A large effort was made to systemically test, identify, and study genetic interactions between regulatory genes for a plant defense metabolism network controlling fitness.
Abstract
Plants use diverse mechanisms influenced by vast regulatory networks of indefinite scale to adapt to their environment. These regulatory networks have an unknown potential for epistasis between genes within and across networks. To test for epistasis within an adaptive trait genetic network, we generated and tested 47 Arabidopsis thaliana double mutant combinations for 20 transcription factors, which all influence the accumulation of aliphatic glucosinolates, the defense metabolites that control fitness. The epistatic combinations were used to test if there is more or less epistasis depending on gene membership within the same or different phenotypic subnetworks. Extensive epistasis was observed between the transcription factors, regardless of subnetwork membership. Metabolite accumulation displayed antagonistic epistasis, suggesting the presence of a buffering mechanism. Epistasis affecting enzymatic estimated activity was highly conditional on the tissue and environment and shifted between both antagonistic and synergistic forms. Transcriptional analysis showed that epistasis shifts depend on how the trait is measured. Because the 47 combinations described here represent a small sampling of the potential epistatic combinations in this genetic network, there is potential for significantly more epistasis. Additionally, the main effect of the individual gene was not predictive of the epistatic effects, suggesting that there is a need for further studies.
INTRODUCTION
To adapt and maximize fitness, plants perceive and respond to a myriad of signals that in combination provide an image of the environment. These signals can arise from the biotic environment, including bacteria, fungi, insects, and other plants, plus stimuli from the abiotic environment, including light, temperature, water, and nutrient availability (Goldwasser et al., 2002; Shinozaki et al., 2003; Jones and Dangl, 2006; Howe and Jander, 2008; Vidal and Gutiérrez, 2008; Harmer, 2009; Chory, 2010; Mengiste, 2012; Xuan et al., 2017). Critically, each specific signal is typically perceived by a separate mechanism that stimulates a downstream regulatory network involving at least tens of genes (Li et al., 2006; Hickman et al., 2017). The current models often suggest that these genetic regulatory networks coalesce around master regulators that are the central controllers for specific pathways and/or phenotypes (Gu et al., 2004; Kazan and Manners, 2013). Often these master regulators are transcription factors (TFs) that are both necessary and sufficient for the changes in expression of genes or pathways that modulate the growth, defense, and metabolic phenotype of the plant to adapt to that specific environment. We call this the master regulator hypothesis. This concept is predominant within developmental regulatory networks that often exhibit switch-like behavior, shifting from one state to another. It is not clear how this concept may translate to metabolic pathways that may instead display a rheostat behavior, where there is a continuous adjustment in response to external and internal stimuli. However, in spite of the advanced knowledge about specific regulatory networks in plants, the exact size and interconnected structure of these genetic networks is a key unanswered question in systems biology (Phillips, 2008). The size of networks is of critical importance for adaptive traits because as genetic networks increase in size and interconnectivity, the concept of a single master regulator at the beginning point of a specific regulatory network is less essential. Additionally, as gene membership increases, there is a concurrent increase in the potential for epistasis between these genes (Mackay, 2014; Gaudinier et al., 2015). In this context, we are defining epistasis as any nonadditive interaction between genotypes at two or more loci influencing a trait. Thus, there is a need to understand how large regulatory networks may be influenced by epistasis, especially for adaptive metabolic traits.
One set of adaptive traits that could be used to study these questions of network scale and epistasis are plant secondary metabolites (Wink, 1988; Burow et al., 2010; Kroymann, 2011). Recent work has shown that plant secondary metabolites have strong epistatic interactions that can influence fitness in the field (Brachi et al., 2015; Kerwin et al., 2015, 2017). Additionally, mechanistic and quantitative genetic studies are showing that plant defense metabolites have vast genetic regulatory networks (Chan et al., 2010, 2011; Harper et al., 2012; Riedelsheimer et al., 2012; Wurschum et al., 2013; Wen et al., 2016). These studies provide an alternative hypothesis where regulation occurs via a promoter integration model. In this model, the pathway is controlled by suites of TFs that interact with distinct subsets of promoters within a metabolic pathway. This promoter integration model leads to a greatly extended gene network influencing a metabolic pathway and allows for potentially increased precision in the regulation of metabolic pathways. Furthermore, this raises the potential for there to be different types of epistasis across a pathway, depending upon the promoter/gene that influences that part of the pathway. For example, if two TFs bind different promoters within a pathway without interacting molecularly, they have the potential to show nonadditive epistasis at the metabolite level, as they are influencing multiple enzymatic reactions within the pathway. In the absence of metabolite-triggered transcriptional feedback, this metabolic epistasis might not be mirrored at the transcript level, which may display an additive model. Thus, metabolic pathways where it is possible to measure different outputs from a single pathway can enable the dissection of genetic networks and epistatic interactions and how they compare at both the metabolic and transcriptional levels.
In this study, we used the aliphatic glucosinolate (GLS) pathway to test the extent of epistasis within an adaptive regulatory network. GLSs are becoming a model system for the study of plant adaption to ever-changing environments (Hopkins et al., 2009; Kliebenstein, 2009; Kroymann, 2011). Aliphatic GLSs are derived from methionine, and genetic variation influencing aliphatic GLS composition is a key mechanism used by plants to adapt to their ecological niches (Lankau and Kliebenstein, 2009; Burow et al., 2010; Züst et al., 2012). Furthermore, the almost complete elucidation of the methionine-derived aliphatic biosynthesis pathway in the model plant Arabidopsis thaliana has provided a unique system to test systems biology concepts (Sønderby et al., 2010a). Combining the full catalog of biosynthetic genes with large-scale systems biology approaches has allowed a rapid characterization of the regulatory networks controlling this pathway to address plants’ defense and survival challenges in connected regulatory networks. Previous studies identified and confirmed the critical importance of transcriptional regulation of the GLS pathway, including the cloning of TF genes MYB28, MYB29, and MYB76, which regulate the accumulation of aliphatic GLS (Gigolashvili et al., 2007, 2008; Hirai et al., 2007; Sønderby et al., 2007; Malitsky et al., 2008; Sønderby et al., 2010c). More recently, TFs in the jasmonate signaling pathway, MYC2, MYC3, and MYC4, were shown to be important regulators of both aliphatic and indolic GLS (Dombrecht et al., 2007; Fernández-Calvo et al., 2011; Schweizer et al., 2013). These key MYB and MYC regulators of GLS pathways are positive regulators and belong to evolutionarily conserved subsets of their corresponding families (Stracke et al., 2001; Fernández-Calvo et al., 2011). Intriguingly, while mutants of these proposed master regulators abolish the accumulation of the GLS metabolites, they only abolish the expression of a few key genes in the biosynthetic pathway and do not affect the expression of other genes in the biosynthetic pathway (Dombrecht et al., 2007; Sønderby et al., 2010b). As such, they do not fit the classical definition of a master transcriptional regulator and suggest the necessary involvement of other TFs. A yeast one-hybrid approach identified numerous additional TFs that bound to promoters of genes involved in GLS biosynthesis and influenced the accumulation of aliphatic GLS, including new TF families with diverse functions (Li et al., 2014a). These new TFs are predominantly negative regulators with pathway-specific GLS effects, allowing them to be clustered into distinct phenotypic modules. How these new TFs interact with each other either within or between phenotypic modules and how they interact with the key aliphatic GLS MYBs to structure the epistatic regulatory network remain to be determined.
Previous work in yeast has shown that the modularity of genes influencing a phenotype, primary metabolism, could be used to predict the presence of epistasis. Specifically, epistatic interactions were predominantly found when studying double mutants involving genes associated with different phenotypic clusters or modules, while genes within the same cluster rarely displayed epistasis (Segrè et al., 2005). This was hypothesized to be caused by genes within a cluster having more redundancy than genes between clusters. This observation was also noted in global studies investigating pairwise interactions among all yeast genes, showing that negative/antagonistic epistasis was predominantly found among genes in the same complex/bioprocess (Costanzo et al., 2010, 2016). In contrast, the molecular underpinnings of positive/synergistic epistasis were less decipherable. These yeast studies focused on growth as the trait, raising the question of how epistasis transitions from gene expression to enzyme to metabolite to fitness. Because we have a large collection of TFs influencing a defense metabolite class, aliphatic GLSs, within Arabidopsis and these TFs cluster into specific groups based on their phenotypic effects on the metabolite accumulation, we can now test if network architecture influences epistasis in adaptive plant defense metabolism, as observed for the yeast genome. The central hypothesis tested is that TFs acting in different phenotypic clusters or modules are more likely to display epistasis than those within a phenotypic cluster or module.
In this study, to explore the potential for epistasis in regulatory networks of the plant secondary metabolite GLSs, we focused on the well-established and highly variable aliphatic GLS pathway by systematically constructing 47 double mutants and 4 triple mutants using 20 selected TFs controlling aliphatic GLS accumulation. These TFs include two key TF regulators, MYB28 and MYB29, and 18 additional TFs that were previously ascribed to specific phenotypic clusters depending upon their single mutant GLS phenotype (Figure 1) (Li et al., 2014a). The 24 GLS phenotypes of the 47 double mutants and 4 triple mutants were systematically explored in 4 contrasting tissue and treatment combinations (Supplemental Data Set 1). Epistatic networks differ for the major aliphatic GLS phenotypic clusters. The absence of aliphatic GLS in myb28 myb29 could not be revived by the tested repressor TFs in triple mutants, even though the expression of biosynthesis genes of aliphatic GLS could be modulated by these repressor TFs, as predicted. Our findings provide insights into epistatic networks, contribute new genetic resources to the community, and elicit research questions on the regulatory networks of the model plant secondary metabolites, GLSs.
