The adaptor proteins AtMIF2 and SlIMA regulate floral meristem termination in Arabidopsis and tomato by joining KNUCKLES, TOPLESS, and a histone deacetylase to repress WUSCHEL gene expression.
Abstract
In angiosperms, the gynoecium is the last structure to develop within the flower due to the determinate fate of floral meristem (FM) stem cells. The maintenance of stem cell activity before its arrest at the stage called FM termination affects the number of carpels that develop. The necessary inhibition at this stage of WUSCHEL (WUS), which is responsible for stem cell maintenance, involves a two-step mechanism. Direct repression mediated by the MADS domain transcription factor AGAMOUS (AG), followed by indirect repression requiring the C2H2 zinc-finger protein KNUCKLES (KNU), allow for the complete termination of floral stem cell activity. Here, we show that Arabidopsis thaliana MINI ZINC FINGER2 (AtMIF2) and its homolog in tomato (Solanum lycopersicum), INHIBITOR OF MERISTEM ACTIVITY (SlIMA), participate in the FM termination process by functioning as adaptor proteins. AtMIF2 and SlIMA recruit AtKNU and SlKNU, respectively, to form a transcriptional repressor complex together with TOPLESS and HISTONE DEACETYLASE19. AtMIF2 and SlIMA bind to the WUS and SlWUS loci in the respective plants, leading to their repression. These results provide important insights into the molecular mechanisms governing (FM) termination and highlight the essential role of AtMIF2/SlIMA during this developmental step, which determines carpel number and therefore fruit size.
INTRODUCTION
The fruit, a specialized organ providing a suitable environment for seed maturation and dispersion, results from the development of the ovary following successful flower pollination and fertilization (Seymour et al., 2013). The ovary, along with the style and stigma, forms single or compound pistils, which constitute the gynoecium. The number of carpels arising from the floral meristem (FM), and consequently the number of fruit locules, is determined during FM termination, a stage at which stem cell activity is arrested (Lenhard et al., 2001).
In Arabidopsis thaliana, the transcription factor WUSCHEL (WUS) specifies the maintenance of stem cell activity in the shoot apical meristem and FM (Mayer et al., 1998). In cooperation with the FM regulator LEAFY, WUS activates the AGAMOUS (AG) gene in stamen and carpel primordia (Lenhard et al., 2001; Lohmann et al., 2001). AG then initiates reproductive development and at the same time antagonizes WUS activity to terminate meristem activity (Lenhard et al., 2001). The complete repression of WUS expression is then essential to promote sharp developmental transitions and regulate the total number of flower organs (Sun et al., 2009). Indeed, the loss of function of WUS results in the production of flowers lacking carpels and most stamens in Arabidopsis, while a gain of function of WUS leads to an increased number in floral organs and in some cases, the reiteration of a flower inside the flower (Xu et al., 2005).
The MADS box transcription factor AG thus plays a central role in the repression of WUS during flower development, according to a two-step process. First, AG directly represses the WUS locus during the early stages of FM termination (stage 3) (Liu et al., 2011). The binding of AG to the WUS locus mediates the recruitment of CURLY LEAF, a core component of the Polycomb Repressive Complex 2 (PRC2), and the subsequent deposition of Histone H3-lysine27 trimethylation (H3K27me3) repressive marks. PRC1 components, namely, TERMINAL FLOWER2/LIKE HETEROCHROMATIN PROTEIN1, then recognize the H3K27me3 marks and promote the compaction of chromatin to ensure the stable repression of WUS. However, this mechanism is insufficient for FM termination. Indeed, the complete arrest of WUS expression at floral stage 6 requires an additional transcription factor belonging to the C2H2 zinc-finger protein family, named KNUCKLES (AtKNU) (Payne et al., 2004; Sun et al., 2009). The expression of AtKNU is directly induced by AG at stages 5 and 6, and a mutation in AtKNU results in an increased number of carpels and stamens due to the prolonged expression of WUS (Sun et al., 2009). Other factors are also required to fine-tune floral stem cell activities (reviewed in Sun and Ito, 2015). However, the mechanism by which AtKNU represses WUS remains unknown, and whether the above mechanisms can be extended to other plant species needs to be investigated.
In tomato (Solanum lycopersicum), nucleotidic polymorphisms in a 15-bp repressor element localized downstream of the SlWUS locus and sharing similarity with the CArG element of Arabidopsis underlie variations in fruit locule number between cultivars (Muños et al., 2011; van der Knaap et al., 2014; Rodríguez-Leal et al., 2017). The increase in fruit locule number associated with this polymorphism results in an approximate 30% yield increase in terms of fruit production. Despite its inferred agronomical interest, the mechanism underlying SlWUS regulation in tomato, and more generally FM termination, is still not well understood. We previously demonstrated that the INHIBITOR OF MERISTEM ACTIVITY (SlIMA) protein, which belongs to the MINI ZINC FINGER (MIF) protein family, is involved in FM termination in tomato by functioning as an indirect repressor of SlWUS (Sicard et al., 2008). To date, the role of AtMIF2, the Arabidopsis homolog of SlIMA, in the FM termination process is unclear.
Despite having a similar organization corresponding to a pistil resulting from the fusion of two carpels, the gynoecium in flowers of Arabidopsis and the wild tomato ancestor (Solanum lycopersicum cv cerasiforme) matures after fertilization into a dry silique and a fleshy berry made of two seed-bearing locules, respectively. Here, to investigate the conservation and/or divergence in the molecular mechanisms regulating FM termination, we used a back-and-forth approach between Arabidopsis and tomato. We demonstrate that AtMIF2 and its tomato ortholog, SlIMA, are activated during flower development by AG and Tomato AGAMOUS1 (TAG1), respectively. AtMIF2 and SlIMA interact with the transcriptional repressors AtKNU and SlKNUCKLES (SlKNU), respectively, allowing for the recruitment of a chromatin remodeling complex at the WUS locus and its subsequent repression. In light of these observations and regarding the specific structure of MIF proteins (Hu et al., 2008), we propose that AtMIF2 and SlIMA act as adaptor proteins between transcriptional regulators and chromatin remodeling proteins to ensure the proper termination of stem cells activity within FM in angiosperm species.
RESULTS
AtMIF2 Is the Arabidopsis Ortholog of SlIMA
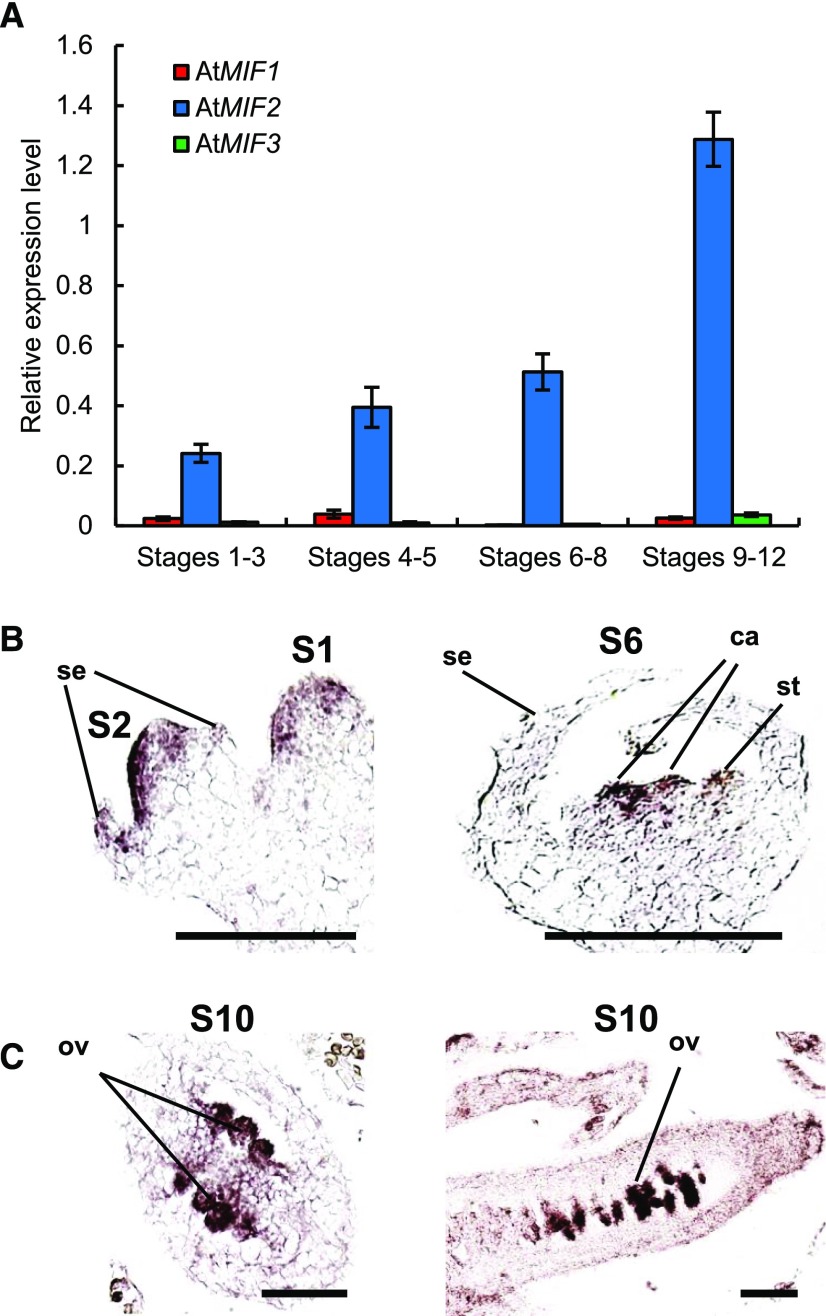
Since SlIMA is a MIF protein involved in the regulation of FM termination in tomato (Sicard et al., 2008), we investigated the putative conservation of this function in Arabidopsis. The MIF protein family in Arabidopsis encompasses three members: AtMIF1, AtMIF2, and AtMIF3 (Hu and Ma, 2006). AtMIF1 and AtMIF3 are mostly expressed in vegetative parts of the plant, whereas AtMIF2 is preferentially expressed in reproductive organs (Arabidopsis eFP browser; Hu and Ma, 2006; Winter et al., 2007). We assessed the expression levels of AtMIF1, AtMIF2, and AtMIF3 in Arabidopsis during floral development using qRT-PCR (Figure 1A). AtMIF2 expression increased gradually during flower development, while AtMIF1 and AtMIF3 expression was barely detectable at all developmental stages examined. This expression pattern suggested that AtMIF2, like SlIMA in tomato (Sicard et al., 2008), is also involved in the regulation of flower development in Arabidopsis.
Figure 1.
Flower-Specific Expression of AtMIF Genes in Arabidopsis.
(A) Expression analysis of AtMIF1, AtMIF2, and AtMIF3 in Col-0 flower buds at various developing stages using qRT-PCR. Error bars represent sd of three biological replicates.
(B) Expression analysis of AtMIF2 by in situ hybridization in Arabidopsis Col-0 developing flower buds at stages 1 and 2 (left panel), and stage 6 (right panel). se, sepal; st, stamen; ca, carpel. Bars = 100 μm.
(C) Expression analysis of AtMIF2 by in situ hybridization in ovary of Arabidopsis Col-0 developing flower buds at stage 10. Transverse section of the ovary (left panel); longitudinal section of the ovary (right panel). ov, ovule. Bars = 100 μm.
AtMIF2 transcripts were detected very early during floral development, from stage 2 until stage 5, in the apical part of the FM and at layers 1 to 3 (Figure 1B). At stage 6, corresponding to the initiation of carpel primordia, the expression signal became more intense within the central cells of the meristem between the two carpel primordia (Figure 1B). Once the formation of the four whorls is initiated, i.e., from stage 6 until the end of floral development, AtMIF2 expression was restricted to the developing ovules in the ovary (Figure 1C). AtMIF2 expression thus appears to be regulated according to a precise pattern during floral development and is mainly associated with early carpel and ovule development.
