Abstract
Purpose
Cardamonin inhibits the proliferation of SKOV3 cells by suppressing the mammalian target of rapamycin complex 1 (mTORC1). However, the mechanism of cardamonin on mTORC1 inhibition has not been well demonstrated. The regulatory-associated protein of TOR (Raptor) is an essential component of mTORC1. Here, we investigated the role of Raptor in the mTORC1 inhibition effect of cardamonin in SKOV3 cells.
Methods
The expression of Raptor was knockdown by small interfering RNA (siRNA). The expressions of specific binding proteins of mTORC1 were analyzed by Western blotting, and the cell proliferation was detected by methyl thiazolyl tetrazolium (MTT) assay.
Results
Rapamycin, AZD8055, and cardamonin inhibited the activity of mammalian target of rapamycin (mTOR). Different from rapamycin and AZD8055, cardamonin suppressed the phosphorylation and protein expression of Raptor. Transfected with Raptor siRNA, the mTOR activation and proliferation of SKOV3 cells were decreased, and these effects were strengthened by cardamonin in Raptor siRNA SKOV3 cells. Cardamonin interfered with the lysosomal colocalization of mTOR with lysosomal associated membrane protein 2 (LAMP2), which was also hindered by Raptor siRNA. Furthermore, cardamonin strengthened the inhibitory effect on the lysosomal localization of mTOR in Raptor siRNA cells.
Conclusion
Our results suggested that Raptor mainly mediated the inhibition of cardamonin on mTORC1 in SKOV3 cells.
Keywords: cardamonin, Raptor, mTORC1, drug target, ovarian cancer
Introduction
The mammalian target of rapamycin (mTOR) is closely associated with the tumorigenesis and development of ovarian cancer.1 Activated mTOR phosphorylates ribosomal protein S6 kinase 1 (S6K1) and 4E-binding protein 1 (4E-BP1), which participates in the regulation of protein translation, and mTOR downregulation inhibits the proliferation of cancer cells and tumor metastasis.2–5 Recently, mTOR inhibitors have achieved a great success in the cancer treatment.6–8 Therefore, mTOR is considered as an important target for cancer therapy.
mTOR exists as two distinct protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 senses nutrition or growth factors and regulates cell growth, metabolism, and autophagy; while mTORC2 directly activates Akt by phosphorylating its hydrophobic motif (Ser473) and regulates cell survival and cytoskeletal organization.9 Both mTOR complexes consisted of different components. The regulatory-associated protein of TOR (Raptor) and proline-rich Akt/PKB substrate 40 kDa (PRAS40) are the specific components of mTORC1. Raptor binds with mTOR to form a nutrient-sensitive complex and mediates the mTOR-catalyzed phosphorylation of S6K1 and 4E-BP1. In addition, Raptor mediates the translocation of mTOR to lysosomes, which is an essential procedure for the activation of mTOR.10 PRAS40 interacts with Raptor and participates in the regulation of mTOR activation and cell proliferation.11,12
mTOR contains a number of distinct functional domains, including HEAT repeat domain, FK506-binding protein 12 kDa (FKBP12)-rapamycin-binding (FRB) domain, and the kinase domain. There exist two generations of mTOR inhibitors. Rapamycin, the first-generation mTOR inhibitor, connects with FKBP12 and binds to the FRB domain of mTOR. The second-generation is the adenosine triphosphate-competitive mTOR inhibitor, which binds to the kinase domain of mTOR and inhibits both mTORC1 and mTORC2.13 However, investigation on novel mTOR inhibitors is continually developing. Several studies revealed that flavonoids compounds could inhibit mTORC1 signaling pathway through disrupting the connection of mTOR and Raptor.14,15
Cardamonin is the main flavonoid extracted from Alpinia katsumadi Hayata. It exhibits a wide range of pharmacological activity, including anti-inflammation, vasorelaxation,16 and enhancement on the therapeutic index of cisplatin.17 Cardamonin has been considered as a chemo-preventive agent in a variety of cancers, including breast, hematological, prostate, and colorectal cancers.18–21 Our previous studies have demonstrated that cardamonin decreases the proliferation of non-small-cell lung cancer A549 cells and ovarian cancer SKOV3 cells through blocking the cell cycle and inducing autophagy;22,23 moreover, it inhibits the metastasis of Lewis lung cancer in vivo.24 These studies demonstrate that the antitumor effect of cardamonin is related to inhibition on mTORC1 signaling pathway.25–27 However, different to rapamycin, cardamonin inhibits mTOR without the assistance of FKBP12.22,25 Therefore, the mechanism of cardamonin on mTORC1 inhibition needs to be clarified. In the present study, we intend to investigate the effect of cardamonin on the specific binding proteins of mTORC1 and the lysosomal localization of mTOR in SKOV3 cells to reveal the underlying mechanism.
