Abstract
We showed previously that mice deficient in astrocyte gap junctions Cx43 and Cx30 exhibit white matter vacuolation and hypomyelination. In this study we tested the hypothesis that loss of astrocytic gap junction proteins leads to exacerbation of the primary demyelinating diseases, using experimental autoimmune encephalomyelitis (EAE) as a model system. To test for this, Cx43 floxed mice were crossed with GFAP:Cre, Cx30 null mice to generate mice lacking astrocytic expression of both Cx43 and Cx30 (dKO). EAE was induced using myelin oligodendrocyte glycoprotein (MOG35-55) peptide, and mice were monitored for acute expression of disease. No statistically significant difference in clinical or pathological expression of EAE was observed. Lesion load and susceptibility of different areas of the CNS to inflammation were similar in all genotypes. Moreover, no differences were noted in blood-brain barrier (BBB) permeability, tissue wet weight, axonal pathology, gliosis or demyelination during acute disease. These data show that loss of the astrocytic connexins, Cx43 and Cx30, and the white matter pathology observed in these mice does not statistically affect clinical or pathological expression of EAE and show that astrocyte gap junctions do not regulate autoimmune inflammation and associated BBB disruption in acute EAE.
INTRODUCTION
In the central nervous system (CNS) astrocytes are extensively connected to other astrocytes by a network of gap junctions (GJ) that involve as many as a thousand cells (Ball et al., 2007). These astrocytic networks contribute to ionic and osmotic buffering (Langer et al., 2012, Wallraff et al., 2006, reviewed in Rash, 2010) and dispersion of glucose metabolites from the vasculature (Gandhi et al., 2009, Rouach et al., 2008). Astrocytes express GJs composed of connexin 43 (Cx43) and Cx30, and in mouse hippocampal slices, loss of Cx43/30 completely abrogates astrocyte:astrocyte GJ connectivity (Wallraff et al., 2006).
Oligodendrocyte:astrocyte gap junctions have also been identified by electron microscopy (Kamasawa et al., 2005, Massa and Mugnaini, 1982), and functionally confirmed by electrophysiology and tracer studies in vitro (Magnotti et al., 2011b, Orthmann-Murphy et al., 2007). Slice preparations from mice with specific targeted deletions of different connexin species have documented oligodendrocyte:astrocyte GJs composed of oligodendrocyte Cx47 and astrocyte Cx43 in deep cortical layers, although this group found no evidence of oliogodendrocyte:astrocyte coupling in the corpus callosum (Wasseff and Scherer, 2011). In contrast, a second group detected a low but significant degree of oligodendrocyte:astrocyte coupling in the corpus callosum which was attenuated by double-deletion of Cx43 and Cx30, or deletion of Cx47, but not deletion of Cx43 alone or oligodendrocyte Cx32 alone (Maglione et al., 2010). Strong genetic evidence for the importance of oligodendrocyte:astrocyte gap junctional connectivity comes from observations that mice deficient in oligodendrocyte GJs (Menichella et al., 2003, Odermatt et al., 2003, Sargiannidou et al., 2009, Tress et al., 2011), astrocyte GJs (Lutz et al., 2009), or combined loss of astrocyte and oligodendrocyte GJs (Magnotti et al., 2011a) exhibit similar white matter pathologies, characterized by oligodendrocyte vacuolation, cell death, myelin blebbing, and hypomyelination (reviewed in Abrams and Scherer, 2011). In mice with compound heterozygosity for oligodendrocyte GJs and the inwardly rectifying potassium channel Kir4.1, pathology is activity dependent, strongly implicating GJ network connectivity in metabolic support to white matter (Menichella et al., 2006). Glial GJ communication thus contributes to homeostatic functions that maintain myelinated and non-myelinated tissues. These studies raise the possibility that loss of astrocyte GJs in reactive astrogliosis could increase susceptibility to pathologic insults directed against myelinated tracts.
A reactive astrogliosis is a common response to CNS injury, and inflammatory mediators such as the cytokine interleukin-1 or toll-like receptor ligands lead to the rapid and persistent loss of Cx43 and Cx30 in astrocytes in culture (Duffy et al., 2000, Esen et al., 2007, Hinkerohe et al., 2005, Meme et al., 2006, Zhao et al., 2006). Consistent with a role for inflammatory cytokines in modulating GJ expression, studies in experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS), showed that Cx43 is downregulated in the CNS at sites of inflammation and was not associated with a compensatory upregulation of other astrocytic connexins (Brand-Schieber et al., 2005, Roscoe et al., 2007b). However, whether loss of Cx43 in astrocytes in EAE contributes to clinical and pathological expression of disease remains to be determined.
In other experimental settings, loss of GJ connectivity has been found to be either beneficial or detrimental (Frantseva et al., 2002a, Frantseva et al., 2002b, Lin et al., 2002, Rami et al., 2001, Rawanduzy et al., 1997). Importantly, in some acute injury models, genetic deletion of Cx43 worsens outcome. For example, mice that are heterozygous for expression of Cx43 (Siushansian et al., 2001; Nakase et al., 2003) and mice with astrocyte-targeted deletion of Cx43 exhibit larger infarct size and greater inflammation four days after middle cerebral artery occlusion (Nakase et al., 2004).