To examine whether AtMIF2 is involved in the regulation of flower development, we studied the effects of gene silencing. T-DNA insertion mutant lines in AtMIF2 have not yet been found in Arabidopsis, nor have constitutive knockdown approaches (RNAi or antisense lines) succeeded in producing transformed plants with a strong reduction in AtMIF2 transcript level. Since AtMIF2 is strongly expressed during ovule/seed development, we reasoned that a complete loss of function would likely impair the development of transgenic seedlings. Therefore, we decided to reduce AtMIF2 expression specifically during flower development but not during ovule/seed development. To this end, we expressed an artificial microRNA (Schwab et al., 2006; Ossowski et al., 2008) specifically directed against AtMIF2 mRNA under the control of an 800-bp-long sequence from the PISTILLATA promoter (ProPI) (Honma and Goto, 2000). The ProPI:amiRNA-AtMIF2 transgenic lines were thus generated. As a prerequisite, we confirmed that ProPI drives strong GUS reporter gene expression in young flower buds and within the inflorescence meristem, notably at the stage when FM termination occurs, whereas no expression was detected during ovule/seed development (Supplemental Figures 1A to 1D).
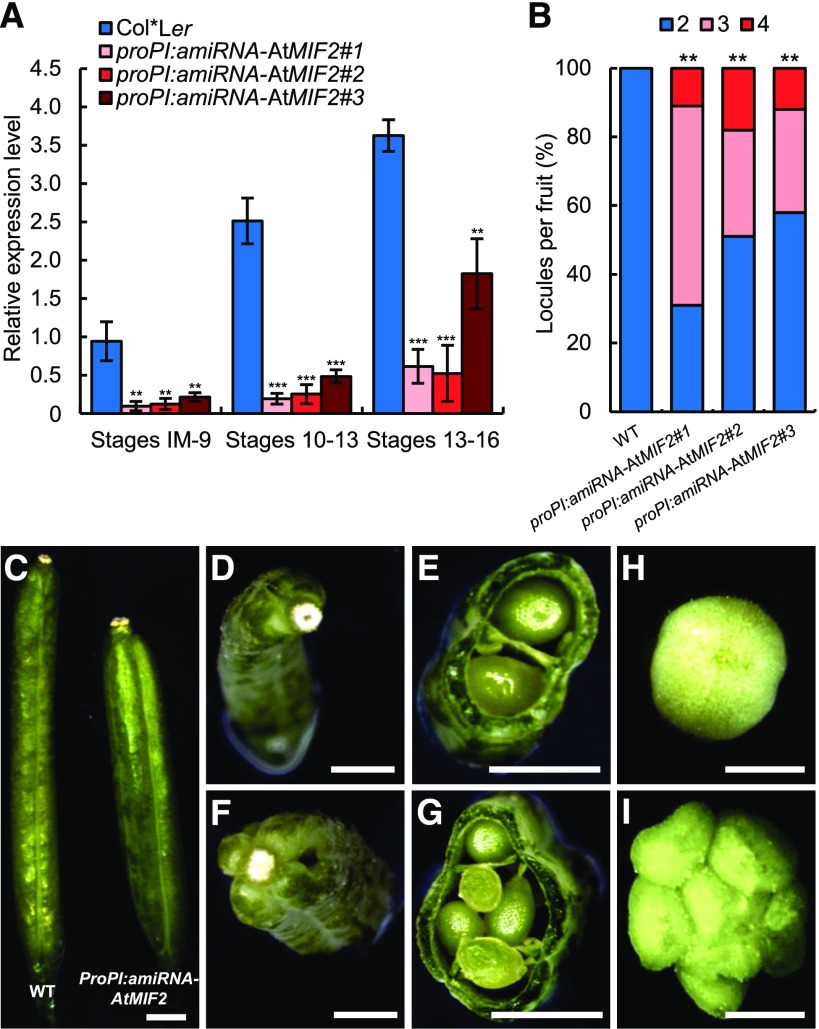
In ProPI:amiRNA-AtMIF2 plants, AtMIF2 expression was strongly reduced compared with wild-type Col-0*Ler plants (∼10-fold before stage 9; ∼19-fold during stages 10–13; ∼4-fold during stages 13–16) (Figure 2A). Flowers from ProPI:amiRNA-AtMIF2 plants displayed an increased number of locules compared with the two locules of the wild type. As a consequence, 30 to 58% and 11 to 20% of developed ProPI:amiRNA-AtMIF2 siliques were trilocular and tetralocular, respectively, containing three and four rows of seeds separated by three or four septa (Figures 2B to 2G). Such an indeterminate phenotype inducing multicarpellar fruit was also obtained in Pro35S:SlIMA-RNAi tomato plants (Figure 2I). In Pro35S:SlIMA-RNAi plants, around 20% of fruits were made of three to eight carpels instead of two in the wild type (Figures 2H and 2I) due to the strong reduction in SlIMA transcript abundance (∼5-fold compared with the wild type) (Supplemental Figure 1E). Taken together, these results indicate that AtMIF2 in Arabidopsis and SlIMA in tomato are involved in determining carpel number.
Figure 2.
Fruit Phenotypes of AtMIF2 and SlIMA Loss-of-Function Plants in Arabidopsis and Tomato, Respectively.
(A) Expression analysis of AtMIF2 in three independent ProPI:amiRNA-AtMIF2 plants compared with Col*Ler wild-type plants at various stages of flower development using qRT-PCR. Error bars represent sd of three biological replicates, and asterisks indicate significant differences from the control (Col*Ler) using two-tailed t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
(B) Percentage of locules per fruit in wild-type and three independent ProPI:amiRNA-AtMIF2 lines (n = 50). **P < 0.01 (Tukey HSD).
(C), (D), and (F) Siliques from wild-type (Col-0*Ler) (left in [C] and [D]) and ProPI:amiRNA-AtMIF2 plants (right in [C] and [F]).
(E) and (G) Cross sections of Col*Ler and ProPI:amiRNA-AtMIF2.
(H) and (I) Young tomato fruits from wild-type (H) and Pro35S:SlIMA-RNAi plants (I).
Bars = 1 mm in (C) and 2 mm in (D) to (I).
Isolation and Characterization of the KNU Ortholog in Tomato
An increase in carpel number was previously described in several Arabidopsis mutants that belong to the WUS repression pathway during FM termination, such as the knu mutant (Payne et al., 2004). The C2H2 ZINC-FINGER protein AtKNU regulates FM termination in Arabidopsis through the repression of WUS (Liu et al., 2011). We next investigated whether the function of KNU is conserved between Arabidopsis and tomato.
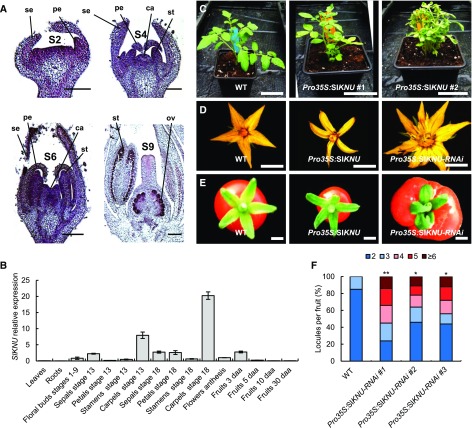
For this purpose, we first searched for putative homologs of AtKNU in the available full tomato genome sequence (Tomato Genome Consortium, 2012). Three distinct homologous sequences were identified: one named Solyc00g014800 at position SL2.50ch00:11060181-11060672, localized on the artificial pseudomolecule composed of scaffolds that could not be placed on either one of the 12 tomato chromosomes defined as chromosome 0 (Tomato Genome Consortium, 2012); one on chromosome 12 at positions SL2.50ch12:51560501-51562100 (annotated C2H2_SL2.50Ch12); and one on chromosome 2 at positions SL2.50ch02:54952316-54952822 (annotated Sl-KNUCKLES) (Supplemental Figures 2A and 2B). These three sequences displayed a low but significant level of identity with AtKNU (27, 27, and 33%, respectively), highlighting the presence of a conserved C2H2 zinc-finger domain (Supplemental Figure 2B). However, the two former sequences did not exhibit a conserved EAR domain, which is one of the characteristics of AtKNU protein (Payne et al., 2004) (Supplemental Figure 2B). Moreover, our own functional analysis of Solyc00g014800, either by overexpression or RNAi silencing, did not lead to any phenotypical alteration in flower development in tomato. Only the third sequence localized on chromosome 2 fit the minimal requirements to be considered a potential AtKNU homolog, i.e., the presence of a C2H2 zinc finger and an EAR domain (Supplemental Figure 2B). In addition, this gene was only expressed during floral and fruit development and more particularly in carpels (Figures 3A and 3B). In young FM, this gene was strongly expressed in the first layers of sepal and petal primordia at stage 2, in all primordia at stage 6, and in ovules and anthers at stage 9 (Figure 3A). Since this expression pattern is consistent with the expression of AtKNU in Arabidopsis, this sequence was then tentatively named SlKNU.
Figure 3.
SlKNU Expression Pattern and Phenotypes of Pro35S:SlKNU and Pro35S:SlKNU-RNAi Tomato Plants.
(A) Expression analysis of SlKNU in developing tomato flower buds analyzed by in situ hybridization. se, sepal; pe, petal; st, stamen; ca, carpel; ov, ovule. Bars = 100 µm (S2 and S4) and 250 μm (S6 and S9).
(B) Expression analysis of SlKNU using qRT-PCR in different tissues and during floral and fruit development. daa, days after anthesis. Error bars represent sd of three biological replicates.
(C) Vegetative phenotypes of wild-type and two Pro35S:SlKNU lines. Bars = 5 cm.
(D) Flowers of wild-type, Pro35S:SlKNU, and Pro35S:SlKNU-RNAi lines. Bars = 5 mm.
(E) Fruits of wild-type, Pro35S:SlKNU, and Pro35S:SlKNU-RNAi lines. Bars = 5 mm.
(F) Number of locules per fruit in wild-type and Pro35S:SlKNU-RNAi lines. Data are presented as percentage of fruits per locule number category (n = 25 fruits). *P < 0.05 and **P < 0.01 (Tukey HSD).
To investigate the function of SlKNU, we generated transgenic tomato with up- or downregulated expression of this gene (Supplemental Figure 2C). The overexpression of SlKNU using a constitutive promoter (Pro35S:SlKNU) was correlated with several phenotypic alterations such as a loss of apical dominance, resulting in a dwarf and bushy phenotype and a reduction in overall flower size (Figures 3C and 3D). Conversely, the Pro35S:SlKNU-RNAi silencing lines did not display any vegetative phenotype but were strongly affected in flower development (Figure 3D, right panel). The number of petals, stamens, and carpels was higher in flowers of the Pro35S:SlKNU-RNAi lines compared with the wild type (Figure 3D). These lines produced multicarpellar fruits (Figure 3E), with up to eight carpels in several fruits in the most extreme line (Figure 3F). Altogether, these results indicate that SlKNU is the likely ortholog of AtKNU.
AtMIF2 and SlIMA Are Members of the Genetic Program Governed by AG/TAG1
We next investigated the transcriptional regulation of AtMIF2 and/or SlIMA to test their involvement within the genetic framework controlling FM termination. First, we used mVISTA software (http://genome.lbl.gov/vista/index.shtml) to perform the alignment of a ∼2.8-kb-long DNA sequence encompassing the 5′ upstream gene and coding sequence of AtMIF2 with the corresponding DNA sequence of the SlIMA locus in order to evaluate the phylogenetic conservation between the two sequences. Within the 2385-bp-long sequence upstream of the translation start codon of AtMIF2 and SlIMA, five conserved DNA regions referred to as domain A to E were identified (Supplemental Figure 3A). We then searched for potential transcription factor binding sites using rVista and MatInspector software (https://www.genomatix.de/matinspector). Based on the phenotypic alterations induced by the silencing of AtMIF2 and SlIMA, we focused on the identification of binding sites for transcription factors involved in carpel identity and FM determination. Putative binding sites for AG and AGAMOUS-LIKE (AGL) proteins were found in the promoter sequences of both AtMIF2 and SlIMA (Supplemental Figure 3B). Among these, AG, AGL1, and AGL3 binding sites, located at around 230 nucleotides upstream of the translation start codon, overlapped with the conserved B domain. A second AG binding site was identified at close proximity to the conserved E domain in both promoter sequences (Supplemental Figure 3B). The sequences of these putative AG binding sites identified in the conserved E (TTCCAAATTAGATA) and B (AACCCTAGATGTC) domains are noncanonical CArG-boxes (Huang et al., 1993) and could be weak binding sites, but their presence, together with the known function of AG and AGL proteins in FM termination and carpel development, suggested that these proteins could regulate SlIMA/AtMIF2 expression during flower development.