Materials and methods
Reagents
Cardamonin (no 110763, purity >99%; National Institutes for Food and Drug Control, Beijing, China), rapamycin (Sigma-Aldrich Co., St Louis, MO, USA), and AZD8055 (Axonmedchem, Groningen, the Netherlands) were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Co.) to prepare the stock solution (2 mM, 10 μM, and 10 μM, respectively) and these solutions were stored at 4°C. Methyl thiazolyl tetrazolium (MTT) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich Co. Lipofectamine 2000 was purchased from Thermo Fisher Scientific (Waltham, MA, USA), and Raptor small interfering RNA (siRNA) was produced by GenePharma Co., Ltd (Shanghai, China). Antibodies against mTOR, p-mTOR (Ser2481), Raptor, p-Raptor (Ser792), PRAS40, p-PRAS40 (Ser183 and Thr246), S6K1, p-S6K1 (Thr389), and β-actin (Cell Signaling Technology, Beverly, MA, USA) were used at a 1:1,000 dilution. The secondary antibodies (anti-rabbit immunoglobulin G, horseradish peroxidase [HRP]-linked antibody) used for detection in all cases were from Cell Signaling Technology. The Alexa 488- and Alexa 647-conjugated secondary antibodies (goat anti-rabbit immunoglobulin G H&L) were from Abcam (Cambridge, MA, USA).
Cell culture
SKOV3 cells were obtained from the Boster Biological Technology Co., Ltd (Wuhan, Hubei, China) and cultured in Mccoy’s 5A medium (M&C Gene Technology, Ltd., Beijing, China) with 10% fetal bovine serum (Hyclone), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Raptor small interfering RNA (siRNA) transfection
The siRNA sequences for Raptor and nonspecific siRNA (negative control) were sense 5′-GUG UCA CAC UGG AUU UGA UTT-3′ and antisense 5′-AUC AAA UCC AGU GUG ACG CTT-3′ and sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense 5′-ACG UGA CAC GUU CGG AGA ATT-3′, respectively. The cells were transfected with siRNA using Lipofectamine 2000 according to the manufacturer’s protocol. The transfection efficiency was measured by Western blotting.
Cell proliferation analysis
Cell proliferation was determined by MTT assay. Cells were seeded in a 96-well plate (5×103 cells per well) and then treated with different drugs for 24 h. A total of 20 μL MTT solution (5 mg/mL) was added to each well and incubated for another 4 h. The supernatant was discarded carefully and 150 μL DMSO was added to each well and then shaken for 10 min. The absorbance was determined at 490 nm by a microplate reader (Model 1680; Bio-Rad Laboratories Inc., Hercules, CA, USA).
Western blotting analysis
After treating with indicated drugs for 2, 16, or 24 h, cells were washed twice with ice-cold phosphate buffered solution (PBS) and resuspended in lysis buffer comprising 50% glycerol, 100 mM NaF2, 10 mM sodium pyrophosphate, 1% Triton X-100, 1 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride, 10 μg/mL antipain, 10 μg/mL leu-peptin, and 10 μg/mL aprotinin for 30 min. Lysates were centrifuged at 14,000× g at 4°C for 20 min, and the supernatant was collected for experiments. Protein content was measured by the bicinchoninic acid method. Proteins (50 μg per lane) were separated on 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for mTOR and p-mTOR and on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for Raptor, p-Raptor, PRAS40, p-PRAS40, S6K1, and p-S6K1 and then transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 at room temperature for 1 h and then incubated with antibodies against mTOR, p-mTOR, Raptor, p-Raptor, PRAS40, p-PRAS40, S6K1, p-S6K1, and β-actin that were diluted in block buffer (1:1,000) at 4°C overnight. Then, the membranes were incubated with the appropriate HRP-linked secondary antibodies. Finally, HRP-enhanced chemiluminescence reagents were added to react with the secondary antibodies for 1–3 min, and the bands of specific proteins on the membranes were developed by autoradiography (KODAK Film, Shanghai, China). Protein bands were quantified by BioImaging Systems as a ratio to actin expression in each sample.
Immunofluorescence assays
The immunofluorescence assay was carried out as previously described.28 A total of 5×105 SKOV3 cells were plated on laser confocal petri dish. Twenty-four hours later, the slides were rinsed with PBS once and fixed with 4% paraformaldehyde in PBS at room temperature for 30 min. The slides were rinsed twice with PBS, and cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min, followed by rinsing with PBS for three times. The slides were incubated with primary antibody in 5% normal goat serum overnight at 4°C and then rinsed four times with PBS, and the slides were then incubated with secondary antibodies (diluted 1:1,000 in PBS) at room temperature in the dark for 1 h and rinsed with PBS for another four times. Finally, the slides were incubated with 4 ng/μL DAPI for 20 min in the dark. After rinsing four times with PBS, images were captured with a confocal microscope (TCS SP8; Leica Microsystems, Wetzlar, Germany). An average of 50 cells was imaged per condition, and the individual channel images were merged and analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA) to estimate the extent of colocalization.
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). All data were expressed as mean ± SD. Differences between groups were evaluated by the Student’s t-test or one-way analysis of variance followed by Dunnett’s post hoc test. P<0.05 was considered statistically significant.