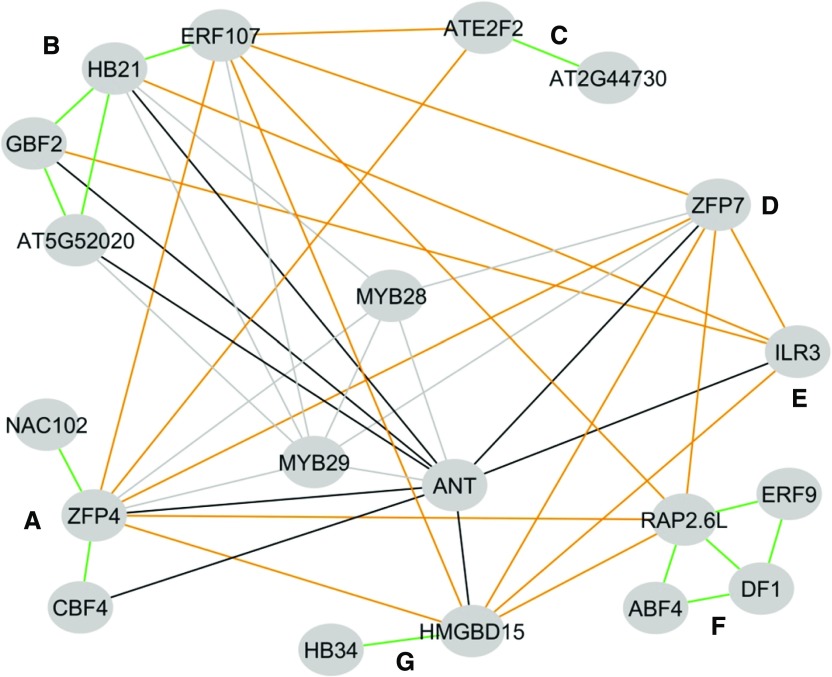
Figure 1.
Genetic Networks under Investigation.
The 20 TFs under investigation are shown as nodes. Lines connecting TFs show where double mutants were generated. The labeled clusters A to G represent the previously identified phenotypic subnetworks for these TFs. Large-effect TFs that were not linked to specific clusters are shown in the centers of the networks as MYB28, MYB29, and ANT. The four different epistatic groups are highlighted with different colors: within-cluster epistatic tests are shown in lime green, between-cluster epistatic tests are shown in dark orange, MYB epistatic tests are shown in gray, and ANT epistatic tests are shown in black.
RESULTS
Selection and Construction of Epistatic Networks
To test our hypothesis, we systematically generated 47 double mutants and 4 triple mutants using 20 representative TFs in Arabidopsis (Figure 1; Supplemental Data Set 2). All of these T-DNA mutants exhibited altered GLS accumulation in single mutants (Li et al., 2014a) (Supplemental Data Set 2). These TFs had previously been grouped based on their mutants’ phenotypic effect on the accumulation of all aliphatic GLS metabolites across multiple tissues and environments. These selected TFs belong to diverse TF families with diverse gene functions, including AINTEGUMENTA (ANT) of the AP2/EREBP family, which controls cell proliferation (Elliott et al., 1996; Krizek et al., 2000; Liu et al., 2000; Mizukami and Fischer, 2000; Horstman et al., 2014), IAA-LEUCINE RESISTANT3 (ILR3) of the bHLH family, which regulates iron deficiency (Rampey et al., 2006; Long et al., 2010), G-BOX BINDING FACTOR2 (GBF2) of the bZIP family, which regulates response to blue light (Schindler et al., 1992; Menkens and Cashmore, 1994; Terzaghi et al., 1997), HMGBD15 of the ARID family, which regulates pollen tube growth (Xia et al., 2014), HOMEOBOX PROTEIN21 (HB21) of the ZF-HD family, which regulates abscisic acid-activated signaling (González-Grandío et al., 2017), NAC102 of NAC, which regulates responses to low oxygen stress (hypoxia) in germinating seedlings (Christianson et al., 2009), and ATE2F2 of E2F/DP, which controls the balance between cell division and endoreduplication and xylem cell development (del Pozo et al., 2006; Berckmans et al., 2011; Taylor-Teeples et al., 2015). The 47 double mutants represent 4 different epistatic test sets: (1) within cluster epistasis, 13 double mutants were obtained by crossing mutants in TFs that were within the same phenotypic cluster; (2) between cluster epistasis, 16 double mutants were obtained by crossing mutants in TFs that were in different clusters; (3) MYB epistasis, 10 double mutants obtained by crossing the new TFs to myb28 or myb29; and (4) ANT epistasis, 8 double mutants obtained by crossing new TFs to ant. The double mutants with the known MYBs and strong-effect TF ANT were included to assess how these new TFs may interact with described master regulators of glucosinolate biosynthesis. We also generated four additional triple mutants to test the consequence of adding mutations in a repressor TF to the double mutant myb28 myb29 that abolishes GLS accumulation, including myb28 myb29 ant, myb28 myb29 zfp4, myb28 myb29 zfp7, and myb28 myb29 hb21. All of the mutants were validated as being homozygous for the specific genotype and grown concurrently with the wild-type and single mutant genotypes to age match all seed stocks.
Epistatic Networks Mediating GLS Traits
To test the genotypes for epistasis, we measured leaf and seed GLS contents for the wild-type control, all single mutants, and all double and triple mutants in two different chambers using a randomized complete block design, as previously described (Li et al., 2014a). The two growth chambers have a controlled abiotic environment but contain different biotic environments. The CEF (controlled environment facility) chamber is maintained as pest free, while the LSA (life sciences addition) chamber has an endogenous pest population provided by continuous propagation of tomato (Solanum lycopersicum) and Brassica napus plants. This generates a mix of mites, aphids, flea beetles, and fungus gnats in the LSA chamber and allows us to test for the effect of a blend of biotic interactions rather than specific biotic interactions. Measuring GLS in two tissues and two environments has previously enhanced our ability to identify significant effects and test if they are tissue or environmentally sensitive (Li et al., 2014a). We then used all measured phenotypes with linear models to specifically test for epistatic interactions (Supplemental Data Sets 3 to 5). For the ensuing analysis, we focused on five summary variables that describe the majority of the variance in aliphatic GLS (Wentzell et al., 2007). These summary GLS traits are as follows: (1) accumulation of short-chain GLS (SC GLS), the sum of the three-carbon and four-carbon side chain aliphatic GLSs; (2) accumulation of long-chain GLS (LC GLS), the sum of the seven-carbon and eight-carbon side chain aliphatic GLSs; (3) accumulation of indolic GLS, the sum of all indolic GLSs; (4) GLS Elong, the percentage of three-carbon GLSs in SC GLS, which is an indication of the enzyme activities of the elongation cycle (Haughn et al., 1991; de Quiros et al., 2000; Kroymann et al., 2003); (5) GLS OX, the percentage of 4-methylthiobutyl glucosinolate to the total of all GLSs with four carbons, an indication of the GLS OX enzyme activities (Hansen et al., 2007; Li et al., 2008, 2011) . These five traits are quantifiable in all tissues and environments and allow an analysis of distinct biochemical processes within the pathway.
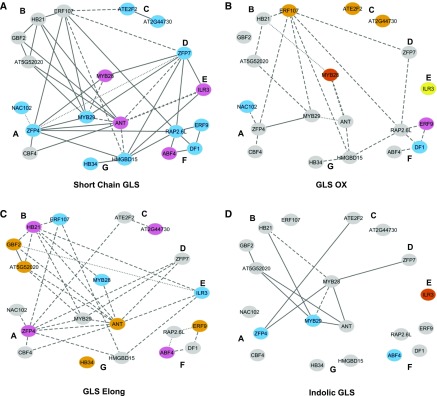
Mapping the epistatic interactions of the TFs based on the different phenotypes highlighted several patterns. First, there were differences in the frequency of epistasis, with SC GLS having significant epistasis for 42 of 47 pairs of interactions compared with only 11 of 42 for indolic GLS (Figures 2A and 2D). Importantly, there were differences in the pattern and conditionality of epistasis between the pathway (SC GLS) and specific enzyme activity estimates of enzymes (GLS OX and GLS Elong). For the whole pathway, most epistatic interactions were independent of the tissue or environment in which the phenotype was measured (Figure 2A). In contrast, the majority of epistatic interactions for the inferred enzymatic activities within the pathway were highly dependent upon the tissue (Figures 2B and 2C). This difference in epistatic patterns between parts of the pathway agrees with the previous observation that the TFs regulate a subset of steps in the pathway and not the whole pathway. Thus, there is significant epistasis in all four phenotypic categories tested in this collection, and this epistasis shows distinct properties and unique patterns from the level of enzyme activity to metabolite accumulation.
Figure 2.
Epistatic Networks Controlling GLS Traits.
The genetic network from Figure 1 is used to represent the significant epistatic interactions for the four major GLS traits. Only connections that show significant epistasis are maintained, while nonsignificant connections are dropped from the network. A solid line shows that only the TF x TF interaction term was significant in the ANOVA model. A dashed line shows epistatic interactions where there was a significant tissue x TF x TF interaction. A dotted line shows epistatic interactions where there was a significant environment x TF x TF interaction. A line of arrows shows that the interaction was conditional on both tissue and environment. The color of the node indicates which main effect terms are significant for the individual TFs: Sky blue indicates only a TF main effect, orange indicates only a tissue x TF interaction, purple indicates both TF and tissue x TF, yellow indicates both TF and treatment x TF, and red indicates that all three terms are significant. Gray indicates that no terms for the individual TF were significant.
(A) Epistatic network for SC GLS.
(B) Epistatic network for GLS OX.
(C) Epistatic network for GLS Elong.
(D) Epistatic network for indolic GLS.
Genetic Variance Controlled by Epistasis
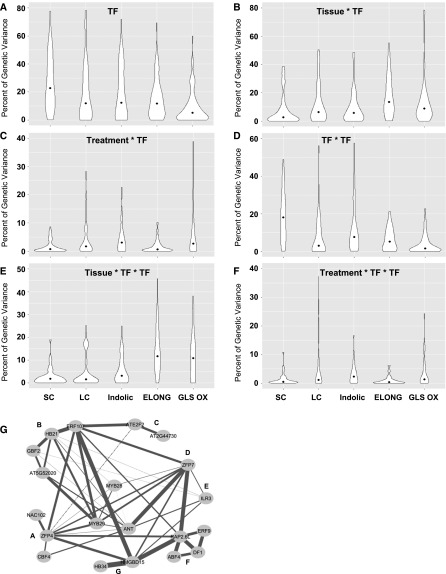
To obtain more quantitative insights into how epistasis influences the traits, we estimated the genetic variance that could be ascribed to epistasis (Figure 3; Supplemental Data Set 4). As expected from the high incidence of epistasis, metabolite accumulation from the SC GLS pathway has the highest fraction of genetic variance present in the TF x TF epistatic term (SC GLS in Figures 3A and 3D). In contrast, most of the variance attributable to epistasis for the enzyme activity traits was in the conditional TF x TF x environment or x tissue terms (GLS Elong and GLS OX in Figures 3B and 3E). To visualize how the epistatic variance was influenced by the network topology, we mapped the epistatic variance for the metabolite accumulation for SC GLS (Figure 3G). This plot showed that the proposed master regulatory TFs were not the key drivers of the epistatic network. For example, the major regulator of SC GLS, MYB28, does not play a key role in shaping the epistatic network. In contrast, MYB29, which is typically considered to have a less significant role than MYB28 in regulating SC GLS accumulation, has a much stronger effect than MYB28 in the epistatic networks (Figure 3G). In contrast, ERF107, RAP2.6L, and ZFP7, which have relatively small single mutant phenotypic effects, had major epistatic roles (Figure 3G). This suggests that the phenotypic consequences of single gene mutants are not sufficient to predict epistatic importance as genes. Thus, to fully understand a genetic network, both large and small effect loci should be analyzed when directly testing for epistasis.
