Abstract
IMPORTANCE
Atrial fibrillation (AF) during sepsis is associated with an increased risk of ischemic stroke during hospitalization, but risks and benefits associated with anticoagulation for AF during sepsis are unclear.
OBJECTIVE
To determine clinician practice patterns and patient risk of stroke and bleeding associated with use of anticoagulation for AF during sepsis.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective cohort study using enhanced administrative claims data from approximately 20% of patients hospitalized in the United States July 1, 2010, to June 30, 2013, examined patients with AF during sepsis who did not have additional indications for therapeutic anticoagulation. Propensity score and instrumental variable analyses were used to evaluate risks of in-hospital stroke and bleeding associated with anticoagulation during sepsis.
EXPOSURES
Parenteral anticoagulants administered in doses greater than those used for prophylaxis of venous thromboembolism.
MAIN OUTCOMES AND MEASURES
Ischemic stroke and clinically significant bleeding events during hospitalization.
RESULTS
Of 113 511 patients hospitalized with AF and sepsis, 38 582 were included in our primary analysis (18 976 men and 19 606 women; mean [SD] age, 74.9 [11.7] years). A total of 13 611 patients (35.3%) received parenteral anticoagulants, while 24 971 (64.7%) did not. Hospital utilization rates of parenteral anticoagulants for AF during sepsis varied (median, 33%; 25th–75th percentile, 25%-43%). CHA2DS2VASc scores (congestive heart failure, hypertension, age ≥ 75 years [doubled], type 1 or type 2 diabetes, stroke or transient ischemic attack or thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65–75 years, sex category [female]) poorly discriminated the risk of ischemic stroke during sepsis (C statistic, 0.526). Among 27 010 propensity score–matched patients, rates of in-hospital ischemic stroke events did not differ significantly between patients who did (174 of 13 505 [1.3%]) and did not (185 of 13 505 [1.4%]) receive parenteral anticoagulation (relative risk [RR], 0.94; 95% CI, 0.77–1.15). Clinically significant bleeding occurred more often among patients who received parenteral anticoagulation (1163 of 13 505 [8.6%]) than patients who did not receive parenteral anticoagulation (979 of 13 505 [7.2%]; RR, 1.21; 95% CI, 1.10–1.32). Risk of ischemic stroke associated with parenteral anticoagulation did not differ significantly between patients with preexisting (RR, 1.12; 95% CI, 0.86–1.44) or newly diagnosed AF (RR, 0.85; 95% CI 0.57–1.27; P = .31 for interaction). Results were robust to multiple sensitivity analyses, including hospital utilization rates of parenteral anticoagulation for AF as an instrument for anticoagulation exposure (RR for stroke, 1.08; 95% CI, 0.62–1.90; RR for bleeding, 1.23; 95% CI, 0.88–1.72).
CONCLUSIONS AND RELEVANCE
Among patients with AF during sepsis, parenteral anticoagulation was not associated with reduced risk of ischemic stroke and was associated with higher bleeding rates.
Sepsis, a dysregulated immune response to infection that results in life-threatening organ dysfunction,1 leads to approximately 1 million hospitalizations in the United States yearly.2 Atrial fibrillation (AF) is the most common arrhythmia to complicate the course of sepsis3; approximately one-fourth of patients 65 years or older hospitalized with sepsis have concomitant AF.4 Although risk of ischemic stroke among patients with AF during sepsis exceeds the risks of both the general population with AF and patients with sepsis who do not experience AF,5 little evidence exists to support the use of anticoagulation for prophylaxis of arterial thromboembolism for patients with AF during sepsis.6,7 Management decisions regarding the use of anticoagulation for prophylaxis of arterial thromboembolism during sepsis are complicated by changes to the coagulation cascade and acute organ dysfunction that may increase risks of bleeding and thrombosis.8,9 Because stroke during sepsis is a relatively rare (eg, 2%-3% of patients with newly diagnosed AF during sepsis)5 but clinically important outcome, we used a large pharmacoepidemiologic database to evaluate associations between anticoagulation use during hospitalization and stroke and bleeding outcomes among patients with sepsis and AF.