Results
Cardamonin decreased the expression and phosphorylation of Raptor
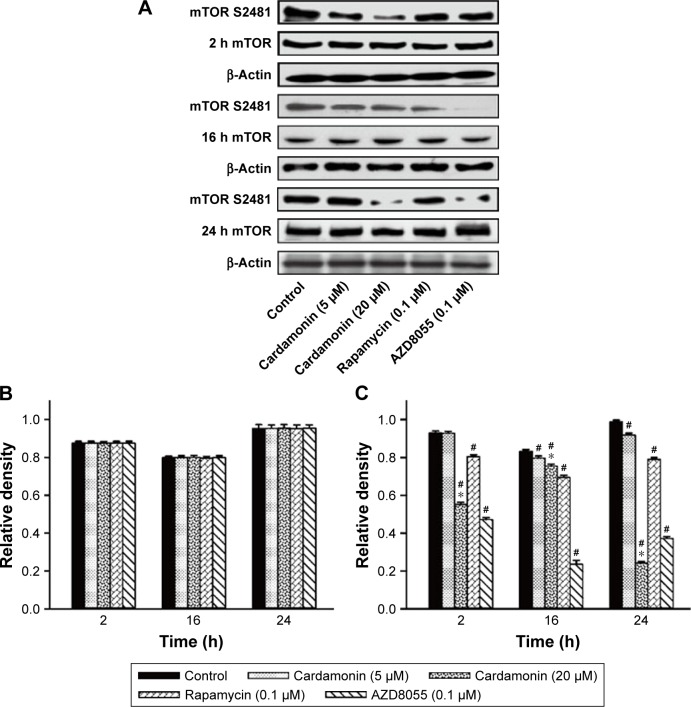
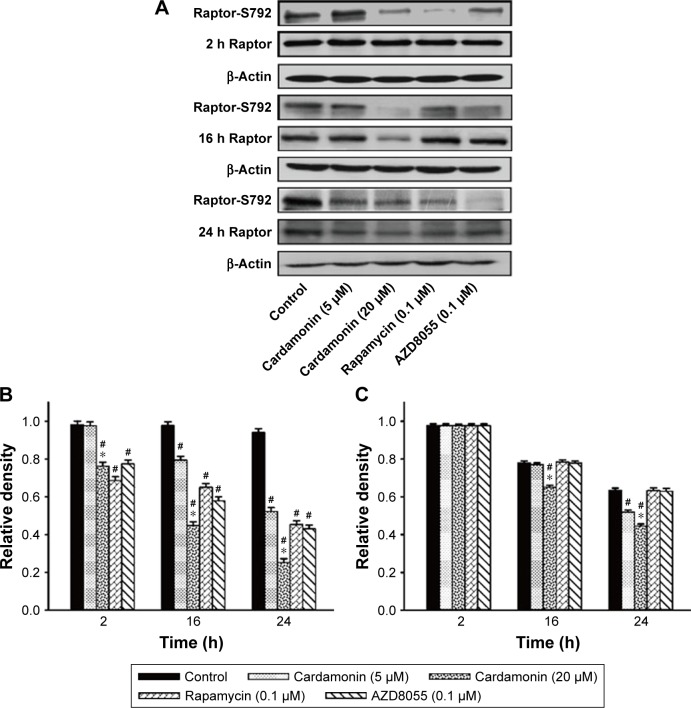
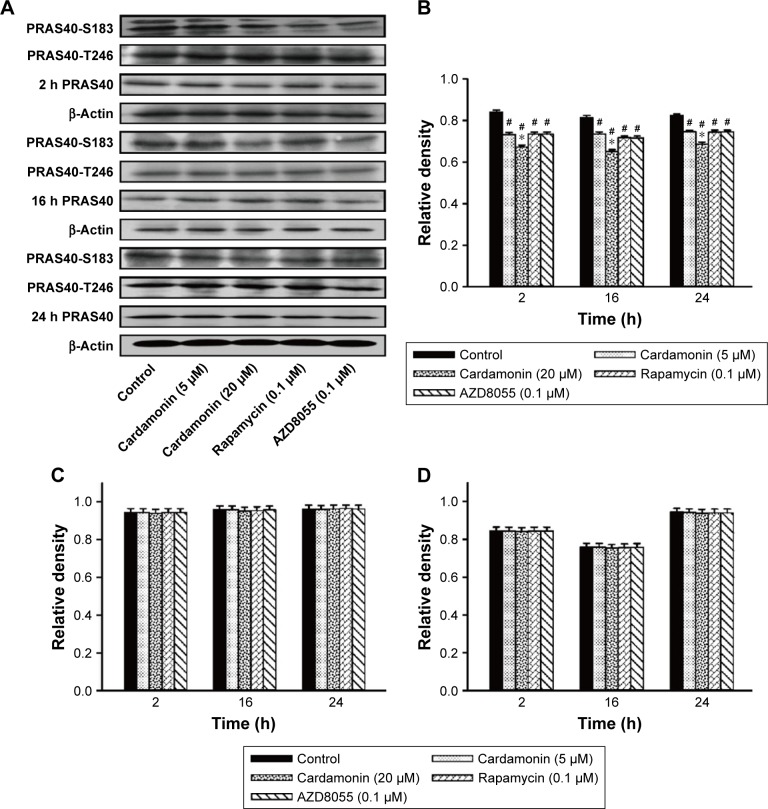
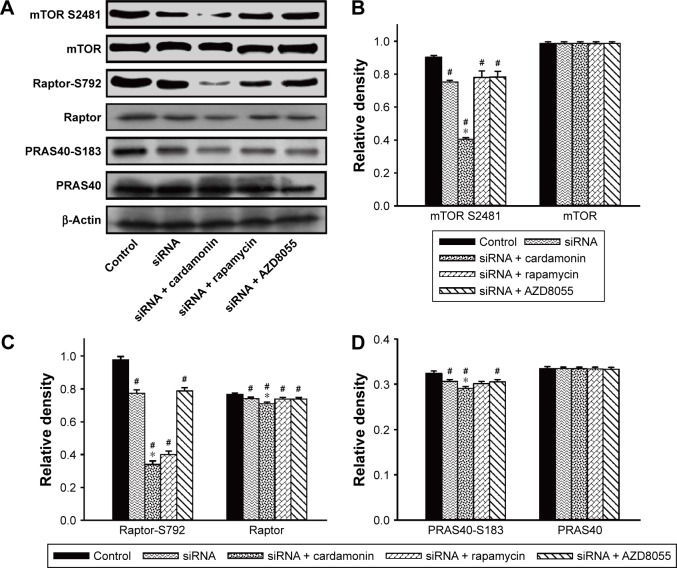
After incubating with cardamonin, rapamycin, and AZD8055 for 2, 16, and 24 h, the phosphorylation of mTOR was decreased in the SKOV3 cells (Figure 1). The phosphorylation of Raptor was reduced by all these drugs, while the protein expression of Raptor was specifically decreased by cardamonin in a time-dependent manner (Figure 2). Cardamonin, rapamycin, and AZD8055 marginally inhibited the phosphorylation of PRAS40 (S183); however, the expression of PRAS40 and PRAS40-T246 was not affected (Figure 3). We speculated that Raptor participated in the mTOR inhibition by cardamonin.
