ABSTRACT
Candida albicans and Streptococcus oralis are ubiquitous oral commensal organisms. Under host-permissive conditions these organisms can form hypervirulent mucosal biofilms. C. albicans biofilm formation is controlled by 6 master transcriptional regulators: Bcr1, Brg1, Efg1, Tec1, Ndt80, and Rob1. The objective of this work was to test whether any of these regulators play a role in cross-kingdom interactions between C. albicans and S. oralis in oral mucosal biofilms, and identify downstream target gene(s) that promote these interactions. Organotypic mucosal constructs and a mouse model of oropharyngeal infection were used to analyze mucosal biofilm growth and fungal gene expression. By screening 6 C. albicans transcription regulator reporter strains we discovered that EFG1 was strongly activated by interaction with S. oralis in late biofilm growth stages. EFG1 gene expression was increased in polymicrobial biofilms on abiotic surfaces, mucosal constructs and tongue tissues of mice infected with both organisms. EFG1 was required for robust Candida-streptococcal biofilm growth in organotypic constructs and mouse oral tissues. S. oralis stimulated C. albicans ALS1 gene expression in an EFG1-dependent manner, and Als1 was identified as a downstream effector of the Efg1 pathway which promoted C. albicans-S. oralis coaggregation interactions in mixed biofilms. We conclude that S. oralis induces an increase in EFG1 expression in C. albicans in late biofilm stages. This in turn increases expression of ALS1, which promotes coaggregation interactions and mucosal biofilm growth. Our work provides novel insights on C. albicans genes which play a role in cross-kingdom interactions with S. oralis in mucosal biofilms.
KEYWORDS: ALS1, Candida, cross-kingdom biofilm, EFG1, oral mucosa, Streptococcus
Introduction
Candida albicans is an important core component of the human oral mycobiota in health.1,2 Under permissive host conditions this organism can form biofilms with oral streptococci on tooth or mucosal surfaces which are associated with oral diseases such as caries and oropharyngeal candidiasis.3,4 The C. albicans core biofilm transcriptional regulatory network is composed of 6 master regulators (Bcr1, Brg1, Efg1, Ndt80, Rob1 and Tec1) that control approximately 1000 downstream target genes.5 Several of these regulators have also been implicated in the control of the yeast-to-hyphal transition and robust biofilm growth almost always requires both morphotypes.5
Most oropharyngeal opportunistic infections are polymicrobial or require “cooperation” by multiple organisms, since they develop in a host habitat that harbors a large number of different bacterial and fungal species.6 Thus, although C. albicans is the most frequently isolated organism in oropharyngeal candidiasis, it is increasingly appreciated that mixed fungal-bacterial biofilms play a role in oral disease.5,7-10 Within mixed biofilm communities, fungal and bacterial cells use metabolites or cell contact-mediated signals to communicate with each other, adjust their population density, change gene expression patterns, modulate host responses and promote disease. Recent work indicated that the introduction of C. albicans to the oral cavity of mice enhances mucosal biofilm formation by S. oralis,3 a ubiquitous commensal of oral mucosal surfaces in healthy humans,11 which lacks the ability to form biofilms on its own in vitro and in vivo.3,12,13 Growth of S. oralis with C. albicans in mucosal biofilms stimulates an increase in TLR2 mucosal expression leading to TLR2-mediated proinflammatory signals and enhanced pathology.3 More recently we discovered that when these 2 commensal microorganisms grow together in mucosal biofilms, they synergize to highjack the epithelial calpain activation pathway, elicit disassembly of intercellular adherens junctions, compromise the integrity of the oral mucosal barrier and promote fungal dissemination.14 It was determined that the ability to enhance C. albicans biofilm pathogenicity was shared among strains of the same streptococcal species.14
In this work, using established models of oral mucosal fungal-bacterial mixed biofilms, we provide novel mechanistic insights into the biofilm interactions between C. albicans with S. oralis. After interrogating the expression of the 6 master regulators of C. albicans biofilm growth, we identified Efg1 as a critical regulator of co-aggregation interactions with S. oralis that increase fungal-bacterial mucosal biofilms. We further illustrate that Als1 is a downstream effector of the Efg1 pathway promoted by S. oralis. This is the first report on the involvement of the Efg1 pathway in regulating cross-kingdom biofilm interactions between C. albicans and oral bacteria.
Results
EFG1 transcription is activated by S. oralis during biofilm growth
To test whether any of the C. albicans master transcriptional regulator(s) were activated by S. oralis during biofilm growth we first screened a panel of reporter strains, each driving the expression of mCherry protein under the control of one of the 6 master transcriptional regulators.5 No differences in the fluorescence signals between single and mixed biofilms were observed after 4 h or 16 h (Fig. S1) of growth, although the mCherry-EFG1p reporter was fluorescing slightly higher at 4 h and 16 h, and the mCherry-NDT80p reporter was fluorescing slightly higher at 16 h in the mixed biofilms. After 36 hours of biofilm growth there was a burst of the red fluorescence signal in the mCherry-EFG1p construct growing with S. oralis. Fluorescence of the mCherry-ROB1p strain increased somewhat, but not to levels seen with the mCherry-EFG1p construct (Fig. 1A). To confirm reporter strain activity, we performed RT-qPCR of the 6 regulator genes in biofilm cultures growing under the same conditions. As seen in Fig. 1B, these data confirmed that S. oralis stimulated a significant increase in EFG1 gene transcription in C. albicans in late biofilm growth stages (p<0.05).
Figure 1.
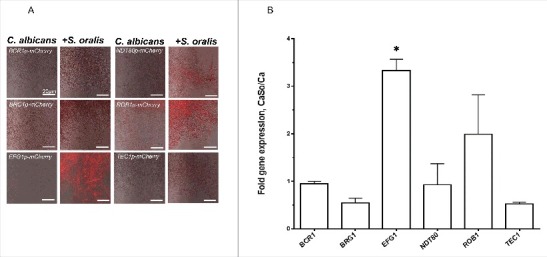
S. oralis activates C. albicans EFG1 gene expression in mixed biofilms. (A) C. albicans mCherry transcriptional regulator reporter strains were grown as biofilms on Permanox® plastic chamber slides, with or without S. oralis 34 in RPMI 10%FBS, 10% BHI media for 36 hours and observed under a fluorescence microscope. A representative of 3 experiments is shown. There was a burst of the red fluorescence signal in the EFG1p-mCherry construct growing with S. oralis, suggesting EFG1 transcriptional activation. Bars: 20 μm. (B) Candida gene mRNA levels from biofilms growing under identical conditions as in (A) were analyzed by RT-qPCR. Results represent fold increase gene expression in C. albicans with S. oralis (CaSo) over C. albicans (Ca) alone. Means ± SD are shown from 3 experiments. S. oralis stimulated a significant increase in EFG1 gene transcripts. *p<0.05 in a comparison to other regulator genes.
Because C. albicans gene expression in biofilms may be affected by the presence of host cells,5 we next tested expression of the 6 master regulators in biofilms growing on organotypic oral mucosal constructs and tongue tissues of orally infected mice. In Candida-streptococcal biofilms growing on the surface of organotypic constructs EFG1 was the only gene that was significantly upregulated by S. oralis (Fig. 2A, p<0.05). Similarly, in most mice infected with both organisms, EFG1 transcripts were higher compared with single infection, although there was variability in the magnitude of this response (Fig. 2B). Collectively these data strongly support the hypothesis that the Efg1 transcriptional regulator may play a role in biofilm interactions between C. albicans and S. oralis.
Figure 2.
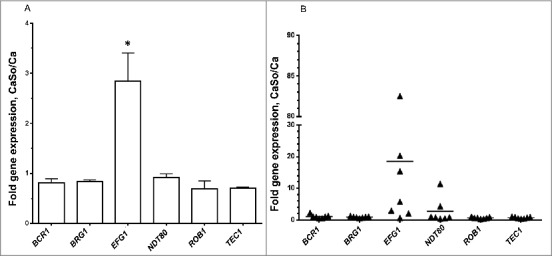
S. oralis increases EFG1 gene expression of C. albicans in organotypic tissue and mouse oral mucosal infection models. (A) Organotypic tissues were infected with C. albicans SC5314 with or without S. oralis 34, for 16h. Candida gene mRNA levels were analyzed by RT-qPCR. Results represent fold increase in gene expression over the single infection group. Means ± SD are shown from twice repeated experiments, with tissue infections set up in duplicate. EFG1 was the only gene that was significantly upregulated by S. oralis. *p<0.05 for a comparison to all other regulators. (B) Mice were infected with C. albicans or C. albicans plus S. oralis 34 for 4 d and Candida gene mRNA levels in tongue samples were analyzed by RT-qPCR. Results represent fold increase gene expression over the single infection group, with 6–7 mice per group. In most mice infected with both organisms, EFG1 transcripts were higher compared with single infection, although variability in the magnitude of this response was noted.
