General de Evaluación and “Fondo Europeo de Desarrollo Regional” (FEDER). This work also received a grant from the Spanish Society of Pneumology and Thoracic Surgery (SEPAR 054/2011) and Instituto de Salud Carlos III: CIBERES (Corporate Research Program on Host-Pathogen interactions).
D. Domínguez-Villanueva is funded by “Plan Nacional de I+D +I” and co-funded by ISCIII- Subdirección General de Evaluación and “Fondo Europeo de Desarrollo Regional” (FEDER). M.Laabei was supported by a joint ERS/SEPAR fellowship (LTRF 2015). A. Lacoma has been a recipient of a grant from Sociedad Española de Microbiologia Clínica y Enfermedades Infecciosas (SEIMC) and from CIBERES (Programa de perfeccionamiento y movilidad).
References
- [1].Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-32. doi: 10.1056/NEJM199808203390806. PMID:9709046 [DOI] [PubMed] [Google Scholar]
- [2].Otto M. Staphylococcus aureus toxins. Curr opin microbiol. 2014;17:32-7. doi: 10.1016/j.mib.2013.11.004. PMID:24581690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Veldkamp KE, van Strijp JA. Innate immune evasion by staphylococci. Adv Exp Med Biol. 2009;666:19-31. doi: 10.1007/978-1-4419-1601-3_2. PMID:20054972 [DOI] [PubMed] [Google Scholar]
- [4].Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S344-9. doi: 10.1086/533590. PMID:18462089 [DOI] [PubMed] [Google Scholar]
- [5].Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603-61. doi: 10.1128/CMR.00134-14. PMID:26016486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weidenmaier C, Goerke C, Wolz C. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol. 2012;20:243-50. doi: 10.1016/j.tim.2012.03.004. PMID:22494802 [DOI] [PubMed] [Google Scholar]
- [7].Parker D, Prince A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin Immunopathol. 2012;34:281-97. doi: 10.1007/s00281-011-0291-7. PMID:22037948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854-62. doi: 10.1378/chest.128.6.3854. PMID:16354854 [DOI] [PubMed] [Google Scholar]
- [9].Chertow DS, Memoli MJ. Bacterial coinfection in influenza: A grand rounds review. JAMA. 2013;309:275-82. doi: 10.1001/jama.2012.194139. PMID:23321766 [DOI] [PubMed] [Google Scholar]
- [10].Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975-1028. doi: 10.1146/annurev.immunol.22.012703.104538. PMID:15771591 [DOI] [PubMed] [Google Scholar]
- [11].Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355-66. doi: 10.1038/nrmicro2128. PMID:19369951 [DOI] [PubMed] [Google Scholar]
- [12].Haas A. The phagosome: Compartment with a license to kill. Traffic. 2007;8:311-30. doi: 10.1111/j.1600-0854.2006.00531.x. PMID:17274798 [DOI] [PubMed] [Google Scholar]
- [13].Sarantis H, Grinstein S. Subversion of phagocytosis for pathogen survival. Cell Host Microbe. 2012;12:419-31. doi: 10.1016/j.chom.2012.09.001. PMID:23084912 [DOI] [PubMed] [Google Scholar]
- [14].Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781-92. doi: 10.1111/j.1462-5822.2005.00665.x. PMID:16611227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102:4033-8. doi: 10.1073/pnas.0409716102. PMID:15753315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garzoni C, Kelley WL. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009;17:59-65. doi: 10.1016/j.tim.2008.11.005. PMID:19208480 [DOI] [PubMed] [Google Scholar]
- [17].Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9:215-22. doi: 10.1038/nrmicro2508. PMID:21297670 [DOI] [PubMed] [Google Scholar]
- [18].Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237-59. doi: 10.1007/s00281-011-0295-3. PMID:22080185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: A biological tug of war. Annu Rev Microbiol. 2013;67:629-50. doi: 10.1146/annurev-micro-092412-155746. PMID:23834243 [DOI] [PubMed] [Google Scholar]
- [20].Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, et al.. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. PMID:18183290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jubrail J, Morris P, Bewley MA, Johnston SA, Foster SJ, Peden AA, Read RC, Marriott HM, Dockrell DH. Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell Microbiol. 2016;18:80-96. doi: 10.1111/cmi.12485. PMID:26248337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Flannagan RS, Heit B, Heinrichs DE. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol. 2016;18:514-35. doi: 10.1111/cmi.12527. PMID:26408990 [DOI] [PubMed] [Google Scholar]