Methods
Sepsis Cohort
As described previously,10 we used an administrative database enhanced with a date-stamped, detailed log of all medications as well as laboratory, diagnostic, and therapeutic services (Premier Inc) to identify a cohort of adult patients 18 years or older hospitalized from July 1, 2010, to June 30, 2013, with sepsis (defined according to the 1992 American College of Chest Physicians and Society of Critical Care Medicine Consensus conference11) present on admission. These data represent approximately 20% of hospitalized patients in nonfederal US hospitals.12 Patients with sepsis present on admission were selected through use of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for sepsis (038.x) with high positive predictive value (>90%)13 combined with receipt of an antibiotic. Atrial fibrillation was identified via ICD-9-CM code 427.31 (positive predictive value, 70%-96%; median, 89%)14 and patients with AF were subclassified as having preexisting AF (eg, diagnosed prevalent AF that was present on admission) or newly diagnosed AF (eg, incident AF that was not present on admission).5 We excluded patients with other potential indications for anticoagulation, including patients with prosthetic heart valves, acute myocardial infarction, or venous thromboembolic disease. To maximize the positive predictive value of our primary AF cohort we included patients with clinically significant AF during sepsis as our primary analysis cohort,10 defined by receipt of intravenous agents to control heart rate or rhythm concomitant with antibiotics. However, sensitivity analyses broadened inclusion criteria to include patients with an ICD-9-CM code for AF, without necessitating receipt of medications to control heart rate or rhythm.
All study procedures were determined as nonhuman participants research due to the deidentified nature of the study data by the Boston University Medical Campus Institutional Review Board.
Anticoagulation
Anticoagulation use during sepsis was defined as anticoagulants given on the same day as an antibiotic during the first 14 days of a hospital admission for sepsis. Anticoagulation data were extracted from pharmacy billing files and included hospital day of administration, quantity, route, and dosing. To attenuate unmeasured confounding by illness severity owing to patients’ ability to take oral anticoagulant medications during sepsis,15 we restricted our definition of anticoagulant exposure in the primary analysis to initial use of parenteral intravenous or subcutaneous administration of anticoagulants in doses greater than those used for prophylaxis of venous thromboembolism (ie, intravenous heparin sodium, >20 000 U daily, subcutaneous enoxaparin sodium twice daily [total daily dose >80 mg], subcutaneous dalteparin sodium, >5000 IU daily, and fondaparinux sodium, >2.5 mg daily). We allowed for oral anticoagulants (eg, warfarin sodium) later during hospitalization among patients who received an intravenous or subcutaneous anticoagulant initially, but excluded patients who received oral anticoagulants as their initial anticoagulant in the primary analysis. Given the clinical importance of understanding the risks and benefits of continuing oral anticoagulation among patients with preexisting AF and sepsis, we performed exploratory analysis evaluating oral anticoagulants as the initial anticoagulant during hospitalization (ie, warfarin, dabigatran, rivaroxaban, and apixaban) among patients with preexisting AF.
Covariates and Subgroups
We included year of hospitalization, patient demographics, co-morbid conditions, acute organ failure present on admission, organ-supportive therapies (given on hospital day 1), source of sepsis, health care professional, and hospital characteristics as covariates (eTable 1 in the Supplement). We performed subgroup analysis and explored interactions between outcomes and anticoagulation status based on whether AF was newly diagnosed vs preexisting.
