ABSTRACT
Plectin involved in activation of kinases in cell signaling pathway and plays important role in cell morphology and migration. Plectin knockdown promotes cell migration by activating focal adhesion kinase and Rac1-GTPase activity in liver cells. Sorafenib is a multi-targeting tyrosine kinase inhibitor that improves patient survival on hepatocellular carcinoma. The aim of this study is to investigate the correlation between the expression of plectin and cell migration as well as the sensitivity of hepatoma cell lines exposing to sorafenib. Hepatoma cell lines PLC/PRF/5 and HepG2 were used to examine the level of plectin expression and cell migration in comparison with Chang liver cell line. In addition, sensitivity of the 3 cell lines to sorafenib treatment was also measured. Expression of plectin was lower in PLC/PRF/5 and HepG2 hepatoma cells than that of Chang liver cells whereas HepG2 and PLC/PRF/5 cells exhibit higher rate of cell migration in trans-well migration assay. Immunohistofluorecent staining on E-cadherin revealed the highest rate of collective cell migration in HepG2 cells and the lowest was found in Chang liver cells. Likewise, HepG2 cell line was most sensitive to sorafenib treatment and Chang liver cells exhibited the least sensitivity. The drug sensitivity to sorafenib treatment showed inverse correlation with the expression of plectin. We suggest that plectin deficiency and increased E-cadherin in hepatoma cells were associated with higher rates of cell motility, collective cell migration as well as higher drug sensitivity to sorafenib treatment.
KEYWORDS: collective cell migration, cytoskeleton, E-cadherin, hepatocellular carcinoma, plectin, sorafenib
Introduction
Microtubules (MTs), intermediate filaments (IFs), and microfilaments (MFs) are major components of the cytoskeleton that are essential to maintain the integrity of eukaryotic cells.1 Proper cross-linking structures among these cytoskeletal proteins are crucial for intracellular architectures and normal cellular morphology. Plectin is a versatile adherent macromolecule expressed in a wide range of mammalian cells with a molecular weight of 500 kDa.2 Plectin exhibits the binding sites accessible to IFs, MTs and MFs rendering the interaction with a variety of cytoskeletal components to maintain the integrity of cytoskeletal network.3 Plectin also participates in regulating mitogen-activated protein (MAP) kinase cascades and the PKC signaling pathway leading to Erk1/2 activation and cell migration.4 Some human diseases such as epidermolysis bullosa simplex with muscular dystrophy are autosomal recessive disorders caused by the mutations of human plectin gene mapped onto chromosome 8q24.13-qter.5
Hepatocellular carcinoma (HCC) is a cancer with high incidence and mortality. HCC cells exhibit different morphological features in divergent shapes and pleomorphic nuclei from normal liver cells. Human liver consists of up to 80% parenchymal cells count of human hepatocytes. Two types of cytokeratins (CKs), CK8 (type II) and CK18 (type I) with molecular weight of 52 kDa and 45 kDa respectively, compose of IFs in human hepatic parenchyma cells.6 Plectin deficiency was characterized in HCC tissues suggesting its involvement in pleomorphism of HCC cells.7,8 Pleomorphism mediated by plectin deficiency is associated with an altered level of CK18 expression in human HCC.7 In addition, plectin deficiency in human liver cells was found in altered cytoskeletal organization in association with changed cell morphology and subsequent tumor transformation.9 Plectin therefore likely regulates cytoskeletal integrity. Changed level of plectin may be associated with diseases such as neoplasm.
Cancer is not only a disease of uncontrolled cell growth, but also a disease of uncontrolled cell migration. Metastasis is a major factor leading to mortality of HCC.10 Many studies have demonstrated two main patterns of cancer cell invasion: collective cell migration and individual cell migration.11 In human cancer pathology studies, cancer cells of epithelial tumors primarily invade collectively.12 Coherent cells are connected by intercellular adhesion molecules such as cadherins and other members of the immunoglobulin adhesion receptor family.13 In tumor progression, cell collectives can be detected at any stage of metastasis. Therefore, collective cell migration may play critical roles in cancer metastasis. In fact, cellular migration requires coordination of cytoskeleton, anchoring proteins and cytolinker proteins.14 It has been demonstrated that transient knockdown-mediated deficiency in plectin alters hepatocellular motility in association with activated focal adhesion kinase (FAK) and Rac1-GTPase activity.15 In this study, the relationship of plectin deficiency with collective cell migration and individual cell migration in human liver and hepatoma cell lines will be analyzed. Moreover, the involvement of E-cadherin expression in collective cell migration will also be studied.