Figure 3.
Distribution of Genetic Variance Ascribed to Distinct Model Terms.
The variances attributable to all terms including a genetic factor were summed together, and the percentage of this total genetic variance ascribed to each genetic term was calculated, as shown in violin plots. The median in each violin is shown as a dot. The phenotypes tested are shown individually, as labeled on the x axis.
(A) Percentage of genetic variation controlled by individual TF main effects.
(B) Percentage of genetic variation controlled by tissue x TF interactions.
(C) Percentage of genetic variation controlled by treatment x TF interactions.
(D) Percentage of genetic variation controlled by TF x TF epistatic interactions.
(E) Percentage of genetic variation controlled by tissue x TF x TF epistatic interactions.
(F) Percentage of genetic variation controlled by treatment x TF x TF epistatic interactions.
(G) Visualization of individual epistatic variance components within the genetic network for SC GLS. The width of the line connecting two TFs is proportional to the variance linked with the TF x TF term for that specific interaction. The highest proportion is the interaction between HMGBD15 and HB34, with 49% of the total genetic variance. Solid lines show that there was a significant interaction term, while dashed lines show combinations with no significant interactions.
Quantification of Epistasis Indicates a Bipartite Regulatory System
The detection of epistasis does not allow us to distinguish between different types of epistatic effects. The epistatic effects on an individual trait could be synergistic, by which the phenotypic value of double mutants was higher than the linear combination of single mutants. Alternatively, the epistatic effects could be antagonistic epistasis, by which the phenotypic value of double mutants was lower than the linear combination of single mutants (Hartman et al., 2001; Segrè et al., 2005; Costanzo et al., 2010, 2016). To differentiate between these forms of epistasis, we subtracted the measured double mutant phenotype from the predicted double mutant phenotype under an additive model. This value was then normalized to the wild-type phenotype to develop an epistasis value. This epistasis value was measured for each pair of mutants for each trait that was measured (Supplemental Figures 1 to 4 and Supplemental Data Set 5). This epistasis value will be positive when there is synergistic epistasis and negative for antagonistic epistasis (Figure 4).
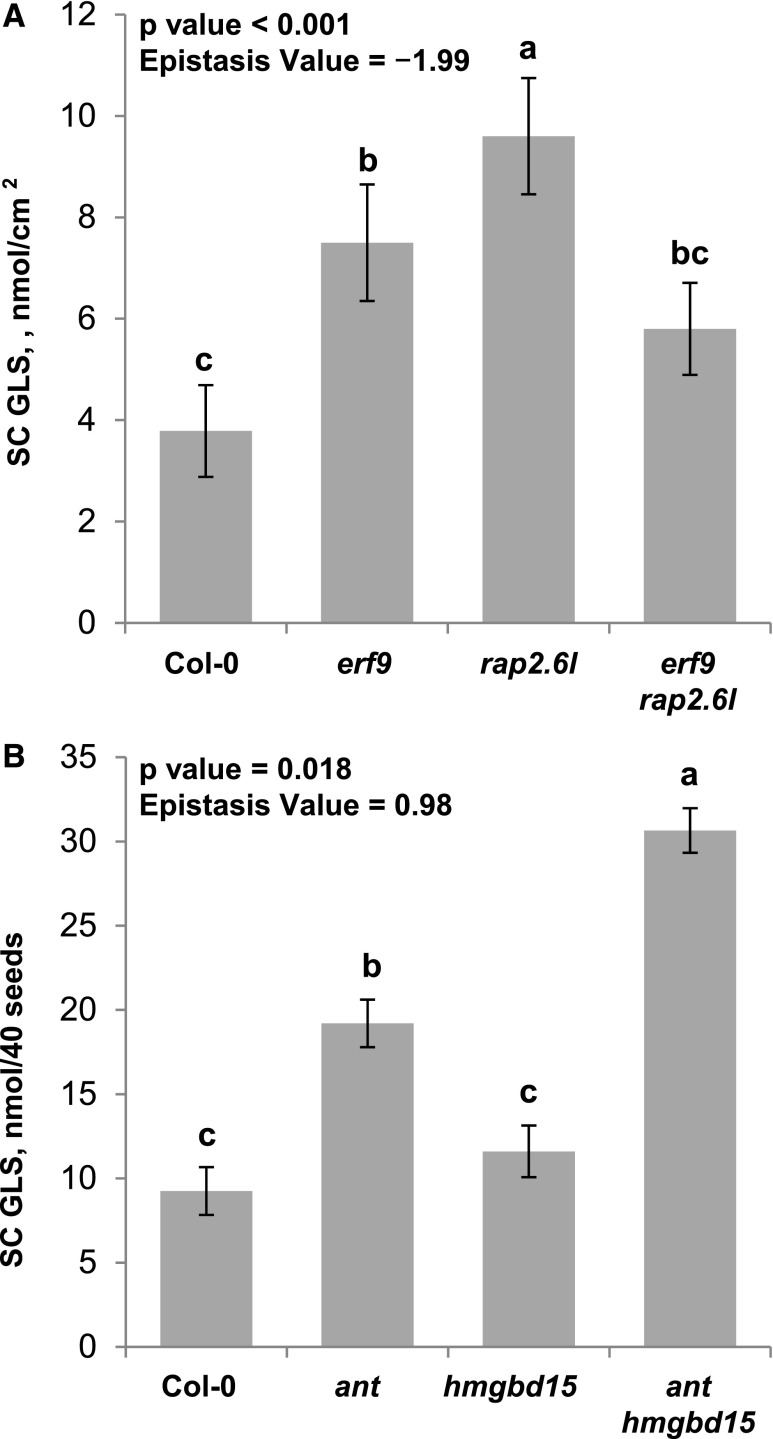
Figure 4.
Synergistic and Antagonistic Epistatic Patterns in SC GLS Accumulation.
The levels of SC GLSs in the corresponding genotypes and tissues are shown. Different letters show genotypes with significantly different SC GLS levels (P < 0.05 using post-hoc Tukey’s test after ANOVA). The P value of the epistatic interaction and the epistasis value are shown on the plots. se is shown with 16 samples across two experiments for each genotype.
(A) erf9 x rap2.6l antagonistic epistasis for SC GLS accumulation in leaves from the stress LSA chamber.
(B) ant x hmgbd15 synergistic epistasis for SC GLS accumulation in seeds from the clean CEF chamber.
Using this approach, we found that for metabolite accumulation in the SC GLS pathway, almost all of the epistasis was antagonistic epistasis, with larger values in leaves versus seeds (Figure 5). Comparing the epistasis value when testing pairs of TFs that come from the same phenotypic cluster versus different phenotypic clusters showed that there was no difference in these groups. This is in contrast to yeast primary metabolism, where there was more epistasis in crosses from different clusters than from crosses involving genes within a cluster (Segrè et al., 2005). Of the comparisons, the only crosses that were significantly different were the crosses to the putative master regulators myb28 or myb29. Thus, the new TFs largely interact additively with the previously identified MYBs while epistatically interacting with each other to control metabolite accumulation (Figure 5). This absence of epistasis between the new TFs and the MYBs suggests that there may be a buffering structure that allows the two groups of TFs to function independently.
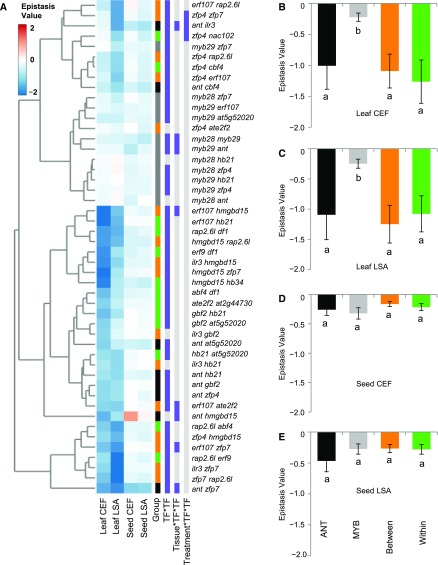
Figure 5.
Epistatic Effects for SC GLS.
Epistasis values were calculated for all pairwise combinations individually in all treatment and tissue combinations. For (B) to (E), different letters indicate statistically different average epistatic values across the cluster tests, as determined by ANOVA at P value of 0.05.
(A) Epistasis values for all pairwise mutant combinations plotted in a heat map using hierarchical clustering; the gene combinations are listed to the right of the diagram. The first vertical column shows if the pairwise mutant interaction is testing epistasis from within a cluster (green), between clusters (orange), MYB (gray), or ANT (black). The next three columns show which epistatic interaction term is significant (purple) or not significant (gray) (ANOVA, P < 0.05).
(B) Average and se of epistatic value for all pairwise mutant combinations measured from leaf samples from the clean CEF chamber.
(C) Average and se of epistatic value for all pairwise mutant combinations measured from leaf samples from the stressed LSA chamber.
(D) Average and se of epistatic value for all pairwise mutant combinations measured from seed samples from the clean CEF chamber.
(E) Average and se of epistatic value for all pairwise mutant combinations measured from seed samples from the stressed LSA chamber.