- [23].Tranchemontagne ZR, Camire RB, O'Donnell VJ, Baugh J, Burkholder KM. Staphylococcus aureus strain USA300 perturbs acquisition of lysosomal enzymes and requires phagosomal acidification for survival inside macrophages. Infect Immun. 2015;84:241-53. doi: 10.1128/IAI.00704-15. PMID:26502911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88-99. doi: 10.1016/j.jim.2014.04.007. PMID:24769066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, et al.. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58:1108-17. doi: 10.1128/AAC.02190-13. PMID:24295977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Munzenmayer L, Geiger T, Daiber E, Schulte B, Autenrieth SE, Fraunholz M, Wolz C. Influence of Sae and Agr regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell Microbiol. 2016;18:1172-83. doi: 10.1111/cmi.12577. PMID:26895738 [DOI] [PubMed] [Google Scholar]
- [27].Lacoma A, Gomes-Fernandes M, Mesalles E, Arméstar F, Villar R, Casas I, Molinos S, Giménez M, Ausina V, Prat C. Persistence of Staphylococcus aureus in lower respiratory tract in patients undergoing mechanical ventilation. Eur Resp J. 2015;46(Suppl 59):PA2640. [Google Scholar]
- [28].Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19-25. doi: 10.1083/jcb.200107069. PMID:11581283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125-31. doi: 10.1038/nprot.2008.75. PMID:18600217 [DOI] [PubMed] [Google Scholar]
- [30].Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): The Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683-93. doi: 10.1093/emboj/20.4.683. PMID:11179213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680-5. doi: 10.1016/S0960-9822(01)00531-0. PMID:11696325 [DOI] [PubMed] [Google Scholar]
- [32].Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: Aging gracefully. Biochem J. 2002;366:689-704. doi: 10.1042/bj20020691. PMID:12061891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lamothe J, Huynh KK, Grinstein S, Valvano MA. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol. 2007;9:40-53. doi: 10.1111/j.1462-5822.2006.00766.x. PMID:16869828 [DOI] [PubMed] [Google Scholar]
- [34].Maurin M, Benoliel AM, Bongrand P, Raoult D. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect Immun. 1992;60:5013-6. PMID:1452331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765-73. PMID:8698506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33:397-405. doi: 10.1016/j.it.2012.03.003. PMID:22560866 [DOI] [PubMed] [Google Scholar]
- [37].Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, Scidmore MA, Grinstein S, Meyer T, Brumell JH. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol. 2007;176:263-8. doi: 10.1083/jcb.200611056. PMID:17261845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cano V, March C, Insua JL, Aguilo N, Llobet E, Moranta D, Regueiro V, Brennan GP, Millan-Lou MI, Martin C, et al.. Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell Microbiol. 2015;17:1537-60. doi: 10.1111/cmi.12466. PMID:26045209 [DOI] [PubMed] [Google Scholar]
- [39].Kuijl C, Savage ND, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJ, Geluk A, et al.. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725-30. doi: 10.1038/nature06345. PMID:18046412 [DOI] [PubMed] [Google Scholar]
- [40].Kuijl C, Neefjes J. New insight into the everlasting host-pathogen arms race. Nat Immunol. 2009;10:808-9. doi: 10.1038/ni0809-808. PMID:19621040 [DOI] [PubMed] [Google Scholar]
- [41].Kyei GB, Vergne I, Chua J, Roberts E, Harris J, Junutula JR, Deretic V. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo J. 2006;25:5250-9. doi: 10.1038/sj.emboj.7601407. PMID:17082769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oviedo-Boyso J, Cortes-Vieyra R, Huante-Mendoza A, Yu HB, Valdez-Alarcon JJ, Bravo-Patino A, Cajero-Juarez M, Finlay BB, Baizabal-Aguirre VM. The phosphoinositide-3-kinase-Akt signaling pathway is important for Staphylococcus aureus internalization by endothelial cells. Infect Immun. 2011;79:4569-77. doi: 10.1128/IAI.05303-11. PMID:21844240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seto S, Tsujimura K, Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic. 2011;12:407-20. doi: 10.1111/j.1600-0854.2011.01165.x. PMID:21255211 [DOI] [PubMed] [Google Scholar]