HCC is often diagnosed at an advanced stage and unable to treat with radical operations.16 Systemic therapy targeting cancer cells is an alternative option for the management of HCC. Sorafenib is a multi-targeting tyrosine kinase inhibitor that blocks the activity of Raf serine/threonine kinase isoforms in consequence to inhibit tumor angiogenesis and tumor cell proliferation.17,18 Large multi-center trials including Phase III Sorafenib HCC Assessment Randomized Protocol (SHARP) trial19 and phase III Sorafenib Asia-Pacific trial performed in China, South Korea, and Taiwan20 have demonstrated positive impact of sorafenib on improved survival and delayed tumor progression. The pivotal trials provided evidences for efficacy and safety of sorafenib for the patients with advanced HCC.21 However, some advanced HCC patients are not well responsive to sorafenib treatment. Since plectin deficiency activates FAK (a kind of tyrosine kinase) activity that is a candidate target of sorafenib,15 we therefore hypothesized that plectin deficiency may enhance the sensitivity to the treatment of sorafenib. In the present study, we investigated the impact of plectin deficiency on the growth inhibitory effect of hepatoma cell lines mediated by sorafenib treatment.
Results
Expression of plectin among liver and hepatoma cells
The results of immunofluorescent staining revealed that plectin was mainly identified in the mesh structure around perinuclear region extending to membrane or forming granular pattern in Chang liver cell. Whereas in PLC/PRF/5 and HepG2 hepatoma cells, weak signals of plectin were detected in a disorganized manner (Fig. 1A). Western blotting analysis demonstrated different levels of plectin expression among Chang liver cells, PLC/PRF/5 cells and HepG2 cells. Chang liver cells exhibited highest amount of plectin and the lowest level was found in HepG2 tumor cells (Fig. 1B). Furthermore, the expression of plectin in PLC/PRF/5 and HepG2 cells was quantitated 70% and 80% less than that of Chang liver cells respectively (p < 0.05) (Fig. 1C).
Figure 1.
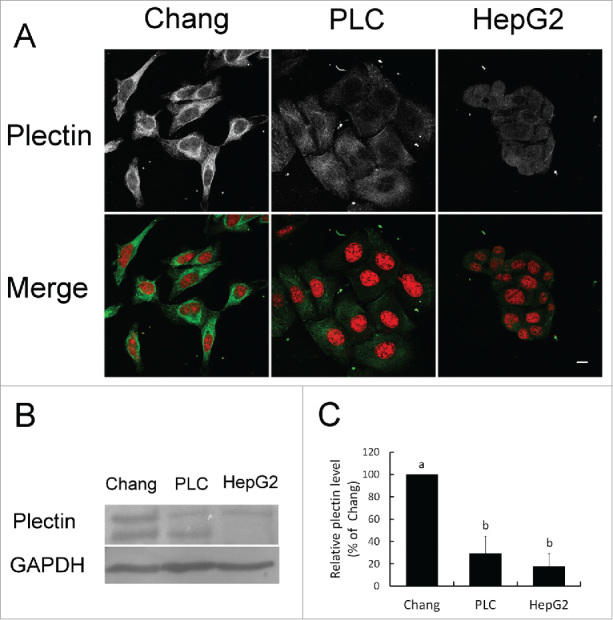
Plectin expression in liver and hepatoma cell lines. (A) Localization of plectin identified by immunofluorescent staining. Red: nucleus. Green: plectin. (B) Western blotting analysis of plectin protein expression in Chang liver cells, PLC/PRF/5, and HepG2 cell lines. (C) Quantification on the level of plectin expression.
Relationship between the level of plectin expression and cell migration
The results of transwell migration assay revealed that the highest rate of cell migration was found in HepG2 hepatoma cells and the lowest rate was identified in Chang liver cells (Fig. 2A). A trend showed that lower amount of plectin is associated with higher individual cell migration despite no significant difference in statistics (p > 0.05) (Fig. 2B). Intriguingly, HepG2 and PLC/PRF/5 cells showed more aggregative migration that is similar to collective cell migration in comparison with Chang liver cells.
Figure 2.

Individual cell migration in liver and hepatoma cell lines. (A) Chang liver cells, PLC/PRF/5, and HepG2 cell lines were subjected to transwell migration assay described in Materials and methods. Cell migration was visualized with DAPI staining. (B) Quantification on the rate of cell migration. HepG2 cells showed higher individual cell migration without significant difference (p > 0.05).