Outcomes
We investigated patient and hospital factors associated with use of parental anticoagulation among patients with AF during sepsis and evaluated in-hospital stroke incidence and risk of bleeding associated with use of anticoagulation. Stroke was defined in the primary analysis using the ICD-9-CM codes for stroke (433.x1, 434.x1, and 436)16 that was not present on admission. Bleeding not present on admission was defined using previously validated algorithms (eTable 2 in the Supplement).17
Statistical Analysis
We used χ2 tests or t tests, as appropriate, as well as standardized differences (the ratio of between-group difference to SD) to assess balance in baseline characteristics between patients receiving or not receiving parenteral anticoagulation. Consistent with prior reports, a standardized difference threshold of 0.1 or greater was chosen to denote potentially important differences between treatment groups.18
To our knowledge, the ability of CHA2DS2-VASc6 scores (congestive heart failure, hypertension, age ≥75 years [doubled], type 1 or type 2 diabetes, stroke or transient ischemic attack or thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65–75 years, sex category [female]) to predict ischemic stroke associated with AF has not previously been evaluated in patients with sepsis. We used C statistics generated from a logistic regression model to summarize the ability of the CHA2DS2-VASc score to discriminate risk of ischemic stroke in our cohort with sepsis.
A propensity score approach was used to adjust for measured confounding in the selection of patients who received parenteral anticoagulation during hospitalization with AF during sepsis. Nonparsimonious propensity scores were calculated using generalized estimating equations with robust SE calculations accounting for within-hospital clustering19 to determine the probability that each patient would receive parenteral anticoagulation, conditional on measured variables. Propensity score models included independent variables representing hospital characteristics, patient demographics, comorbid conditions, use of intensive care, measures of acute organ dysfunction, source of infection, and year of hospitalization (eTable 1 in the Supplement). Our primary analysis used the propensity score to match patients with AF during sepsis who did and did not receive parenteral anticoagulation based on each patient’s predicted probability of receiving anticoagulation. We determined risk-standardized, between-hospital variation in the use of parenteral anticoagulation for AF during sepsis using the hospital random-effects output from hierarchical logistic regression models.
Sensitivity Analysis
We performed sensitivity analyses to evaluate the robustness of our findings to different specifications of our cohort definition for AF during sepsis, stroke and bleeding outcomes, and analytic methods to adjust for confounding. We performed a sensitivity analysis including all patients with ICD-9 codes for AF during sepsis, regardless of whether they received medication for AF to control heart rate or rhythm. Another analysis used the timing of computed tomographic scans of the head (for stroke) and transfusion of blood products (for bleeding) before or after the anticoagulation start date. A further analysis used inverse probability of treatment weighting among all eligible patients.20 We performed 2-level analysis using hospital-level rates of parenteral anticoagulation among patients with AF during sepsis as an ecological-level exposure instrument in logistic regression (with robust SEs), using patient-level outcomes and covariates.21,22
Exploratory Analyses of Initial Oral Anticoagulants
We explored practice patterns associated with oral anticoagulants as initial anticoagulants during hospitalization among patients with preexisting AF. Stroke and bleeding outcomes associated with initial use of oral anticoagulants during sepsis were examined using a propensity score–matching approach as well as an ecological exposure instrument approach using hospital-level rates of oral anticoagulation among patients with preexisting AF during sepsis.
We used SAS, version 9.3 (SAS Institute), for all analyses and selected a 2-sided P < .05 for statistical significance.
Results
Among 113 511 patients with sepsis and AF, we identified 38 582 eligible patients in our primary analysis cohort (18 976 men [49.2%] and 19 606 women [50.8%]; mean [SD] age, 74.9 [11.7] years), of whom 13 611(35.3%) received an initial intravenous or subcutaneous anticoagulant in doses greater than those generally used for prophylaxis of venous thromboembolism and 24 971 (64.7%) did not receive such an anticoagulant (Figure 1). Hospital risk-adjusted use of intravenous or subcutaneous anticoagulation varied (median, 33%; 25th-75th percentile, 25%-43%); 170 of 520 hospitals (32.7%) had rates of anticoagulation utilization that differed significantly from the mean hospital rate (Figure 2). Patients received the initial intravenous or subcutaneous anticoagulant dose on a median of hospital day 2 (25th–75th percentile, day 1–4). Enoxaparin was the most commonly selected initial parenteral anticoagulant (6991 of 14 121 [49.4%]) and warfarin (8289 of 9294 initial anticoagulants [89.2%]) was the most commonly chosen initial oral anticoagulant (eTable 3 in the Supplement).
Figure 1. Flowchart of Patient Inclusion Into Primary Analysis Cohort.