- [44].Grosz M, Kolter J, Paprotka K, Winkler AC, Schafer D, Chatterjee SS, Geiger T, Wolz C, Ohlsen K, Otto M, et al.. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin alpha. Cell Microbiol. 2014;16:451-65. doi: 10.1111/cmi.12233. PMID:24164701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lam TT, Giese B, Chikkaballi D, Kuhn A, Wolber W, Pane-Farre J, Schafer D, Engelmann S, Fraunholz M, Sinha B. Phagolysosomal integrity is generally maintained after Staphylococcus aureus invasion of nonprofessional phagocytes but is modulated by strain 6850. Infect Immun. 2010;78:3392-403. doi: 10.1128/IAI.00012-10. PMID:20530231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4:295-305. doi: 10.1038/nrmicro1384. PMID:16541137 [DOI] [PubMed] [Google Scholar]
- [47].Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, et al.. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3:129-41. doi: 10.1002/emmm.201000115. PMID:21268281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leimer N, Rachmuhl C, Palheiros Marques M, Bahlmann AS, Furrer A, Eichenseher F, Seidl K, Matt U, Loessner MJ, Schuepbach RA, et al.. Nonstable Staphylococcus aureus small-colony variants are induced by low pH and sensitized to antimicrobial therapy by phagolysosomal alkalinization. J Infect Dis. 2016;213:305-13. doi: 10.1093/infdis/jiv388. PMID:26188074 [DOI] [PubMed] [Google Scholar]
- [49].Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, Klumperman J, Scheller RH. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218-29. doi: 10.1091/mbc.E03-10-0777. PMID:15004230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bhuin T, Roy JK. Rab proteins: The key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328:1-19. doi: 10.1016/j.yexcr.2014.07.027. PMID:25088255 [DOI] [PubMed] [Google Scholar]
- [51].Garg A, Wu LP. Drosophila Rab14 mediates phagocytosis in the immune response to Staphylococcus aureus. Cell Microbiol. 2014;16:296-310. doi: 10.1111/cmi.12220. PMID:24119134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jarry TM, Memmi G, Cheung AL. The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cell Microbiol. 2008;10:1801-14. doi: 10.1111/j.1462-5822.2008.01166.x. PMID:18466345 [DOI] [PubMed] [Google Scholar]
- [53].Giese B, Dittmann S, Paprotka K, Levin K, Weltrowski A, Biehler D, Lam TT, Sinha B, Fraunholz MJ. Staphylococcal alpha-toxin is not sufficient to mediate escape from phagolysosomes in upper-airway epithelial cells. Infect Immun. 2009;77:3611-25. doi: 10.1128/IAI.01478-08. PMID:19564384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81:1830-41. doi: 10.1128/IAI.00095-13. PMID:23509138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chi CY, Lin CC, Liao IC, Yao YC, Shen FC, Liu CC, Lin CF. Panton-valentine leukocidin facilitates the escape of Staphylococcus aureus from human keratinocyte endosomes and induces apoptosis. J Infect Dis. 2014;209:224-35. doi: 10.1093/infdis/jit445. PMID:23956440 [DOI] [PubMed] [Google Scholar]
- [56].Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: Live-in and let die. Front Cell Infect Microbiol. 2012;2:43. doi: 10.3389/fcimb.2012.00043. PMID:22919634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sinha B, Fraunholz M. Staphylococcus aureus host cell invasion and post-invasion events. Int J Med Microbiol. 2010;300:170-5. doi: 10.1016/j.ijmm.2009.08.019. PMID:19781990 [DOI] [PubMed] [Google Scholar]
- [58].Krut O, Utermohlen O, Schlossherr X, Kronke M. Strain-specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates. Infect Immun. 2003;71:2716-23. doi: 10.1128/IAI.71.5.2716-2723.2003. PMID:12704146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Prat C, Lacoma A. Bacteria in the respiratory tract-how to treat? Or do not treat? Int J Infect Dis. 2016;51:113-22. doi: 10.1016/j.ijid.2016.09.005. PMID:27776777 [DOI] [PubMed] [Google Scholar]
- [60].Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008;190:300-10. doi: 10.1128/JB.01000-07. PMID:17951380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sun Q, Westphal W, Wong KN, Tan I, Zhong Q. Rubicon controls endosome maturation as a Rab7 effector. Proc Natl Acad Sci U S A. 2010;107:19338-43. doi: 10.1073/pnas.1010554107. PMID:20974968 [DOI] [PMC free article] [PubMed] [Google Scholar]