Figure 1.
Cardamonin downregulated the expression of mTOR S2481.
Notes: SKOV3 cells were incubated with cardamonin (5 and 20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 2, 16, and 24 h. Western blotting was used to detect the expression of mTOR S2481 and total mTOR. (A) Autoradiograph of expression of mTOR S2481 and total mTOR. The density ratios of each band by mTOR/β-actin (B) and mTOR S2481/β-actin (C). n=3; #P<0.05 vs control group; *P<0.05 vs cardamonin (5 μM) group.
Abbreviation: mTOR, mammalian target of rapamycin.
Figure 2.
Cardamonin downregulated the expression of Raptor-S792 and total Raptor.
Notes: SKOV3 cells were incubated with cardamonin (5 and 20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 2, 16, and 24 h. Western blotting was used to detect the expression of Raptor-S792 and total Raptor. (A) Autoradiograph of expression of Raptor-S792 and total Raptor. The density ratios of each band by Raptor-S792/β-actin (B) and Raptor/β-actin (C). n=3; #P<0.05 vs control group; *P<0.05 vs cardamonin (5 μM) group.
Figure 3.
Cardamonin downregulated the expression of PRAS40-S183.
Notes: SKOV3 cells were incubated with cardamonin (5 and 20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 2, 16, and 24 h. Western blotting was used to detect the expression of PRAS40-S183, PRAS40-T246, and total PRAS40. (A) Autoradiograph of expression of PRAS40-S183, PRAS40-T246, and total PRAS40. The density ratios of each band by PRAS40-S183/β-actin (B), PRAS40-T246/β-actin (C), and PRAS40/β-actin (D). n=3, #P<0.05 vs control group; *P<0.05 vs cardamonin (5 μM) group.
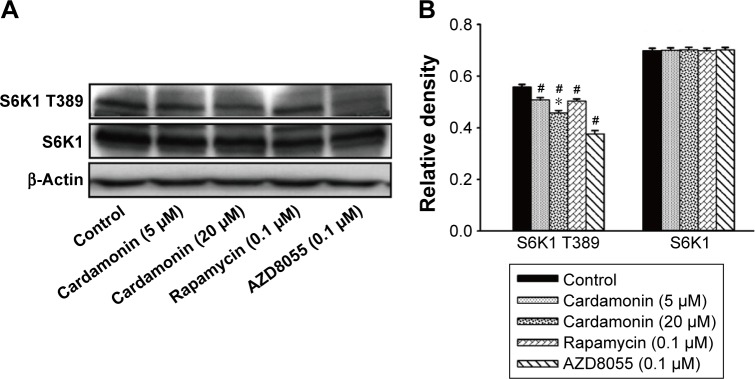
Cardamonin inhibited the activity of mTORC1 signaling
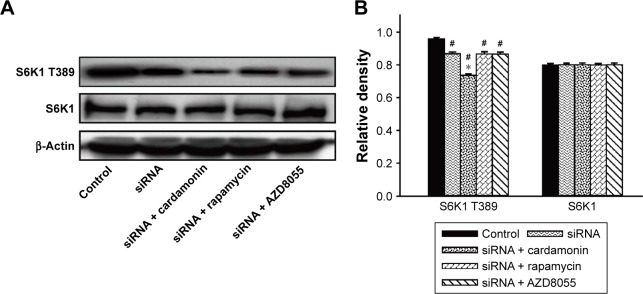
As shown in Figure 4, the phosphorylation of S6K1 was significantly decreased by cardamonin, rapamycin, and AZD8055. However, the expression of S6K1 was not changed. Since S6K1 is a sensitive sensor of mTORC1, the result indicated that cardamonin inhibited the mTORC1 signaling.
Figure 4.
Cardamonin downregulated the expression of p-S6K1.
Notes: SKOV3 cells were incubated with cardamonin (5 and 20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 24 h. Western blotting was used to detect the expression of S6K1 T389 and total S6K1. (A) Autoradiograph of expression of S6K1 T389 and total S6K1. (B) The density ratios of each band by S6K1 T389/β-actin and S6K1/β-actin. n=3; #P<0.05 vs control group; *P<0.05 vs cardamonin (5 μM) group.