Increased expression of E-cadherin in the collective cell migration of hepatoma cells
Involvement of E-cadherin expression has been reported in collective cell migration. In this study, it was found that the level of E-cadherin was very low in Chang liver cells and it was higher in HepG2 and PLC/PRF/5 cells demonstrated in Western blotting analysis (Fig. 3A). The quantitative analysis revealed that the highest level of E-cadherin was found in HepG2 cells in comparison PLC/PRF/5 and Chang cells (p < 0.05) (Fig. 3B). Immunofluorescent staining identifies cell nuclei and E-cadherin in migrated cells. Cell areas with more than 3 cells connected with E-cadherin were counted as collective cell migration (Fig. 3C). The difference in quantification of collective cell migration was significant in statistics among 3 tested cell lines (p < 0.05) (Fig. 3D). The results revealed that the rate of collective cell migration was correlated with the expression of E-cadherin in Chang liver cells, PLC/PRF/5 cells and HepG2 cells.
Figure 3.
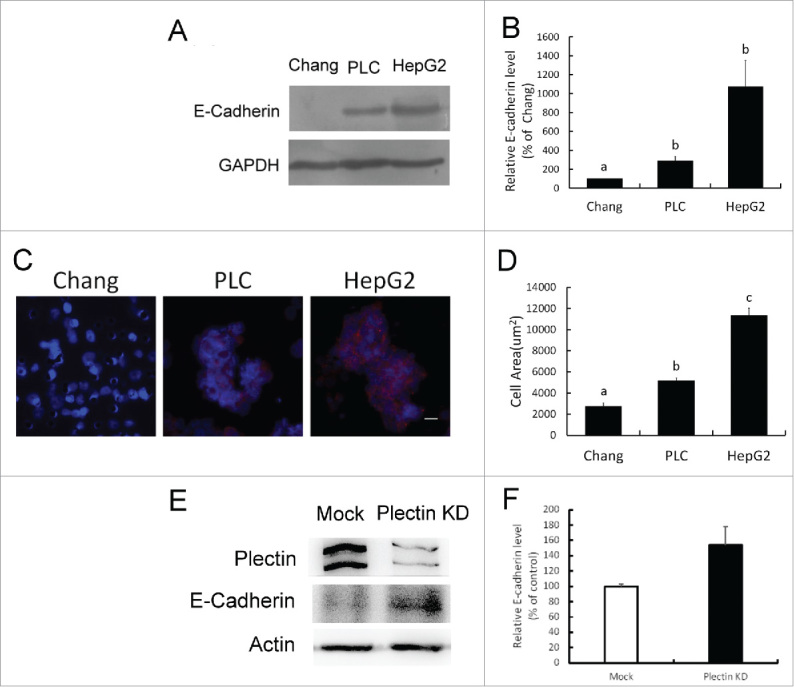
Collective cell migration associated with E-cadherin expression and plectin deficiency. (A) Western blotting assay of E-cadherin expression in Chang liver cells, PLC/PRF/5, and HepG2 cell lines. (B) Quantification data of E-cadherin expression obtained from Western blotting analysis. HepG2 cells showed highest level of E-cadherin expression compared with Chang and PLC/PRF/5 cells (p < 0.05). (C) Immunofluorescent staining for detecting the distribution of E-cadherin in collective migrated cells. Blue: nucleus. Red: E-cadherin. (D) Quantification of collective cell migration in transwell assay. Cell area appearing more than 3 cells connected with E-cadherin was calculated as collective cell migration. The result of quantification showed significant differences (p < 0.05). (E) Western blotting assay of plectin and E-cadherin expression in mock (Mock) and plectin knockdown-Chang liver cells (Plectin KD), respectively. (F) Quantification data of E-cadherin expression obtained from Western blotting analysis. Plectin knockdown-cells showed higher level of E-cadherin expression compared with mock cells (p < 0.05).