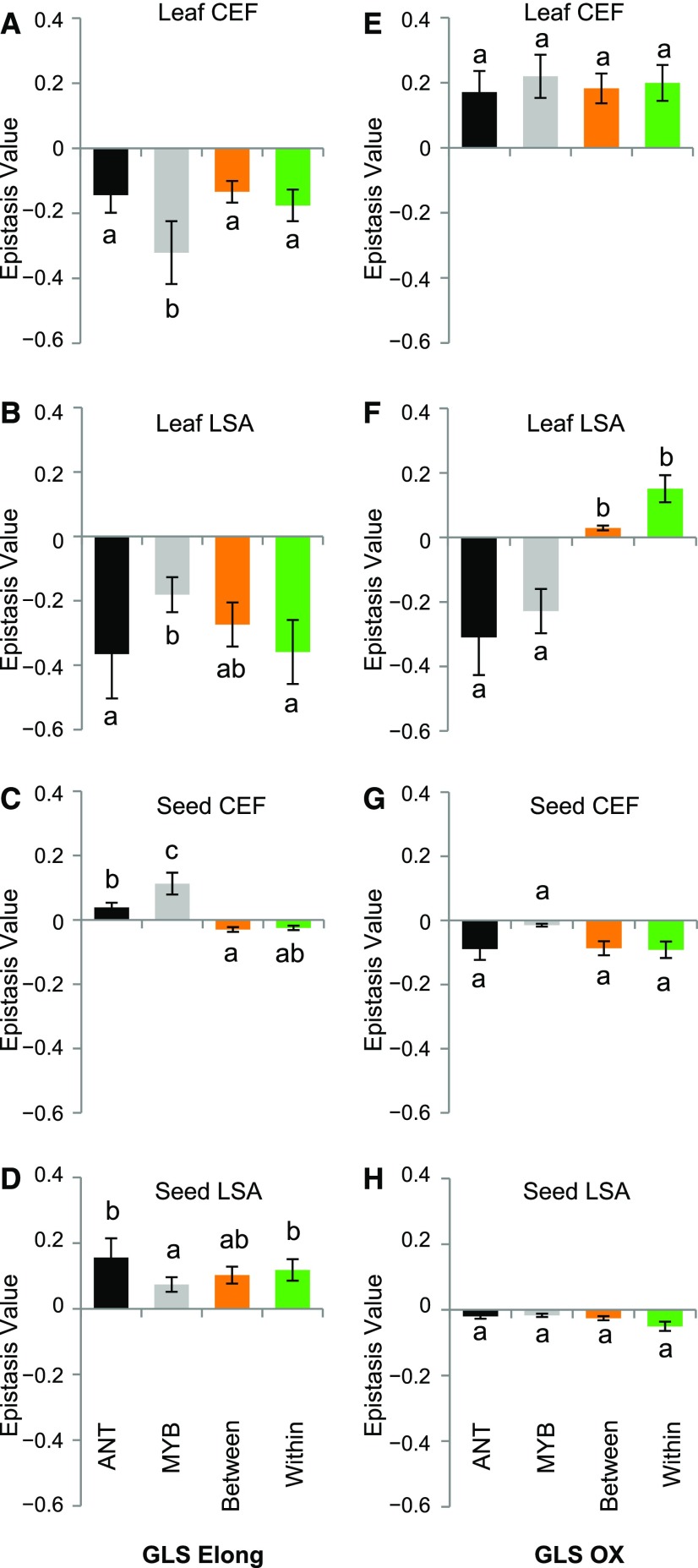
In contrast to the exclusively antagonistic epistasis for SC GLS, for GLS Elong and GLS OX, epistatic effects could shift from synergistic to antagonistic depending on the tissue and the enzyme activity being measured (Figure 6). For GLS Elong, there were conditional effects with predominantly antagonistic epistasis in leaf tissue and synergistic epistasis in seed tissue, while GLS OX showed antagonistic versus synergistic epistasis within the leaf in the two different environments (Figure 6). Similar to the situation for metabolite accumulation, there was no difference in the level of epistasis within and between TF clusters (Figure 6). One possible explanation for the difference in epistatic patterns is that the enzymatic activities are expressed as ratios while metabolite accumulation is expressed as absolute abundance. Arguing that these ratios are providing biological insight is the observation that both ratio traits, GLS Elong and GLS OX, show differential epistasis in the leaf samples between the two environments. If this were solely a mathematical issue, these traits should show similar behaviors. This argues that the same set of TFs have distinct patterns of epistatic effects upon different components of the same metabolic pathway, as the two estimated enzyme activities have opposing patterns and the resulting total accumulation of the compounds in this pathway have a distinct antagonistic epistasis. This suggests that epistasis of TFs is frequent within the aliphatic GLS pathway and that the effects of this epistasis change depending upon the specific portion of the pathway being measured.
Figure 6.
Epistatic Effects for GLS Elong and GLS OX.
Epistasis values were calculated for all pairwise combinations individually in all treatment and tissue combinations. Different letters indicate statistically different average epistatic values across the cluster tests, as determined by ANOVA at P value of 0.05. The average and se of the epistatic values for each group of epistatic groups are shown in all box plots, with orange showing between-cluster crosses, green showing within-cluster crosses, gray showing crosses involving MYB, and black showing crosses involving ANT.
(A) GLS Elong epistatic value for all pairwise mutant combinations measured from leaf samples from the clean CEF chamber.
(B) GLS Elong epistatic value for all pairwise mutant combinations measured from leaf samples from the stressed LSA chamber.
(C) GLS Elong epistatic value for all pairwise mutant combinations measured from seed samples from the clean CEF chamber.
(D) GLS Elong epistatic value for all pairwise mutant combinations measured from seed samples from the stressed LSA chamber.
(E) GLS OX epistatic value for all pairwise mutant combinations measured from leaf samples from the clean CEF chamber.
(F) GLS OX epistatic value for all pairwise mutant combinations measured from leaf samples from the stressed LSA chamber for GLS OX.
(G) GLS OX epistatic value for all pairwise mutant combinations measured from seed samples from the clean CEF chamber for GLS OX.
(H) GLS OX epistatic value for all pairwise mutant combinations measured from seed samples from the stressed LSA chamber for GLS OX.
Absence of Aliphatic GLS in Triple Mutants between Repressors and Activators
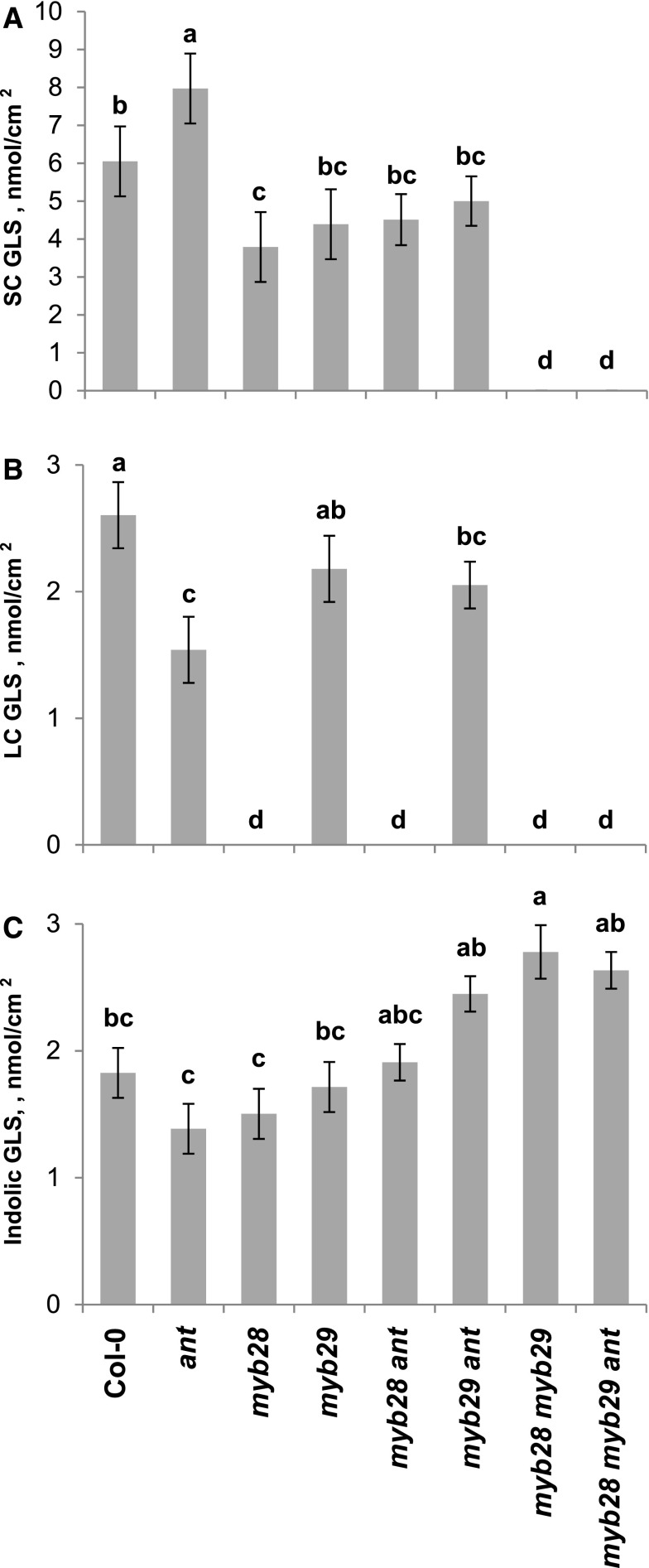
The observation that the putative master regulators, MYB28 and MYB29, had limited epistasis with the rest of the TFs suggested that they function largely additively with the other TFs. Considering that the MYBs are activators, as the myb28 myb29 double mutant has no aliphatic GLS while the other TFs are largely repressors (mutants have higher GLS), we hypothesized that triple mutants should be additive. Thus, the triple mutant under this hypothesis should restore detectable aliphatic GLS. To test this hypothesis and to assess if it is possible to roughly predict triple mutant interactions from pairwise combinations, we generated four triple mutants, myb28 myb29 ant, myb28 myb29 zfp4, myb28 myb29 zfp7, and myb28 myb29 hb21 because ANT, ZFP4, ZFP7, and HB21 are negative regulators that function in different parts of the pathway and have strong effects on aliphatic GLS accumulation. Measuring aliphatic GLS accumulation in the triple, double, and single mutants in all four contrasting conditions showed that none of the triple mutants rescued the accumulation of aliphatic GLS in any tissue or condition. This included 4MSO GLS, the most abundant aliphatic GLS in leaf tissue, and 4-methylthiobutyl glucosinolate GLS, the most abundant aliphatic GLS in seeds (Figure 7; Supplemental Figure 5 and Supplemental Data Set 5). In contrast to the pairwise epistasis analysis, which suggested additivity, this indicates that all four TFs show classical recessive epistasis to the master regulators and require a functional MYB28 and MYB29 to induce GLS metabolite accumulation. Thus, these additional TFs do genetically interact with both MYB28 and MYB29 to influence SC GLS accumulation, in contrast to the pairwise mutant evidence, and they appear to function downstream of the MYBs.
Figure 7.
Leaf GLS Accumulation in All myb28/myb29/ant Combinatorial Genotypes.
Leaf GLS levels in the corresponding genotypes, as measured in the clean CEF chamber. Different letters indicate genotypes with significantly different SC GLS levels (P < 0.05 using post-hoc Tukey’s test after ANOVA). se is shown with 16 samples across two experiments for each genotype.
(A) SC GLS.
(B) LC GLS.
(C) Indolic GLS.