In EAE, inhibition of reactive astrogliosis increases leukocyte entry into the CNS parenchyma, and is associated with a more fulminant disease course, suggesting that astrocytes regulate blood-brain barrier (BBB) changes associated with inflammation (Voskuhl et al., 2009). Astrocytes are known to play an important role in BBB formation and maintenance, and astrocytic foot processes encompassing the cerebral vasculature form a major structural barrier that inflammatory cells must cross to enter the CNS parenchyma (Lutz et al., 2012). Astrocyte perivascular endfeet are extensively connected by tightly docked GJs composed of Cx43 and Cx30 (Ball et al., 2007, Simard et al., 2003, Yamamoto et al., 1990). Thus, if Cx43 down-regulation is characteristic of a reactive astrocytic phenotype, it is predicted that reduced astrocytic Cx43 expression may exacerbate the spread of inflammatory damage that starts at the BBB. Additionally, loss of metabolic support from astrocytic GJs may exacerbate immune-mediated damage to oligodendrocytes and myelin. We therefore hypothesized that loss of astrocytic GJ proteins leads to exacerbation of the primary demyelinating diseases. To test this, we sensitized mice with a global loss of Cx30 and an astrocyte-targeted loss of Cx43 using myelin oligodendrocyte glycoprotein (MOG35-55) and assessed the clinical and pathological expression of acute EAE in double and single knock-out mice, and in age- and sex-matched wild type and Cre or Cx43 floxed only mice. We found that neither loss of astrocytic GJs nor the resulting CNS pathology significantly altered the clinical and pathological expression of acute EAE.
METHODS
Animals
Cx43F/F (gene Gja1) Cx30-/- (gene Gjb6) mice on a C57Bl/6 background were kindly provided by Dr. Klaus Willecke (Teubner et al., 2003, Theis et al., 2003). Mice expressing Cre under the control of a promoter cassette containing the full sequence of the murine GFAP gene (line 73.120) were kindly provided by Dr. Michael Sofroniew (Garcia et al., 2004, Herrmann et al., 2008). Crossing these mice led to global deletion of Cx30 and astrocyte-directed loss of Cx43 (Cx43/Cx30 dKO). A distinct line of Cx43F/F mice kindly supplied by Dr. Brian Duling (Liao et al., 2001) on the C57Bl/6 background was also bred with mGFAP-Cre C57Bl/6 mice to give Cx43 single knock-out (sKO) mice and Cre+ littermate controls.
In each experiment, genotype and appropriate protein expression were confirmed using PCR and a combination of western blotting and immunostaining as described previously (Lutz et al., 2009). Genotyping for mGFAP-Cre was performed using the following primers: 5′ ACC AGC CAG CTA TCA ACT C; 5′ TAT ACG CGT GCT AGC GAA GAT CTC CAT CTT CCA GCA G; reaction product 350bp. Primers for Cx30 were: 5′ GGT ACC TTC TAC TAA TTA GCT TGG; 5′ AGG TGG TAC CCA TTG TAG AGG AAG; 5′ AGC GAG TAA CAA CCC GTC GGA TTC; transgenic band 460bp, WT band 544bp. Primers for Cx43 in the dKO colony were: 5′ GGC ATA CAG ACC CTT GGA CTC C; 5′ TCA CCC CAA GCT GAC TCA ACC G; transgenic band 650bp, WT band 500bp. Primers for Cx43 in the single KO colony were: 5′ CTT TGA CTC TGA TTA CAG AGC TTA A; 5′ GTC TCA CTG TTA CTT AAC AGC TTG A; transgenic band 600bp, WT band 500bp. Mice were housed in an SPF AAALAC approved facility and allowed access to food and water ad libitum. Experimental protocols and number of animals were approved by the IACUC of Albert Einstein College of Medicine.
Experimental autoimmune encephalomyelitis
EAE was induced in mice at 9-11 wk of age by subcutaneous immunization with 300 μg of myelin oligodendrocyte glycoprotein MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK; Celtek Bioscience) in a 200 μl emulsion comprised of equal parts MOG (in dH2O) and Incomplete Freund’s Adjuvant (Difco cat# 263910) supplemented with heat-killed Mycobacterium tuberculosis H37Ra (Difco cat# 231141) at 10 mg/mL. The day of MOG immunization was designated day 0. On day 0 and day 2 post immunization (dpi), mice were injected intraperitoneally with 500 ng Pertussis toxin (List cat# 181). Mice were examined for clinical signs of EAE every 1-2 days using the scale: 0, no signs; 1, flaccid tail; 2, hind limb paresis; 3, hind limb paralysis; 4, hind limb and forelimb paralysis; 5, moribund (Chen and Brosnan, 2006). Each experiment was gender-matched. No statistically significant differences were noted in cumulative trends of male versus female mice.
Histopathology
Animals were deeply anesthetized with ether vapor prior to transcardial perfusion with phosphate buffered saline (PBS). Brain and spinal cords were removed and immersion fixed overnight in cold 4% neutral buffered formalin then embedded in paraffin.
Immunohistochemistry
Paraffin sections (5 to 7 μm) were dewaxed and rehydrated in xylene and graded alcohols. Antigen retrieval was performed in sodium citrate buffer for 25 minutes in a water bath heated to 100°C. For br ight field immunohistochemistry, endogenous peroxidatic activity was quenched using 3% H2O2 for 30min. Non-specific binding was blocked with 2.5% horse serum for 20 min, and sections were incubated with primary antibody in PBS for 1 hour room temperature, incubated with Immpress anti-mouse or anti-rabbit Ig (Vector Laboratories), developed with diaminobenzidine (Dako), counterstained, dehydrated, cleared, and mounted in Permount. Sections were examined using a Leica microscope with an attached Olympus DP12 camera.