Plectin-knockdown affected the E-cadherin expression and promoted the cell migration in the continuing parallel experiments confirmed the above results. In Fig. 3E, the results of Western blotting assay showed an increase of E-cadherin expression in the lysate of siRNA-transfected Chang liver cells, suggesting that the depletion of plectin might be associated with the E-cadherin expression. The difference in quantification of E-cadherin expression was significant in statistics (p < 0.05) (Fig. 3F). The cell migration rate of Chang liver cells promoted by plectin-knockdown has been identified in our previous report. In that study, we found that plectin-deficient Chang liver cells exhibit higher cell motility associated with increase in focal adhesion kinase activity that are comparable to the properties of invasive HCC.15
Plectin deficiency sensitizes the hepatoma cells and plectin-knockdown liver cells to sorafenib treatment
The growth inhibitory effects of sorafenib on human liver cell line (Chang cell) and on human hepatoma cell lines (PLC/PRF/5 and HepG2) were shown in Fig. 4A. Dose-dependent manners with a maximum 90% reduction of cell growth among 3 cell lines were demonstrated for 72-hour sorafenib treatment in the concentration range of 0 – 10 μM. The growth inhibitory effect mediated by sorafenib treatment was the highest one in HepG2 cell whereas the lowest one was found in Chang liver cell. In Fig. 4C, the growth inhibitory effect of sorafenib treatment was higher in the plectin knockdown-Chang liver cells in comparison with mock cells (p < 0.05). The level of cellular plectin inversely correlates with the sensitivity to sorafenib treatment.
Figure 4.
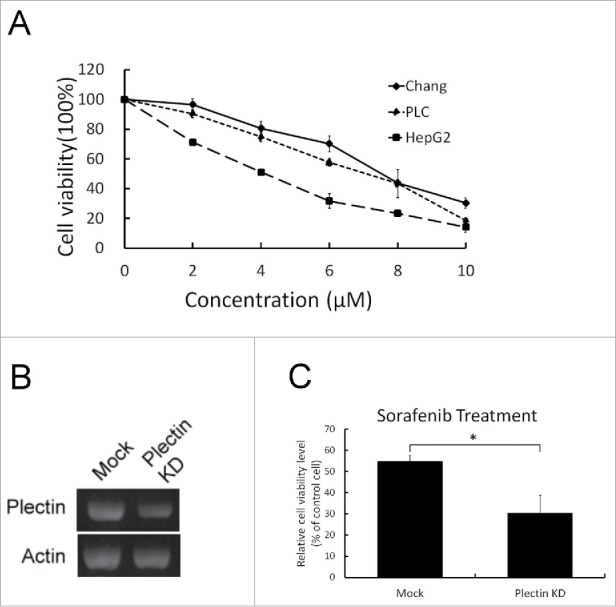
Sorafenib treatment in liver cells, hepatoma cells, and plectin knockdown-liver cells. (A) Sorafenib (from 0 to 10 μM) was applied in Chang liver cells, PLC/PRF/5 and HepG2 cell lines. Cell viabilities were determined in 72 hours. The data showed that HepG2 cells were more sensitive to sorafenib treatment than Chang liver cells and PLC/PRF/5 cell lines were. (B) Reduction in mRNA expression of Chang liver cells was associated with transient knockdown of plectin. (C) Sorafenib (10 μM) was applied in mock and plectin knockdown-Chang liver cell respectively. Plectin knockdown-cells revealed significantly higher sensitivity to sorafenib treatment (p < 0.05).
Discussion
Plectin is one of the cytolinker proteins that play a crucial role in maintaining the integrity of cellular architecture. Previous studies demonstated that plectin was deficiency in human HCC tissues.7,8 Plectin knockdown in human HCC leads to changed expression and organization of CK18. In consequence, partial augmentation of cytoskeleton is associated with pleomorphic changes.7,9 In this study, we provided evidences that plectin is deficient in hepatoma cell lines in association with divergent and pleomorphic morphology, while the hepatic cells appear uniform and in polygonal shape. The results are consistent with the former reports.
HCC is one of the most common cancers with high mortality while metastasis is a hallmark of cancer and the major cause of cancer-related mortality. Identification of the risk factors associated with cancer metastasis is an important issue for improving clinical management. Plectin plays several pivotal roles to maintain cellular architecture by connecting cytoskeleton and/or by modulating activity of kinases.3 Previous study found that plectin knockdown in human hepatic cells exhibits higher cell motility in association with increased FAK activity that was comparable to the properties of invasive HCC.15 In this study, we demonstrated that plectin expression in hepatoma cells (PLC/PRF/5 and HepG2) was lower than that in hepatic cells (Chang liver cells). Meanwhile, plectin deficiency in hepatoma cells correlates with the ability of cell migration. We therefore hypothesize that plectin deficiency might be a risk factor associated with metastasis of HCC. The relationship between the level of plectin and clinical outcomes of HCC patients interest us to investigate in future.