Presence of Aliphatic GLS Transcripts in Triple Mutants between Repressors and Activators
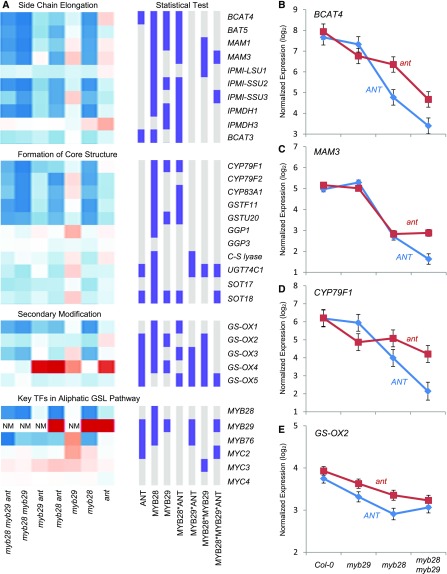
The above epistatic analysis is solely built upon measuring the accumulation of the metabolites, raising the question of how this may be reflected in transcript accumulation, which should be more proximal to the TFs. To test if the underlying transcriptional changes mirrored the metabolic consequences, we conducted transcriptomic analysis of the ant myb28 myb29 genotypes. We used RNA-seq to measure the transcript abundance of all known enzyme-encoding genes in the GLS pathway in the wild type and all single, double, and triple mutants using leaf tissue. This was done using leaf tissue from the CEF chamber where the strongest three-way epistasis between these genes occurs (Figure 8; Supplemental Data Set 6). The transcript analysis showed that, as previously observed, myb28 had a stronger effect than myb29 on pathway transcript abundance (Sønderby et al., 2007, 2010c) In contrast to the observation that ANT is a repressor of the metabolite, ant had contrasting effects on the transcripts, with some showing higher and some lower accumulation in the single ant mutant versus the wild type (Figures 8B to 8D; i.e., compare GS-OX2 versus GS-OX3). Supporting the idea that epistasis can change dependent on the molecular traits (i.e., transcripts versus metabolites), ant showed more epistasis with myb28 for the transcripts, while ant was mainly epistatic to myb29 for the metabolites (Figure 8). Further support came from the observation that ant is able to induce the transcription of pathway genes in the myb28 myb29 double mutant in contrast to its dependency for metabolite accumulation (Figures 8B to 8E). Thus, ANT can function at least in parallel to MYB28 MYB29 to regulate transcript levels while genetically, it appears to occupy a downstream role in metabolite accumulation. This suggests that the epistasis between these TFs is likely a complex blend of potential direct interactions and indirect interactions that could be caused by the metabolites’ accumulation being constrained by the structure of the biosynthetic pathway and the relative fluxes of the different enzymes. As such, the epistasis measured at the metabolite level would be the result of how the promoter-level epistasis is translated to enzyme activity epistasis and correspondingly how this equates to the accumulation of the final metabolite. This suggests that molecular models of epistasis at the level of how TFs do or do not interact with each other at a single promoter may not translate to the prediction of metabolite accumulation within a single pathway.
Figure 8.
Transcript Levels for Aliphatic Glucosinolate Biosynthesis Genes in All myb28 myb29 ant Combinatorial Genotypes.
se for each data point is shown from three independent biological samples.
(A) The heat map displays the fold change in transcript levels in the mutants compared with the Col-0 control, with red showing increased accumulation and blue showing decreased accumulation. The columns on the right display the statistical significance (purple, significant P < 0.05; gray, not significant) for each term in the ANOVA model, as listed at the bottom. The expression of MYB29 in the myb29 background lines is shown as NM (not measurable) due to the T-DNA insertion.
(B) Transcript levels of BCAT4. Lines show the values in the ANT (red) and ant (blue) genotypes across the four different myb28 myb29 backgrounds.
(C) Transcript levels of MAM3. Lines show the values in the ANT (red) and ant (blue) genotypes across the four different myb28 myb29 backgrounds.
(D) Transcript levels of CYP79F1. Lines show the values in the ANT (red) and ant (blue) genotypes across the four different myb28 myb29 backgrounds.
(E) Transcript levels of GS-OX2. Lines show the values in the ANT (red) and ant (blue) genotypes across the four different myb28 myb29 backgrounds.
DISCUSSION
In this work, we used a large network of TFs that regulate aliphatic GLS biosynthesis to measure pairwise and higher-order epistatic interactions. This pathway had previously been identified as having master regulators of GLS biosynthesis: MYB28 and MYB29 (Hirai et al., 2007; Traka et al., 2013). In contrast, we previously identified a large collection of TFs that had much more modest impacts on the pathway via binding distinct subsets of the promoters in the pathway (Li et al., 2014a; Gaudinier et al., 2015). Within this network, the vast majority of TFs showed pairwise epistasis. The strongest interactions involved interactions among TFs with smaller single mutant effects, while the putative master regulatory MYB had the lowest level of epistasis. For a related pathway that involves some overlap but also a distinct set of TFs and enzyme-encoding genes, indolic GLS, there was far lower epistasis, suggesting that the prevalence of epistasis is not caused by indirect pleiotropies but is a general property of the specific network being studied. The epistasis detected changed in terms of frequency, direction, and strength depending on how the network’s phenotype was measured, initial transcript abundance, intermediate enzyme activity estimates, or final metabolite accumulation. This suggests that epistasis may be a common feature of large regulatory networks that influence adaptive traits. Furthermore, the idea of a master regulator does not appear to translate into a central function within an epistatic network. Instead, it appears that epistasis is favored within a collection of small to moderate effect TFs that interact with partially overlapping sets of promoters within the pathway. This epistasis needs to be assessed when working to develop predictive models that translate from single gene studies to higher order studies on entire networks.
Epistasis Varies across a Network’s Molecular Hierarchy from Transcript to Metabolite
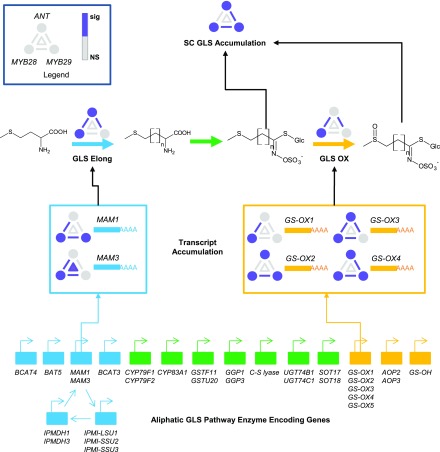
Biochemical pathways can be considered to have multiple traits that can be measured across the hierarchy proceeding from a gene encoding an enzyme to the accumulation of the final metabolite. This includes measuring the transcript abundance for each and every gene in the pathway, approximating enzyme activities linked to these transcripts, and finally measuring the detectable metabolites produced by the pathway. Frequently, regulatory studies focus on the TF to transcript link, with the implicit assumption that the other steps in this hierarchy will inherently follow the same logic. However, with posttranscriptional regulatory processes at the RNA, protein, and activity level in addition to the competition by other metabolic processes for the same precursor compounds and energy, this is not inherently the case. This is especially the case when most TFs are not limited to regulating a single metabolic pathway but instead have numerous other regulatory links. As such, it is an open question how regulatory processes may translate from gene to enzyme to metabolite in a complex multicellular organism. We measured transcripts and metabolites and inferred enzymatic activities to test how genetic epistasis changes through the molecular hierarchy of the aliphatic GLS pathway. This showed that the epistasis was highly dependent upon the specific molecular step being measured. While the metabolite and enzymatic activity steps had a similar frequency of epistasis, they showed differing sensitivity to the environment and development. The metabolites revealed epistatic effects that were consistent across tissues and environment, while the enzymatic efficiencies were largely conditional (Figures 4 and 6). As a more focused example, we summarized all the epistatic information linked to the ant myb28 myb29 combination of genotypes (Figure 9). This illustrates how main effects and epistatic interactions shift from transcript to metabolite. ANT has no main effect on methylthioalkylmalate synthase transcripts but is a key player in the resulting GLS Elong activity and SC GLS accumulation. Similarly, ANT had significant main effects on GS-OX transcripts, but this was only displayed as epistatic interactions with no main effect on GLS OX efficiency. This shows that epistasis within large regulatory networks can have contrasting effects, depending upon the specific molecular output being measured.
Figure 9.
Shifting Epistatic Interactions of ANT, MYB28, and MYB29 across the Molecular Aliphatic GLS Accumulation Processes.
The bottom of the figure shows the genes involved in the synthesis of aliphatic GLS within Arabidopsis. The different molecular phenotypes measured within this study that pertain to the SC GLS, GLS Elong, and GLS OX processes are shown, from the key transcripts involved in each process to the estimated enzyme activity to the final accumulation of SC GLS. A summary of the statistical effects of the ANT, MYB28, and MYB29 main effects (dots), pairwise interactions (lines between dots), and three-way interaction (triangle) are shown as per the legend. Purple indicates that the specific term was significant for the represented phenotype, and gray indicates nonsignificance. The different processes in aliphatic GLS biosynthesis are shown as follows: blue for elongation-related steps, green for core structure synthesis, and yellow for side chain modification.
Naive Pairwise Tests Find High Levels of Epistasis
Within this study, we attempted to cross the majority of available TF mutants known to affect aliphatic GLS regardless of mechanistic or homology information. In most published epistasis tests, the choice of mutants to cross is frequently guided by the genes having a known function in the same regulatory pathway. Alternatively, the genes may be chosen based on their membership in a gene family, and the mutants are crossed to test for redundancy. While these guided approaches frequently reveal epistasis, they do not test for how often epistasis occurs outside of these guiding rules. In this work, the majority of the interactions involve TFs belonging to different TF families, and there is little to no mechanistic information suggesting that they function in a single pathway (Figure 1). Thus, the explicit goal of this design was to test for epistasis between TFs that may function independently and are only connected by influencing the same trait. This naive design identified a high level of epistasis, including connections between processes not typically linked. For example, ANT and ILR3 show significant epistasis but are typically considered to function in different biological processes. ANT belongs to the AP2/EREBP TF family and controls cell proliferation (Elliott et al., 1996; Krizek et al., 2000; Liu et al., 2000; Mizukami and Fischer, 2000; Horstman et al., 2014), while ILR3 belongs to the bHLH TF family and controls responses to iron deficiency (Rampey et al., 2006; Long et al., 2010). Our results suggest that these two TFs somehow have regulatory effects that interact to modulate GLS accumulation. More intriguing is the idea that if this interaction is not specific to GLS, is it possible that ANT and ILR3 have epistatic interactions affecting the regulation of cell proliferation and/or responses to iron deficiency? This raises the potential that conducting naive crosses of TF mutants may open up a unique avenue to investigate how processes such as growth, nutrient acquisition, and biotic resistance are coordinated across large regulatory networks that are frequently studied in isolation.