Cardamonin decreased the phosphorylation of mTORC1-specific binding proteins in Raptor knockdown cells
After transfecting with Raptor siRNA, the protein expression of Raptor was decreased as expected. However, cardamonin had an additional inhibitory effect on the expression of Raptor in the Raptor siRNA cells (Figure 5). Raptor siRNA also decreased the phosphorylation of mTOR, Raptor, PRAS40-S183 (marginally), and S6K1, which were further inhibited by cardamonin in the Raptor siRNA SKOV3 cells (Figures 6 and 7), whereas rapamycin and AZD8055 had no additional inhibitory effect on these proteins.
Figure 5.
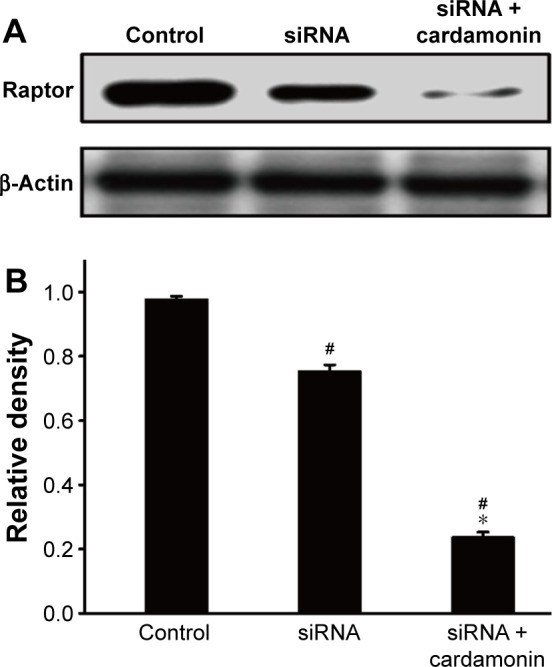
Analysis of Raptor interference.
Notes: SKOV3 cells were transfected with Raptor siRNA for 24 h and then treated with cardamonin (20 μM) for 24 h. Cell extracts were analyzed by Western blotting for the expression of total Raptor. (A) Expression of total Raptor in Raptor RNAi SKOV3 cells. (B) The density ratios of each protein expression band by Raptor/β-actin in Raptor RNAi SKOV3 cells. n=5; #P<0.05 vs control group; *P<0.05 vs siRNA group.
Abbreviation: siRNA, small interfering RNA.
Figure 6.
Cardamonin downregulated the phosphorylation of mTORC1-specific binding proteins.
Notes: SKOV3 cells were transfected with Raptor siRNA for 24 h and then treated with cardamonin (20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 24 h. Expression of protein extracted from total cells was assayed by Western blotting. (A) The level of phosphorylation and total protein of mTORC1-specific binding proteins in Raptor RNAi SKOV3 cells. (B) The density ratios of each band by mTOR S2481/β-actin and mTOR/β-actin in Raptor RNAi SKOV3 cells. (C) The density ratios of each band by Raptor-S792/β-actin and Raptor/β-actin in Raptor RNAi SKOV3 cells. (D) The density ratios of each band by PRAS40-S183/β-actin and PRAS40/β-actin in Raptor RNAi SKOV3 cells. n=3; #P<0.05 vs control group; *P<0.05 vs siRNA group.
Abbreviations: mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; siRNA, small interfering RNA.
Figure 7.
Cardamonin downregulated the phosphorylation of S6K1.
Notes: SKOV3 cells were transfected with Raptor siRNA for 24 h and then treated with cardamonin (20 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 24 h. Protein expression of phosphorylation and total S6K1 extracted from the total cell extracts were assayed by Western blotting. (A) The level of phosphorylation and total S6K1 in Raptor RNAi SKOV3 cells. (B) The density ratios of each band by S6K1 T389/β-actin and S6K1/β-actin in Raptor knock-down SKOV3 cells. n=3; #P<0.05 vs control group; *P<0.05 vs siRNA group.
Abbreviation: siRNA, small interfering RNA.
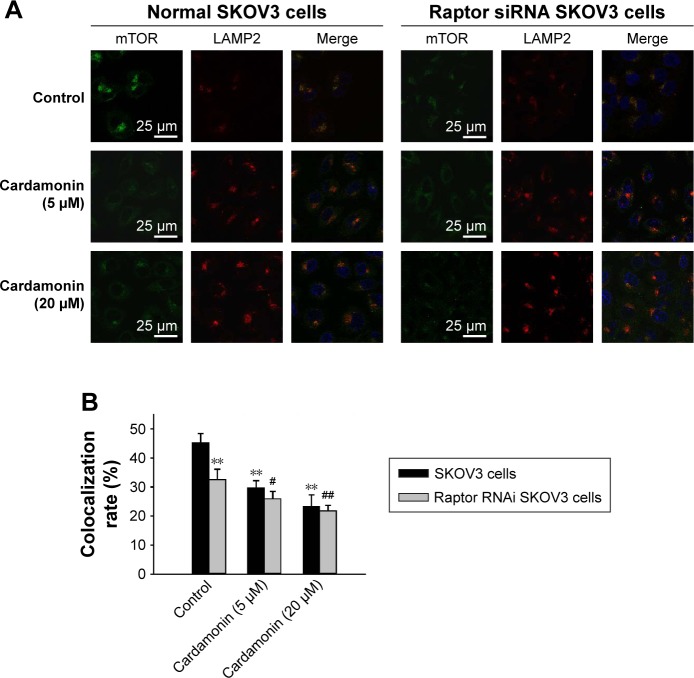
Cardamonin inhibited the lysosomal localization of mTOR
Cardamonin suppressed the lysosomal colocalization of mTOR with LAMP2, an established lysosomal membrane protein. As expected, Raptor siRNA decreased the localization of mTOR to the lysosomal surface. Furthermore, cardamonin strengthened the inhibitory effect on lysosomal localization of mTOR in Raptor siRNA cells (Figure 8).