S.oralis promotes EFG1-dependent hyphal morphogenesis in synthetic saliva medium (SSM)
Because Efg1 regulates filamentation under many environmental conditions15 we hypothesized that activation of EFG1 by S. oralis may promote C. albicans hyphae when growing together in the biofilm state. We first grew biofilms in nutrient-rich media, emulating the conditions that induced strong mCherry-EFG1p reporter activity. Under these conditions C. albicans formed long hyphae and we were unable to detect differences in hyphal length in C. albicans growing with or without S. oralis microscopically (not shown), presumably because nutrient rich media alone provide ample environmental cues for robust filamentous growth. Since growth of C. albicans in SSM at 37°C occurs exclusively in the yeast form,16 we wondered whether S. oralis can promote filamentous growth of C. albicans in this physiologically relevant medium and whether this effect might be Efg1-mediated. Initially, we quantified the expression of each of the 6 Candida biofilm transcriptional regulators by RT-qPCR when growing together with S. oralis in SSM to confirm that EFG1 is upregulated under these conditions. As shown in Fig. 3A, under these nutrient-limiting conditions EFG1 transcripts were significantly increased (p<0.05) in the presence of S. oralis, whereas transcription of the other regulators was either repressed or unaltered.
Figure 3.
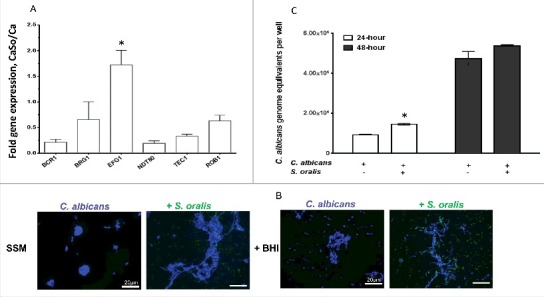
S. oralis promotes C. albicans hyphae in synthetic saliva. (A) C. albicans SC5314 (Ca) transcriptional regulator gene mRNA levels in biofilms growing in 6-well polystyrene plates with or without S. oralis 34 (So) for 24h, with SSM as the sole nutrient source. RNA samples were analyzed by RT-qPCR, after 24 h of co-culture. Results represent fold increase gene expression in mixed (CaSo) over C. albicans alone (Ca) biofilms. Means ± SD are shown from 3 experiments. EFG1 transcripts were significantly increased in the presence of S. oralis, whereas transcription of other regulators was either repressed or essentially unaltered. *p<0.05 in comparison with other regulator genes. (B) C. albicans SC5314 (blue) or C. albicans with teal protein expressing S. oralis 34 (green) were cultured on glass slides with SSM, supplemented with or without 10% BHI for 48 hours. Candida cells were stained with Calcoflour White® and cultures observed under a fluorescence microscope. Co-culture with S. oralis promoted hyphal growth and cell-cell aggregation under both conditions. Bars: 20 μm. (C) Fungal biomass expressed as “genome equivalents” of C. albicans SC5314 in biofilms with or without S. oralis 34. Biofilms were grown in 6-well polystyrene plates with SSM as the sole nutrient source for 24 h or 48 h. Genome equivalents were extrapolated by analyzing 18 S rRNA gene copy numbers in each biofilm well with qPCR and comparing to a standard curve. Means ± SD are shown from 3 experiments. Fungal biomass was higher in biofilms with S. oralis consistent with hyphal growth (B), although this effect was small and reached statistical significance only at the 24 h time-point. *p < 0.05, for a comparison to C. albicans alone.
Consistent with other reports,16 C. albicans remained almost exclusively in the yeast form even after 48-hours of incubation at 37°C in SSM. However, as expected, co-culture with S. oralis promoted hyphal growth and cell-cell aggregation (Fig. 3B). C. albicans growing in SSM supplemented with 10% BHI (to promote streptococcal metabolic activity) formed short hyphae which were considerably elongated in the presence of S. oralis. To provide a more quantitative measure of the positive effect of S. oralis on hyphal growth we performed qPCR of the 18 S rRNA gene and compared C. albicans biomass in biofilms with and without S. oralis under the same conditions. As anticipated, the fungal biomass, expressed as genome equivalents in each biofilm, was higher in biofilms with S. oralis although this effect was small and reached statistical significance only at the 24 h time-point (Fig. 3C).
To test whether EFG1 is required for this effect, an efg1Δ/Δ strain was tested. As expected, this strain was unable to form hyphae in 10% BHI-supplemented SSM with S. oralis (Fig. 4A). In contrast, the revertant strain formed a mix of yeast and short hyphae in SSM, which were elongated when growing with S. oralis (Fig. 4A). These results were corroborated by the higher Candida biomass in biofilms with S. oralis and the revertant but not the efg1Δ/Δ strains, as assessed by qPCR after 24 h-48 h of growth (Fig. 4B). Collectively, these data show that S. oralis can promote C. albicans hyphal growth in SSM in vitro, and that this effect is Efg1-dependent.
Figure 4.
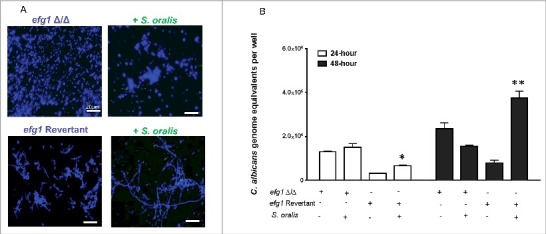
Hyphal growth stimulated by S. oralis is dependent on the Efg1 transcriptional regulator. (A) C. albicans efg1 homozygous deletion mutant (efg1Δ/Δ) and efg1 revertant were grown with or without teal protein expressing S. oralis 34 (green) in 10% BHI-supplemented SSM medium, on Permanox® plastic chamber slides, for 48 hours. Candida cells were stained with Calcoflour White® (blue) and cultures were observed under a fluorescence microscope. The revertant strain formed a mix of yeast and short hyphae in SSM, which were elongated when growing with S. oralis. Bars: 20 μm. (B) Fungal biomass expressed as “genome equivalents” of the efg1Δ/Δ mutant and efg1 revertant strains growing in biofilms with or without S. oralis 34. Biofilms were grown in 6-well polystyrene plates with SSM as the sole nutrient source for 24 h or 48 h. Genome equivalents were extrapolated by analyzing 18 S rRNA gene copy numbers with qPCR in each biofilm well and comparing to a standard curve. Means ± SD are shown from 3 experiments. A higher Candida biomass was noted in biofilms with S. oralis and the efg1 revertant, but not the efg1Δ/Δ mutant, in agreement with the hyphal elongation observed microscopically in the revertant (A). *p < 0.05 and **p < 0.01, for a comparison to C. albicans alone.
Efg1 promotes cross-kingdom mucosal biofilms in organotypic construct and mouse models
Given the activation of EFG1 by S. oralis we next hypothesized that Efg1 plays a role in mucosal biofilms formed by the 2 organisms. Indeed, an efg1Δ/Δ mutant was not able to form thick biofilms with S. oralis on oral mucosal organotypic constructs, as neither organism exhibited robust growth in the presence of the other (Fig. 5A). In contrast, the efg1 revertant strain promoted growth of S. oralis which resulted in a robust mixed mucosal biofilm (Fig. 5A). In the presence of S. oralis the biofilm formed by this strain was denser with longer hyphae observed extending into the submucosal compartment, compared with the single species biofilm (Fig. 5A, bottom panel, arrows). Similarly, tongue biofilms were more robust (Fig. 5B) and the oral fungal and streptococcal burdens in mice co-infected with S. oralis were significantly greater with the revertant strain compared with the efg1Δ/Δ strain (Fig. 5C, p<0.05 and p<0.01, respectively). No S. oralis was retrieved from oral tissues (not shown) unless animals were also infected with C. albicans, consistent with our published work.3,14 Despite the stimulation of hyphal growth by S. oralis in SSM in vitro, S. oralis did not cause an increase in the CFU counts of the EFG1-revertant strain in vivo (Fig. 5C), consistent with our published work with the wild type strain.3 This finding may reflect the fact that CFU methods underestimate hyphal organisms.17
Figure 5.