Immunofluorescence
Sections were blocked in 10% normal goat or donkey serum for one hour prior to incubation with primary antibody in 5% serum/PBS overnight at 4°C followed by Alexa-conjugated goat or donkey secondary antibodies (Invitrogen) for 1.5 hours at 22°C and 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) to visualize nuclei. Sections were mounted using Prolong Gold AntiFade Reagent (Invitrogen) and examined using an Olympus IX 81 microscope with motorized stage, 20x N.A. 0.4 or 60X N.A.O. 1.4 optics, and a Cooke Sensicam QE air-cooled CCD camera in the Einstein Analytic Imaging Facility.
Antibodies
Primary antibodies were as follows: CD45 (Serotec rat monoclonal 1:500), mouse anti-GFAP (1:100, Dako, for DAB), rabbit anti-Iba1 (1:500, Wako), SMI32 (1:2000, Covance, for nonphosphorylated neurofilament H), MBP (1:2000, Covance, clone SMI99), rabbit anti-Cx43 (1:1000, Sigma); rabbit anti-Cx43 (1:100, Cell Signaling); mouse anti-Cx43 (1:250, Chemicon); mouse anti-GFAP (1:2000, Cell Signaling cat. #3670), rat anti-GFAP (Invitrogen cat. #130300, 1:100). Control sections were processed using species- and isotype-matched irrelevant primary antibodies, IgG fractions, or no primary antibody.
Image Quantification and Cell Counting
Image fields were selected for examination in an unbiased manner, randomized with respect to X, Y, and Z planes. To ensure cells were counted only once regardless of their size, we used the “optical dissector” stereological technique (Mouton, 2002) to probe the tissue. We only counted cells for which the top of the nucleus was in focus within the z-planes of the dissector field. Images were collected with IPLab 4.0.8. Pseudocolor images were assembled in Adobe Photoshop 7.0 using global manipulations to optimize signal-to-noise ratios.
Real-time quantitative PCR (Q-PCR)
Animals were perfused with PBS under ether anesthesia, spinal cords removed by insufflation and total RNA isolated from fresh or snap-frozen tissues using TRIzol (Invitrogen), treated to remove contaminating genomic DNA with RNase-free recombinant DNase I (Roche), re-extracted, and precipitated. Concentration was determined by OD values at 260 nm, and 10 μg total RNA reverse transcribed in a reaction volume of 20μl (SuperscriptIII, Invitrogen). Quantitative PCR was performed in 384-well reaction plates. Each reaction sample comprised a 1:1 mixture of diluted (1/100) RT-PCR sample to SYBR Green Q-PCR Master Mix (Applied Biosystems) combined with a 1:1 mixture of gene-specific forward and reverse primers to the same SYBR Green Master Mix, resulting in a total volume of 8 μl/reaction. Plates were processed in an ABI PRISM 7000 light cycler (Applied Biosystems). All runs were accompanied by the internal control gene GAPDH. Samples were normalized using a cycle threshold-based algorithm to give arbitrary units representing relative expression levels between samples. The following primers were used: RANTES_F GTG CCC ACG TCA AGG AGT AT; RANTES_R CCC ACT TCT TCT CTG GGT TG; IL-1b_F CAG GCA GGC AGT ATC ACT CA; IL-1b_R TGT CCT CAT CCT GGA AGG TC; IL-1Ra_F TTG TGC CAA GTC TGG AGA TG; IL-1Ra_R AAG CGC TTG TCT TCT TCT TTG; TNFa_FACG GCA TGG ATC TCA AAG AC; TNFa_R GTG GGT GAG GAG CAC GTA GT; CCL2_F CAA GAA GGA ATG GGT CCA GA; CCL2_R GCT GAA GAC CTT AGG GCA GA; Iba1_F TGA TGA GGA TCT GCC GTC CAA ACT; Iba1_R TCT CCA GCA TTC GCT TCA AGG ACA; IL-6_F CCG GAG AGG AGA CTT CAC AG; Il-6_R TCC ACG ATT TCC CAG AGA AC. The CXCL10 (IP-10) QPCR primer was purchased from SA Biosciences.
Evan’s Blue extravasation assay
To visualize and quantify blood brain barrier disruption, Evans blue (Sigma) was prepared at 0.5mg/ml in saline, sterile filtered, and 200μl injected into the intraperitoneal cavity of mice sensitized for EAE or naïve controls. One hour later, mice were perfused with PBS to clear the dye from blood, such that only dye in the tissue parenchyma was left. Brain, cerebellum and kidneys were photographed, dye was extracted overnight in dimethylformamide (Sigma), and absorbance read colorimetrically at 610 nm. Absorbance at 710nm was used for background subtraction. Data are presented as ng Evans blue per mg tissue.
Ex vivo Proliferation Assay
WT, Cx43F/F mice and Cx43 KO mice were sensitized with MOG as above, and lymphocytes harvested from the spleen and draining lymph nodes on day 21. Cell suspensions were prepared and cultured in vitro with 0, 10, or 20μg/ml MOG35-55 peptide. After 2 days, low-toxicity 3H-thymidine (Amersham) was added to the cultures for an additional 48 hrs and then cells harvested and assayed for thymidine uptake.
Statistical analysis
Statistical analysis was performed with Prism 4 software (GraphPad, San Diego, CA), using ANOVA followed by Newman-Keuls post-hoc test for multiple comparisons, or two-tailed student’s T-test. A value of p< 0.05 was considered significant.