To determine whether AG interacts with these sequences in the AtMIF2 promoter, we tested the relevance of these putative AG binding site using electrophoretic mobility shift assay (EMSA) (Figure 4A). First, soluble AG proteins were mixed with an oligonucleotide sequence (Supplemental Data Set 1) used as a positive control probe for AG fixation as previously reported (Riechmann et al., 1996). A mobility shift was observed which could not be obtained when the control probe was mixed in the presence of soluble proteins from cells that did not express AG (used as negative control protein cell extracts) (Figure 4A, left panel). We then tested two oligonucleotide sequences, designated as pAtMIF2-E and pAtMIF2-B (Supplemental Data Set 1), respectively, corresponding to the E and B conserved domains identified as putative AG binding sites (Supplemental Figure 3B). In the presence of AG proteins, a mobility shift with either pAtMIF2-E or pAtMIF2-B probe was obtained, similar to that observed with the positive control probe (Figure 4A, center and right panels), thus indicating that AG can bind to the two pAtMIF2 oligonucleotide sequences examined.
Figure 4.
AtMIF2/SlIMA Expression Is Activated by AG/TAG1.
(A) In vitro binding of AG to the AtMIF2 promoter sequences analyzed by EMSA. The black arrow points to the mobility shift corresponding to AG binding to the probe; the white arrow indicates an unspecific binding of bacterial proteins to the probe. Control probe is a conserved CArG sequence as described by Riechmann et al. (1996). pAtMIF2-E and pAtMIF2-B: sequences from the AtMIF2 promoter (Supplemental Figure 3).
(B) Expression analysis of AtMIF2 in wild-type Ler and ag-3 floral buds at various stages of floral development using qRT-PCR.
(C) In situ hybridization against AtMIF2 mRNA in floral bud of ag-3 plant. Bars = 100 µm.
(D) Expression analysis of SlIMA in wild-type and Pro35S:TAG1 tomato floral buds at various stages of development using qRT-PCR.
(E) GUS staining in fruits of ProSlIMA:GUS and ProSlIMA:GUS Pro35S:TAG1 double-transgenic lines. Bars = 5 mm.
(F) CRISPR/Cas9-induced deletions in the CArG motif (CArG-box) of the SlIMA promoter in three independent T0 plants. The three CR-ProSlIMA plants were homozygous for the deletion. Red font highlights sgRNA targets, and bold indicates protospacer-adjacent motif (PAM) sequences.
(G) Cross sections of fruit from wild-type, CR#1-ProSlIMA, CR#2-ProSlIMA, and CR#3-ProSlIMA lines.
(H) Number of locules per fruit produced by wild-type and CR#ProSlIMA lines. Data are presented as percentage of fruits per locule number category (n = 12 fruits). **P < 0.01 (Tukey HSD).
(I) Expression analysis of SlIMA in wild-type and CR#-ProSlIMA floral buds using qRT-PCR.
(B), (D), (H), and (I) Error bars represent sd of three biological replicates and asterisks indicate significant differences from the control (WT) using two-tailed t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
To evaluate the involvement of AG/TAG1 (Pnueli et al., 1994) in the regulation of AtMIF2/SlIMA expression, we determined the relative abundance of AtMIF2 and SlIMA transcripts in plants with modified expression of AG and TAG1. The expression of AtMIF2 was totally abolished in flower buds of the ag-3 Arabidopsis mutant (Figures 4B and 4C), while that of SlIMA was upregulated in tomato Pro35S:TAG1 plants overexpressing TAG1 (Figure 4D; Supplemental Figure 4). The results of GUS reporter gene assays were in full agreement with these results: When expressed in the Pro35S:TAG1 background, GUS expression driven by the SlIMA promoter (ProSlIMA:GUS) was greatly enhanced in tomato fruits (Figure 4E).
To analyze whether the conserved CArG-box sequence in the SlIMA promoter has any relevance to its expression during flower and fruit development in tomato, we designed two single-guide RNAs (sgRNAs) targeting the region of the putative CArG-box to induce mutations using CRISPR/Cas9 technology. After transformation, we obtained three independent T0 transgenic lines displaying homozygous mutations within the SlIMA promoter, as revealed by sequencing (Figure 4F). Two of these lines were mutated at the putative CArG-box site (CR#1-ProSlIMA and CR#2-ProSlIMA), and the third line was mutated further downstream (CR#3-ProSlIMA). These three lines did not display any vegetative defects. Interestingly, the CR#1-ProSlIMA and CR#2-ProSlIMA lines displayed an alteration in fruit development, with a significantly increased number of locules compared with the wild type (Figures 4G and 4H). The third line, CR#3-ProSlIMA, did not display any alteration in fruit development (Figures 4G and 4H). Using qRT-PCR, we found that SlIMA expression was repressed in CR#1-ProSlIMA and CR#2-ProSlIMA flowers by 5- and 2-fold, respectively, compared with wild-type plants, while SlIMA expression was not significantly affected in CR#3-ProSlIMA (Figure 4I), indicating that the CArG-box is necessary for SlIMA expression in tomato flowers. Altogether, these results suggest that AG and TAG1 regulate AtMIF2 and SlIMA expression, respectively, either directly or indirectly through the activation of other MADS box protein(s) that could act as positive regulators of AtMIF2 and SlIMA expression.
The Misexpression of AtMIF2/SlIMA or AtKNU/SlKNU Affects the Expression Pattern of WUS and SlWUS in Arabidopsis and Tomato
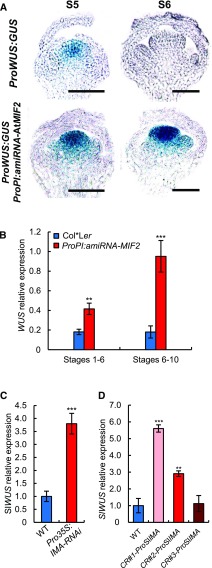
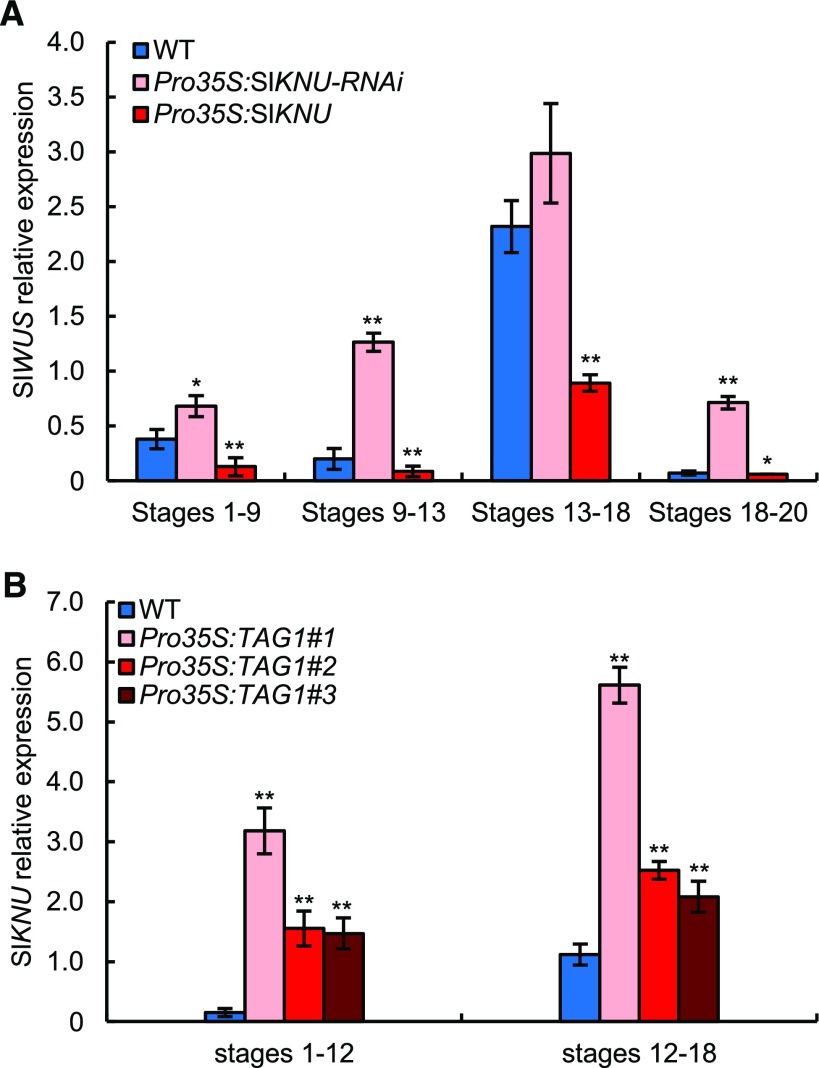
In mutants producing additional carpels, such as clavata, FM termination is often impaired, which is related to the ectopic expression of WUS (Schoof et al., 2000). We therefore investigated the origin of the formation of additional carpels in Arabidopsis ProPI:amiRNA-AtMIF2 and in tomato Pro35S:RNAi-SlIMA silencing lines. This phenotype could be linked to an impairment in FM termination, likely due to ectopic WUS expression. We therefore analyzed the temporal and spatial expression of WUS and SlWUS (Reinhardt et al., 2003) in AtMIF2 and SlIMA silencing plants. In ProWUS:GUS plants (Gross-Hardt et al., 2002), which were used to report the endogenous expression pattern of WUS, WUS was expressed in flower buds at stage 5 within the organizing center of FM harboring the stem cells; at stage 6, its expression totally disappeared in this region (Figure 5A), as previously described (Mayer et al., 1998). WUS expression was expected to start again in later floral developmental stages during anther and ovule development (Gross-Hardt et al., 2002), which could explain the detection of WUS transcripts in these stages using qRT-PCR (Figure 5B). In the ProPI:amiRNA-AtMIF2 lines, WUS was expressed at a level 2-fold higher in early flower buds at stages 1 to 6 and ∼5-fold higher at later stages 6 to 10 compared with the wild type (Figure 5B). These results are fully consistent with the persistence of a strong GUS signal in flower buds in pWUS:GUS reporter lines at stage 6 (Figure 5A). Similar data were obtained in tomato, as SlWUS expression was strongly induced in flower buds of Pro35S:SlIMA-RNAi plants compared with the wild type (Figure 5C; Sicard et al., 2008) but also in the CRISPR CR#1-ProSlIMA and CR#2-ProSlIMA lines (Figure 5D).
Figure 5.
AtMIF2 and SlIMA Repress WUS and SlWUS Expression during FM Termination in Arabidopsis and Tomato, Respectively.
(A) and (B) Expression analysis of WUS in wild-type (Col*Ler) and AtMIF2 loss-of-function (ProPI:amiRNA-AtMIF2 and ProPI:amiRNA-AtMIF2 pWUS:GUS) plants in floral bud at various developmental stages using ProWUS:GUS staining and qRT-PCR. Bars = 100 µm.
(C) and (D) Expression analysis of SlWUS in the wild type, SlIMA silencing line (Pro35S:SlIMA-RNAi), and CR#-ProSlIMA flower buds at stage 1-6 using RT-PCR (C) and qRT-PCR (D).
(B) to (D) Error bars represent sd of three biological replicates and asterisks indicate significant differences from the control (WT) using two-tailed t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
Next, we examined whether SlKNU may play a similar role to AtKNU in the repression of WUS by analyzing SlWUS expression in SlKNU misexpressing tomato plants. In Pro35S:SlKNU plants, the expression of SlWUS was reduced compared with wild-type plants at all stages of flower development examined (Figure 6A). Conversely, the expression of SlWUS was enhanced compared with the wild type in Pro35S:SlKNU-RNAi plants (Figure 6A). In addition, the expression of SlKNU was greatly enhanced as a result of TAG1 overexpression (Figure 6B), indicating that TAG1 positively regulates SlKNU.