Epistasis, Heritability, and Fitness
As GLS accumulation is an adaptive trait, it is tempting to attempt and translate these results directly to their potential fitness consequences. Mathematically, this is relatively simple using the equation R = h2S, where the response to any selection, R, is the additive heritability, h2, multiplied by the strength of selection, S (Falconer and Mackay, 1996; Mackay, 2001, 2014). In our system, the total variance controlled by any genetic term averaged ∼30% across the models, but this included all terms, both additive and nonadditive. The typical single gene additive heritability was ∼5%, which would suggest that this system may have small effects on fitness in Arabidopsis. However, this direct comparison is complicated by several factors. The first is that by measuring GLS in multiple environments and multiple tissues, we are constraining our estimate of heritability. In these models, tissue and environment and their interaction averaged ∼50% of the total variance, which places a constraint on additive heritability. The second complication is that the above equation largely relies on the species being a random outbreeding population, whereas Arabidopsis is species that has a low level of outbreeding, which typically occurs within narrow local populations (Charlesworth et al., 1997; Nordborg et al., 2002). As such, in this species, nonadditive epistatic variance may actually contribute more to selection responses than the simple equation would suggest (Rieseberg et al., 1999, 2003).
A final complication in this direct comparison is that in this analysis, there is a built-in assumption that because the GLS are the same compounds in the leaf and seed, they must be a single trait. However, fitness effects in a complex environment may actually suggest that leaf and seed GLS are distinct traits. This is best exemplified by the nonrandom distribution of biotic attackers across the different tissues and their potential differential sensitivity to specific glucosinolates (Jander et al., 2001; Kim and Jander, 2007; Hansen et al., 2008). For example, most lepidopteran larvae focus on eating the leaves of Arabidopsis, while granivores, i.e., seed-predators such as weevils, solely focus on the developing seed. As such, if fitness was driven by lepidopteran larvae, then only the leaf GLS effects would contribute to fitness. In contrast, if granivores were the dominant selective pressure, then only the seed GLS would contribute to fitness. In both cases, the heritability estimated across both tissues would be inaccurate for predicting responses to selection in these environments. Thus, there is a significant amount of work needed to understand the complexity of the biotic interaction driving plant adaptation and equally, the complexity of the underlying genetic network controlling this response.
Genetic Network Size for a Single Metabolic Pathway?
Within this project, we tested the epistasis of 20 TFs that have been linked to the accumulation of aliphatic GLS. While this is a large collection, this does not represent anywhere near the true scope of the regulatory network. The original yeast one-hybrid analysis that focused solely on root stele-expressed TFs suggested that there were likely dozens of other TFs that influence the accumulation of this metabolite but that were not able to be tested (Gaudinier et al., 2011; Li et al., 2014). Furthermore, genome-wide association studies performed to estimate the size of the genetic networks influencing GLS accumulation regardless of gene activity suggested that there were at least hundreds of likely genes that causally influence this pathway (Chan et al., 2010, 2011). In the natural variation studies, there are also extensive epistatic interactions among the identified loci (Kliebenstein et al., 2002a, 2002b; Wentzell et al., 2007). Together, these findings suggest that the genetic network influencing this metabolite is vastly larger than 20 TFs and 34 promoters. Yet these crosses identified a high level of epistasis within this subnetwork. This raises the question of how this might translate to a complete genetic network for aliphatic glucosinolates and all possible epistatic combinations within that network. This would go far beyond three-way epistatic interactions and require vast experimental populations. The other key question is how this translates to other metabolic pathways or other biological processes. Is this a unique property of metabolites that provide adaptation to biotic stresses, or is this a general property of metabolism and/or genetic networks in a multicellular organism?
METHODS
Plant Materials
The Arabidopsis thaliana T-DNA insertion lines of the 20 TFs were initially ordered from the Arabidopsis Biological Resource Center (Sussman et al., 2000; Alonso et al., 2003) and validated as homozygous in previous studies (Supplemental Data Set 2) (Sønderby et al., 2010c; Li et al., 2014). The 47 double mutants and four triple mutants were generated by crossing the corresponding single mutants and validating the double mutant homozygosity in the F2 generation using PCR-based markers for each mutant. The confirmed homozygous double and triple mutants were grown together with single mutants and the wild type to bulk seeds and provide matching seed batches for the downstream GLS profiling experiments.
Plant Growth Conditions
The Arabidopsis plants were grown in two independent chambers with 16 h light at 100- to 120-µE light intensity for the GLS profiling experiment. Both growth chambers were set at a continuous 22°C and utilized high-output fluorescent bulbs. These were the same growth chambers and conditions as utilized in the original report of these TFs. The use of two growth chambers allowed for a test of biotic environmental effects. The two growth chambers were set to identical abiotic environments but contain dramatically different biotic environments, one pest free, CEF, and one with an endogenous pest population, LSA. The endogenous pest population is provided by continuous propagation of tomato (Solanum lycopersicum) and Brassica napus plants that generates a mix of mites, aphids, flea beetles, and fungus gnats. This is not meant to test a specific biotic interaction but the general effect of biotic interactions with a blend of biotic interactions. This use of the clean chamber CEF and stress chamber LSA increases our ability to detect significant GLS phenotypes conditioned on the variation in environmental factors (Li et al., 2014a). Briefly, seeds were imbibed in water at 4°C for 3 d and sown into Sunshine Mix 1 (Sun Gro Horticulture). Seedlings were thinned to one plant per pot (6 × 5 cm) at 7 d after planting. For each experiment, at least eight replicates of Col-0, single, double, and triple mutants were planted using a randomized complete block design. Each flat had one plant per genotype leading to eight flats per replication. This experiment was conducted independently in the clean CEF and stress LSA chamber to generate a minimum of 16 biological repeats in total for most of the genotypes. This design means that each individual biological replicate is derived from a single independent plant that was planted in a randomized design.
GLS Extraction and Analysis
The harvest and collection of plant samples for GLS analysis were performed as described before (Kliebenstein et al., 2001a, 2001b, 2001c). Briefly, one fully mature leaf from each 4-week-old plant was removed, placed in 400 μL 90% (v/v) methanol, and stored at −20°C before extraction. The plants finished their life cycle and the seeds were harvested. Forty seeds from each plant were counted and stored at −20°C before extraction. The samples were broken with two 2.3-mm metal ball bearings in a paint shaker at room temperature and incubated at room temperature for 1 h. The tissues were pelleted by centrifugation for 15 min at 2500g and the supernatant was used for anion exchange chromatography in 96-well filter plates. After methanol and water washing steps, the columns were incubated with sulfatase solution overnight. Desulfo-GLSs were eluted and analyzed by HPLC according to a previously described method (Kliebenstein et al., 2001c).
Statistics
To test for epistasis of the TFs in controlling GLS biosynthesis, the GLS phenotypes for each epistatic combination were separately analyzed by ANOVA using a general linear model by SAS. The following model was used to test for the epistasis for the GLS phenotypes in the double mutants, with each double mutant having both single mutants and the wild type grown concurrently: yabtc = µ + Aa +Bb + Tt + Chc + AaxBb + AaxTt + AaxChc + BbxTt + BbxCc + Tt xChc + AaxBbxTt + AaxBbxChc + BbxTtxChc+ AaxTtxChc + AaxBbxTtxChc + εabtc, where εrgt is the error term and is assumed to be normally distributed with mean 0 and variance σε2. In this model, yabtc denotes the GLS phenotype in each plant, genotype A represents the presence or absence of a T-DNA insert in one TF gene (wild type versus mutant of locus A), and genotype B represents the presence or absence of a T-DNA insert in another TF gene (wild type versus mutant of locus B) in the double mutant from tissue Tt (leaf or seed) and chamber Chc (clean CEF chamber or stress LSA chamber). The ANOVA table, least-square (LS) means, and se for each genotype x tissue x treatment combinations were obtained using SAS. The type III sums of squares from this model were used to calculate the variance and percent variance attributable to each term in the model. For the percentage of variance, this was calculated by comparing to the total variance in the model as the denominator. All network representations were generated using Cytoscape.v2.8.3 (Shannon et al., 2003).
For all metabolites, we utilized the absolute abundance for all calculations, as the residuals for these tests were normally distributed in all but the model testing myb28/myb29 epistasis, which displays classical recessive epistasis. Adjusting to a log scale did not improve the residuals in the myb28/myb29 epistatic model and as such, we maintained the most direct link to the absolute metabolite abundance for the other pairwise models using the absolute abundance. In addition, we utilized an additive rather than a multiplicative scale for the epistatic tests because natural Arabidopsis accessions and recombinant inbred lines generated with Col-0 crosses can accumulate up to 20-fold more glucosinolate metabolites than the Col-0 accession. This suggests that there is not a physiological maxima that we are approaching in our data that would necessitate a multiplicative scale (Kliebenstein et al., 2001a, 2001b, 2002c; Chan et al., 2010, 2011). Transcripts were tested for epistasis using log-adjusted normalized expression values, as is the standard requirement for transcriptomic analysis. The ratio tests for the GS-OX and GS-Elong locus were also conducted using the unscaled data, as like for the metabolite, the residuals in these tests were largely normally distributed. These ratios are derived by having the value in the numerator (4-methylthiobutyl for GS-OX) also in the denominator to force the ratio to be between 0 and 1. In our previous work, we found that these two ratios are largely uncorrelated with the absolute content of any specific glucosinolate, allowing for them to behave as independent variables focused on the specific enzymatic step in question (Kliebenstein et al., 2001a, 2002c; Wentzell et al., 2007; Chan et al., 2010, 2011).
Calculation of Epistasis Value
To study the effect of epistasis, we utilized an algebraic approximation describing the direction and strength of the epistasis by normalizing the difference of observed double mutant phenotype versus the predicted double mutant phenotype, assuming additivity of the single mutants. This epistasis value was then normalized to the wild type, as done with other epistasis terms (Segrè et al., 2005). The phenotype for the wild type was set as w, mutant TFa as a, mutant TFb as b, and double mutant TFa/TFb as ab. The epistasis value is calculated as (ab − (w + (a-w) + (b-w))/w). If the epistasis value is positive, this shows evidence for synergistic epistasis, while antagonistic epistasis is reflected in negative values. The larger the epistasis value, the stronger the epistasis effects. The epistasis values were further visualized using the iheatmapr package in R software (R Core Team, 2015).