Although plectin-deficient hepatoma cells behave higher ability of individual cell migration than hepatic cells do, there is no significant difference in quantitative results. In contrast, collective cell migration was significantly increased in hepatoma cells compared with hepatic cells. For adjacent epithelial cells, E-cadherin molecules render strong mechanical attachment between adherent junctions. E-cadherin plays an essential role in orientating collective migration of large epithelial sheets.22 It also mediates mechanical feedback for sensing the direction during collective cell migration.23 In our study, hepatoma cells exhibited higher E-cadherin expression compared with hepatic cells. Likewise, higher rate of collective cell migration was found in hepatoma cells rather than hepatic cells. Based on these findings, we suggest that the higher degree of collective cell migration in hepatoma cells is associated with the increased expression of E-cadherin and decrease in plectin. Cell morphology may be regulated by the expression of plectin and in consequence to determine either individual or collective migration.
In tumor mass, cell's collective and individual migration is concurrent. Switch individual to collective migration was suggested a critical factor determining the invasive and metastatic potential of cancers.11 Collective cell migration has been characterized as a crucial characteristic in metastasis of many cancers including oral squamous cell carcinoma, colorectal carcinoma, breast cancer, endometrial carcinoma, melanoma, and pancreatic cancer.24 However, collective cell migration in HCC was rarely reported up to now. During collective cell migration, rearrangement of actin is crucial for filopodia formation and the maintenance of VASP/Mena-dependent cell–cell adhesion25 while pseudopodia and filopodia are controlled by Rac and Cdc42, respectively.26 A previous study demonstrated that Rac1-GTPase activity was activated and actin rearrangement was found in the plectin knockdown hepatic cells.15 Recently we found that Rab11 (a small GTPase of the Rab family) collaborates E-cadherin to promote collective cell migration in colorectal carcinoma.27 Therefore we hypothesize that plectin deficiency promotes collective cell migration of hepatoma cells involving the mechanisms such as increase in E-cadherin expression, activation of Rac1-GTPase activity and actin rearrangement.
Clinical studies demonstrated that sorafenib exhibits benefits on the time to progression and overall survival for the patients with HCC but its efficacy is moderate.19 Biomarkers indicating individual sensitivity to sorafenib may help to predict prognosis for HCC patients. Up to now, however, biologic parameters specific for measuring the outcomes of HCC treatment have not yet been well developed.28 Godin and colleagues investigated the correlation between the clinical efficacy of sorafenib and the induction of apoptotic regulatory factors M30 and M65 but significant association was not found.29 A recent study reported that the expression of histone methylation-related genes is likely to be used as biomarkers indicating the sensitivity to sorafenib treatment of HCC patients. Meanwhile they are candidate therapeutic targets for the development of adjuvant therapy in association with sorafenib.30 In this study, we found that HepG2 cells expressing high level of E-cadherin and small amount of plectin are more sensitive to sorafenib treatment. In addition, plectin knockdown in hepatic cells were also more sensitive to sorafenib treatment than mock hepatic cells. In accordance, we hypothesize that individual levels of plectin and E-cadherin in patients with HCC may be correlated with their sensitivity to sorafenib exposure. The future study will be planned to develop cell lines expressing different levels of plectin by molecular manipulation and to examine their sensitivity to sorafenib treatment.
In conclusion, higher cell motility and collecting migration are associated with deficiency in plectin and increased expression of E-cadherin in hepatoma cells. Plectin may be involved in the regulation of cell motility that is related to the invasiveness of hepatocellular carcinoma. We also suggest that hepatoma cells with plectin deficiency and high level of E-cadherin are more sensitive to sorafenib treatment.
Materials and methods
Materials
Goat anti-plectin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-E-cadherin monoclonal antibodies were purchased from BD Transduction Laboratories™ (USA). Secondary antibodies including Rhodamine-conjugated anti-mouse IgG and Fluorescein-conjugated anti-goat IgG used for immunofluorescence microscopy, horseradish peroxidase-conjugated goat and mouse antibodies used for Western blotting analysis were purchased from Jackson Immuno Research Laboratories (West Grove, PA, USA). Sorafenib (Nexavar) was purchased from Bayer Pharmaceuticals (USA).
Cell cultures
Chang liver cells, PLC/PRF/5 hepatoma cells, and HepG2 hepatoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Dulbecco's minimum essential medium (Gibco, USA) supplemented with 10% fetal bovine serum, 50 unit/ml penicillin, non-essential amino acids and HEPES was used for culture at 37°C in presence of 5% CO2. Medium was replaced and confluent growth was split regularly.