Figure 6.
SlKNU Expression Is Promoted by TAG1 to Repress SlWUS in Tomato Floral Bud.
(A) Expression analysis of SlWUS in Pro35S:SlKNU-RNAi and Pro35S:SlKNU plants, compared with wild-type plants, using qRT-PCR.
(B) Expression analysis of SlKNU in three Pro35S:TAG1 overexpressing lines, compared with wild-type plants, using qRT-PCR.
Error bars represent sd of three biological replicates and asterisks indicate significant differences from the control (WT) using two-tailed t test (*P < 0.05 and **P < 0.01).
Therefore, these data show that both SlKNU/AtKNU and SlIMA/AtMIF2 are required to repress WUS expression during FM termination.
AtMIF2 and SlIMA Interact Physically with AtKNU and SlKNU
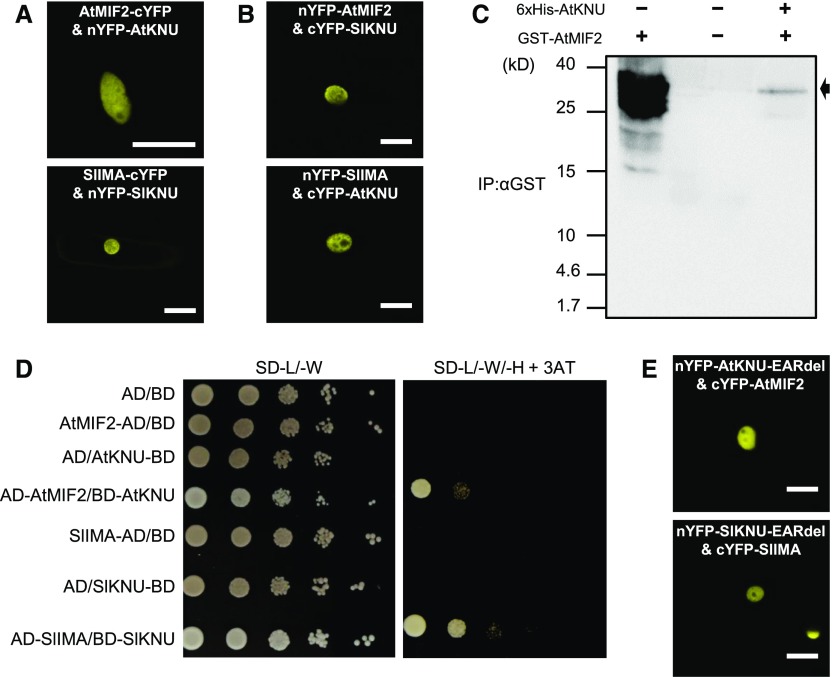
Proteins from the MIF family are characterized by the presence of a unique domain, a noncanonical zinc finger involved in protein-protein interaction (Hu and Ma, 2006; Hong et al., 2011), suggesting that these proteins may act through interaction with transcription factors. Therefore, we assayed the ability of AtMIF2 and SlIMA to interact with AtKNU and SlKNU using bimolecular fluorescence complementation (BiFC) experiments. As revealed by the fluorescent signal resulting from the reconstruction of a functional YFP, AtMIF2 and SlIMA were able to interact with AtKNU and SlKNU (Figures 7A and 7B). The interacting proteins were exclusively localized to the nucleus in this heterologous system, like AtMIF2/SlIMA and AtKNU/SlKNU, which accumulated inside the nucleus in homologous systems (Supplemental Figure 5). We made use of truncated forms of KNU and SlKNU to investigate which part is involved in the KNU-MIF interaction. When the C2H2 zinc-finger domain and its immediate surrounding region were removed, a nucleocytoplasmic localization of the protein was found, whereas when this region was present, the nuclear localization of the protein was observed (Supplemental Figure 6A). Interestingly, SlIMA and AtMIF2 interacted with the truncated proteins SlKNUΔC43-168 and AtKNUΔC39-161, respectively (Supplemental Figure 6B), but not with any of the N-terminal truncated forms, thus suggesting that SlIMA and AtMIF2 interact specifically with the N-terminal part of SlKNU and AtKNU, respectively.
Figure 7.
Interaction Analyses between AtMIF2 and AtKNU and between SlIMA and SlKNU.
(A) BiFC analysis testing the interaction between AtMIF2 and AtKNU (upper panel) and SlIMA and SlKNU (lower panel) in onion epidermal cells. Bars = 25 µm.
(B) BiFC analysis testing the interaction between AtMIF2 and SlKNU (upper panel) and SlIMA and AtKNU (lower panel) in onion epidermal cells. Bars = 50 µm.
(C) In vitro pull-down of GST-AtMIF2 with 6xHis-AtKNU as a bait bound to Ni-NTA resin. GST-AtMIF2 was detected using anti-GST antibody. The black arrow indicates GST-AtMIF2.
(D) Yeast two-hybrid interactions were tested by transforming fusions of either AtMIF2 or SlIMA with the Gal4 activation domain (AD) and fusions of AtKNU or SlKNU to the Gal4 binding domain (BD). Serial dilutions of yeast cells from 105 to 10 on nonselective SD medium lacking leucine and tryptophan (-L/-W) show normal yeast growth. Only positive interactors are able to grow on restrictive growth medium supplemented with 25 mM 3-AT and lacking leucine, tryptophan, and histidine (-L/-W/-H).
(E) BiFC analysis testing the interaction between AtMIF2 and AtKNU-EARdel (upper panel) and SlIMA and SlKNU-EARdel (lower panel) in onion epidermal cells. Bars = 50 µm.
We also investigated this interaction using an in vitro pull-down assay. The purified recombinant bait protein 6xHis-AtKNU was attached to a Ni-NTA column to trap the GST-AtMIF2 prey protein. The GST-AtMIF2 protein was effectively pulled down in the presence of 6xHis-AtKNU (Figure 7C). The interactions between AtMIF2 and AtKNU, as well as SlIMA and SlKNU, were also confirmed in a heterologous system using a yeast two-hybrid assay (Figure 7D). These interactions likely occurred via the C2H2 zinc-finger domain within the N-terminal part of AtKNU and SlKNU, since when the EAR domain was deleted from the C-terminal parts of these proteins, the AtMIF2-AtKNU and SlIMA-SlKNU interactions were conserved (Figure 7E; Supplemental Figure 7). Taken together, these data demonstrate that AtMIF2/SlIMA directly interact with AtKNU/SlKNU both in vitro and in vivo.
AtMIF2/SlIMA Mediate AtKNU/SlKNU and TPL/SlTPL1 Interactions
The presence of an EAR domain at the C terminus of Arabidopsis KNU is an important characteristic in the mediation of transcriptional repression (Kagale and Rozwadowski, 2011). This repressive activity of AtKNU is thought to require the interaction with a transcriptional corepressor such as TOPLESS (TPL), which can indeed interact (although weakly) with AtKNU, as shown in targeted yeast two-hybrid experiments (Causier et al., 2012) but no fluorescence indicating in vivo interaction could be observed using BiFC (Supplemental Figure 8). As proposed by Kagale and Rozwadowski (2011), the presence of an additional protein could be required for the stabilization of the interaction. To investigate whether AtMIF2 could play such a role as an adaptor protein, we investigated the interaction between AtKNU and TPL and between SlKNU and the tomato ortholog of the Arabidopsis TPL protein, SlTPL1 (Hao et al., 2014), in the presence of AtMIF2 or SlIMA, respectively, by BiFC analysis. A BiFC YFP fluorescent signal indicating the formation of an AtKNU-TPL or SlKNU-SlTPL1 complex was solely observed in the presence of AtMIF2 or SlIMA, here fused to RFP as a second reporter protein in the assay (Figure 8A). The BiFC signal was localized in small subnuclear speckles exhibiting RFP signals, suggesting the colocalization of AtMIF2-RFP with the AtKNU/TPL interacting proteins and SlIMA-RFP with SlKNU/SlTPL1.
Figure 8.
AtMIF2/SlIMA Bridges the KNU/SlKNU and TPL/SlTPL1 Interaction and Directly Interacts with HDA19/SlHDA1.
(A) BiFC analysis of the interactions between Arabidopsis KNU and TPL (upper panels) and tomato SlKNU and SlTPL1 (lower panels) in the presence of AtMIF2 and SlIMA, respectively, in onion epidermal cells. Bars = 25 µm.
(B) Yeast three-hybrid assay demonstrating the formation of AtMIF2-KNU-TPL and SlIMA-SlKNU-SlTPL1 trimeric complexes. Serial dilutions of yeast cells from 105 to 10 on nonselective SD medium lacking leucine, tryptophan, and uracil (-L/-W/-U) show normal growth. Only positive interactions were able to grow on restrictive growth medium supplemented with 75 mM 3-AT and lacking leucine, tryptophan, and histidine (-L/-W/-H).
(C) BiFC analysis of the interactions between TPL and HDA19 (left panel) and SlTPL1 and SlHDA1 (right panel) in onion epidermal cells. Bars = 50 µm.
(D) BiFC analysis of the interactions between AtMIF2 and HDA19 (left panel) and SlIMA and SlHDA1 (right panel) in onion epidermal cells. Bars = 50 µm.
We then tested if AtMIF2/SlIMA indeed forms a tripartite complex using a yeast three-hybrid assay. When cotransformed with the empty pYES2 plasmid (pYES), AD-TPL with BD-AtKNU and AD-SlTPL1 with BD-SlKNU were unable to induce yeast growth on selective medium (Figure 8B). However, the growth of yeast colonies was observed in the presence of pYES2 plasmids expressing either AtMIF2 (pYES-AtMIF2) or SlIMA (pYES-SlIMA) (Figure 8B). These results suggest that AtMIF2 or SlIMA likely stabilize the AD-TPL/BD-AtKNU or AD-SlTPL1/BD-SlKNU complexes, allowing for the growth of yeast on selective medium. In addition, the supplementation of 3-aminotriazole (3-AT; 75 mM), a competitive inhibitor of the HIS3 gene product, in the selective medium indicated that the tripartite complexes AD-TPL/BD-AtKNU/AtMIF2 and AD-SlTPL1/BD-SlKNU/SlIMA are stable. These results indicate that AtMIF2/SlIMA act as an adaptor/stabilizer to allow the bridging between TPL/SlTPL1 and the transcription factor AtKNU/SlKNU.
The mechanism by which TPL mediates gene repression was recently studied, revealing its ability to interact with a HISTONE DEACETYLASE (HDA)-like HDA19 protein, suggesting that TPL-mediated regulation of gene expression involves a deacetylation mechanism (Szemenyei et al., 2008; Kagale and Rozwadowski, 2011). BiFC experiments with Arabidopsis TPL and HDA19 proteins or their orthologs in tomato, SlTPL1 and SlHDA1 (Zhao et al., 2015), confirmed this interaction (Figure 8C). We also demonstrated that AtMIF2 and SlIMA were able to interact with HDA19 and SlHDA1, respectively (Figure 8D), arguing for the hypothesis that they function as adaptor proteins, thereby enabling the formation of a chromatin remodeling complex.
AtMIF2/SlIMA Bind to the WUS/SlWUS Locus in a KNU-Dependent Manner
To examine whether AtMIF2/SlIMA binds directly to the WUS/SlWUS locus, we performed chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analyses using transgenic Arabidopsis and tomato lines overexpressing AtMIF2-3HA and SlIMA-YFP, respectively. Commercial antibodies directed against the 3HA- or YFP-protein tag were used to immunoprecipitate AtMIF2- or SlIMA-chromatin complexes from flower buds (a mixture of harvested buds from stages 1–12). Five and eight DNA sequences spread along the WUS locus (Liu et al., 2011) and the SlWUS locus, respectively, were used to identify the chromatin-immunoprecipitated sequences (Figures 9A to 9C; primers listed in Supplemental Data Set 1). There was no apparent enrichment of the genomic fragments in wild-type samples or with control anti-IgG antibodies (Figures 9B to 9D). In contrast, in Arabidopsis, among the five targeted sequences, the P1 and P4 sequences were amplified after chromatin enrichment from AtMIF2-HA expressing floral buds, demonstrating that AtMIF2 is able to bind to this sequence within the WUS locus (Figures 9B to 9D). In tomato, three of the eight targeted sequences (P1, P7, and P8) were amplified in SlIMA-YFP-expressing floral buds (Figure 9C). Notably, these regions are at similar locations in both species (Figure 9A).