RESULTS AND DISCUSSION
Clinical expression of EAE
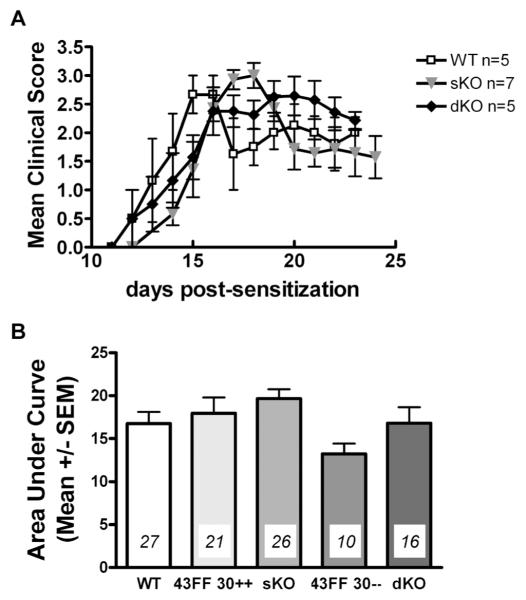
Clinical expression of acute EAE in WT, sKO and dKO for one representative experiment is shown in figure 1A, and cumulative data for four experiments in figure 1B. A typical pattern of disease expression for MOG-induced EAE in C57Bl/6 mice was noted in all genotypes tested, characterized by an ascending paralysis with acute peak of clinical signs followed by partial remission. No statistically significant change in disease activity in sKO or dKO mice was detected. Similarly, no difference was detected in day of onset, peak clinical expression, or recovery (Table I).
Figure 1. EAE in control, Cx43 sKO, and Cx43/Cx30 dKO mice.
A, results of one experiment are shown and are expressed as mean ± SEM daily clinical index (CI) for each group (* p<0.05). B, cumulative data for multiple experiments are expressed as area under the curve as an index of disease burden. Number of animals per group is indicated in italics. See also Table I.
TABLE I. EAE in Cx43 and Cx30 deficient mice.
| Genotype | n | AUCa | Peak Score | Date of Onset | Date of Peak Score | Incidence (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| WT | 27 | 16.8 | 1.4 | 2.5 | 0.1 | 12.6 | 0.5 | 15.1 | 0.6 | 81.6 | 4.4 |
| Cx43F/F Cx30+/+ | 21 | 18.0 | 1.8 | 2.8 | 0.1 | 12.7 | 0.7 | 15.4 | 0.8 | 91.6 | 5.3 |
| Cre Cx43F/F (sKO) | 26 | 19.7 | 1.1 | 3.0 | 0.1 | 12.6 | 0.5 | 15.3 | 0.5 | 91.6 | 5.2 |
| Cx43F/F Cx30−/− | 10 | 13.2 | 1.2 | 2.3 | 0.2 | 12.7 | 0.7 | 16.9 | 1.0 | 100.0 | 5.2 |
| Cre Cx43F/F Cx30−/− (dKO) | 16 | 16.8 | 1.9 | 2.7 | 0.2 | 12.6 | 0.5 | 15.8 | 0.7 | 83.6 | 7.5 |
Area under the curve (AUC) was calculated for days 10-25. Animals were excluded from analysis which did not attain a clinical score of at least 1 for two consecutive days, or which died or were sacrificed before 25 days post immunization. n, number of animals per group included in analysis. SE, standard error.
Pathological expression of inflammation and reactive gliosis
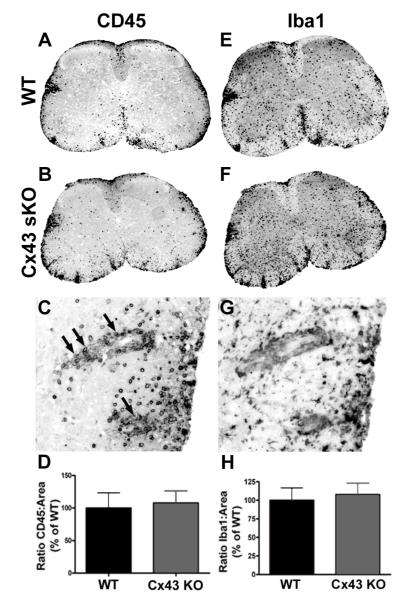
To assess extent of inflammation in the CNS, tissue was collected on day 21, and sections were taken throughout the neuraxis and stained with H&E. Quantitative assessment of inflammation showed that lesions were more prevalent in spinal cord as compared to brain in all groups of mice, and that extent of pathology correlated with clinical score (data not shown). Extent of spinal cord inflammation did not differ between genotypes. We quantified glial activation in WT and sKO spinal cord sections using immunoreactivity for GFAP (not shown), and the ionized calcium binding adaptor protein 1 (Iba1) that is specifically expressed in cells of the macrophage lineage and preferentially upregulated in microglia following activation (Figure 2E, F, G and H). We also assessed immunoreactivity for CD45 for cells of hematopoietic lineage (Figure 2, A, B, C and D). Quantitative assessment of glial reactivity and CD45 indicated no differences between genotypes at this timepoint. Using stereotactic morphometry, we assessed axonal damage in serial sections of the anterior columns of the lumbar spinal cord. There was no significant difference in extent of axonal pathology, as determined by immunoreactivity for SMI32 (not shown).
Figure 2. Leukocyte and microglial immunostaining in spinal cord sections from WT and Cx43 sKO EAE mice.
Sections were stained for CD45 (panels A C) or Iba1 (panel E-G) and the extent of immunoreactivity was quantified using ImageJ (D, H). Panels A-B, low power images from WT (A) and Cx43 sKO (B) mice exhibit CD45+ leukocytes in inflammatory lesions. The submeningeal location in white matter is characteristic of lesion distribution in EAE. C, A higher power view of the lateral column from the Cx43 sKO mouse shows perivascular cuffing (arrows) and parenchymal infiltration in an EAE lesion. D, quantification of immunostaining showed no difference between WT (n=9) and Cx43 sKO (n=7) EAE mice. E-F, serial sections stained for the microglial calcium-binding protein Iba1. Low power view (4x) shows strong immunostaining in submeningeal lesions and gray matter. G, A higher power (20x) view shows that immunoreactivity is associated with small process bearing cells consistent with a microglial phenotype. Serial section from panel C. H, quantification of immunostaining showed no difference in EAE between WT and Cx43 sKO mice.