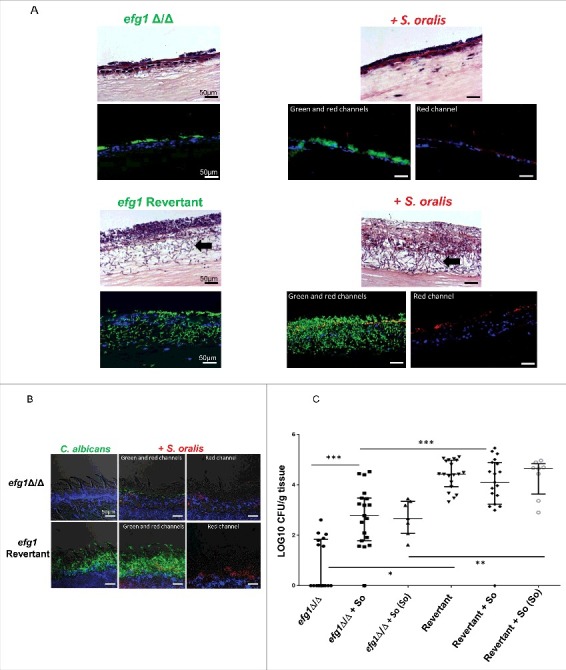
Efg1 promotes cross-kingdom mucosal biofilms in organotypic construct and animal models. (A) Mucosal biofilms formed by C. albicans (left panel) or C. albicans with S. oralis (right panel). Biofilms of the efg1 mutant and efg1 revertant strain were grown with or without S. oralis 34 on the surface of organotypic models of the oral mucosa for 16h, the time required for a well-organized biofilm to form on mucosal surfaces.7 H&E staining (top panels) and fluorescence images (bottom panels) of tissue sections labeled with a FITC-conjugated anti-Candida antibody (green), an Alexa Fluor 568-labeled streptococcal FISH probe for S. oralis (red), and counterstained with the nucleic acid stain Hoechst 33258 (blue), are shown. Red and blue channel only images are used to illustrate biofilm growth by S. oralis in the presence of C. albicans. Unlike the efg1 mutant, the efg1 revertant promoted growth of S. oralis on mucosal constructs which resulted in a robust mixed mucosal biofilm. The efg1 revertant formed longer hyphae extending into the submucosal compartment, in mixed compared with single species biofilms (bottom panel, arrows). No growth of S. oralis was observed when inoculated alone (not shown), consistent with previous work.13,14 Bar: 50 µm. (B) Representative tongue tissue sections from mice infected with the efg1 mutant (efg1Δ/Δ) and efg1 revertant, with or without S. oralis 34 for 4 d. C. albicans (green) labeled with a FITC-conjugated anti-Candida antibody and S. oralis (red) labeled with an Alexa Fluor 568-labeled FISH probe. Mucosal cell nuclei were counterstained with the nucleic acid stain Hoechst 33258 (blue). In tissues infected with both organisms (middle and right panels), overlay of 3-color fluorescence and overlay of red and blue channels only, are used to illustrate biofilm growth of S. oralis with C. albicans. In these tissues, little biofilm growth can be seen in the efg1 mutant with S. oralis. In contrast, the revertant strain formed a more robust biofilm and promoted stronger biofilm growth of S. oralis compared with the efg1 mutant. No biofilm growth in mice infected with S. oralis alone was observed (not shown), consistent with previous work.3,14 Bar: 50 µm (C) Fungal and bacterial burdens in mice infected with the efg1 mutant (efg1Δ/Δ) and efg1 revertant (Revertant), with or without S. oralis 34 (So) for 4 d. Data represent log CFU of fungi, or bacteria (labeled as “So” in parentheses, on the X-axis) per gram of tongue tissue, in 3 independent mouse experiments, with 5–8 animals per group. Candida and streptococcal burdens in mice infected with both organisms were significantly greater with the revertant strain compared with the efg1Δ/Δ strain. S. oralis was not retrieved from oral tissues of mice infected with bacteria alone (not shown). *p < 0.0001, **p<0.01, ***p < 0.05.
In both organotypic and mouse mucosae the efg1Δ/Δ mutant grew almost entirely in the yeast form regardless of the presence of S. oralis. In agreement with work published by other groups,18 the efg1Δ/Δ strain was severely deficient in colonizing the oral mucosa, as evidenced by the CFU data (Fig. 5C). Surprisingly, S. oralis promoted the mouse oral fungal burdens and tongue biofilm formation of the efg1Δ/Δ mutant (Fig. 5B,C), suggesting that additional regulators of biofilm development, are activated by S. oralis in the oral cavity of mice to compensate for the loss of Efg1. In summary, these results demonstrate that Efg1 plays a key role in promoting C. albicans-S. oralis mucosal biofilms in vitro and in vivo.
Als1 is a downstream effector of the Efg1 pathway in dual biofilms
We next set out to discover genes, under the transcriptional control of Efg1, that play a role in the interactions between the 2 organisms in mucosal biofilms. We first compared the expression of 3 genes with a well-established role in C. albicans-oral epithelial cell interactions (ALS1, ALS3, HWP1) between the efg1 mutant and revertant strains, in biofilms growing on mucosal constructs. Of the 3 genes tested ALS1 was the only gene with expression significantly increased by S. oralis in the revertant but not in the efg1Δ/Δ strain, suggesting that ALS1 is an Efg1-dependent gene that may play a role in cross-kingdom interactions (Fig. 6A, p<0.01). These results were further substantiated in the mouse model of oral infection where ALS1 expression was enhanced by S. oralis in the efg1 revertant but not the efg1Δ/Δ strain (Fig. 6B).
Figure 6.
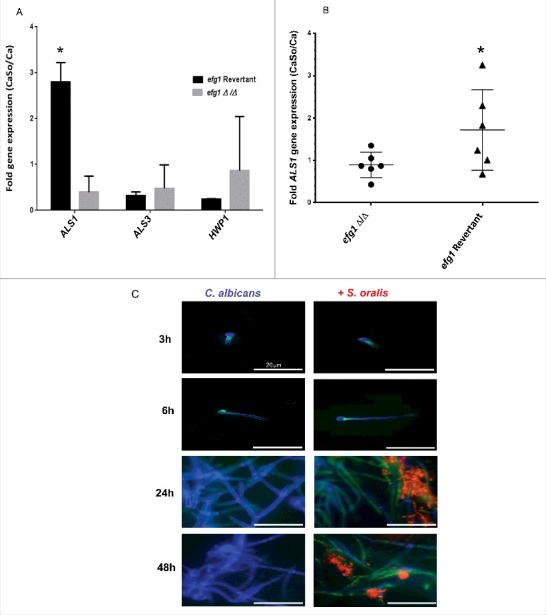
C. albicans ALS1 expression is increased by S. oralis. (A) Biofilms of the efg1 mutant and efg1 revertant were grown with or without S. oralis 34 on the surface of organotypic oral mucosal constructs for 16h. mRNA levels of 3 Candida genes known to play a role in the interactions with oral mucosal epithelium (ALS1, ALS3 and HWP1) were analyzed by RT-qPCR. Results represent fold increase gene expression of each Candida strain with S. oralis over Candida alone. Means ± SD are shown from technical triplicates, in 2 independent experiments. ALS1 was the only gene with expression significantly increased by S. oralis in the efg1 revertant but not in the efg1Δ/Δ strain. *p<0.01 in a comparison between the efg1Δ/Δ and efg1 revertant strains. (B) ALS1 gene mRNA expression levels in tongue tissues of infected mice analyzed by RT-qPCR. Mice were infected with the efg1 mutant (efg1Δ/Δ) and efg1 revertant strain with or without S. oralis 34 for 4 d. Results show ALS1 gene expression levels of the mixed infection group (CaSo) relative to Candida (Ca) infection alone, in 6 mice per group. ALS1 expression was enhanced by S. oralis in the efg1 revertant but not the efg1Δ/Δ infection group. p<0.05 for a comparison between mutant and revertant strains. (C) Als1 protein expression in single (C. albicans) and mixed (C. albicans with S. oralis) biofilms. C. albicans SC5314 was grown on Permanox® plastic chamber slides with or without S. oralis 34 in RPMI 10%FBS, 10% BHI media, for 3h-48 hours. Biofilms were labeled with a monoclonal antibody against Als1, followed by a secondary FITC-conjugated antibody (green). S. oralis (red) was labeled with an Alexa Fluor 568-labeled FISH probe and C. albicans (blue) was stained with Calcofluor White®. A representative of 3 independent experiments is shown. S. oralis increased C. albicans Als1 protein expression on the surface of hyphae after 24–48 h of co-culture. Bars: 50 μm.