Immunofluorescent assay
A number of 5 × 104 cells per well were grown in a 24-well plate for 48 hours. Cells were washed with ice-cooled phosphate buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 1.5 mM KH2PO4, pH7.4) and fixed with 3.7% paraformaldehyde (in PBS) at room temperature for 20 minutes. The fixed cells were washed with PBS for 3 times and then treated with 0.1% Triton X-100 for 2 minutes. After 3 times washing with PBS, mouse anti-E-cadherin (1:100 in PBS) and goat anti-plectin (1:100 in PBS) antibodies were simultaneously added to the pre-treated cells for 1 hour. For fluorescent imaging, cells were incubated with secondary antibodies (Cy2-conjugated anti-mouse IgG and Cy3-conjugated anti-goat IgG) and Cy5- conjugated Phalloidin dye (1:100 in PBS) for 1 hour. After 3-time washing with PBS, fluorescent images were viewed under the Zeiss LSM 510 (German) confocal microscope.
Western blotting analysis
Total cellular lysate of each sample was separately loaded for running a 10% sodium dodecyl sulfate polyacrylamide gel. Followed by the completion of electrophoresis, the gel was blotted onto a polyvinylidene difluoride (PVDF) membrane by using semi-dry transferring method (Bio-Rad, USA). The blotted PVDF membrane was blocked with 5% non-fat milk in PBS with Tween-20 (PBST) buffer for 1 hour and then washed 3 times for 5 minutes with PBST before adding the diluted primary antibody (1:1000 in PBST). The hybridization continued for one hour and the hybridized membrane was washed 5 minutes with PBST for 3 times before adding the diluted secondary antibody (1:5000 in PBST). At last, the chemiluminescent reagent was added to the washed membrane and the image was developed by the Chemiluminescence Imaging System (Fuji, Japan) as manual's instruction.
Transwell migration assay
Transwell migration assay was examined by using QCM™ 24-Well Colorimetric Cell Migration Assay kit (Millipore, USA). Cell lines including Chang liver cells, PLC/PRF/5 hepatoma cells, and HepG2 hepatoma cells (5 × 104 cells) were harvested and then transferred into the upper chamber of 24-well plate inserted with trans-well. The lower chamber was filled with culture medium containing fetal bovine serum. After incubation for 4 hours, cells in the upper chamber were carefully removed with a cotton swab. Cells that passed through the membrane to the lower chamber surface were fixed with 3.7% formaldehyde (in PBS) in for 20 minutes, then treated with 0.1% Triton X-100 (in PBS) for 1 minutes, and finally stained with 1:1000 DAPI (in PBS). Cells from each well were counted in 9 random high-power fields under a microscope (ZEISS Axiovert 200M, German). Each experiment was repeated in triplicate.
Transient knockdown of plectin in chang liver cells
Transient knockdown was performed by using RNA interference techniques in Chang liver cells. Small interfering RNA (siRNA) duplex oligonucleotides specific for plectin transcripts (5′-GAG CUA AUG UGG CUG AAU G-3′; 5′-GCA CUG CGU AGG AAA UAC A-3′ 5′-CGG AAU ACU UCA CGG CAG A-3′; 5′-GUG CCA GAG GUU UGC GAA A-3′) was purchased from Dharmacon, Inc. (Lafayette, CO). Chang liver cells were grown on a 60 mm petri dish to 50% confluence. For introducing the siRNA into cells, the siRNA was diluted with serum free medium (1:100, v/v) and then gently mixed with pre-diluted (1:50, v/v) Lipofectamine 2000 (Invitrogen, USA). The mixture was left at room temperature for 15 minutes. Afterwards the mixture was added to the Petri dishes with gentle shaking. The transfected culture will be available for further assays after 48 hours since siRNA was added.
Reverse transcription-PCR
The mRNA was isolated by TRIZOL isolation kit (Invitrogen, USA) according to manufactory's protocol. The amount of cellular mRNA was determined by measuring the light absorbance at 260 nm. The mRNA was reverse transcribed to cDNA with Super Script First-strand RT-PCR kit (Invitrogen, USA). The cDNA were amplified via PCR kit (Amplicon, USA) according to manufactory's protocol. The reaction mixtures (40 μl) contained 1μM of 5″ and 3′ primers. Primers for PCR are the following sequences. Plectin, forward: 5′-CACTGGCTACAAGGACCCCT-3′, reverse: 5′-AAACCTCTGGCACTCCTCGAC-3′; GAPDH (as internal control), forward: 5′-GAAGGTGAAGGTCGGAGTC-3′, reverse: 5′-GAAGATGGTGATGGGATTTC-3′.