Immunologic parameters of EAE
We considered the possibility that other immunological parameters might provide a more sensitive measure of differences between genotypes in the autoimmune response within the CNS. To test this, we used Q-PCR to determine levels of cytokines and chemokines associated with inflammation in the spinal cord. Q-PCR data showed no significant differences between sKO, Cx43F/F and WT mice for IL-6, CCL2, CCL5, CXCL10, and TNFα, as well as IL-1β, IL-12 and IL-23 as markers of activation of macrophages/microglia and dendritic cells, on spinal cord tissue harvested from astrocyte-Cx43 KO and WT mice at 21 days post-sensitization for EAE. Rather, there was a correlation between clinical index and chemokine/cytokine levels, with a significant positive correlation between clinical index and expression of IL-12, CCL5, CXCL10, and TNFα, and a significant negative correlation between clinical index and expression of IL-23 (data not shown).
For T cell activity, we tested lymphocyte proliferative responses to MOG on day 27 to determine if the immune response to MOG was altered in Cx43 KO mice. No differences were detected between WT, Cx43F/F and Cx43 dKO mice (data not shown), indicating that T cell responsiveness to MOG was not affected either by floxing Cx43 or deleting the gene in astrocytes.
Myelin pathology in dKO mice
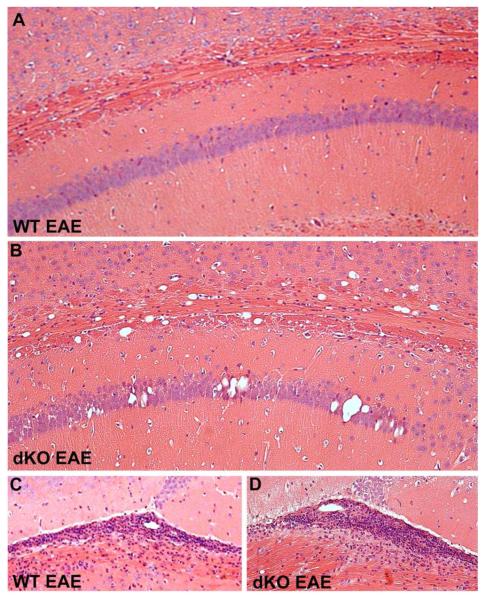
We showed previously that Cx43/Cx30 dKO mice develop widespread intramyelinic edema and vacuolation of both cerebral white matter and gray matter, particularly in the corpus callosum and CA1 region of the hippocampus (Lutz et al., 2009). We hypothesized that this pathology might increase susceptibility of affected structures to EAE-mediated inflammation and demyelination. To test for this, twelve sagittal serial sections through the hippocampus from Cx43/Cx30 dKO and control mice were stained with H&E. In all genotypes, inflammation was detected in the hippocampal fissure and in the stria medullaris of the thalamus, a white matter structure immediately ventral to the hippocampal fissure (Figure 3). However, there was no evidence of enhanced perivascular cuffing or leukocyte infiltration associated with the abnormal pathology found in the corpus callosum and hippocampus of the dKO mice (Figure 3).
Figure 3. Distribution of inflammatory lesions in the corpus callosum and hippocampus in WT and Cx43/Cx30 dKO mice.
Hematoxylin and eosin stained sagittal brain sections from WT (A) and dKO (B) mice sensitized with EAE illustrate the pathology associated with Cx43/Cx30 dKO phenotype. As previously reported, Cx43/Cx30 dKO brains exhibited abnormal morphology in the corpus callosum and hippocampus (B). However, perivascular inflammatory foci were not associated with this pathology. Original magnification, 10x. C-D, In contrast, extensive inflammation typical of EAE was observed in the hippocampal fissure and underlying stria medullaris in all genotypes. Magnification, 20x.
Blood brain barrier permeability
To examine if astrocytes lacking GJs might affect the integrity of the BBB and thereby increase leakage of serum proteins into the CNS, we injected Evan’s Blue dye into mice of differing genotypes sensitized for EAE. Control, naïve mice were injected in parallel. Evan’s Blue dye extracted from PBS-perfused brain and spinal cord was measured spectrophotometrically. To verify that mice received equivalent systemic doses of dye, dye load from kidneys was compared. No genotype differences were noted in extent of Evan’s Blue extravasation into the CNS in either naive animals or in animals sensitized for EAE (Figure 4).
Figure 4. Analysis of blood-brain barrier permeability in EAE.
Evans blue dye was injected IP and 1 hr later tissues were collected from the brain (A) and spinal cord (B) after perfusion with PBS. Quantification of dye extracted from the tissue showed increased levels in mice sensitized for EAE compared with naive (no EAE) WT, and in animals with EAE higher levels of dye in spinal cord (B) compared with brain (A). However no significant differences were noted between genotypes. C, typical distribution of dye in the brain and spinal cord is shown.