Figure 8.
Raptor interference enhanced the inhibitory effect of cardamonin on the lysosomal localization of mTOR.
Notes: Both normal and Raptor siRNA SKOV3 cells were treated with cardamonin (5 and 20 μM) for 150 min. The lysosomal localization of mTOR was determined by immunofluorescence assays. (A) Images of SKOV3 cells coimmunostained for mTOR (green), LAMP2 (red), and nucleus (blue). (B) Quantitation of the colocalization. n=3; **P<0.01 vs SKOV3 control group; #P<0.05, ##P<0.05 vs Raptor siRNA SKOV3 control group.
Abbreviations: mTOR, mammalian target of rapamycin; siRNA, small interfering RNA.
Cardamonin decreased the proliferation of Raptor siRNA SKOV3 cells
Cell viability was gradually reduced by cardamonin in both normal and Raptor siRNA cells in a dose-dependent manner. In addition, the antiproliferative effect of cardamonin was stronger on Raptor siRNA cells than that on normal cells (Figure 9). This inhibitory effect of cardamonin on cell proliferation was consistent with that on the expression of Raptor, whereas the inhibitory effect of cardamonin or AZD8055 in normal cells and Raptor siRNA cells were similar. These results suggested that Raptor mainly mediated the antiproliferation effect of cardamonin.
Figure 9.
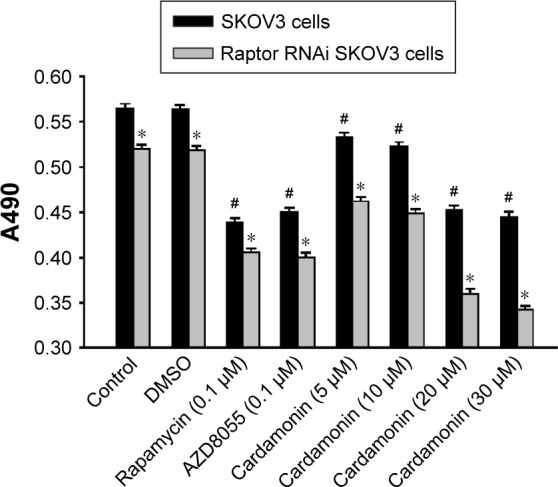
Inhibitory effect of cardamonin on the proliferation of SKOV3 cell-transfected Raptor-siRNA.
Notes: SKOV3 cells were seeded in a 96-well plate and then treated with DMSO (0.1%), cardamonin (5, 10, 20, and 30 μM), rapamycin (0.1 μM), and AZD8055 (0.1 μM) for 24 h. A total of 20 μL MTT solution (0.5 mg/mL) was added to each well, incubated at 37°C for 4 h, then the supernatant was discarded, 150 μL DMSO added into each well, and shaken for 15 min. Finally, the absorbance was determined at 490 nm by a microplate reader. n=6; #P<0.05 vs control group; *P<0.05 vs nontransfection groups.
Abbreviations: DMSO, dimethyl sulfoxide; MTT, methyl thiazolyl tetrazolium; siRNA, small interfering RNA.
Discussion
Ovarian cancer is the main cause of death among the gynecologic malignancies. Platinum-based adjuvant chemotherapy following cytoreductive surgery is the common treatment for ovarian cancer. However, the clinical outcome is unsatisfactory due to the severe adverse reaction and drug resistance of cancer cells. Thus, it will gain great clinical benefits to explore targeted drugs with better curative effects for ovarian cancer.
mTOR is a promising drug target for cancer. It accelerates the tumorigenesis, development, and platinum resistance of ovarian cancer.1 Our previous studies have demonstrated that cardamonin inhibits the proliferation and angiogenesis in SKOV3 cells through suppressing mTOR;29 in addition, it has no effect on the specific component of mTORC2 and its downstream substrate Akt (Shi et al, unpublished data, 2018). It speculates that cardamonin is an mTORC1 inhibitor.23,29 As Raptor and PRAS40 are important in the activation of mTORC1 and S6K1, we investigated the effect of cardamonin on the phosphorylation and expression of these proteins. In the present study, the activity of mTOR and S6K1 was significantly decreased by cardamonin. After treating with cardamonin for 2, 16, and 24 h, the expression of Raptor, Raptor-S792, and PRAS40-S183 was reduced in SKOV3 cells, whereas the expression of PRAS40-T246 was not affected. Since the phosphorylation of PRAS40 at Ser183 rather than Thr246 was regulated by mTORC1,30 we further confirmed that cardamonin inhibited mTORC1. Interestingly, both rapamycin and AZD8055 have no effect on the expression of Raptor. It suggests that Raptor plays a critical role in mTORC1 inhibition by cardamonin, which is different from rapamycin and AZD8055.