Measurement of effect for sorafenib treatment on cell growth and viability
Sorafenib were dissolved in dimethyl sulfoxide (DMSO) and stored at −20 °C. The working solution contains DMSO in a concentration below 10 μM to avoid background effect on cell growth. The mock Chang liver cells, plectin-knockdown Chang liver cells, PLC/PRF/5 hepatoma cells, and HepG2 hepatoma cells were seeded in 24-well plates in a density of 5 × 104 cells per well and supplemented with various concentrations (0, 2, 4, 6, 8, 10 μM) of sorafenib for 72-hour incubation. The cultures were incubated with MTS reagent according to the manufacturer's protocol then read the OD values at 490 nm by an ELISA reader. Cell viability was determined by dividing the average absorbance of each well with that of the control wells. Each experiment was repeated in triplicate.
Statistical analysis
Quantitative results were presented as mean ± standard deviation. The statistical analysis was performed by using 2-tailed unpaired t test (between 2 groups) or one-way analysis of variance (ANOVA) (among 3 groups) with the computer software SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A p value smaller than 0.05 was considered as significance in statistics.
Funding Statement
This work was supported by the Chang Bing Show Chwan Memorial Hospital under grants RD104005, RD105050 and RD105051.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly appreciate Miss Chen, You-Yin for her skillful assistance in laboratory.
References
- [1].Huber F, Boire A, Lopez MP, Koenderink GH. Cytoskeletal crosstalk: when three different personalities team up. Curr Opin Cell Biol 2015; 32:39-47; PMID:25460780; https://doi.org/ 10.1016/j.ceb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- [2].Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci 1998; 111(Pt 17):2477-86; PMID:9701547 [DOI] [PubMed] [Google Scholar]
- [3].Castanon MJ, Walko G, Winter L, Wiche G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol 2013; 140(1):33-53; PMID:23748243; https://doi.org/ 10.1007/s00418-013-1102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol 2006; 174(4):557-68; PMID:16908671; https://doi.org/ 10.1083/jcb.200605172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Winter L, Wiche G. The many faces of plectin and plectinopathies: pathology and mechanisms. Acta Neuropathol 2013; 125(1):77-93; PMID:22864774; https://doi.org/ 10.1007/s00401-012-1026-0 [DOI] [PubMed] [Google Scholar]
- [6].Omary MB, Ku NO, Toivola DM. Keratins: guardians of the liver. Hepatology 2002; 35(2):251-7; PMID:11826396; https://doi.org/ 10.1053/jhep.2002.31165 [DOI] [PubMed] [Google Scholar]
- [7].Cheng CC, Liu YH, Ho CC, Chao WT, Pei RJ, Hsu YH, Yeh KT, Ho LC, Tsai MC, Lai YS. The influence of plectin deficiency on stability of cytokeratin18 in hepatocellular carcinoma. J Mol Histol 2008; 39(2):209-16; PMID:18038249; https://doi.org/ 10.1007/s10735-007-9155-9 [DOI] [PubMed] [Google Scholar]
- [8].Liu YH, Ho CC, Cheng CC, Chao WT, Pei RJ, Hsu YH, Lai YS. Cytokeratin 18-mediated disorganization of intermediate filaments is induced by degradation of plectin in human liver cells. Biochem Biophys Res Commun 2011; 407(3):575-80; PMID:21420381; https://doi.org/ 10.1016/j.bbrc.2011.03.066 [DOI] [PubMed] [Google Scholar]
- [9].Liu YH, Cheng CC, Ho CC, Chao WT, Pei RJ, Hsu YH, Ho LC, Shiu BH, Lai YS. Plectin deficiency on cytoskeletal disorganization and transformation of human liver cells in vitro. Med Mol Morphol 2011; 44(1):21-6; PMID:21424933; https://doi.org/ 10.1007/s00795-010-0499-y [DOI] [PubMed] [Google Scholar]
- [10].Roessler S, Long EL, Budhu A, Chen Y, Zhao X, Ji J, Walker R, Jia HL, Ye QH, Qin LX, et al.. Integrative genomic identification of genes on 8p associated with hepatocellular carcinoma progression and patient survival. Gastroenterology 2012; 142(4):957-66 .e12; PMID:22202459; https://doi.org/ 10.1053/j.gastro.2011.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM. Cancer Invasion: Patterns and Mechanisms. Acta Naturae 2015; 7(2):17-28; PMID:26085941 [PMC free article] [PubMed] [Google Scholar]