Discussion
Taken together, the results of these experiments show that loss of neither one (Cx43) nor both (Cx43, Cx30) of the major GJ proteins found in astrocytes had any significant effect on the development of clinical or pathological expression of MOG peptide-induced acute EAE. No difference in BBB permeability in Cx43/Cx30 dKO mice was detected. The data presented herein for astrocyte-specific Cx43 sKO are remarkably similar to the findings of Roscoe, Kidder and Karlik (Roscoe et al., 2007a), who also failed to detect clinical or pathological differences in Cx43 heterozygotes sensitized for EAE. Importantly, both Roscoe (Roscoe et al., 2007b) and Brand-Schrieber (Brand-Schieber et al., 2005) showed that Cx43 protein is downregulated during inflammation in EAE. Thus, the absence of significant effect of Cx43/30 gene deletion on EAE may simply reflect the fact that these proteins are physiologically downregulated during acute EAE. No evidence of altered Cx26 expression, which is expressed at low levels in astrocytes (Nagy et al., 2011), was detected in these mice (Lutz et al., 2009).
Loss of GJ connectivity between astrocytes and oligodendrocytes and myelin leads to white matter vacuolation and glial cell death (Lutz et al., 2009, Magnotti et al., 2011a). In astrocyte Cx43/Cx30 dKO mice, white matter pathology was more profound in the brain as compared to the spinal cord (Lutz et al., 2009). EAE is known to be a disease that affects mainly spinal cord and cerebellum but what renders certain areas of white matter more vulnerable than others remains poorly understood. We hypothesized that these fundamental changes in the structure of cerebral myelinated tracts would predispose to more severe regional damage in response to immunologic attack. For example, the loss of myelin structural integrity, and the presence of intramyelinic edema, might increase the spread of soluble inflammatory factors. Additionally, because the amount of myelin is already decreased in Cx43/Cx30 dKOs (Lutz et al., 2009), the amount of autoimmune demyelination consequent to EAE might reduce the overall level of myelination below the threshold for animal survival. However, because our data did not indicate increased disease severity nor increased myelin pathology in GJ KO mice, we did not investigate further these possibilities. In our previous study, no BBB breakdown was observed in naïve dKO mice (Lutz et al 2009). Our current data show that myelin pathology in the absence of BBB injury does not exacerbate signs in the MOG35-55 acute EAE model.
With respect to multiple sclerosis (MS), proteomic and gene array analyses of MS tissue detected increased Cx43 expression in chronic active MS lesions over that found in normal controls, but only very low level expression in chronic silent lesions (Chabas et al., 2001, Han et al., 2008, Lock et al., 2002). Western blot and immunohistochemical analyses of MS lesions demonstrated Cx43 downregulation in chronic silent lesions, and considerable variability in Cx43 expression in chronic active lesions (Lutz et al., unpublished observations).
The best documented link between Cx43 and myelin pathology in humans is found in individuals with oculodentodigital dysplasia (ODDD), a rare autosomal-dominant disorder associated with germ-line mutations in Cx43. ODDD patients display congenital craniofacial and limb abnormalities. Neurologic manifestations are frequent in ODDD and include dysarthria, neurogenic bladder disturbances, spastic paraparesis, ataxia, seizures and a slowly progressive leukodystrophy (Loddenkemper et al., 2002). Brain magnetic resonance imaging studies of ODDD patients show diffuse bilateral abnormalities in the subcortical cerebral white matter (Paznekas et al., 2003).
In contrast to the data for EAE, studies that have addressed a role for Cx43 and Cx30 in gray matter damage have shown that astrocyte GJs play a critical role in neuroprotection. So, for example, in mice with a specific deletion of the Cx43 genomic sequence in GFAP-expressing cells, middle cerebral artery occlusion resulted in enhanced stroke volume and increased neuronal apoptosis, as well as accelerated spreading depression (Nakase et al., 2003, Nakase et al., 2004, Theis et al., 2003). It remains possible, therefore, that a study of the chronic stage of EAE or viral models of inflammatory demyelination where neuronal damage is prominent may be more informative, but at the present time our data do not indicate a causal link between the dynamic regulation of astrocyte GJ proteins in EAE and disease outcome.
Acknowledgements
Supported by the National Multiple Sclerosis Society USA grants RG 3827A5/1 to CFB and RG1001-K-11 to CSR, USPHS grant NS11920 to CSR and CFB, and SEL was suported by NINDS training grant T32 NS07439. CSR is the Wollowick Family Foundation Professor for Multiple Sclerosis Research. Mice were kindly provided by Dr. Michael Sofroniew (Dept. of Neurobiology, UCLA, California), Dr. Brian Duling (Dept. of Biomedical Engineering, University of Virginia, Virginia), and Dr. Klaus Willecke (Dept. of Genetics, Universitat Bonn).
Footnotes
Authors’ contributions
SEL and CFB induced EAE in mice and monitored them for clinical expression of disease. SEL and CSR perfused the animals and collected tissues. SEL performed immunohistochemistry and quantitative microscopy. SEL, CSR, and CFB reviewed the slides and wrote the manuscript.