Figure 9.
AtMIF2/SlIMA Bind WUS/SlWUS Locus to Repress Their Expression via Histone Deacetylation.
(A) Schematic representation of the WUS and SlWUS loci (5′ and 3′UTR are in gray, introns in white, and exons in black) with the different targeted regions (P1 to P8).
(B) ChIP assay at the WUS locus using HA- or IgG-antibodies in Col-0, knu, Pro35S:AtMIF2-3HA in Col-0, and Pro35S:AtMIF2-3HA in knu plants. The y axis shows enrichment relative to input using IgG as a control. Error bars represent sd of three biological replicates, and asterisks indicate significant differences from the control (Col-0) using two-tailed t test (*P < 0.05 and **P < 0.01).
(C) ChIP assay at the SlWUS locus using GFP or IgG antibodies in wild-type and Pro35S:IMA-YFP plants. The y axis shows relative enrichment to input using IgG as a control. Error bars represent sd of three biological replicates, and asterisks indicate significant differences from the control (Col-0) using two-tailed t test (*P < 0.05 and **P < 0.01).
(D) DamID ratios at different regions of the WUS locus prior (NI) and after 24 h of ethanol induction (I) in lines expressing Arabidopsis MIF2 or KNU fused to DAM (Dam-AtMIF2 and Dam-KNU, respectively).
(E) Expression analysis of WUS in Col-0, Pro35S:AtMIF2, and hda19 floral buds (stages 1–12) treated or not with 0.5 µM TSA, using qRT-PCR. Error bars represent sd of three biological replicates and asterisks indicate significant differences from the control (Col-0) using two-tailed t test (*P < 0.05 and **P < 0.01).
(F) Expression analysis of WUS using GUS staining of ProWUS:GUS Pro35S:AtMIF2 double transgenic plants treated (upper panel) or not (lower panel) with 0.5 µM of TSA. Bars = 10µm.
When AtMIF2-HA was overexpressed in the knu mutant genetic background, qPCR following chromatin immunoprecipitation did not lead to any amplification for the different DNA sequences targeted (Figure 9B), indicating the requirement of AtKNU expression for AtMIF2 binding to the WUS locus.
To confirm the binding of AtMIF2 and AtKNU to the WUS locus, we also made use of DNA adenine methyltransferase identification (DamID) technology (Germann et al., 2006), a technique used to map the binding sites of DNA binding proteins based on the expression of a protein fused with the DNA adenine methyltransferase (Dam) and the detection of adenine methylation using a methylation-specific qPCR protocol (Germann and Gaudin, 2011). DNA was extracted from young inflorescences of Arabidopsis lines expressing AtMIF2 or AtKNU fused to the Dam (Dam-AtMIF2 and Dam-AtKNU, respectively) under an ethanol-inducible promoter or the Dam alone, prior to and after 24 h of ethanol induction (NI and I). Using the same five targeted sequences as those used for ChIP-qPCR experiments as well as another one (P3), qPCR was performed to determine the enrichment of methylation at the different sites indicating the binding of the Dam-fusion protein. P1 and P4 sequences were enriched in both Dam-AtMIF2 and Dam-AtKNU, indicating the binding of AtMIF2-Dm and AtKNU-Dam fusion proteins on these regions of the WUS locus (Figure 9D). Together, these suggest that AtMIF2/SlIMA participate in the direct repression of WUS/SlWUS.
To test the hypothesis that the repression of WUS by an AtKNU-AtMIF2-TPL-HDA complex requires histone deacetylation, we evaluated the effects of trichostatin A (TSA), a specific inhibitor of histone deacetylase activity, on WUS expression in Arabidopsis Col-0 wild-type plants and Pro35S:AtMIF2-overexpressing plants. TSA treatment led to a strong increase in WUS expression in the wild type and (more importantly) partially rescued WUS expression to wild-type levels in AtMIF2 overexpressing plants (Figure 9E), indicating that the repression of WUS requires a deacetylation mechanism putatively involving AtMIF2. The high level of WUS expression in the hda19 mutant strongly supports this hypothesis (Figure 9E). To examine whether this increased expression level is associated with an extended WUS expression pattern, we treated the ProWUS:GUS Pro35S:AtMIF2 reporter line with or without TSA before GUS staining. After TSA treatment, the GUS signal was observed in an enlarged zone of the shoot apical meristem compared with that obtained in TSA-untreated plants (Figure 9F), thus indicating a derepression of WUS in conjunction with the inhibition of HDA activity.
These findings indicate that the repression of WUS mediated by AtMIF2 in Arabidopsis requires the activity of a histone deacetylase such as HDA19, suggesting that AtMIF2 acts as an adaptor protein to form a chromatin remodeling complex to epigenetically repress WUS through histone deacetylation.
DISCUSSION
Floral stem cell termination involves a complex regulatory network that has been extensively described in the model Arabidopsis, but much less is known about the conservation of (or potential differences in) this mechanism in other plant species. Here, combining information from Arabidopsis and tomato, we characterized an actor in this process, AtMIF2/SlIMA, and showed its conserved function in FM termination and carpel number control.
AtMIF2, the Floral Actor of the MIF Family in Arabidopsis, Is Involved in Controlling Locule Number, Like SlIMA in Tomato
The three genes of the MIF family in Arabidopsis were originally described as important actors in hormonal regulation. AtMIF1 and AtMIF3 are expressed in vegetative parts of plants (leaves and root), and AtMIF2 is expressed in stems and inflorescences (Hu and Ma, 2006). Here, we precisely described the expression pattern of AtMIF2, which is expressed during early stages of floral development. The lack of AtMIF2 knockout lines and the observation that knockdown approaches (RNAi or antisense lines) failed to produce transformed plants displaying a strong reduction in AtMIF2 transcript level has hampered the study of the biological function of AtMIF2, as acknowledged by Hu and Ma (2006). The only other MIF gene studied so far is SlIMA in tomato (Sicard et al., 2008), for which partial loss-of-function plants could be obtained using both RNAi and RNA antisense strategies (Sicard et al., 2008), revealing the role of this gene in floral development. Here, using the ProPI:amiRNA-AtMIF2 line, with reduced expression of AtMIF2 during young floral bud development, we demonstrated that AtMIF2, like SlIMA in tomato, functions early in carpel number determination.
AtMIF2 and SlIMA Are Directly or Indirectly Regulated by AG/TAG1 to Repress WUS/SlWUS Expression
Since both the biological functions and expression patterns of AtMIF2 in Arabidopsis and SlIMA in tomato are conserved, we hypothesized that their transcriptional regulation should also be conserved and that conserved regulatory elements could well be identified within the promoters of the two genes. Using in silico analysis, we identified several conserved regulatory boxes in the SlIMA and AtMIF2 promoters corresponding to the noncanonical CArG-box, which is the general target of MADS box proteins (Riechmann et al., 1996). Next, we demonstrated that AG can physically bind to two of these conserved boxes in vitro by EMSA, and we demonstrated that in vivo, mutation in one of these boxes strongly reduced the expression of SlIMA. Moreover, the ectopic expression of TAG1 in tomato led to an increase in SlIMA expression. Altogether, these results demonstrate that AtMIF2 and SlIMA act downstream of AG and TAG1, respectively. Transcription factors such as AG are believed to act in a high order complex in which MADS box domain proteins with specific functions in organ identity determination are recruited through their interaction with more generic MADS box proteins such as SEPALLATA3 (SEP3) (Kaufmann et al., 2009; Pajoro et al., 2014). The genome-wide interaction of SEP3 with DNA has been extensively studied in various MADS box mutant backgrounds, allowing complex, specific downstream targets to be identified (Kaufmann et al., 2009; Pajoro et al., 2014). We therefore surveyed the published data from AG and SEP3 ChIP-seq experiments performed in the wild-type and ag-3 mutant backgrounds. AG ChIP-seq experiments did not reveal any binding site inside the AtMIF2 locus sequence (Ó’Maoiléidigh et al., 2013), but SEP3 binds to the AtMIF2 promoter in an AG-dependent manner (Kaufmann et al., 2009), suggesting that AG itself or its targets promote the binding of the MADS box protein complex to the AtMIF2 promoter.
Increased expression of WUS in Arabidopsis results in increased floral organ number, particularly locule number (Mayer et al., 1998; Schoof et al., 2000), and silencing of SlWUS in tomato leads to a reduction in locule number (Li et al., 2017). We found that in AtMIF2/SlIMA-overexpressing and silencing plants, the expression of WUS/SlWUS was downregulated and upregulated, respectively, suggesting that AtMIF2/SlIMA is involved in determining locule number through the regulation of WUS/SlWUS expression. These findings provide evidence that AtMIF2 and SlIMA as representative members of the MIF protein family, functioning as regulatory elements within the AG-WUS pathway controlling FM termination in both Arabidopsis and tomato. Our results also point to a general genetic network controlling FM termination that is conserved between Arabidopsis and tomato.
The AG-KNU-WUS Pathway Is Conserved in Tomato
Previous studies defined the AG-KNU-WUS pathway as important for regulating floral termination (Sun and Ito, 2015), but the conservation of this pathway in other model plants had been unclear. Here, we identified the functional homolog of AtKNU in tomato, SlKNU, and demonstrated its involvement in repressing SlWUS. We also showed that ectopic expression of TAG1 strongly increased SlKNU expression in tomato, indicating that SlKNU acts downstream of TAG1. These results provide evidence for the conservation of the AG-KNU-WUS pathway in tomato. The similar regulation of AtMIF2/SlIMA and AtKNU/SlKNU, together with the similarity of their partial loss-of-function phenotypes, indicates that SlKNU and SlIMA are required to control organ number during tomato flower development according to a common molecular regulatory mechanism.
AtMIF2/SlIMA May Form a Chromatin Remodeling Complex Including AtKNU/SlKNU, TPL/SlTPL1, and HDA19/SlHDA1 to Repress WUS/SlWUS
Another interesting question raised by our study is how AtMIF2/SlIMA acts to repress WUS/SlWUS. It has been suggested that MIF proteins could act as small interfering peptides/microProteins to inhibit the function of zinc-finger homeodomain (ZHD) proteins through the formation of inactive heterodimers (Hong et al., 2011; Seo et al., 2011; Staudt and Wenkel, 2011; Eguen et al., 2015). Similarly, the C2H2 zinc-finger AtKNU protein harbors an EAR transcriptional repressive motif at its C terminus. As reviewed by Kagale and Rozwadowski (2011), recent discoveries of corepressors interacting with EAR motifs, such as TPL, led to the proposal of a model for EAR motif-mediated transcriptional repression in plants. In this model, EAR repressors suppress the expression of target genes through chromatin modification of regulatory regions by histone deacetylation. TPL, together with HDA19, is thought to form part of a repressor complex that drives the epigenetic regulation of gene expression. However, the exact composition of the repressor complex remains unknown. In addition, the microprotein, miP1a/b, has been described as an adaptor protein that functions between a zinc-finger transcription factor, CONSTANS, and a TPL-related protein (Graeff et al., 2016). Based on these findings, we wondered whether MIF proteins could also act as adaptor proteins in such interactions and evaluated the potential interaction between AtKNU and TPL in the presence of AtMIF2. We demonstrated the required involvement of AtMIF2 and SlIMA as adaptor microproteins engaging AtKNU and SlKNU, respectively, in a ternary complex that involves TPL and HDA19. Our findings confirmed the existence of an adaptor protein that facilitates the association between TPL and its partners. We identified this protein as AtMIF2 in Arabidopsis and SlIMA in tomato. We further demonstrated that AtMIF2/SlIMA bind to two distinct regulatory regions located at −1494 to −1403 and +335 to +489 for AtMIF2, and −1614 to −1424 and +186 to +594 in SlIMA (+1 being the transcription start site), to repress WUS/SlWUS in a KNU-dependent manner. These two binding sites are localized at similar position in the two species, suggesting a conserved regulatory mechanism. Further work is needed to determine whether histone deacetylation on the WUS locus occurs directly or indirectly to reduce its expression during floral termination in Arabidopsis and tomato.