To provide evidence that S. oralis can increase Als1 protein expression levels we performed a time-dependent analysis of Als1 in biofilms growing on Permanox® plastic chamber slides, by indirect immunofluorescence using an anti-Als1 mAb. As seen in Fig. 6C at 24 h of biofilm growth in nutrient-rich media (RPMI, 10%FBS, 10% BHI) S. oralis increased C. albicans Als1 protein expression on the surface of hyphae, which persisted after 48 h of co-culture (Fig. 6C). In comparison, Als3 expression levels were not elevated in biofilms with S. oralis, compared with biofilms formed by C. albicans alone (Fig. S2).
Because Als1 functions as an adhesin that binds to streptococcal cell wall proteins and promotes co-aggregation interactions with C. albicans,19 we hypothesized that an als1Δ/Δ mutant would be deficient in co-aggregation interactions with S. oralis and form a mixed species biofilm with a reduced S. oralis biomass. Deletion of the ALS1 gene significantly decreased the number of hyphae co-aggregating with S. oralis compared with the reference strain, as assessed using a co-aggregation assay (Fig. 7A, p<0.0001). A significant reduction in co-aggregation interactions was also observed with an als3 Δ/Δ mutant (p<0.05), in line with previous observations in a related oral streptococcal species showing that both Als1 and Als3 participate in adhesion to bacterial cells and promote co-aggregation interactions.19 To explore the possibility that Als1 and Als3 may function as complementary co-aggregation-promoting adhesins to S. oralis on oral mucosal surfaces we hypothesized that whereas mutants lacking either ALS1 or ALS3 form defective mucosal biofilms with S. oralis, mixing the 2 mutants together would restore the mixed biofilm. As expected the biofilm mass of S. oralis growing on the surface of organotypic mucosal constructs was reduced in biofilms formed by each mutant alone, compared with biofilms formed with the reference strain. However, as predicted, a 1:1 mixture of the 2 mutant strains restored the S. oralis biofilm on mucosal surfaces (Fig. 7B), supporting a complementary function of these adhesins in cross-kingdom co-aggregation interactions on mucosal surfaces.
Figure 7.
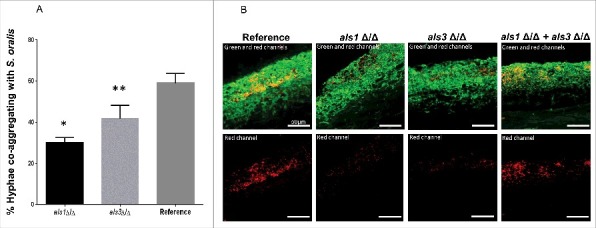
Als1 promotes co-aggregation interactions between C. albicans and S. oralis. (A) Co-aggregation assays between S. oralis 34 and C. albicans reference strain or strains lacking either ALS1 (als1Δ/Δ) or ALS3 (als3Δ/Δ) were performed as described in methods. Results represent percentage of hyphae co-aggregating with S. oralis in each microscopic field, at 40X magnification. Means ± SD are shown from 3 independent experiments, with 8 microscopic fields analyzed per condition in each experiment. Deletion of ALS1 or ALS3 significantly decreased the number of hyphae co-aggregating with S. oralis compared with the reference strain. *p<0.0001, **p<0.05 for a comparison with the reference strain. (B) Mucosal biofilms of C. albicans with S. oralis growing for 16 h on the surface of oral mucosal organotypic constructs. Tissues were inoculated with S. oralis 34 together with a C. albicans reference strain, als1Δ/Δ mutant, als3Δ/Δ mutant or a 1:1 mixture of the 2 mutants. Fluorescence images (top panel) show biofilms labeled with a FITC-conjugated anti-Candida antibody (green) and an Alexa Fluor 568-labeled streptococcal FISH probe for S. oralis (red). The red channel is individually shown in the lower panel to better visualize the bacterial biomass. Mixing the 2 mutants restored the S. oralis biofilm on mucosal surfaces, supporting a complementary functional role of Als1 and Als3 adhesins in cross-kingdom co-aggregation interactions. Bars: 50 µm.
The ALS1 overexpressing strain in the efg1Δ/Δ background forms relatively short pseudohyphae in multiple filamentation-promoting media20 and co-culture with teal-expressing S. oralis on plastic bottom chamber slides did not affect this phenotype (Fig. 8AB). However, abundant fungal-bacterial cell co-aggregation interactions were observed both in early (Fig. 8A) and late (Fig. 8B) stages of mixed biofilm development with the ALS1 overexpressing strain, compared with the efg1Δ/Δ mutant, possibly due to high constitutive expression levels of Als1 in this strain. In co-aggregation assays in shaking flasks, flocculation was so pronounced with this strain that accurate microscopic quantitative assessments could not be made.
Figure 8.
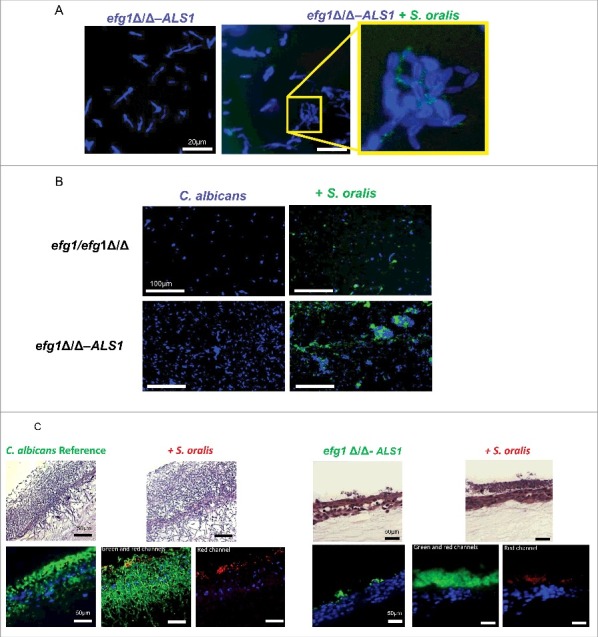
Overexpression of the ALS1 gene in the efg1Δ/Δ background partially rescues the cross-kingdom mucosal biofilm phenotype (A-B) Co-culture of a C. albicans ALS1-overexpressing strain in the efg1Δ/Δ background (efg1Δ/Δ-ALS1) with a teal protein expressing S. oralis 34 strain for 45 minutes. (A) or 24 hours (B) on Permanox® plastic chamber slides, in RPMI 10%FBS, 10%BHI media. Candida cells were stained with Calcofluor White (blue) and filamentation pattern and co-aggregation interactions were observed under a fluorescence microscope. A representative of 2 experiments is shown, with conditions set up in duplicate. Fungal-bacterial cell co-aggregation interactions were observed both in early (Fig. 8A) and late (Fig. 8B) stages of mixed biofilm development with the ALS1 overexpressing strain. Bars: 20 μm (A); 100 μm (B). (C) Sixteen-hour mucosal biofilms of C. albicans (left panels) or C. albicans with S. oralis (right panels). Biofilms of the reference strain and the efg1Δ/Δ-ALS1 overexpressing strain, with or without S. oralis 34, were grown on the surface of organotypic oral mucosal surfaces. H&E staining (top panels) and fluorescence images of biofilms labeled with a FITC-conjugated anti-Candida antibody (green), an Alexa Fluor 568-labeled streptococcal FISH probe for S. oralis (red), and counterstained with the nucleic acid stain Hoechst 33258 (blue) to visualize mucosal cells, are shown. Green and red channels are individually shown in the efg1Δ/Δ-ALS1 plus S. oralis panels to better visualize the fungal and bacterial signals. A representative of 2 experiments is shown, with conditions set up in duplicate. Overexpression of ALS1 in the efg1Δ/Δ strain background restored S. oralis biofilm to levels similar to the reference and efg1 revertant strains (see Fig. 5A for comparison to the revertant strain). Bars: 50 µm.
Because we observed more co-aggregation interactions between the ALS1 overexpressing strain and S. oralis during biofilm growth on plastic bottom chamber slides, we hypothesized that the mixed biofilm phenotype is at least partially rescued on organotypic constructs inoculated with these organisms. As seen in Fig. 8C, S. oralis promoted biofilm growth of the ALS1 overexpressing strain, however this biofilm was less robust than the biofilm formed by the reference strain, and no hyphae were observed extending into the submucosal compartment. This suggests that, in addition to ALS1, other downstream genes in the Efg1 transcriptional pathway are required to completely rescue the biofilm phenotype. Perhaps more importantly though, whereas S. oralis did not form a mucosal biofilm with the efg1Δ/Δ strain (Fig. 5A), overexpression of ALS1 in this strain background restored S. oralis biofilm to levels similar with the ones observed with the reference and efg1 revertant strains (Figs. 5A and 8C). This finding further supports the conclusion that Als1 has an important functional role in cross-Kingdom mucosal biofilm interactions between C. albicans and S. oralis.