Competing Interests
The funding agency had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors have no financial or non-financial competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- ABRAMS CK, SCHERER SS. Gap junctions in inherited human disorders of the central nervous system. Biochimica et Biophysica Acta. 2011 doi: 10.1016/j.bbamem.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL KK, GANDHI GK, THRASH J, CRUZ NF, DIENEL GA. Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking. Journal of Neuroscience Research. 2007;85:3267–83. doi: 10.1002/jnr.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAND-SCHIEBER E, WERNER P, IACOBAS DA, IACOBAS S, BEELITZ M, LOWERY SL, SPRAY DC, SCEMES E. Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. Journal of Neuroscience Research. 2005;80:798–808. doi: 10.1002/jnr.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABAS D, BARANZINI SE, MITCHELL D, BERNARD CC, RITTLING SR, DENHARDT DT, SOBEL RA, LOCK C, KARPUJ M, PEDOTTI R, HELLER R, OKSENBERG JR, STEINMAN L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–5. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- CHEN L, BROSNAN CF. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R-/- mice: evidence for loss of apoptotic activity in lymphocytes. Journal of Immunology. 2006;176:3115–26. doi: 10.4049/jimmunol.176.5.3115. [DOI] [PubMed] [Google Scholar]
- DUFFY HS, JOHN GR, LEE SC, BROSNAN CF, SPRAY DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. Journal of Neuroscience. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESEN N, SHUFFIELD D, SYED MM, KIELIAN T. Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus. Glia. 2007;55:104–17. doi: 10.1002/glia.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANTSEVA MV, KOKAROVTSEVA L, NAUS CG, CARLEN PL, MACFABE D, PEREZ VELAZQUEZ JL. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. Journal of Neuroscience. 2002a;22:644–53. doi: 10.1523/JNEUROSCI.22-03-00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANTSEVA MV, KOKAROVTSEVA L, PEREZ VELAZQUEZ JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. Journal of Cerebral Blood Flow and Metabolism. 2002b;22:453–62. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- GANDHI GK, CRUZ NF, BALL KK, THEUS SA, DIENEL GA. Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. Journal of Neurochemistry. 2009;110:857–69. doi: 10.1111/j.1471-4159.2009.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA AD, DOAN NB, IMURA T, BUSH TG, SOFRONIEW MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature Neuroscience. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- HAN MH, HWANG SI, ROY DB, LUNDGREN DH, PRICE JV, OUSMAN SS, FERNALD GH, GERLITZ B, ROBINSON WH, BARANZINI SE, GRINNELL BW, RAINE CS, SOBEL RA, HAN DK, STEINMAN L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–81. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- HERRMANN JE, IMURA T, SONG B, QI J, AO Y, NGUYEN TK, KORSAK RA, TAKEDA K, AKIRA S, SOFRONIEW MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. Journal of Neuroscience. 2008;28:7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKEROHE D, SMIKALLA D, HAGHIKIA A, HEUPEL K, HAASE CG, DERMIETZEL R, FAUSTMANN PM. Effects of cytokines on microglial phenotypes and astroglial coupling in an inflammatory coculture model. Glia. 2005;52:85–97. doi: 10.1002/glia.20223. [DOI] [PubMed] [Google Scholar]
- JOHN GR, LEE SC, BROSNAN CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- KAMASAWA N, SIK A, MORITA M, YASUMURA T, DAVIDSON KG, NAGY JI, RASH JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGER J, STEPHAN J, THEIS M, ROSE CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia. 2012;60:239–52. doi: 10.1002/glia.21259. [DOI] [PubMed] [Google Scholar]
- LIAO Y, DAY KH, DAMON DN, DULING BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9989–94. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN JH, TAKANO T, COTRINA ML, ARCUINO G, KANG J, LIU S, GAO Q, JIANG L, LI F, LICHTENBERG-FRATE H, HAUBRICH S, WILLECKE K, GOLDMAN SA, NEDERGAARD M. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. Journal of Neuroscience. 2002;22:4302–11. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCK C, HERMANS G, PEDOTTI R, BRENDOLAN A, SCHADT E, GARREN H, LANGER-GOULD A, STROBER S, CANNELLA B, ALLARD J, KLONOWSKI P, AUSTIN A, LAD N, KAMINSKI N, GALLI SJ, OKSENBERG JR, RAINE CS, HELLER R, STEINMAN L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Medicine. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- LODDENKEMPER T, GROTE K, EVERS S, OELERICH M, STOGBAUER F. Neurological manifestations of the oculodentodigital dysplasia syndrome. Journal of Neurology. 2002;249:584–95. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- LUTZ SE, ZHAO Y, GULINELLO M, LEE SC, RAINE CS, BROSNAN CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. Journal of Neuroscience. 2009;29:7743–52. doi: 10.1523/JNEUROSCI.0341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTZ SE, RAINE CS, BROSNAN CF. Astrocyte Involvement in the Acquired Demyelinating Diseases. In: SCEMES E, SPRAY DC, editors. Astrocytes Wiring the Brain. Taylor & Francis Group, LLC; Boca Raton: 2012. [Google Scholar]
- MAGLIONE M, TRESS O, HAAS B, KARRAM K, TROTTER J, WILLECKE K, KETTENMANN H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia. 2010;58:1104–17. doi: 10.1002/glia.20991. [DOI] [PubMed] [Google Scholar]