Mini zinc-finger proteins, which constitute conserved components of the floral termination pathway, play key roles in the determination of carpel number, and hence fruit locule number, an important agronomic trait controlling fruit size and yield. Our results provide important insights into the conservation of the molecular regulatory network of FM termination in Arabidopsis and tomato. Based on our data, we propose a model integrating MIF2 in the conserved regulatory pathway of FM termination in Arabidopsis that can be transposed to tomato (Figure 10). A future challenge will be to determine how well conserved the protein interactions and transcriptional regulation revealed in this study are in floral termination events outside of Arabidopsis and tomato. Characterizing such conservation or variation will provide valuable insight into the molecular mechanisms underlying the complex regulation of floral stem cell termination.
Figure 10.
Model Illustrating the Conserved Role of MIF2 in the Regulation of FM Termination.
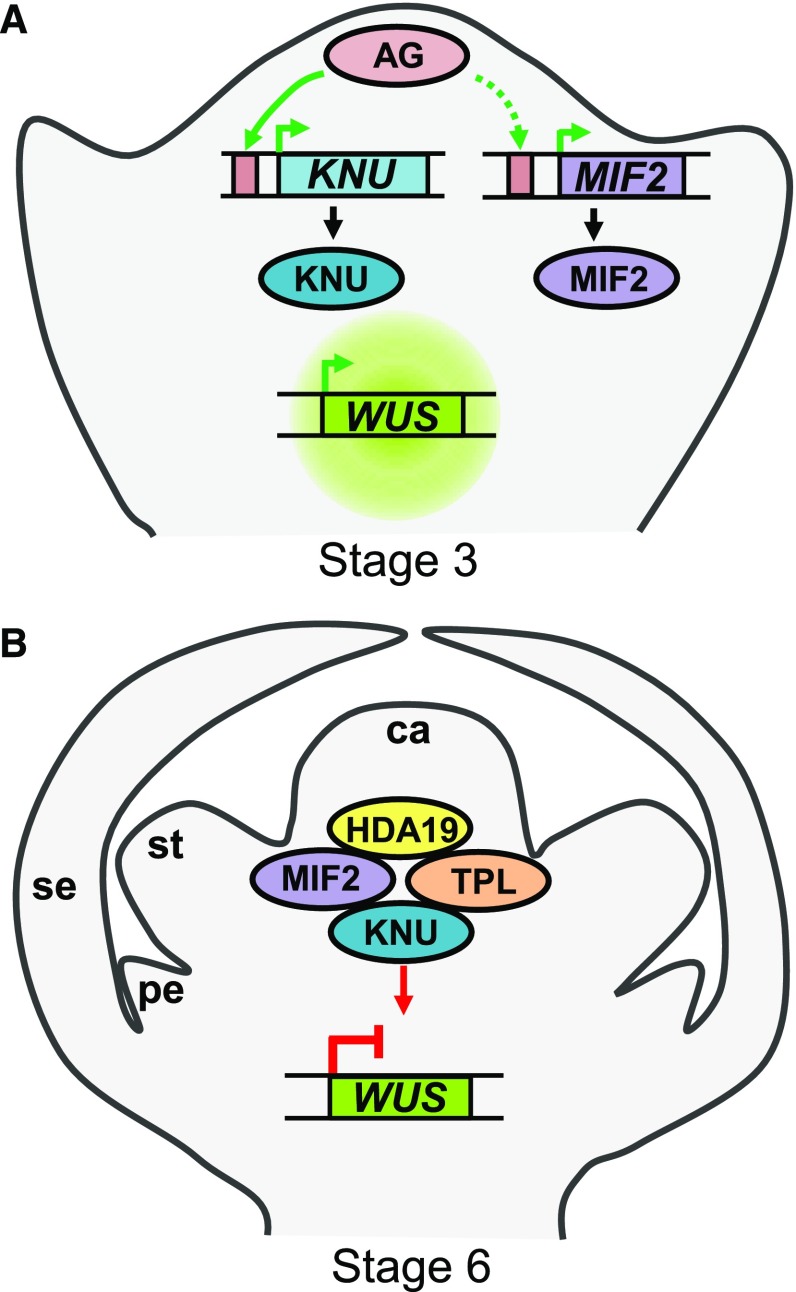
(A) The expression of MIF2 and KNU is activated by AG directly (green arrows) or indirectly (dashed arrow) in floral buds at stage 3.
(B) MIF2 and KNU associate with HDA19 and TPL to form a chromatin remodeling complex that binds to the first intron of the WUS locus, enabling the complete repression of WUS (red arrows) and leading consequently to the termination of stem cell activity at stage 6. se, sepal; pe, petal; st, stamen; ca, carpel.
METHODS
Plant Material and Growth Conditions
Tomato (Solanum lycopersicum cv West Virginia106-Wva106) plants were grown in soil in a greenhouse supplemented with light for a 16-h photoperiod using a set of 100-W warm white LED projectors. The spectrum is constituted by equivalent levels of blue irradiation (range 430–450 nm) and red irradiation (640–660 nm). The irradiance was close to 100 µmol m−2 s−1 at the canopy level.
For transformation, tomato cotyledons were cultivated in vitro in MS medium (Murashige and Skoog, 1962) in a culture chamber under a thermoperiod of 22°C/20°C and a photoperiod of 16 h/8 h (day/night) using white light (Osram L36 W/77 Fluora 1400 Im) resulting in a photon flux density at the stirring plate of 80 to 100 μE m−2 s−1.
The wild-type Arabidopsis thaliana accessions used in this study are Col-0, Landsberg erecta (Ler), and the cross-product Col-0*Ler. The ag-3 mutant (generous gift from R. Sablowski, John Innes Centre, Norwich, UK) was in the Ler background. Seeds were surface sterilized for 15 min in 12.5% (v/v) sodium hypochlorite and 0.02% (v/v) Triton X-100, rinsed at least five times, and sown in Petri dishes containing 0.5× MS growth medium for germination. After cold treatment at 4°C for 2 d in the dark, the plates were incubated in a growth chamber at 22°C with a 16-h-light/8-h-dark cycle. After 10 d of growth, the plantlets were transferred to soil in a growth chamber under the same light regime. For TSA treatments, Arabidopsis plantlets were grown in Petri dishes containing 0.5× MS in the presence of 0.5 µM of TSA (Merck). Fourteen-day-old plantlets were maintained in vitro in the same medium until flowering.
RNA Extraction and qRT-PCR Analysis
Total RNA was isolated from pools of flower buds harvested at different stages using TRIzol reagent (Life Technologies) following the manufacturer’s instructions. RQ1 RNase-free DNase (Promega) treatment was performed for each sample. Reverse transcription was performed using an iScript One-Step RT-PCR Kit for Probes (Bio-Rad). For each tissue sample, three biological replicates and three technical replicates per biological replicate were analyzed using a CFX96 real-time system (Bio-Rad) and GoTaq qPCR Master Mix (Promega). For Figures 1A, 2A, 4B, 4D, 5B, 6A, and Supplemental Figure 2C, each biological replicate was an independent pool of tissues from 10 plants. For Figures 3B, 5C, and Supplemental Figures 1F and 4, each biological replicate was an independent pool of tissues from three plants. For Figures 2B, 4I, 4J, and 6B, each biological replicate corresponded to a pool of tissues from the same plant. The transcript levels of the genes were normalized to that of the housekeeping genes ELONGATION FACTOR1-α (AT5G60390) and TUBULIN β-2/β-3 chain (AT5G62690) in Arabidopsis samples and SlACTIN2 and SlEiF4e in tomato samples. For the qRT-PCR experiments, total RNA was purified from various tomato organs from three plants using an RNeasy Plant Mini Kit (Qiagen). The RT-PCR experiments and analyses were performed as described by Joubès et al. (2001). Data were presented as mean and sd of biological replicates. Supplemental Data Set 1 lists all the primer sequences used in this study.
In Situ Hybridization
In situ hybridizations of mRNA using digoxygenin-UTP-labeled RNA probes were performed as described (Bisbis et al., 2006). To synthesize the riboprobes, specific cDNA fragments were amplified using forward and reverse oligonucleotides for AtMIF2 (At3G28917) and SlKNU (Supplemental Data Set 1). The cDNA fragments were then amplified by nested PCR using the forward or reverse oligonucleotides (Supplemental Data Set 1) to generate the DNA matrix for antisense and sense riboprobe synthesis, respectively. Antisense or sense RNA probes were obtained by transcription using T7 RNA polymerase. To be as quantitative as possible, all samples originating from different Arabidopsis or tomato lines were processed in the same way at the same time and prepared on the same slide for in situ hybridizations.
Production and Purification of Recombinant Protein
To produce AG, AtKNU, and AtMIF2 recombinant proteins, the cDNA fragment encoding each protein was amplified by PCR from cDNA obtained after reverse transcription of RNA extracted from Arabidopsis flowers. Coding sequences were cloned into the pET28a, pET300, and pDEST15 expression vectors to generate the pET28a-AG, pET300-AtKNU, and pDEST15-AtMIF2 constructs, respectively. These constructs were then used to transform Escherichia coli BL-21 (DE3) cells to produce a 6xHis-tagged version of AG and AtKNU and a GST-tagged version of AtMIF2. The production of the recombinant protein AG was induced with 0.5 mM IPTG for 30 min at 20°C. Under these conditions, satisfactory levels of AG were recovered from the soluble protein fraction and subsequently used in EMSA experiments. The production of the recombinant AtMIF2 and AtKNU proteins was induced with 0.1 mM IPTG for 16 h at 20°C. Under these conditions, satisfactory levels of GST-AtMIF2 and 6xHis-AtKNU were recovered from the soluble protein fraction and subsequently used in pull-down experiments. For purification of 6xHis-tagged protein, bacteria were lysed for 30 min in a buffer containing 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 10 mM imidazole, 1 mg mL−1 of lysozyme, and an EDTA-free proteinase inhibitor cocktail (Roche; 11836153001) and sonicated five times during a 15-s period. Soluble proteins were then purified using Protino Ni-NTA Agarose (Macherey-Nagel) according to the manufacturer’s protocol.
EMSA
The double-stranded probes were 5′-end labeled using the T4 polynucleotide kinase (Promega) in the presence of [γ-32P]-ATP according to the manufacturer’s instructions. DNA binding assays and gel electrophoresis were performed as described by Riechmann et al. (1996).
DamID Assay
DamID was performed as described by Germann and Gaudin (2011). Adenine methylation was assayed using a methylation-specific qPCR protocol (Germann and Gaudin, 2011) with DNA extracted from young inflorescences prior to and after 24 h of ethanol induction (I and NI) in lines expressing AtMIF2 or AtKNU fused to Dam (Dam-AtMIF2 and Dam-AtKNU, respectively) and in lines expressing Dam alone. Extracted DNA was digested by DpnII methylation-sensible endonuclease, which cuts only GATCs recognition sites where A is not methylated. The DpnII-digested DNA was diluted before use for qRT-PCR. DamID ratio (DIR) was calculated at each GATC site according to Germann and Gaudin (2011).
CRISPR/Cas9 Gene Editing and Genotyping of the Resulting Mutants
CRISPR/Cas9 mutagenesis was performed as described (Xu et al., 2015). Briefly, constructs were designed to produce defined deletions within each target gene-coding sequence using two sgRNAs alongside the Cas9 endonuclease gene. The sgRNA target sequences were designed using CRISPR-P 2.0 web software (http://crispr.hzau.edu.cn/CRISPR2/; Lei et al., 2014) (see Supplemental Data Set 1 for a list of the sgRNAs used in this study). For genotyping of each first-generation (T0) transgenic line, three different leaf samples were collected, and genomic DNA was extracted using DNAzol (Invitrogen) according to the manufacturer’s instructions. Each plant was genotyped by PCR for the presence of the construct with primers designed to amplify a region spanning the 3′ end of the construct containing the 3′ end of the cas9 sequence and the two sgRNA sequences. The CRISPR/Cas9 T-DNA-positive lines were further genotyped for indel mutations using a forward primer to the left of sgRNA1 and a reverse primer to the right of sgRNA2 (Supplemental Data Set 1). PCR products from selected plants were purified for cloning into the pDONR201 vector using Gateway cloning technology (Invitrogen). Ten clones per PCR product were sequenced. Only homozygous plants in which all sequenced alleles were mutated were phenotyped to ensure that quantification and comparison of locule number were based on effectively null mutants. For quantification of the locule number of the genotyped mutants, we randomly collected ∼15 fruits from each line and counted the number of locules. The data from three different mutants were then pooled to compare with the locule number of wild-type plants grown under the same conditions.