Discussion
Several members of the mitis streptococcal group have been implicated in pathogenic synergy when forming polymicrobial biofilms with other bacterial or fungal organisms.3,21,22 This work builds on previously published evidence that S. oralis, a mitis group member, potentiates the virulence of C. albicans oral mucosal biofilms and that C. albicans promotes S. oralis biofilm formation.3,13 Because both S. oralis and C. albicans are ubiquitous colonizers of the oral cavity in healthy humans,1,2,11 it is important to understand how these organisms modulate the capacity of each other to form robust biofilms that enable their transition from commensals to opportunistic pathogens.
In this study we identified Efg1 and its target gene ALS1 as important regulators of cross-kingdom biofilm interactions between C. albicans and S. oralis, particularly in late stages of biofilm growth. We previously reported that the master regulator Bcr1, and its downstream effector Hyr1, play a role in oral mucosal biofilms formed by C. albicans,23 however BCR1 gene expression was not activated in Candida-streptococcal biofilms in this work. This is showcasing the fact that fungal genes turned on during mucosal biofilm growth do not respond to a single central regulator but to different regulators, depending on the presence of other microorganisms and the stage of biofilm growth. In this work none of the 6 C. albicans master transcriptional regulators was strongly activated in response to early contact with bacterial cells, suggesting that they may not play a role in the initial inter-kingdom cell-cell interactions that promote mixed biofilm growth. It is possible that other, as yet unidentified C. albicans transcriptional regulators play a role in early mixed biofilm development steps, such as adhesion to the substratum surface. The fact that EFG1 is strongly activated by S. oralis in late stages of biofilm development may suggest that a critical bacterial biomass is needed to trigger this response, perhaps through release of a quorum sensing molecule.
The Efg1 transcription regulator is one of the best-studied, multifunctional regulators in C. albicans.15,24-30 Homozygous deletion of the EFG1 gene in C. albicans strongly attenuates its response to filamentation-inducing stimuli in most liquid and solid media.30,31 The Efg1 regulator not only controls filamentous growth and biofilm formation but also the ability of C. albicans to adhere to the epithelial matrix protein laminin, to adhere to and invade epithelial cells, and to induce epithelial cell damage in vitro.18,27 This work highlights a novel functional role for Efg1 in promoting co-aggregation interactions between C. albicans and oral streptococci during late stages of mixed biofilm growth, via induction of the adhesin Als1. This was not surprising since Als1 has been shown to play a role in C. albicans adhesion to, and co-aggregation with S. gordonii, another member of the oral streptococcal mitis group.19 Since expression of Als1 promotes inter-kingdom co-aggregation interactions, this may be an important mechanism for S. oralis mucosal biofilm formation, as this bacterial species does not form single species biofilms.3,12,13
Late biofilm stage activation of ALS1 gene transcription in C. albicans biofilms has also been observed by others32 and may have a distinct functional role from early activation, in promoting cell-cell interactions within biofilms, rather than promoting adhesion to the substratum. Although recognized as an important adhesin to biofilm substrates,5 our finding that Als1 is upregulated in late biofilm growth stages with S. oralis suggests that in these biofilms the most important function of Als1 is to promote co-aggregation interactions between bacteria and hyphae. Consistent with a role in promoting co-aggregation interactions, Als1 has been shown to play a positive role in both filamentation and flocculation.20,25
ALS1 expression is completely dependent on Efg1 under many in vitro conditions20,31 and Efg1-dependent ALS1 gene expression has been shown to mediate cell-cell aggregation in C. albicans.33 However, in biofilms growing on abiotic surfaces Als1 was found to be regulated principally by Bcr1.34 Our finding that ALS1 is instead regulated by Efg1 in C. albicans-S. oralis biofilms underscores the significance of environmental cues, such as the presence of bacteria in the mucosal environment, in dictating Candida biofilm regulatory signaling pathways.
Expression of ALS1 from an EFG1-independent promoter in the efg1Δ/Δ strain does not restore the ability to form hyphae,20 thus our findings that S. oralis only partly restored the Candida biofilm phenotype of this strain are not surprising. Work from Mitchell and colleagues showed that expression of ALS1 from an RHR2-independent promoter fully restored biofilm formation of a biofilm-deficient rhr2Δ/Δ mutant. However, unlike the efg1Δ/Δ mutant, this mutant is not defective in hyphal formation.35 Importantly, almost complete restoration of S. oralis biofilm growth was noted in mixed mucosal biofilms when ALS1 was expressed from an EFG1-independent promoter in the efg1Δ/Δ strain. This further underscores the importance of Als1 in regulating co-aggregation interactions and S. biofilm growth, in the context of the Efg1 signaling pathway.
In addition to Als1, which is not hypha-specific,36,37 hyphal adhesins such as Hwp1 and Als3 play a role in co-aggregation interactions with streptococci of the mitis group.38 Our work with the als1 and als3 homozygous deletion mutants showed that both of these adhesins promote S. oralis mucosal biofilm growth and that their function may be complementary, if not overlapping. This finding is consistent with previous work showing that Als1 and Als3 have complementary functions in vitro and in vivo.39 The fact that Als1, but not Als3, was upregulated in an Efg1-dependent manner by S. oralis in mucosal biofilms, underlines the central role of ALS-gene regulation in identifying the manifested, as opposed to plausible function of Als adhesins in each biofilm model, an idea also supported by work from others.39
An efg1 homozygous deletion mutant grows as short pseudohyphae with oral epithelial monolayers.18 Early work from our group established that hyphal growth is essential in oral epithelial adhesion, injury and induction of pro-inflammatory cytokine responses.40 This explains the attenuated mucosal colonization of the efg1Δ/Δ strain in this work and the work of others.18 Along these lines, in the C. albicans mouse vaginitis model the efg1Δ/Δ strain grew exclusively as yeast, with mucosal damage and inflammatory responses considerably reduced, suggesting that similar to the oral mucosa, virulence in this mucosal site depends primarily on the Efg1 pathway.41 The fact that S. oralis promotes Efg1-mediated hyphal growth supports a possible role of this transcription pathway in the exaggerated mucosal inflammatory response and increased virulence in co-infected mice, reported previously.3 The role of this pathway in fungal-bacterial synergy was also demonstrated in an intra-abdominal staphylococcal-C. albicans mixed infection model where Efg1 was required for synergistic virulence.42
The finding that filamentation was not promoted by S. oralis in the efg1 mutant shows that this regulatory pathway is required for hyphal growth in Candida-streptococcal biofilms. Interestingly, S. oralis was able to promote the oral colonization of the efg1Δ/Δ strain, suggesting that the requirement for Efg1 in these interactions in vivo is modified by the mucosal environment and perhaps by the presence of commensal microorganisms other than S. oralis. This is exemplified also by the fact that although the efg1Δ/Δ strain colonizes the oral mucosa of mice poorly (this work and ref. 18), it colonizes the vaginal and gastrointestinal mucosae in high numbers.41,43 Efg1 is involved in the regulation of SAP 4,5,6 genes in the GI tract,43 genes that are also upregulated in the Candida-streptococcal oral biofilm mouse model,14 further lending support to a central role of this transcription pathway in mixed infection. However, it is also important to note that, although these genes play a role in oral mucosal invasion in single-species biofilms44 they were not required for the increased C. albicans tissue invasion in biofilms with S. oralis.14 This suggests that some genes identified as strong virulence determinants in single infection models may be dispensable in mixed biofilms of C. albicans with certain bacterial species.
We conclude that S. oralis modulates C. albicans virulence by increasing EFG1 gene expression. This, in turn increases filamentation and expression of the downstream gene ALS1, which promotes coaggregation interactions and biofilm growth. Exploring the transcriptional regulation of Candida-bacterial biofilms is novel and important, since most mucosal fungal infections occur in a polymicrobial environment. Our work provides novel insights on the regulatory control of cross-kingdom mucosal biofilms which may facilitate the transition of C. albicans and S. oralis from commensals to opportunistic pathogens.