Since cardamonin decreases the indispensable binding protein of mTORC1, it is worthwhile investigating the effect of cardamonin in Raptor knockdown cells. In accordance with other studies, we successfully knocked down the expression of Raptor by siRNA in SKOV3 cells.31,32 Moreover, the phosphorylation of mTOR and S6K1 was decreased. However, the expression of Raptor was partially downregulated by siRNA. After treating with cardamonin, the protein expression of Raptor and the phosphorylation of mTOR and S6K1 were further reduced. We speculated that the further inhibitory effect of cardamonin on mTORC1 signal pathway in Raptor siRNA SKOV3 cells was due to the incomplete knockdown of Raptor. It was worth noting that both rapamycin and AZD8055 had no additional inhibitory effect on the expression of Raptor.
Mechanism of mTORC1 activation remains mysterious. It is expected that proteins that signal the availability of nutrition to mTORC1 are also likely to interact with it, but so far, no good candidates have been identified. Recent studies have demonstrated that Raptor-mediated translocation of mTOR to lysosomes is an essential procedure for the activation of mTOR.33–35 Cardamonin and Raptor siRNA decreased the localization of mTOR to the lysosomal surface. Furthermore, the inhibitory effect of cardamonin on mTOR lysosomal localization was strengthened in Raptor siRNA cells. It suggested that Raptor was the potential target of cardamonin on mTORC1 inhibition. Nevertheless, more studies are needed to confirm whether cardamonin has a direct effect on mTOR.
Previous studies have shown that the proliferation is suppressed by cardamonin and mTOR inhibitors in various cancer cells.36,37 To illustrate whether the antiproliferative effect of cardamonin was associated with Raptor downregulation, we performed MTT assay in both normal cells and Raptor siRNA cells. The results showed that the cell viability was decreased by cardamonin. Similar to Raptor inhibition, the antiproliferation effect of cardamonin in Raptor siRNA-transfected cells was stronger than that in normal SKOV3 cells. However, the effective dose of cardamonin is much higher than that of rapamycin and AZD8055. It indicated that Raptor was involved in the antiproliferative effect of cardamonin in SKOV3 cells. Several studies have demonstrated that cardamonin downregulates the proliferation of cancer cells through inhibiting nuclear factor-κB, signal transducers and activators of transcription 3, and c-Jun N-terminal kinase.38–40 The effective concentration of cardamonin (10–20 μM) in these studies is higher than that of rapamycin, which is consistent with our results in the present and previous studies.23,29 Although cardamonin interferes with other signaling pathways, our results in this study suggest that Raptor mainly mediates the antiproliferative effect of cardamonin in SKOV3 cells.
Conclusion
Our results reveal that Raptor is the potential target of cardamonin on antiproliferation by suppressing the mTORC1 activity in ovarian cancer SKOV3 cells.
Acknowledgments
The authors gratefully thank Dr Peng Huang (University of Calgary, Calgary, AB, Canada) for helpful comments. This study was supported by the Natural Science Foundation of Fujian Province (2015J01368 and 2016J01492) and Innovative Medical Foundation of Fujian Provincial Health and Family Planning Commission (2016-CX-13), China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137(1):173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Feng X, Li L, Jiang H, Jiang K, Jin Y, Zheng J. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: involvement of apoptosis and autophagy. Biochem Biophys Res Commun. 2014;444(3):376–381. doi: 10.1016/j.bbrc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Kashiyama T, Oda K, Ikeda Y, et al. Antitumor activity and induction of TP53-dependent apoptosis toward ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor DS-7423. PLoS One. 2014;9(2):e87220. doi: 10.1371/journal.pone.0087220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwasnicki A, Jeevan D, Braun A, Murali R, Jhanwar-Uniyal M. Involvement of mTOR signaling pathways in regulating growth and dissemination of metastatic brain tumors via EMT. Anticancer Res. 2015;35(2):689–696. [PubMed] [Google Scholar]
- 5.Zhu M, Guo J, Xia H, et al. Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2(1):59–70. doi: 10.18632/oncoscience.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck JT, Mantooth R. A case of disease improvement after treatment with everolimus plus exemestane in a patient with hormone receptor-positive metastatic breast cancer with bone metastases. Case Rep Oncol. 2015;8(1):101–105. doi: 10.1159/000375119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czarnecka AM, Kornakiewicz A, Lian F, Szczylik C. Future perspectives for mTOR inhibitors in renal cell cancer treatment. Future Oncol. 2015;11(5):801–817. doi: 10.2217/fon.14.303. [DOI] [PubMed] [Google Scholar]