- MAGNOTTI LM, GOODENOUGH DA, PAUL DL. Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia. 2011a;59:1064–74. doi: 10.1002/glia.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNOTTI LM, GOODENOUGH DA, PAUL DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia. 2011b;59:26–34. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSA PT, MUGNAINI E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience. 1982;7:523–38. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- MEME W, CALVO CF, FROGER N, EZAN P, AMIGOU E, KOULAKOFF A, GIAUME C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB Journal. 2006;20:494–6. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- MENICHELLA DM, GOODENOUGH DA, SIRKOWSKI E, SCHERER SS, PAUL DL. Connexins are critical for normal myelination in the CNS. Journal of Neuroscience. 2003;23:5963–73. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENICHELLA DM, MAJDAN M, AWATRAMANI R, GOODENOUGH DA, SIRKOWSKI E, SCHERER SS, PAUL DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. Journal of Neuroscience. 2006;26:10984–91. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUTON PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. The Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- NAGY JI, LYNN BD, TRESS O, WILLECKE K, RASH JE. Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice. European Journal of Neuroscience. 2011;34:263–71. doi: 10.1111/j.1460-9568.2011.07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKASE T, FUSHIKI S, SOHL G, THEIS M, WILLECKE K, NAUS CCG. Neuroprotective role of astrocytic gap junctions in ischemic stroke. Cell Communication and Adhesion. 2003;10:413–417. doi: 10.1080/cac.10.4-6.413.417. [DOI] [PubMed] [Google Scholar]
- NAKASE T, SOHL G, THEIS M, WILLECKE K, NAUS CCG. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. American Journal of Pathology. 2004;164:2067–2075. doi: 10.1016/S0002-9440(10)63765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODERMATT B, WELLERSHAUS K, WALLRAFF A, SEIFERT G, DEGEN J, EUWENS C, FUSS B, BUSSOW H, SCHILLING K, STEINHAUSER C, WILLECKE K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. Journal of Neuroscience. 2003;23:4549–59. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTHMANN-MURPHY JL, FREIDIN M, FISCHER E, SCHERER SS, ABRAMS CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. Journal of Neuroscience. 2007;27:13949–57. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNASCH U, VARGOVA L, REINGRUBER J, EZAN P, HOLCMAN D, GIAUME C, SYKOVA E, ROUACH N. Astroglial networks scale synaptic activity and plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8467–72. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZNEKAS WA, BOYADJIEV SA, SHAPIRO RE, DANIELS O, WOLLNIK B, KEEGAN CE, INNIS JW, DINULOS MB, CHRISTIAN C, HANNIBAL MC, JABS EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. American Journal of Human Genetics. 2003;72:408–18. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMI A, VOLKMANN T, WINCKLER J. Effective reduction of neuronal death by inhibiting gap junctional intercellular communication in a rodent model of global transient cerebral ischemia. Experimental Neurology. 2001;170:297–304. doi: 10.1006/exnr.2001.7712. [DOI] [PubMed] [Google Scholar]
- RASH JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience. 2010;168:982–1008. doi: 10.1016/j.neuroscience.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWANDUZY A, HANSEN A, HANSEN TW, NEDERGAARD M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. Journal of Neurosurgery. 1997;87:916–20. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- ROSCOE WA, KIDDER GM, KARLIK SJ. Experimental allergic encephalomyelitis in connexin 43-heterozygous mice. Cell Commun Adhes. 2007a;14:57–73. doi: 10.1080/15419060701459569. [DOI] [PubMed] [Google Scholar]
- ROSCOE WA, MESSERSMITH E, MEYER-FRANKE A, WIPKE B, KARLIK SJ. Connexin 43 gap junction proteins are up-regulated in remyelinating spinal cord. Journal of Neuroscience Research. 2007b;85:945–53. doi: 10.1002/jnr.21194. [DOI] [PubMed] [Google Scholar]
- ROUACH N, KOULAKOFF A, ABUDARA V, WILLECKE K, GIAUME C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- SARGIANNIDOU I, VAVLITOU N, ARISTODEMOU S, HADJISAVVAS A, KYRIACOU K, SCHERER SS, KLEOPA KA. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. Journal of Neuroscience. 2009;29:4736–49. doi: 10.1523/JNEUROSCI.0325-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMARD M, ARCUINO G, TAKANO T, LIU QS, NEDERGAARD M. Signaling at the gliovascular interface. Journal of Neuroscience. 2003;23:9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIUSHANSIAN R, BECHBERGER JF, CECHETTO DF, HACHINSKI VC, NAUS CC. Connexin43 null mutation increases infarct size after stroke. Journal of Comparative Neurology. 2001;440:387–94. doi: 10.1002/cne.1392. [DOI] [PubMed] [Google Scholar]
- TEUBNER B, MICHEL V, PESCH J, LAUTERMANN J, COHEN-SALMON M, SOHL G, JAHNKE K, WINTERHAGER E, HERBERHOLD C, HARDELIN JP, PETIT C, WILLECKE K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Human Molecular Genetics. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- THEIS M, JAUCH R, ZHUO L, SPEIDEL D, WALLRAFF A, DORING B, FRISCH C, SOHL G, TEUBNER B, EUWENS C, HUSTON J, STEINHAUSER C, MESSING A, HEINEMANN U, WILLECKE K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. Journal of Neuroscience. 2003;23:766–76. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRESS O, MAGLIONE M, ZLOMUZICA A, MAY D, DICKE N, DEGEN J, DERE E, KETTENMANN H, HARTMANN D, WILLECKE K. Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans. PLoS Genet. 2011;7:e1002146. doi: 10.1371/journal.pgen.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOSKUHL RR, PETERSON RS, SONG B, AO Y, MORALES LB, TIWARIWOODRUFF S, SOFRONIEW MV. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. Journal of Neuroscience. 2009;29:11511–22. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLRAFF A, KOHLING R, HEINEMANN U, THEIS M, WILLECKE K, STEINHAUSER C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. Journal of Neuroscience. 2006;26:5438–47. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSEFF SK, SCHERER SS. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiology of Disease. 2011;42:506–13. doi: 10.1016/j.nbd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO T, OCHALSKI A, HERTZBERG EL, NAGY JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. Journal of Comparative Neurology. 1990;302:853–83. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- ZHAO Y, RIVIECCIO MA, LUTZ S, SCEMES E, BROSNAN CF. The TLR3 ligand polyI: C downregulates connexin 43 expression and function in astrocytes by a mechanism involving the NF-kappaB and PI3 kinase pathways. Glia. 2006;54:775–85. doi: 10.1002/glia.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]