Methods
Strains and growth conditions
C. albicans and S. oralis strains used in this study are listed in Table 1. C. albicans strains were routinely maintained in yeast extract peptone dextrose (YPD) agar and grown in YPD broth, aerobically, at room temperature, on a rotor shaker. S. oralis 34 was routinely grown from frozen stocks in brain heart infusion (BHI) medium (BD Cat. No. 211059) under aerobic, static conditions, at 37°C, 5% CO2, one day before each experiment. A teal fluorescence protein expressing- S. oralis 34 strain (emitting green fluorescence at excitation wavelength 492 nm) was constructed by transforming strain 34 with the streptococcal replicative plasmid pVMTeal carrying a streptococcal codon-optimized gene for TFP1 fluorescent protein.45 The plasmid was transformed into serum-competent strain 34 cells, plated on Todd Hewitt agar medium and excited colonies were screened for fluorescence intensity on a ChemiDoc MP Imaging System (Bio-Rad). After 2 successive passages in Todd Hewitt broth and selection, plasmid DNA was recovered from one of the brightest colonies and retransformed into parental S. oralis 34 cells to create strain S. oralis 34 teal.
Table 1.
Organisms used in this study.
| Strain | Genotype | Note |
|---|---|---|
| SC5314 | Blood clinical isolate | 51 |
| SN425 | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 | 52 |
| ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 | ||
| efg1Δ/Δ/ | ura3Δ::λimm434::URA3-IRO1arg4::hisGhis1::hisGleu2::hisG::CdARG4efg1Δ::CmLEU2 | 5 |
| CJN2302 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG efg1Δ::CdHIS1 | |
| efg1 revertant/ | ura3Δ::λimm434::URA3-IRO1arg4::hisGhis1::hisGleu2::hisG::EFG1-CdARG4efg1Δ::CmLEU2 | 5 |
| CJN 2318 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG efg1Δ::CdHIS1 | |
| EFG1p-mCherry/ | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 EFG1p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2619 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 EFG1 | |
| ROB1p-mCherry | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1ROB1p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2629 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 ROB1 | |
| BRG1p-mCherry/ | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 BRG1p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2621 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 BRG1 | |
| TEC1p-mCherry/ | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 TEC1p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2616 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 TEC1 | |
| BCR1p-mCherry/ | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 BCR1p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2614 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG::CmLEU2 BCR1 | |
| NDT80p-mCherry/ | ura3Δ::λimm434::URA3-IRO1arg4::hisG::CdARG4his1::hisGleu2::hisG::CdHIS1 NDT801p-mCherry-FRT-FLP-SAT1-FRT | 5 |
| CJN2672 | ura3Δ::λimm434 rg4::hisG his1::hisG leu2::hisG::CmLEU2 NDT80 | |
| efg1Δ/Δ−ALS1/ | ura3Δ::λimm434::URA3-IRO1arg4::hisGhis1::hisGleu2::hisGefg1Δ::CmLEU2ALS1::AgTEF1p-NAT1-AgTEF1UTR-TDH3p-ALS1 | 5 |
| CJN2479 | ura3Δ::λimm434 arg4::hisG his1::hisG leu2::hisG efg1Δ::CdHIS1 ALS1 | |
| als1Δ/Δ/ | ura3Δ::λimm434::URA3-IRO1als1::hisG | 34 |
| CAYC2YF1U | ura3Δ::λimm434 als1::hisG | |
| als3Δ/Δ/ | ura3Δ::λimm434::URA3-IRO1als3::ARG4arg4::hisGhis1::hisG | 34 |
| CAYF178U | ura3Δ::λimm434 als3::HIS1 arg4::hisG his1::hisG | |
| S. oralis 34 | Oral clinical isolate from healthy mucosa | Dr. A. Rickard |
| S. oralis 34 teal | S. oralis 34 carrying replicative plasmid expressing mTFP1 (fluorescent Teal Protein) | This work |
Biofilm growth
Growth on abiotic surfaces
To prepare the microbial inocula for biofilm growth, overnight broth cultures of each microorganism were used to inoculate new cultures in YPD (for C. albicans) or BHI (for S. oralis) broth, that were allowed to grow until late logarithmic phase. For fluorescence imaging of biofilms, microorganisms from these cultures were inoculated in 4-well Permanox® plastic chamber slides (ThermoFisher, Cat. No. 177437). Each chamber was inoculated with 104 cells of C. albicans and 105 cells of S. oralis. For biofilms used for DNA or RNA extraction, 105 C. albicans and 106 S. oralis cells were seeded in 6-well polystyrene plates. Biofilms were grown in RPMI 1640 media containing 10% FBS and 10% BHI or in SSM with or without 10% BHI. SSM consisted of 0.625 g L−1 type II porcine gastric mucin, 0.5 g L−1, peptone, 0.5 g L−1 tryptone, 0.25 g L−1 yeast extract, 0.088 g L−1 NaCl, 0.05 g L−1 KCl, 0.05 g L−1 CaCl2, and 0.25 mg mL−1 haemin, pH 7.0 supplemented with 2.5 mM DTT and 0.5 g L−1 sucrose.15 Biofilms were grown for 4–48 hours at 37°C, in a 5%CO2 incubator.
Growth on oral mucosal constructs
The development of 3-dimensional oral mucosal constructs which faithfully mimic non-keratinized human oral mucosa have been described in detail elsewhere.46 Briefly the organotypic constructs consist of a TERT-2-immortalized human oral keratinocyte cell line (OKF6) seeded on collagen type I-embedded fibroblasts (3T3 fibroblasts, ATCC). Tissues are airlifted to ensure epithelial differentiation and stratification. Microbial inocula were prepared as done for abiotic surface biofilms. Each tissue was inoculated with 106 cells of C. albicans or 107 cells of streptococci or a combination, in 25 μL of infection medium. When 2 strains of C. albicans were used to simultaneously inoculate a tissue surface, 5 × 105 cells of each strain were used/tissue. Infection media consist of DMEM, supplemented with L-glutamine, hydrocortisone, ITES, O-phosphorylethanolamine, adenine and triiodothyronine. Mucosal biofilms grew at the air-liquid interface at 37°C in a 5% CO2 incubator for 16h. These conditions have been optimized to result in well-organized, mature mucosal biofilms.7
Mouse model of co-infection
Six- to 12-week-old female C57BL/6 mice were purchased from the Jackson Laboratory and used in these experiments (Animal protocol #100358–0215). The C. albicans-S. oralis 34 mouse oral co-infection model has been described in detail elsewhere.3,14 Briefly, mice were immunosuppressed with cortisone acetate (225 mg/kg, subcutaneously, Sigma Cat. No. C3130–5G), on the first and third day of the infection period. Microbial inocula were prepared as described above. A cotton pellet, saturated with 100 μL microbial suspension of each organism or their combination (yeast cells [6 × 108/mL] and/or bacteria [2.5 × 109/mL]), was placed sublingually, under general anesthesia for 2 h. Fresh suspensions of microorganisms were added daily in the drinking water. Mice were killed on day 4 post-inoculation and tissues were harvested for mRNA, CFU or histological analysis. For CFU determinations tongues were excised, weighed and homogenized. Undiluted and diluted homogenates were plated on Sabouraud dextrose agar (Becton-Dickinson, Cat. No. 211584) supplemented with chloramphenicol (Sigma, Cat. No. C0378), or Mitis-Salivarius® agar supplemented with 1% Tellurite (Difco Cat. No. 229810), for C. albicans and S. oralis CFU counts, respectively. Tongue homogenates plated from uninfected animals show no fungal or bacterial colony growth on these solid media.3,14
RNA extraction and RT-qPCR
Fungal RNA from abiotic biofilms, mucosal constructs or mouse tongue tissues was extracted as described previously, with minor modifications.14,47 Mouse tongues were dissected sagittally along the mid line and one half was used to extract fungal RNA. Tissues were homogenized using a POLYTRON® homogenizer and the supernatants were beat by using 0.5 mm zirconium beads (BeadBug® prefilled tubes, Sigma-Aldrich, Cat. No. Z763772), mixed with 4°C UltraPure Phenol:Chloroform:Isoamyl Alcohol (Invitrogen, Cat. No. 15593–031) in 1:1 vol:vol ratio. RNA was purified using the RNeasy Mini Kit® which includes a DNAse treatment step (QIAgen Cat. No. 74104). A second DNase treatment was performed by using the TURBO DNA-free® Kit (Thermo Fisher Scientific Cat. No. AM1907). RNA concentrations and quality were determined using a NanoDrop®. cDNA was synthesized with SuperScript III CellDirect cDNA Synthesis kits® (Invitrogen Cat. No. 18080–051).