- 8.Mazan-Mamczarz K, Peroutka RJ, Steinhardt JJ, et al. Distinct inhibitory effects on mTOR signaling by ethanol and INK128 in diffuse large B-cell lymphoma. Cell Commun Signal. 2015;13:15. doi: 10.1186/s12964-015-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke R, Cook KL, Hu R, et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72(6):1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey JW, Jacobs BL, Goodman CA, Hornberger TA. A role for raptor phosphorylation in the mechanical activation of mTOR signaling. Cell Signal. 2014;26(2):313–322. doi: 10.1016/j.cellsig.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrik-Outmezguine VS, Okaniwa M, Yao Z, et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272–276. doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69(3):1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundin T, Peffley DM, Hentosh P. Disruption of an hTERT-mTOR-RAPTOR protein complex by a phytochemical perillyl alcohol and rapamycin. Mol Cell Biochem. 2013;375(1–2):97–104. doi: 10.1007/s11010-012-1532-3. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves LM, Valente IM, Rodrigues JA. An overview on cardamonin. J Med Food. 2014;17(6):633–640. doi: 10.1089/jmf.2013.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Naga RN. Pre-treatment with cardamonin protects against cisplatin-induced nephrotoxicity in rats: impact on NOX-1, inflammation and apoptosis. Toxicol Appl Pharmacol. 2014;274(1):87–95. doi: 10.1016/j.taap.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Jia D, Tan Y, Liu H, et al. Cardamonin reduces chemotherapy-enriched breast cancer stem-like cells in vitro and in vivo. Oncotarget. 2016;7(1):771–785. doi: 10.18632/oncotarget.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Gwak J, Han SJ, et al. Cardamonin suppresses the proliferation of colon cancer cells by promoting beta-catenin degradation. Biol Pharm Bull. 2013;36(6):1040–1044. doi: 10.1248/bpb.b13-00158. [DOI] [PubMed] [Google Scholar]
- 20.Pascoal AC, Ehrenfried CA, Lopez BG, et al. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules. 2014;19(2):1843–1855. doi: 10.3390/molecules19021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Y, Sun CY, Lu FR, et al. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-kappaB pathway in vitro. Leuk Res. 2012;36(4):514–520. doi: 10.1016/j.leukres.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Heng X, Shi D, Niu P, et al. Study on the inhibitory effect of cardamonin on ovarian cancer SKOV3 cells by regulation of autophagy. Prog Modern Biomed. 2014;14:663–667. [Google Scholar]
- 23.Tang Y, Fang Q, Shi D, Niu P, Chen Y, Deng J. mTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2014;99(1–2):44–51. doi: 10.1016/j.lfs.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 24.Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY, Deng J. Cardamonin inhibits metastasis of lewis lung carcinoma cells by decreasing mTOR activity. PLoS One. 2015;10(5):e0127778. doi: 10.1371/journal.pone.0127778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Q, Shi DH, Zheng W, Xu XJ, Yu YH. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur J Pharmacol. 2010;641(2–3):179–186. doi: 10.1016/j.ejphar.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Niu P, Zhang Y, Shi D, Chen Y, Deng J. Cardamonin ameliorates insulin resistance induced by high insulin and high glucose through the mTOR and signal pathway. Planta Med. 2013;79(6):452–458. doi: 10.1055/s-0032-1328325. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Shi D, Ji X, Han Y, Liao Q. Antiproliferation of cardamonin associated with mRNA expression of mTOR, raptor and rictor. Zhongguo Zhong Yao Za Zhi. 2010;35(17):2318–2323. Chinese. [PubMed] [Google Scholar]
- 28.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue ZG, Niu PG, Shi DH, Liu Y, Deng J, Chen YY. Cardamonin inhibits angiogenesis by mTOR downregulation in SKOV3 cells. Planta Med. 2016;82(1–2):70–75. doi: 10.1055/s-0035-1557901. [DOI] [PubMed] [Google Scholar]
- 30.Nascimento EB, Snel M, Guigas B, et al. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal. 2010;22(6):961–967. doi: 10.1016/j.cellsig.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Yang ZJ, Yu C, Sinicrope FA. Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1. J Biol Chem. 2011;286(46):40002–40012. doi: 10.1074/jbc.M111.297432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Wang Z, Zhang J, et al. Rapamycin attenuates endothelial apoptosis induced by low shear stress via mTOR and sestrin1 related redox regulation. Mediators Inflamm. 2014;2014:769608. doi: 10.1155/2014/769608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai Q, Xie F, Han Y, et al. Inactivation of regulatory-associated protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem. 2017;292:196–204. doi: 10.1074/jbc.M116.764761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elghazi L, Blandino-Rosano M, Alejandro E, Cras-Méneur C, Bernal-Mizrachi E. Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Mol Metab. 2017;6(6):560–573. doi: 10.1016/j.molmet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun D, Liu H, Dai X, et al. Aspirin disrupts the mTOR-raptor complex and potentiates the anti-cancer activities of sorafenib via mTORC1 inhibition. Cancer Lett. 2017;406:105–115. doi: 10.1016/j.canlet.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Andrade-Vieira R, Goguen D, Bentley HA, Bowen CV, Marignani PA. Pre-clinical study of drug combinations that reduce breast cancer burden due to aberrant mTOR and metabolism promoted by LKB1 loss. Oncotarget. 2014;5(24):12738–12752. doi: 10.18632/oncotarget.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hortobagyi GN. Everolimus plus exemestane for the treatment of advanced breast cancer: a review of subanalyses from BOLERO-2. Neoplasia. 2015;17(3):279–288. doi: 10.1016/j.neo.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YJ, Kang KS, Choi KC, Ko H. Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells. Bioorg Med Chem Lett. 2015;25(12):2559–2564. doi: 10.1016/j.bmcl.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 39.Wu N, Liu J, Zhao X, et al. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol. 2015;36(12):9667–9676. doi: 10.1007/s13277-015-3673-y. [DOI] [PubMed] [Google Scholar]
- 40.Yadav VR, Prasad S, Aggarwal BB. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br J Pharmacol. 2012;165(3):741–753. doi: 10.1111/j.1476-5381.2011.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]