RNA-Seq Analysis
Arabidopsis plants, including Col-0, myb28, myb29, ant, myb28 ant, myb29 ant, and myb28 myb29 ant, were grown in CEF clean chambers in a randomized complete block design using two independent experiments. Leaves were harvested from two individual plants per genotype from each experiment and used to make four independent RNA-seq libraries per genotype. Total leaf RNA was extracted using Trizol (Invitrogen) and stored at −70°C before constructing the library. The RNA sequencing libraries were created with a QuantSequation 3′ mRNA-Seq Library Prep Kit (Lexogen). Each library had unique indexing primers, and the libraries were pooled and sequenced on the HiSeq 4000 platform at UC Davis DNA Technologies Core Facility. Fastq files from individual HiSeq lane were separated by adapter index into individual libraries. The alignment and gene counting were done with the BlueBee pipeline accompanying the Lexogen kit using the Arabidopsis (TAIR10) Lexogen QuantSeq 2.2.1 FWD reference genome. Statistical analysis of the RNA-seq data was conducted using the R V3.4.1 statistical environment (R Core Team, 2015). The gene count data from RNA-seq were subjected to a previously described statistical approach (Zhang et al., 2017). Normalization on gene counts was first conducted using the TMM method in function calcNormFactors() from the edgeR package (Robinson and Smyth, 2008; Robinson et al., 2010; Robinson and Oshlack, 2010; Nikolayeva and Robinson, 2014), and normalized pseudo-counts were then obtained for downstream analysis. The linear model was conducted on normalized gene counts using function glm.nb() from the MASS package (Venables and Ripley, 2002). Model-corrected means and standard errors for each transcript were determined using the lsmeans V2.19 package (Lenth, 2016). Raw P values for F- and χ2 tests were determined by type III sums of squares using the function ANOVA() from the car package (Fox and Weisberg, 2011). Transcript P values were false discovery rate (P value < 0.05) corrected for multiple tests of significance (Benjamini et al., 2001; Strimmer, 2008).
Accession Numbers
Transcriptomics data from this article could be found in Supplemental Data Set 6. The accession numbers for the genes analyzed are as follows: MYB28, AT5G61420; MYB29, AT5G07690; ANT, AT4G37750; ILR3, AT5G54680; ZFP4, AT1G66140; ERF107, AT5G61590; CBF4, AT5G51990; GBF2, AT4G01120; HMGBD15, AT1G04880; ZFP7, AT1G24625; HB21, AT2G02540; RAP2.6L, AT5G13330; HB34, AT3G28920; NAC102, AT5G63790; ERF9, AT5G44210; ATE2F2, AT1G47870; ABF4, AT3G19290; DF1, AT1G76880; MYB76, AT5G07700; MYC2, AT1G32640; MYC3, AT5G46760; and MYC4, AT4G17880.
Supplemental Data
Supplemental Figure 1. Epistatic effects for GLS Elong.
Supplemental Figure 2. Epistatic effects for GLS OX.
Supplemental Figure 3. Epistatic effects for LC GLS.
Supplemental Figure 4. Epistatic effects for indolic GLS.
Supplemental Figure 5. The absence of aliphatic GLS in triple mutants between repressor TFs and myb28/myb29.
Supplemental Data Set 1. Glucosinolate abbreviations, descriptions, and chemical structures.
Supplemental Data Set 2. The selected transcription factors and their T-DNA insertion lines.
Supplemental Data Set 3. P values of the ANOVA of the double and triple mutants.
Supplemental Data Set 4. The sum of squares of the ANOVA of the double and triple mutants.
Supplemental Data Set 5. LS means of the ANOVA of the double and triple mutants.
Supplemental Data Set 6. RNA-seq analysis of the full set of ant-related mutants.
Acknowledgments
We thank Wei Zhang for the discussions and help with the use of the pipeline to analyze the RNA-seq data. This work was funded by National Science Foundation Grants MCB1330337 to S.M.B. and D.J.K. and DBI 0820580 to D.J.K., by the USDA National Institute of Food and Agriculture, Hatch Project CA-D-PLS-7033-H to D.J.K., by Danish National Research Foundation Grant DNRF99 to D.J.K., by the National Science Foundation GRFP to M.T. via NSF DGE 1148897 to Jeffery C. Gibeling, Dean and Vice Provost of Office of Graduate Studies at UC Davis, and by the UC Davis Department of Plant Sciences Jastro Shields Research Award to M.T.
AUTHOR CONTRIBUTIONS
B.L. and D.J.K. conceived and designed the experiments. B.L., M.T., A.N., H.C., X.Z., C.C.-W., and R.N. performed the experiments. B.L. and D.J.K. analyzed the data. B.L., M.T., S.M.B., and D.J.K. wrote the article.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. (2001). Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125: 279–284. [DOI] [PubMed] [Google Scholar]
- Berckmans B., Lammens T., Van Den Daele H., Magyar Z., Bögre L., De Veylder L. (2011). Light-dependent regulation of DEL1 is determined by the antagonistic action of E2Fb and E2Fc. Plant Physiol. 157: 1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachi B., Meyer C.G., Villoutreix R., Platt A., Morton T.C., Roux F., Bergelson J. (2015). Coselected genes determine adaptive variation in herbivore resistance throughout the native range of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 112: 4032–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow M., Halkier B.A., Kliebenstein D.J. (2010). Regulatory networks of glucosinolates shape Arabidopsis thaliana fitness. Curr. Opin. Plant Biol. 13: 348–353. [DOI] [PubMed] [Google Scholar]
- Chan E.K., Rowe H.C., Corwin J.A., Joseph B., Kliebenstein D.J. (2011). Combining genome-wide association mapping and transcriptional networks to identify novel genes controlling glucosinolates in Arabidopsis thaliana. PLoS Biol. 9: e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E.K.F., Rowe H.C., Kliebenstein D.J. (2010). Understanding the evolution of defense metabolites in Arabidopsis thaliana using genome-wide association mapping. Genetics 185: 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., Nordborg M., Charlesworth D. (1997). The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided populations. Genet. Res. 70: 155–174. [DOI] [PubMed] [Google Scholar]
- Chory J. (2010). Light signal transduction: an infinite spectrum of possibilities. Plant J. 61: 982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J.A., Wilson I.W., Llewellyn D.J., Dennis E.S. (2009). The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 149: 1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., et al. (2010). The genetic landscape of a cell. Science 327: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., et al. (2016). A global genetic interaction network maps a wiring diagram of cellular function. Science 353: aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo J.C., Diaz-Trivino S., Cisneros N., Gutierrez C. (2006). The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18: 2224–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quiros H.C., Magrath R., McCallum D., Kroymann J., Schnabelrauch D., Mitchell-Olds T., Mithen R. (2000). α-Keto acid elongation and glucosinolate biosynthesis in Arabidopsis thaliana. Theor. Appl. Genet. 101: 429–437. [Google Scholar]
- Dombrecht B., Xue G.P., Sprague S.J., Kirkegaard J.A., Ross J.J., Reid J.B., Fitt G.P., Sewelam N., Schenk P.M., Manners J.M., Kazan K. (2007). MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19: 2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R.C., Betzner A.S., Huttner E., Oakes M.P., Tucker W.Q., Gerentes D., Perez P., Smyth D.R. (1996). AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D.S., Mackay T.F.C. (1996). Introduction to Quantitative Genetics. (Essex, UK: Longman, Harlow; ). [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Weisberg S. (2011). An R Companion to Applied Regression. (Thousand Oaks, CA: SAGE; ). [Google Scholar]
- Gaudinier A., Tang M., Kliebenstein D.J. (2015). Transcriptional networks governing plant metabolism. Curr. Plant Biol. 3–4: 56–64. [Google Scholar]
- Gaudinier A., et al. (2011). Enhanced Y1H assays for Arabidopsis. Nat. Methods 8: 1053–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigolashvili T., Yatusevich R., Berger B., Müller C., Flügge U.I. (2007). The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 51: 247–261. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Engqvist M., Yatusevich R., Müller C., Flügge U.I. (2008). HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol. 177: 627–642. [DOI] [PubMed] [Google Scholar]
- Goldwasser Y., Westwood J.H., Yoder J.I. (2002). The Use of Arabidopsis to Study Interactions between Parasitic Angiosperms and Their Plant Hosts. Arabidopsis Book 1: e0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E., Pajoro A., Franco-Zorrilla J.M., Tarancón C., Immink R.G., Cubas P. (2017). Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 114: E245–E254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Wang Z., Yang Z. (2004). ROP/RAC GTPase: an old new master regulator for plant signaling. Curr. Opin. Plant Biol. 7: 527–536. [DOI] [PubMed] [Google Scholar]
- Hansen B.G., Kliebenstein D.J., Halkier B.A. (2007). Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 50: 902–910. [DOI] [PubMed] [Google Scholar]
- Hansen B.G., Kerwin R.E., Ober J.A., Lambrix V.M., Mitchell-Olds T., Gershenzon J., Halkier B.A., Kliebenstein D.J. (2008). A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol. 148: 2096–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer S.L. (2009). The circadian system in higher plants. Annu. Rev. Plant Biol. 60: 357–377. [DOI] [PubMed] [Google Scholar]
- Harper A.L., Trick M., Higgins J., Fraser F., Clissold L., Wells R., Hattori C., Werner P., Bancroft I. (2012). Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nat. Biotechnol. 30: 798–802. [DOI] [PubMed] [Google Scholar]
- Hartman J.L. IV, Garvik B., Hartwell L. (2001). Principles for the buffering of genetic variation. Science 291: 1001–1004. [DOI] [PubMed] [Google Scholar]
- Haughn G.W., Davin L., Giblin M., Underhill E.W. (1991). Biochemical genetics of plant secondary metabolites in Arabidopsis thaliana. The glucosinolates. Plant Physiol. 97: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman R., et al. (2017). Architecture and Dynamics of the Jasmonic Acid Gene Regulatory Network. Plant Cell 29: 2086–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M.Y., et al. (2007). Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA 104: 6478–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.J., van Dam N.M., van Loon J.J. (2009). Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 54: 57–83. [DOI] [PubMed] [Google Scholar]
- Horstman A., Willemsen V., Boutilier K., Heidstra R. (2014). AINTEGUMENTA-LIKE proteins: hubs in a plethora of networks. Trends Plant Sci. 19: 146–157. [DOI] [PubMed] [Google Scholar]
- Howe G.A., Jander G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66. [DOI] [PubMed] [Google Scholar]
- Jander G., Cui J., Nhan B., Pierce N.E., Ausubel F.M. (2001). The TASTY locus on chromosome 1 of Arabidopsis affects feeding of the insect herbivore Trichoplusia ni. Plant Physiol. 126: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D.G., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kazan K., Manners J.M. (2013). MYC2: the master in action. Mol. Plant 6: 686–703. [DOI] [PubMed] [Google Scholar]