All RT-qPCR was performed with a BIO-RAD CFX96 cycler and the IQTM SYBR® Green Supermix kit (BIO-RAD Cat. No. 1708880). Mastermix, cDNA and primers (0.5 μM) were mixed in a total volume of 20 μL per reaction. The amplification program included an initial denaturation step at 95°C for 5 min, followed by 40 cycles of 95°C for 45 s and 58°C for 30 s. A minus-reverse transcriptase control was used in all PCR reactions to confirm that amplification of any residual genomic DNA is negligible. The primers used to amplify Candida genes were, Als1-F AGAACTGATTTGCAGTGATGG, Als1-R TGAGGATTCATTGCTATCTGG; Hwp1-F TGGTCCAGGTGCTTCTTCTT, Hwp1-R GGTTGCATGAGTGGAACTGA; Als3-F CCACTTCACAATCCCCAT, Als3-R CAGCAGTAGTAGTAACAGTAGTAGTTTCAT; Ndt80-R GGTTGTCTTGCTGGTTGAGGC, Ndt80-F ACACCTCAGCAGCCACATTTG; Efg1-F TATCACGTGAATATTTCCAGGG, Efg1-R CTGACTGTTCGTTGTGATTTGG; Tec1-F GCTCAGTAGCTTCACAACTGC, Tec1-R AGTAGGTGGAACAAAAGTGCC, Brg1-F GGGTTATTCCACGCTAAATTG, Brg1-R TATTCTTCGACCGTTCCTCCC, Rob1-F AGCCAAAACATGAATACCACG, Rob1-R TTCTTGTGGTTGTGGTTCGTC Bcr1-F CCCCCAGTATCAAGCATAACAG, Bcr1-R ATCGTGAAGTTCGATACTTTGG. The EFB1 translation elongation factor gene was used as an internal control and data were normalized and calculated by ΔΔCq method.
DNA extraction and Candida quantification by qPCR
For quantitative assessment of C. albicans hyphae in biofilms growing on abiotic surfaces, qPCR was performed. Fungal DNA was extracted using the Fungi DNA Isolation Kit® (NORGEN Cat. No. E5038), according to the manual. Primers specific for the 18 S rRNA gene of C. albicans were used (18S-F GGATTTACTGAAGACTAACTACTG, 18S-R GAACAACAACCGATCCCTAGT), with an annealing temperature of 59°C. The amplification protocol and genome equivalent calculations were described in detail elsewhere.48 Briefly, the qPCR was performed using the thermocycling instructions recommended for the SYBR green PCR Master Mix (95°C for 30 s and 40 cycles of 30 s at 95°C and 30 s at 59°C). Since the organism has variable numbers of 18S rRNA gene copies per genome,49 genome equivalents (or fungal biomass) were calculated based on standard curves obtained after amplifying 10-fold serial dilutions of fungal DNA isolated from overnight 37°C YPD broth cultures (strain SC5314). To convert nanograms of DNA in overnight cultures to genome equivalents, we took into account that the C. albicans SC5314 haploid genome size is ∼15 Mbp (www.candidagenome.org). Assuming the average base pair weight in fungal cells to be 650 Daltons, cell numbers were calculated according to the formula: cell number = (ng of DNA* 6.022 × 1023) / (15 × 106 * 1 × 109 * 650). PCR was performed with a BIO-RAD CFX96 cycler and the IQTM SYBR® Green Supermix kit (BIO-RAD Cat. No. 1708880).
Fluorescence imaging
Protocols for immunofluorescence labeling of C. albicans and S. oralis biofilms were described in detail previously.3,7,14 To visualize epithelial cell nuclei and C. albicans simultaneously, Hoechst 33258 and a FITC-conjugated anti-Candida antibody (Meridian Life Science Cat. No. B65411F) were used, respectively. In mucosal biofilms S. oralis was visualized by FISH with the Streptococcus-specific oligonucleotide probe STR405, conjugated to Alexa 546. In abiotic surface biofilms Candida cells were stained with Calcofluor White® (Sigma Cat. No. 18909–100ML-F) for 5 minutes. Als1 and Als3 protein expression in single-species and mixed abiotic surface biofilms were tested by indirect immunofluorescence labeling using monoclonal anti-Als1 and anti-Als3 antibodies with established specificities and protocols developed by the Hoyer laboratory.19,36,37 Briefly, biofilms were fixed in 4% paraformaldehyde, followed by washing and a blocking step with 15 μl/ml normal goat serum for 15 min. Immuno-labeling was performed with 18 μg/ml of purified anti-Als antibody, followed by a FITC-conjugated goat anti-mouse IgG F(ab')2 fragment-specific antibody (Jackson ImmunoResearch Cat. No. 115–096–006). Als protein immuno-labeling was followed by FISH with the Streptococcus-specific oligonucleotide probe STR405, conjugated to Alexa 546 to visualize S. oralis, and Calcofluor White® to visualize C. albicans hyphae. Fluorescence images were captured using a Zeiss Axio Imager M1 microscope, using a 63x oil immersion objective.
Co-aggregation assays
Experiments to quantitatively assess co-aggregation between C. albicans strains and streptococci were performed as originally described by the Jenkinson laboratory,50 and later modified by Hoyer and colleagues,19 with the exception that instead of FITC-labeled bacteria we used a teal-expressing S. oralis strain. Briefly, C. albicans yeast cells from an overnight YPD broth culture were inoculated into RPMI1640–10% FBS medium for 3 h to form hyphae. S. oralis late-log phase inocula were prepared as described in biofilm growth assays. Harvested bacterial and fungal cells (10:1 ratio) were suspended in co-aggregation buffer (pH 8.0) which consisted of the following: 1 mM Tris HCl, 0.15 M NaCl, 0.1 mM MgCl2, and 0.1 mM CaCl2. Co-aggregation interactions were allowed to take place for 1 h, at 37°C, in a shaking flask. 150 μl of cell suspension from each flask was transferred into a well of an 8-well Permanox® plastic chamber slide, hyphae were stained with Calcofluor White® and observed under a fluorescence microscope. The numbers of hyphae with bacterial binding were expressed as percentages of the total number of hyphae counted, as determined in 8–10 microscopic fields (40X)/per condition, in 3 independent experiments.
Statistical analyses
Animal experiments used groups of 5–8 mice and were independently repeated 3 times, unless noted otherwise. Pair-wise comparison of gene expression data was performed using the non-parametric Mann-Whitney test, or Student's t-test when data points were normally distributed. Fungal and bacterial burdens with different combinations of strains were analyzed using ANOVA or the Kruskal-Wallis test, when data did not pass the normality test. Analyses were performed using the Graph-Pad Prism® software. Statistical significance for all tests was set at P < 0.05.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Alexander Johnson for providing C. albicans strains used in this study and Dr. Lois Hoyer for providing the monoclonal antibodies for Als proteins.
Funding
This work was supported by Public Health Service from the National Institute of Dental and Craniofacial Research under grant number [RO1 DE013986].