- [12].Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol 2015; 36:13-22; PMID:26183445; https://doi.org/ 10.1016/j.ceb.2015.06.004 [DOI] [PubMed] [Google Scholar]
- [13].Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol 2004; 48(5–6):441-9; PMID:15349818; https://doi.org/ 10.1387/ijdb.041821pf [DOI] [PubMed] [Google Scholar]
- [14].Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 2009; 23(4):397-418; PMID:19240129; https://doi.org/ 10.1101/gad.1758709 [DOI] [PubMed] [Google Scholar]
- [15].Cheng CC, Lai YC, Lai YS, Hsu YH, Chao WT, Sia KC, Tseng YH, Liu YH. Transient knockdown-mediated deficiency in plectin alters hepatocellular motility in association with activated FAK and Rac1-GTPase. Cancer Cell Int 2015; 15:29-35; PMID:25861244; https://doi.org/ 10.1186/s12935-015-0177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379(9822):1245-55; PMID:22353262; https://doi.org/ 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- [17].Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al.. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64(19):7099-109; PMID:15466206; https://doi.org/ 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- [18].Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43–9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst 2006; 98(5):326-34; PMID:16507829; https://doi.org/ 10.1093/jnci/djj069 [DOI] [PubMed] [Google Scholar]
- [19].Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al.. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359(4):378-90; PMID:18650514; https://doi.org/ 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- [20].Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al.. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10(1):25-34; PMID:19095497; https://doi.org/ 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- [21].Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, et al.. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012; 57(4):821-9; PMID:22727733; https://doi.org/ 10.1016/j.jhep.2012.06.014 [DOI] [PubMed] [Google Scholar]
- [22].Li L, Hartley R, Reiss B, Sun Y, Pu J, Wu D, Lin F, Hoang T, Yamada S, Jiang J, et al.. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell Mol Life Sci 2012; 69(16):2779-89; PMID:22410739; https://doi.org/ 10.1007/s00018-012-0951-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical Feedback through E-Cadherin Promotes Direction Sensing during Collective Cell Migration. Cell 2014; 157(5):1146-59; PMID:24855950; https://doi.org/ 10.1016/j.cell.2014.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 2011; 728(1–2):23-34; PMID:21605699; https://doi.org/ 10.1016/j.mrrev.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol 2001; 13(1):76-84; PMID:11163137; https://doi.org/ 10.1016/S0955-0674(00)00177-0 [DOI] [PubMed] [Google Scholar]
- [26].Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 2008; 9(6):446-54; PMID:18464790; https://doi.org/ 10.1038/nrm2406 [DOI] [PubMed] [Google Scholar]
- [27].Chung YC, Wei WC, Hung CN, Kuo JF, Hsu CP, Chang KJ, Chao WT. Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. Eur J Clin Invest 2016; 46(12):1002-11; PMID:27696383; https://doi.org/ 10.1111/eci.12683 [DOI] [PubMed] [Google Scholar]
- [28].Galmiche A, Chauffert B, Barbare JC. New biological perspectives for the improvement of the efficacy of sorafenib in hepatocellular carcinoma. Cancer Lett 2014; 346(2):159-62; PMID:24380851; https://doi.org/ 10.1016/j.canlet.2013.12.028 [DOI] [PubMed] [Google Scholar]
- [29].Godin C, Louandre C, Bodeau S, Diouf M, Saidak Z, Conte MA, Chauffert B, Barbare JC, Barget N, Trinchet JC, et al.. Biomarkers of apoptosis and necrosis in patients with hepatocellular carcinoma treated with sorafenib. Anticancer Res 2015; 35(3):1803-8; PMID:25750346 [PubMed] [Google Scholar]
- [30].Li GM, Wang YG, Pan Q, Wang J, Fan JG, Sun C. RNAi screening with shRNAs against histone methylation-related genes reveals determinants of sorafenib sensitivity in hepatocellular carcinoma cells. Int J Clin Exp Pathol 2014; 7(3):1085-92; PMID:24696725 [PMC free article] [PubMed] [Google Scholar]