In Vitro Pull-Down Assay and Immunoblot
Approximately 2 µg of purified prey protein (GST-AtMIF2) was added to 6xHis-AtKNU bait protein bound on Protino Ni-NTA agarose beads and incubated for 2 h at 4°C. Six further vigorous washes were performed with NPI-20/wash buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, and 20 mM imidazole), and the next elution was performed using NPI-250/elution buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, and 250 mM imidazole). GST-AtMIF2 pulled-down proteins were resolved by 12% SDS-PAGE and detected by immunoblotting using 1:2000 dilution of mouse monoclonal anti-GST antibody (Santa Cruz Sc-138). A secondary anti-mouse IgG, HRP-linked antibody 7076 (Cell Signaling Technology) was used with a 1:10,000 dilution, and an ECL RevelBlot Intense kit (Ozyme) was used for visualization of membrane-associated peroxidase activity.
Vector Constructs and Plant Transformation
ProPI:amiRNA-AtMIF2 constructs were produced as follows: the 1735-bp ProPI:amiRNA-AtMIF2-t35S sequence (containing 800 bp of the PI promoter, 701 bp of amiRNA, and 222 bp of the Pro35S terminator surrounded by EcoRI and BamHI restriction sites) was synthesized by Eurofins Genomics into a vector. The entire cassette was cloned into the pPZP212 destination vector using EcoRI and BamHI endonucleases.
The IMA RNAi construct was obtained from the IMA cDNA by amplifying a 321-bp fragment corresponding to 103 nucleotides from the 3′ open reading frame followed by 118 nucleotides of the 3′ untranslated region (UTR) using the following oligonucleotides: AAAAAGCAGGCTTGAGATATGTTGAGTGCGAG and AGAAAGCTGGGTCACACCTTATTCACACACAC. The amplified DNA fragment was cloned using the Gateway cloning system (Clontech) as described by Karimi et al. (2002), with pDONR 201 as the entrance vector and pK7GWIWG2(1) as the destination vector.
For CRISPR/Cas9 mutagenesis, constructs were designed to create defined deletions using two sgRNAs. All constructs were assembled using the Golden Gate cloning method (Weber et al., 2011). Level 1 constructs carrying sgRNAs placed under the control of the Arabidopsis U6 promoter were assembled as described (Belhaj et al., 2013). Level 1 construct pICSL11024 (pICH47732:NOSp-NPTII-OCST) was a gift from Jonathan D. Jones (Addgene plasmid 51144). Level 1 construct pICH47742:2x35S-5′UTR-hCas9(STOP)-NOST was a gift from Sophien Kamoun (Addgene plasmid 49771). Level 1 constructs pICH47751:AtU6p:sgRNA1, pICH47761:AtU6p:sgRNA2, and the linker pICH41780 were gifts from Sylvestre Marillonnet (Addgene plasmids 48002, 48003, and 48019, respectively). The level 1 constructs were assembled into the level 2 vector pAGM4723 (gift from Sylvestre Marillonnet; Addgene plasmid 48015) as described (Weber et al., 2011).
For DamID assays, fusions between the AtMIF2 and AtKNU coding sequence and the Dam coding sequence were produced by PCR using the pCMycDam vector (thanks to the Van Steensel lab), and the constructs were cloned into a pDONR entry vector. Next, these sequence were cloned into the pBIN:AlcR vector (provided to us as a generous gift from Valérie Gaudin, Institut Jean-Pierre Bourgin, UMR1318 INRA-AgroParisTech) in order to create an ethanol-inducible version of the fusion proteins.
The different constructs were introduced into Agrobacterium tumefaciens strain GV3101 pMP90 (Koncz and Schell, 1986) using electroporation (2.5 kV, 400 liters). Arabidopsis plants were transformed by the floral dip method (Clough and Bent, 1998). After transformation, seeds were harvested from T0 plants, pooled, and sown on MS medium plates containing kanamycin (50 μg mL−1) or hygromycin (15 μg mL−1), depending on the transformation vector used. T1 transformed plants were selected and grown in a growth chamber until mature seeds were obtained. The agrotransformation of tomato cotyledons was performed as described (Cortina and Culiáñez-Macià, 2004). Plant regeneration, selection, and elimination of agrobacteria were performed on MS medium supplemented with 0.1 mg L−1 of IAA, 1 mg L−1 of 6-BA, 150 mg L−1 of kanamycin, and 300 mg L−1 of mixed ticarcillin and clavulanic acid (DUCHEFA Biochemie). Transformed plants were grown on hormone-free MS medium supplemented with 150 mg L−1 of kanamycin and transferred into the greenhouse.
Yeast Two- and Three-Hybrid Assays
The AtMIF2, SlIMA, TPL, SlTPL1, AtKNU, and SlKNU coding sequences were recombined with the pDEST22 and pDEST32 Gateway vectors for fusion with the AD or BD domains, respectively, at their N termini. The pYES-DEST52 yeast expression vector was used for the expression of AtMIF2 or SlIMA during the yeast three-hybrid assays.
The empty vectors or vectors containing the respective coding sequences were cotransformed into the pJ69-4α yeast strain, and positive colonies were selected on dropout medium without Trp and Leu for yeast two-hybrid analysis or without Trp, Leu, and Ura for yeast three-hybrid analysis. The presence of the plasmids in the strains was verified by PCR, and three positive strains for each transformation were screened on selective medium without Trp, Leu, and His for yeast two-hybrid analysis or without Trp, Leu, Ura, and His for yeast three-hybrid analysis with additional 75 mM 3-AT. For each experiment, the different possible orientations of the protein fusions were tested to assess interaction, and cotransformation with empty plasmid was performed as a negative control.
Colocalization and in Cellulo Protein Interaction Assays
Gateway vectors were used for the fusion with YFP- and RFP-tagged proteins at the N- or C-terminal parts of proteins of interest. Plasmids for the BiFC experiments were kindly provided by Tsuyoshi Nagakawa (Shimane University, Japan). Transient expression assays were performed using a homologous system, namely, leaf epidermis from tomato or Arabidopsis, and onion epidermal cells as a heterologous system. To perform biolistic transformations, slices of onion epidermis were placed on MS medium in Petri dishes. For subsequent bombardment, 2 mg of 1.6-μm gold particles was coated with 5 µg plasmid DNA in the presence of 1 M CaCl2 and 150 mM spermidine. Pelleted gold particles were washed consecutively with 70% and 100% ethanol and resuspended in 10 μL of 100% ethanol before loaded onto macrocarriers for transformation with the particle delivery system using an 1100 p.s.i. (7.58 MPa) rupture disc (PDS-1000He; Bio-Rad). The distance between the macrocarrier and tissue was 6 cm. After gene delivery, onion, tomato, or Arabidopsis epidermal tissues were incubated overnight on MS medium at room temperature in the dark prior to analysis. Each transformation assay was performed in triplicate, and each experiment was replicated at least twice. Concerning the BiFC experiment, all possible orientations of the protein fusions were tested to assess interaction. To test spontaneous YFP reconstitution as a negative control, the N- or C-terminal YFP fragment fused to each protein was coexpressed with the unfused C- or N-terminal YFP fragment and the absence of fluorescent signal was assessed (Kudla and Bock, 2016). Supplemental pictures of the BiFC interactions are presented in Supplemental Figure 7.
Microscopy Techniques and Imaging
Images for protein subcellular localization were obtained using the TCS SP2 AOBS confocal scanning microscope from Leica. YFP and RFP tags were excited and the respective emissions were scanned using a sequential scan setting to prevent overlapping fluorescence signals. All images were processed using Leica Confocal Software and ImageJ software.
For histological observations, tissues were fixed, embedded in paraffin wax, sectioned, and stained as described (Bereterbide et al., 2002).
GUS staining was performed as described (Mudunkothge and Krizek, 2014), observed on paraffin sections under a Zeiss Axioplan microscope, and recorded using a Moticam 3 camera (Motic).
ChIP-qPCR
ChIP assays were performed on young tomato (WVA106 WT and Pro35S:SlIMA-YFP) and Arabidopsis (Col-0, Pro35S:AtMIF2-3HA, and Pro35S:AtMIF2-3HA in knu mutant background) floral buds (stages 1–9) using polyclonal anti-HA (Roche; ref. 11867423001, lot number 14553800), anti-GFP (Abcam; ab290, lot number GR3062151), or control IgG (Millipore; lot number 2896738) antibodies, using a procedure adapted from Jégu et al. (2013). Briefly, after fixation of the plant material in 1% (v/v) formaldehyde, tissues were homogenized; nuclei were then isolated and lysed. Cross-linked chromatin was sonicated in a Bioruptor UCD-200 water bath (Diagenode) using the following parameters: 30-s-on/30-s-off pulses, at high intensity for 60 min. Protein/DNA complexes were immunoprecipitated with antibodies overnight at 4°C with gentle shaking and subsequently incubated for 1 h at 4°C with 50 mL of Dynabeads Protein A (100-02D; Invitrogen). Immunoprecipitated DNA was then recovered using the ChIP DNA Clean and Concentrator (Zymo Research). An aliquot of untreated sonicated chromatin was processed in parallel for each sample and used as total input DNA control. Concerning qPCR, for each tissue sample, three biological replicates, each resulting from the tissue pools from 48 plants for Arabidopsis samples and from five plants for tomato samples, and three technical replicates per biological replicate were analyzed. For each locus, the fold enrichment was calculated by comparing the Ct values of triplicate measurements between immunoprecipitates from transgenic and wild-type plants, relative to the Ct value of the chromatin input control.
Promoter Sequence Analyses
mVISTA software with 70% identity and a sliding window of 20 bases as parameters (Mayor et al., 2000) was used to compare the 2400-bp region encompassing the tomato SlIMA promoter sequence with the 2400-bp upstream region of Arabidopsis AtMIF2 (At3g28917) in order to identify conserved regions. The presence of putative transcription factor binding sites was then analyzed using rVISTA and MatInspector software (Loots and Ovcharenko, 2004; Cartharius et al., 2005).
Multiple Sequence Alignments and Phylogenetic Analysis
Phylogenetic analysis was performed with C2H2 proteins from Arabidopsis and tomato. Multiple sequence alignments were generated via Geneious software (http://www.geneious.com/) (Supplemental File 1) using BLOSUM matrix with default parameter setting (gap cost between 0.1 and 10). A phylogenetic tree was produced with Geneious Tree Builder from 1000 bootstrap replicates by applying the neighbor-joining method with Jukes-Cantor-like genetic distance model. Parameter settings were the following: no gap penalty, no outgroup, random seed of 1000, and support threshold of 10%.
Accession Numbers
All Arabidopsis genes used in this study are referenced in the Arabidopsis Genome Initiative database under the following accession numbers: AG, At4g18960; AtKNU, At5g14010; AtMIF2, At3g28917; AtMIF1, At1g74660; AtMIF3, At1g18835; WUS, At2g17950; TPL, At1g15750; HDA19, At4g38130; ACTIN2 (ACT), At3g18780; and EF1α (EF1), At5g60390. Tomato sequence information can be found in the Sol Genomics Network (http://solgenomics.net; Fernandez-Pozo et al., 2015) under the following IDs: TAG1, Solyc02g071730; SlKNU, Solyc02g094428; SlWUS, Solyc02g083950; SlIMA, Solyc02g087970; SlTPL1, Solyc01g100050; and SlHDA1, Solyc09g091440.
Supplemental Data
Supplemental Figure 1. Expression of the ProPI:GUS reporter gene and floral phenotypes of Arabidopsis ProPI:amiRNA-AtMIF2 lines.
Supplemental Figure 2. SlKNUCKLES phylogenetic and expression analyses of SlKNUCKLES.