- Kerwin R., et al. (2015). Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. eLife 4: e05604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R.E., et al. (2017). Epistasis × environment interactions among Arabidopsis thaliana glucosinolate genes impact complex traits and fitness in the field. New Phytol. 215: 1249–1263. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Jander G. (2007). Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 49: 1008–1019. [DOI] [PubMed] [Google Scholar]
- Kliebenstein D., Pedersen D., Barker B., Mitchell-Olds T. (2002a). Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 161: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D., Lambrix V., Reichelt M., Gershenzon J., Mitchell-Olds T. (2001a). Gene duplication and the diversification of secondary metabolism: side chain modification of glucosinolates in Arabidopsis thaliana. Plant Cell 13: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D.J. (2009). A quantitative genetics and ecological model system: understanding the aliphatic glucosinolate biosynthetic network via QTLs. Phytochem. Rev. 8: 243–254. [Google Scholar]
- Kliebenstein D.J., Figuth A., Mitchell-Olds T. (2002b). Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics 161: 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D.J., Gershenzon J., Mitchell-Olds T. (2001b). Comparative quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 159: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D.J., Kroymann J., Brown P., Figuth A., Pedersen D., Gershenzon J., Mitchell-Olds T. (2001c). Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126: 811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek B.A., Prost V., Macias A. (2000). AINTEGUMENTA promotes petal identity and acts as a negative regulator of AGAMOUS. Plant Cell 12: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroymann J. (2011). Natural diversity and adaptation in plant secondary metabolism. Curr. Opin. Plant Biol. 14: 246–251. [DOI] [PubMed] [Google Scholar]
- Kroymann J., Donnerhacke S., Schnabelrauch D., Mitchell-Olds T. (2003). Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc. Natl. Acad. Sci. USA 100 (suppl 2.): 14587–14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau R.A., Kliebenstein D.J. (2009). Competition, herbivory and genetics interact to determine the accumulation and fitness consequences of a defence metabolite. J. Ecol. 97: 78–88. [Google Scholar]
- Lenth R.V. (2016). Least-squares means: TheRPackagelsmeans. J. Stat. Softw. 69: 1–33. [Google Scholar]
- Li B., Gaudinier A., Tang M., Taylor-Teeples M., Nham N.T., Ghaffari C., Benson D.S., Steinmann M., Gray J.A., Brady S.M., Kliebenstein D.J. (2014). Promoter-based integration in plant defense regulation. Plant Physiol. 166: 1803–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kristiansen K.A., Hansen B.G., Halkier B.A. (2011). Cellular and subcellular localization of flavin-monooxygenases involved in glucosinolate biosynthesis. J. Exp. Bot. 62: 1337–1346. [DOI] [PubMed] [Google Scholar]
- Li J., Hansen B.G., Ober J.A., Kliebenstein D.J., Halkier B.A. (2008). Subclade of flavin-monooxygenases involved in aliphatic glucosinolate biosynthesis. Plant Physiol. 148: 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Assmann S.M., Albert R. (2006). Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol. 4: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Franks R.G., Klink V.P. (2000). Regulation of gynoecium marginal tissue formation by LEUNIG and AINTEGUMENTA. Plant Cell 12: 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T.A., Tsukagoshi H., Busch W., Lahner B., Salt D.E., Benfey P.N. (2010). The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22: 2219–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T.F.C. (2001). The genetic architecture of quantitative traits. Annu. Rev. Genet. 35: 303–339. [DOI] [PubMed] [Google Scholar]
- Mackay T.F.C. (2014). Epistasis and quantitative traits: using model organisms to study gene-gene interactions. Nat. Rev. Genet. 15: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malitsky S., Blum E., Less H., Venger I., Elbaz M., Morin S., Eshed Y., Aharoni A. (2008). The transcript and metabolite networks affected by the two clades of Arabidopsis glucosinolate biosynthesis regulators. Plant Physiol. 148: 2021–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T. (2012). Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 50: 267–294. [DOI] [PubMed] [Google Scholar]
- Menkens A.E., Cashmore A.R. (1994). Isolation and characterization of a fourth Arabidopsis thaliana G-box-binding factor, which has similarities to Fos oncoprotein. Proc. Natl. Acad. Sci. USA 91: 2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Fischer R.L. (2000). Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 97: 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolayeva O., Robinson M.D. (2014). edgeR for differential RNA-seq and ChIP-seq analysis: an application to stem cell biology. Methods Mol. Biol. 1150: 45–79. [DOI] [PubMed] [Google Scholar]
- Nordborg M., Borevitz J.O., Bergelson J., Berry C.C., Chory J., Hagenblad J., Kreitman M., Maloof J.N., Noyes T., Oefner P.J., Stahl E.A., Weigel D. (2002). The extent of linkage disequilibrium in Arabidopsis thaliana. Nat. Genet. 30: 190–193. [DOI] [PubMed] [Google Scholar]
- Phillips P.C. (2008). Epistasis--the essential role of gene interactions in the structure and evolution of genetic systems. Nat. Rev. Genet. 9: 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing). [Google Scholar]
- Rampey R.A., Woodward A.W., Hobbs B.N., Tierney M.P., Lahner B., Salt D.E., Bartel B. (2006). An Arabidopsis basic helix-loop-helix leucine zipper protein modulates metal homeostasis and auxin conjugate responsiveness. Genetics 174: 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedelsheimer C., Czedik-Eysenberg A., Grieder C., Lisec J., Technow F., Sulpice R., Altmann T., Stitt M., Willmitzer L., Melchinger A.E. (2012). Genomic and metabolic prediction of complex heterotic traits in hybrid maize. Nat. Genet. 44: 217–220. [DOI] [PubMed] [Google Scholar]
- Rieseberg L.H., Archer M.A., Wayne R.K. (1999). Transgressive segregation, adaptation and speciation. Heredity (Edinb.) 83: 363–372. [DOI] [PubMed] [Google Scholar]
- Rieseberg L.H., Widmer A., Arntz A.M., Burke J.M. (2003). The genetic architecture necessary for transgressive segregation is common in both natural and domesticated populations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Smyth G.K. (2008). Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9: 321–332. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., Oshlack A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Menkens A.E., Beckmann H., Ecker J.R., Cashmore A.R. (1992). Heterodimerization between light-regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J. 11: 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrè D., Deluna A., Church G.M., Kishony R. (2005). Modular epistasis in yeast metabolism. Nat. Genet. 37: 77–83. [DOI] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K., Seki M. (2003). Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6: 410–417. [DOI] [PubMed] [Google Scholar]
- Sønderby I.E., Geu-Flores F., Halkier B.A. (2010a). Biosynthesis of glucosinolates--gene discovery and beyond. Trends Plant Sci. 15: 283–290. [DOI] [PubMed] [Google Scholar]
- Sønderby I.E., Burow M., Rowe H.C., Kliebenstein D.J., Halkier B.A. (2010b). A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 153: 348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Burow M., Rowe H.C., Kliebenstein D.J., Halkier B.A. (2010c). A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 153: 348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Hansen B.G., Bjarnholt N., Ticconi C., Halkier B.A., Kliebenstein D.J. (2007). A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2: e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R., Werber M., Weisshaar B. (2001). The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 4: 447–456. [DOI] [PubMed] [Google Scholar]
- Strimmer K. (2008). fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 24: 1461–1462. [DOI] [PubMed] [Google Scholar]
- Sussman M.R., Amasino R.M., Young J.C., Krysan P.J., Austin-Phillips S. (2000). The Arabidopsis knockout facility at the University of Wisconsin-Madison. Plant Physiol. 124: 1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Teeples M., et al. (2015). An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517: 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi W.B., Bertekap R.L. Jr., Cashmore A.R. (1997). Intracellular localization of GBF proteins and blue light-induced import of GBF2 fusion proteins into the nucleus of cultured Arabidopsis and soybean cells. Plant J. 11: 967–982. [DOI] [PubMed] [Google Scholar]
- Traka M.H., et al. (2013). Genetic regulation of glucoraphanin accumulation in Beneforté broccoli. New Phytol. 198: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. (2002). Modern Applied Statistics with S, Statistics and Computing, Vol. 12. (New York: Springer). [Google Scholar]
- Vidal E.A., Gutiérrez R.A. (2008). A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Curr. Opin. Plant Biol. 11: 521–529. [DOI] [PubMed] [Google Scholar]
- Wen W., Liu H., Zhou Y., Jin M., Yang N., Li D., Luo J., Xiao Y., Pan Q., Tohge T., Fernie A.R., Yan J. (2016). Combining quantitative genetics approaches with regulatory network analysis to dissect the complex metabolism of the maize kernel. Plant Physiol. 170: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzell A.M., Rowe H.C., Hansen B.G., Ticconi C., Halkier B.A., Kliebenstein D.J. (2007). Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet. 3: 1687–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. (1988). Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75: 225–233. [Google Scholar]
- Wurschum T., Maurer H.P., Dreyer F., Reif J.C. (2013). Effect of inter- and intragenic epistasis on the heritability of oil content in rapeseed (Brassica napus L.). Theor. Appl. Genet. 126: 435–441. [DOI] [PubMed] [Google Scholar]
- Xia C., Wang Y.J., Liang Y., Niu Q.K., Tan X.Y., Chu L.C., Chen L.Q., Zhang X.Q., Ye D. (2014). The ARID-HMG DNA-binding protein AtHMGB15 is required for pollen tube growth in Arabidopsis thaliana. Plant J. 79: 741–756. [DOI] [PubMed] [Google Scholar]
- Xuan W., Beeckman T., Xu G. (2017). Plant nitrogen nutrition: sensing and signaling. Curr. Opin. Plant Biol. 39: 57–65. [DOI] [PubMed] [Google Scholar]
- Zhang W., Corwin J.A., Copeland D., Feusier J., Eshbaugh R., Chen F., Atwell S., Kliebenstein D.J. (2017). Plastic transcriptomes stabilize immunity to pathogen diversity: The jasmonic acid and salicylic acid networks within the Arabidopsis/Botrytis pathosystem. Plant Cell 29: 2727–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züst T., Heichinger C., Grossniklaus U., Harrington R., Kliebenstein D.J., Turnbull L.A. (2012). Natural enemies drive geographic variation in plant defenses. Science 338: 116–119. [DOI] [PubMed] [Google Scholar]