References
- [1].Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. Plos Pathog 2010; 6:e1000713; PMID:; https://doi.org/ 10.1371/journal.ppat.1000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI, Strausbaugh LD. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One 2014; 9:e90899; PMID:; https://doi.org/ 10.1371/journal.pone.0090899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, Cervantes J, Diaz PI, Dongari-Bagtzoglou A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol 2014; 16:214-31; PMID:; https://doi.org/ 10.1111/cmi.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, et al.. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun 2014; 82:1968-81; PMID:; https://doi.org/ 10.1128/IAI.00087-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012; 148:126-38; PMID:; https://doi.org/ 10.1016/j.cell.2011.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 2012; 25:193-213; PMID:; https://doi.org/ 10.1128/CMR.00013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. Characterization of mucosal Candida albicans biofilms. Plos One 2009; 4:e7967; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu H, Jenkinson HF, Dongari-Bagtzoglou A. Innocent until proven guilty: mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol Oral Microbiol 2014; 29:99-116; PMID:; https://doi.org/ 10.1111/omi.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Johnson CC, Yu A, Lee H, Fidel PL Jr., Noverr MC. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect Immun 2012; 80:1736-43; PMID:; https://doi.org/ 10.1128/IAI.00019-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nett JE, Marchillo K, Spiegel CA, Andes DR. Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 2010; 78:3650-9; PMID:; https://doi.org/ 10.1128/IAI.00480-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diaz PI, Dupuy AK, Abusleme L, Reese B, Obergfell C, Choquette L, Dongari-Bagtzoglou A, Peterson DE, Terzi E, Strausbaugh LD. Using high throughput sequencing to explore the biodiversity in oral bacterial communities. Mol Oral Microbiol 2012; 27(3):182-201; PMID:; https://doi.org/ 10.1111/j.2041-1014.2012.00642.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rickard AH, Jr Palmer RJ, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol 2006; 60(6):1446-56; PMID:; https://doi.org/ 10.1111/j.1365-2958.2006.05202.x [DOI] [PubMed] [Google Scholar]
- [13].Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, Ikonomou L, Dongari-Bagtzoglou A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun 2012; 80:620-32; PMID:; https://doi.org/ 10.1128/IAI.05896-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu H, Sobue T, Bertolini M, Thompson A, Dongari-Bagtzoglou A. Streptococcus oralis and Candida albicans synergistically activate μ-Calpain to degrade E-cadherin from oral epithelial junctions. J Infect Dis 2016; 214(6):925-34; PMID:; https://doi.org/ 10.1093/infdis/jiw201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell 1997; 90:939-49; PMID:; https://doi.org/ 10.1016/S0092-8674(00)80358-X [DOI] [PubMed] [Google Scholar]
- [16].Arzmi MH, Dashper S, Catmull D, Cirillo N, Reynolds EC, McCullough M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res 2015; 15:fov038; PMID:; https://doi.org/ 10.1093/femsyr/fov038 [DOI] [PubMed] [Google Scholar]
- [17].Brinkman NE, Haugland RA, Wymer LJ, Byappanahalli M, Whitman RL, Vesper SJ. Evaluation of a rapid, quantitative real-time PCR method for enumeration of pathogenic Candida cells in water. Appl Environ Microbiol 2003; 69(3):1775-82; PMID:; https://doi.org/ 10.1128/AEM.69.3.1775-1782.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards J, Filler SG. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 2005; 7:499-510; PMID:; https://doi.org/ 10.1111/j.1462-5822.2004.00476.x [DOI] [PubMed] [Google Scholar]
- [19].Hoyer LL, Oh SH, Jones R, Cota E. A proposed mechanism for the interaction between the Candida albicans Als3 adhesin and streptococcal cell wall proteins. Front Microbiol 2014; 5:e564; https://doi.org/ 10.3389/fmicb.2014.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, Filler SG, Edwards JE Jr.. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol 2002; 44(1):61-72; PMID:; https://doi.org/ 10.1046/j.1365-2958.2002.02873.x [DOI] [PubMed] [Google Scholar]
- [21].Ramsey MM, Rumbaugh KP, Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. Plos Pathog 2011; 7:e1002012; PMID:; https://doi.org/ 10.1371/journal.ppat.1002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol 2011; 81:305-14; PMID:; https://doi.org/ 10.1111/j.1365-2958.2011.07707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, Dongari-Bagtzoglou A. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. Plos One 2011; 6:e16218; PMID:; https://doi.org/ 10.1371/journal.pone.0016218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lassak T, Schneider E, Bussmann M, Kurtz D, Manak JR, Srikantha T, Soll DR, Ernst JF. Target specificity of the Candida albicans Efg1 regulator. Mol Microbiol 2011; 82:602-18; PMID:; https://doi.org/ 10.1111/j.1365-2958.2011.07837.x [DOI] [PubMed] [Google Scholar]
- [25].Gregori C, Glaser W, Frohner IE, Reinoso-Martin C, Rupp S, Schuller C, Kuchler K. Efg1 Controls caspofungin-induced cell aggregation of Candida albicans through the adhesin Als1. Eukaryot Cell 2011; 10:1694-704; PMID:; https://doi.org/ 10.1128/EC.05187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nie X, Liu X, Wang H, Chen J. Deletion of EFG1 promotes Candida albicans opaque formation responding to pH via Rim101. Acta Biochim Biophys Sin (Shanghai) 2010; 42:735-44; PMID:; https://doi.org/ 10.1093/abbs/gmq076 [DOI] [PubMed] [Google Scholar]
- [27].Saville SP, Thomas DP, Lopez Ribot JL. A role for Efg1p in Candida albicans interactions with extracellular matrices. FEMS Microbiol Lett 2006; 256:151-8; PMID:; https://doi.org/ 10.1111/j.1574-6968.2006.00109.x [DOI] [PubMed] [Google Scholar]
- [28].Sohn K, Urban C, Brunner H, Rupp S. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol Microbiol 2003; 47:89-102; PMID:; https://doi.org/ 10.1046/j.1365-2958.2003.03300.x [DOI] [PubMed] [Google Scholar]
- [29].Srikantha T, Tsai LK, Daniels K, Soll DR. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J Bacteriol 2000; 182:1580-91; PMID:; https://doi.org/ 10.1128/JB.182.6.1580-1591.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J 1997; 16:1982-91; PMID:; https://doi.org/ 10.1093/emboj/16.8.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 2000; 155:57-67; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fox EP, Bui CK, Nett JE, Hartooni N, Mui MC, Andes DR, Nobile CJ, Johnson AD. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol Microbiol 2015; 96(6):1226-39; PMID:; https://doi.org/ 10.1111/mmi.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bastidas R, Heitman J, Cardenas ME. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog 2009; 5(2): e1000294; PMID:; https://doi.org/ 10.1371/journal.ppat.1000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2006; 2(7):e63; PMID:; https://doi.org/ 10.1371/journal.ppat.0020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Desai JV, Cheng S, Ying T, Nguyen MH, Clancy CJ, Lanni F, Mitchell AP. Coordination of Candida albicans invasion and infection functions by phosphoglycerol phosphatase Rhr2. Pathogens 2015; 4:573-89; PMID:; https://doi.org/ 10.3390/pathogens4030573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coleman DA, Oh SH, Zhao X, Hoyer LL. Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody. Microbiology 2010; 156:3645-59; PMID:; https://doi.org/ 10.1099/mic.0.043851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coleman DA, Oh SH, Zhao X, Hutchins JT, Vernachio JH, Patti JM, Hoyer LL. Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J Microbiol Methods 2009; 78:71-8; PMID:; https://doi.org/ 10.1016/j.mimet.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bamford CV, d'Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun 2009; 77:3696-704; PMID:; https://doi.org/ 10.1128/IAI.00438-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. Complementary adhesin function in C. albicans biofilm formation. Current Biology 2008; 18:1017-24; PMID:; https://doi.org/ 10.1016/j.cub.2008.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Villar CC, Kashleva H, Dongari-Bagtzoglou A. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol 2004; 19:262-9; PMID:; https://doi.org/ 10.1111/j.1399-302X.2004.00150.x [DOI] [PubMed] [Google Scholar]
- [41].Peters BM, Palmer GE, Nash AK, Lilly EA, PLJr Fidel, Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun 2014; 82:532-43; PMID:; https://doi.org/ 10.1128/IAI.01417-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nash EE, Peters BM, Fidel PL, Noverr MC. Morphology-independent virulence of Candida species during polymicrobial intra-abdominal infections with Staphylococcus aureus. Infect Immun 2016; 84:90-8; https://doi.org/ 10.1128/IAI.01059-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pierce JV, Dignard D, Whiteway M, Kumamoto CA. Normal adaptation of Candida albicans to the murine gastrointestinal tract requires Efg1p-dependent regulation of metabolic and host defense genes. Eukaryot Cell 2013; 12:37-49; PMID:; https://doi.org/ 10.1128/EC.00236-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 2007; 75:2126-35; PMID:; https://doi.org/ 10.1128/IAI.00054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vickerman MM, Mansfield JM, Zhu M, Walters KS, Banas JA. Codon-optimized fluorescent mTFP and mCherry for microscopic visualization and genetic counterselection of streptococci and enterococci. J Microbiol Methods 2015; 116:15-22; PMID:; https://doi.org/ 10.1016/j.mimet.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dongari-Bagtzoglou A, Kashleva H. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat Protoc 2006; 1:2012-8; PMID:; https://doi.org/ 10.1038/nprot.2006.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xie Z, Thompson A, Kashleva H, Dongari-Bagtzoglou A. A quantitative real-time RT-PCR assay for mature C. albicans biofilms. BMC Microbiol 2011; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sztajer H, Szafranski SP, Tomasch J, Reck M, Nimtz M, Rohde M, Wagner-Döbler I. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J 2014; 8:2256-71; PMID:; https://doi.org/ 10.1038/ismej.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rustchenko EP, Curran TM, Sherman F. Variations in the number of ribosomal DNA units in morphological mutants and normal strains of Candida albicans and in normal strains of Saccharomyces cerevisiae. J Bactereriol 1993; 175(22):7189-99; https://doi.org/ 10.1128/jb.175.22.7189-7199.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun 2010; 78:4644-52; PMID:; https://doi.org/ 10.1128/IAI.00685-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 1984; 198:179-82; PMID:; https://doi.org/ 10.1007/BF00328721 [DOI] [PubMed] [Google Scholar]
- [52].Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 2010; 42(7):590-8; PMID:; https://doi.org/ 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