Supplemental Figure 3. The SlIMA and AtMIF2 promoters contain highly conserved sequences.
Supplemental Figure 4. Expression analysis of TAG1 in the wild type, ProSlIMA:GUS, and Pro35S:TAG1-overexpressing line using qRT-PCR.
Supplemental Figure 5. Subcellular localization of the proteins studied.
Supplemental Figure 6. Characterization of the interaction domain involved in the interaction between IMA/MIF2 and KNU/SlKNU.
Supplemental Figure 7. Additional BiFC analysis
Supplemental Figure 8. BiFC analysis of the interactions between AtKNU and TPL and between SlKNU and SlTPL1 in the presence of tagRFP in onion epidermal cells.
Supplemental Data Set 1. List of primer sequences used in this study.
Supplemental File 1. Alignment used to produce the phylogenetic tree shown in Supplemental Figure 2.
Acknowledgments
We thank Valérie Gaudin for valuable advice and protocols on DamID. We thank Catherine Perrot-Rechenmann and Thierry Desnos for the pbinSRNACatN plasmid. The microscopy was done in the Bordeaux Imaging Center, Plant Imaging Plateform, UMS 3420, INRA-CNRS-INSERM-University of Bordeaux, member of the national infrastructure France BioImaging. This work was financed by a grant from the Ministère de l’Enseignement Supérieur et de la Recherche (for N.B., PhD).
AUTHOR CONTRIBUTIONS
F.D., N.B., A.S., C.C., and M.H. conceived the project and designed the research. N.B., A.S., J.L., F.D., and M.H. conducted the experiments. A.S. performed EMSA and rVISTA analyses. N.B., F.D., and M.H. produced the transgenic plant material. N.B., D.L., M.B., and C.R. contributed to ChIP studies. N.G., F.G., and M.L. helped analyze the results. All authors discussed the results. N.B., N.G., C.C., M.H., and F.D. wrote the manuscript with input from the other authors.
References
- Belhaj K., Chaparro-Garcia A., Kamoun S., Nekrasov V. (2013). Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereterbide A., Hernould M., Farbos I., Glimelius K., Mouras A. (2002). Restoration of stamen development and production of functional pollen in an alloplasmic CMS tobacco line by ectopic expression of the Arabidopsis thaliana SUPERMAN gene. Plant J. 29: 607–615. [DOI] [PubMed] [Google Scholar]
- Bisbis B., Delmas F., Joubès J., Sicard A., Hernould M., Inzé D., Mouras A., Chevalier C. (2006). Cyclin-dependent kinase (CDK) inhibitors regulate the CDK-cyclin complex activities in endoreduplicating cells of developing tomato fruit. J. Biol. Chem. 281: 7374–7383. [DOI] [PubMed] [Google Scholar]
- Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. (2005). MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21: 2933–2942. [DOI] [PubMed] [Google Scholar]
- Causier B., Ashworth M., Guo W., Davies B. (2012). The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol. 158: 423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cortina C., Culiáñez-Macià F.A. (2004). Tomato transformation and transgenic plant production. Plant Cell Tissue Organ Cult. 76: 269–275. [Google Scholar]
- Eguen T., Straub D., Graeff M., Wenkel S. (2015). MicroProteins: small size-big impact. Trends Plant Sci. 20: 477–482. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pozo N., Menda N., Edwards J.D., Saha S., Tecle I.Y., Strickler S.R., Bombarely A., Fisher-York T., Pujar A., Foerster H., Yan A., Mueller L.A. (2015). The Sol Genomics Network (SGN)--from genotype to phenotype to breeding. Nucleic Acids Res. 43: D1036–D1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann S., Gaudin V. (2011). Mapping in vivo protein-DNA interactions in plants by DamID, a DNA adenine methylation-based method. Methods Mol. Biol. 754: 307–321. [DOI] [PubMed] [Google Scholar]
- Germann S., Juul-Jensen T., Letarnec B., Gaudin V. (2006). DamID, a new tool for studying plant chromatin profiling in vivo, and its use to identify putative LHP1 target loci. Plant J. 48: 153–163. [DOI] [PubMed] [Google Scholar]
- Graeff M., Straub D., Eguen T., Dolde U., Rodrigues V., Brandt R., Wenkel S. (2016). MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet. 12: e1005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Hardt R., Lenhard M., Laux T. (2002). WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 16: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Wang X., Li X., Bassa C., Mila I., Audran C., Maza E., Li Z., Bouzayen M., van der Rest B., Zouine M. (2014). Genome-wide identification, phylogenetic analysis, expression profiling, and protein-protein interaction properties of TOPLESS gene family members in tomato. J. Exp. Bot. 65: 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.Y., Kim O.K., Kim S.G., Yang M.S., Park C.M. (2011). Nuclear import and DNA binding of the ZHD5 transcription factor is modulated by a competitive peptide inhibitor in Arabidopsis. J. Biol. Chem. 286: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma T., Goto K. (2000). The Arabidopsis floral homeotic gene PISTILLATA is regulated by discrete cis-elements responsive to induction and maintenance signals. Development 127: 2021–2030. [DOI] [PubMed] [Google Scholar]
- Hu W., dePamphilis C.W., Ma H. (2008). Phylogenetic analysis of the plant-specific zinc finger-homeobox and mini zinc finger gene families. J. Integr. Plant Biol. 50: 1031–1045. [DOI] [PubMed] [Google Scholar]
- Hu W., Ma H. (2006). Characterization of a novel putative zinc finger gene MIF1: involvement in multiple hormonal regulation of Arabidopsis development. Plant J. 45: 399–422. [DOI] [PubMed] [Google Scholar]
- Huang H., Mizukami Y., Hu Y., Ma H. (1993). Isolation and characterization of the binding sequences for the product of the Arabidopsis floral homeotic gene AGAMOUS. Nucleic Acids Res. 21: 4769–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégu T., et al. (2013). Multiple functions of Kip-related protein5 connect endoreduplication and cell elongation. Plant Physiol. 161: 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubès J., Lemaire-Chamley M., Delmas F., Walter J., Hernould M., Mouras A., Raymond P., Chevalier C. (2001). A new C-type cyclin-dependent kinase from tomato expressed in dividing tissues does not interact with mitotic and G1 cyclins. Plant Physiol. 126: 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S., Rozwadowski K. (2011). EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193–195. [DOI] [PubMed] [Google Scholar]
- Kaufmann K., Muiño J.M., Jauregui R., Airoldi C.A., Smaczniak C., Krajewski P., Angenent G.C. (2009). Target genes of the MADS transcription factor sepallata3: Integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 7: e1000090. [DOI] [PMC free article] [PubMed]
- Koncz C., Schell J. (1986). The promoter of T L-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204: 383–396. [Google Scholar]
- Kudla J., Bock R. (2016). Lighting the way to protein-protein interactions: Recommendations on best practices for bimolecular fluorescence complementation analyses. Plant Cell 28: 1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Lu L., Liu H.Y., Li S., Xing F., Chen L.L. (2014). CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 7: 1494–1496. [DOI] [PubMed] [Google Scholar]
- Lenhard M., Bohnert A., Jürgens G., Laux T. (2001). Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105: 805–814. [DOI] [PubMed] [Google Scholar]
- Li H., Qi M., Sun M., Liu Y., Liu Y., Xu T., Li Y., Li T. (2017). Tomato transcription factor SlWUS plays an important role in tomato flower and locule development. Front. Plant Sci. 8: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kim Y.J., Müller R., Yumul R.E., Liu C., Pan Y., Cao X., Goodrich J., Chen X. (2011). AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. Plant Cell 23: 3654–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann J.U., Hong R.L., Hobe M., Busch M.A., Parcy F., Simon R., Weigel D. (2001). A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105: 793–803. [DOI] [PubMed] [Google Scholar]
- Loots G.G., Ovcharenko I. (2004). rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 32: W217–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Mayor C., Brudno M., Schwartz J.R., Poliakov A., Rubin E.M., Frazer K.A., Pachter L.S., Dubchak I. (2000). VISTA : visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 16: 1046–1047. [DOI] [PubMed] [Google Scholar]
- Mudunkothge J.S., Krizek B.A. (2014). The GUS reporter system in flower development studies. In Methods in Molecular Biology (Methods and Protocols), Vol. 1110, J. Riechmann and F. Wellmer, eds (New York: Humana Press), pp. 295–304. [DOI] [PubMed]
- Muños S., Ranc N., Botton E., Bérard A., Rolland S., Duffé P., Carretero Y., Le Paslier M.C., Delalande C., Bouzayen M., Brunel D., Causse M. (2011). Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 156: 2244–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Ó’Maoiléidigh D.S., Wuest S.E., Rae L., Raganelli A., Ryan P.T., Kwasniewska K., Das P., Lohan A.J., Loftus B., Graciet E., Wellmer F. (2013). Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell 25: 2482–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S., Schwab R., Weigel D. (2008). Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 53: 674–690. [DOI] [PubMed] [Google Scholar]
- Pajoro A., et al. (2014). Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 15: R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne T., Johnson S.D., Koltunow A.M. (2004). KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development 131: 3737–3749. [DOI] [PubMed] [Google Scholar]
- Pnueli L., Hareven D., Rounsley S.D., Yanofsky M.F., Lifschitz E. (1994). Isolation of the tomato AGAMOUS gene TAG1 and analysis of its homeotic role in transgenic plants. Plant Cell 6: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D., Frenz M., Mandel T., Kuhlemeier C. (2003). Microsurgical and laser ablation analysis of interactions between the zones and layers of the tomato shoot apical meristem. Development 130: 4073–4083. [DOI] [PubMed] [Google Scholar]
- Riechmann J.L., Wang M., Meyerowitz E.M. (1996). DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Nucleic Acids Res. 24: 3134–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Leal D., Lemmon Z.H., Man J., Bartlett M.E., Lippman Z.B. (2017). Engineering quantitative trait variation for crop improvement by genome editing. Cell 171: 470–480.e8. [DOI] [PubMed] [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K.F., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo P.J., Hong S.Y., Kim S.G., Park C.M. (2011). Competitive inhibition of transcription factors by small interfering peptides. Trends Plant Sci. 16: 541–549. [DOI] [PubMed] [Google Scholar]
- Seymour G.B., Østergaard L., Chapman N.H., Knapp S., Martin C. (2013). Fruit development and ripening. Annu. Rev. Plant Biol. 64: 219–241. [DOI] [PubMed] [Google Scholar]
- Sicard A., Hernould M., Chevalier C. (2008). The INHIBITOR OF MERISTEM ACTIVITY (IMA) protein: The nexus between cell division, differentiation and hormonal control of development. Plant Signal. Behav. 3: 908–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt A.-C., Wenkel S. (2011). Regulation of protein function by ‘microProteins’. EMBO Rep. 12: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Xu Y., Ng K.H., Ito T. (2009). A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 23: 1791–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Ito T. (2015). Regulation of floral stem cell termination in Arabidopsis. Front. Plant Sci. 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei H., Hannon M., Long J.A. (2008). TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. [DOI] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012). The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E., Chakrabarti M., Chu Y.H., Clevenger J.P., Illa-Berenguer E., Huang Z., Keyhaninejad N., Mu Q., Sun L., Wang Y., Wu S. (2014). What lies beyond the eye: the molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 5: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Engler C., Gruetzner R., Werner S., Marillonnet S. (2011). A modular cloning system for standardized assembly of multigene constructs. PLoS One 6: e16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G.V., Provart N.J. (2007). An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., et al. (2015). A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 47: 784–792. [DOI] [PubMed] [Google Scholar]
- Xu Y.Y., Wang X.M., Li J., Li J.H., Wu J.S., Walker J.C., Xu Z.H., Chong K. (2005). Activation of the WUS gene induces ectopic initiation of floral meristems on mature stem surface in Arabidopsis thaliana. Plant Mol. Biol. 57: 773–784. [DOI] [PubMed] [Google Scholar]
- Zhao L., Lu J., Zhang J., Wu P.-Y., Yang S., Wu K. (2015). Identification and characterization of histone deacetylases in tomato (Solanum lycopersicum). Front. Plant Sci. 5: 760. [DOI] [PMC free article] [PubMed] [Google Scholar]