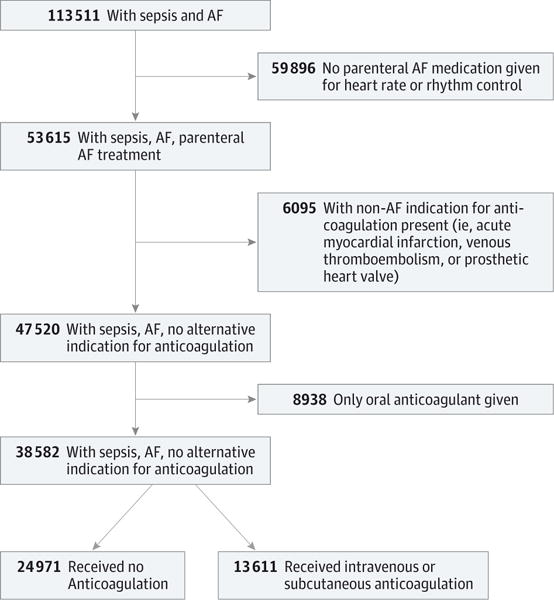
Among 113 511 patients with sepsis and atrial fibrillation (AF), 38 582 were included in the primary analysis cohort.
Figure 2. Variation in Hospital Risk-Standardized Rates of Anticoagulation for Atrial Fibrillation During Sepsis.
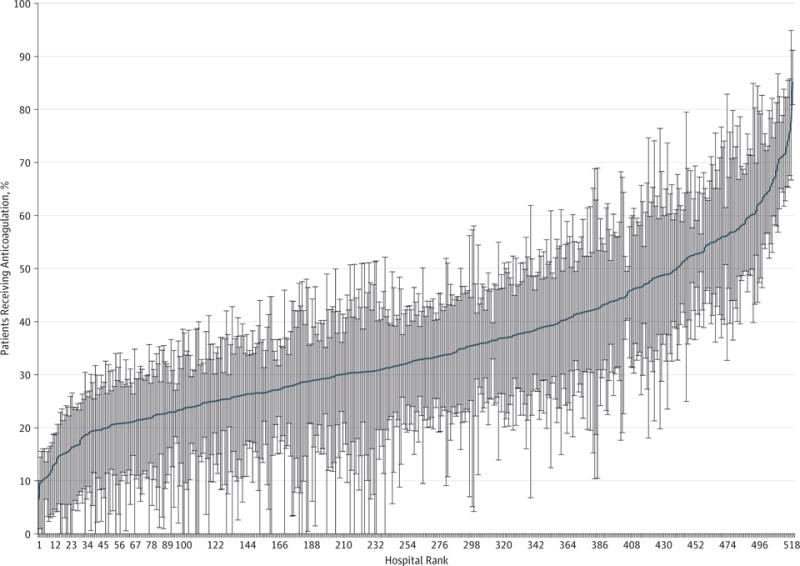
Hospitals are ranked in order of increasing rate of anticoagulation utilization.
Patient Characteristics, Anticoagulation, and Stroke
Patients with AF during sepsis who received intravenous or subcutaneous anticoagulation differed from patients who did not receive any anticoagulation on multiple baseline factors, and anticoagulation use decreased over time (Table 1). For example, patients receiving parenteral anticoagulation during sepsis were younger (mean [SD] age, 73.2 [11.7] vs 75.8 [11.7] y; P < .001), and were less likely to have prior bleeding events (930 [6.8%] vs 2845 [11.4%]; P < .001), acute hematologic failure (1839 [13.5%] vs 4552 [18.2%]; P < .001), acute kidney failure (7612 [55.9%] vs 15 814 [63.3%]; P < .001), chronic kidney disease (3971 [29.2%] vs 8696 [34.8%]; P < .001), cancer (1540 [11.3%] vs 3786 [15.2%]; P < .001), or metabolic acidosis (3296 [24.2%] vs 6756 [27.1%]; P = .002). The ability of CHA2DS2-VASc scores to predict ischemic stroke during sepsis was nominally greater than chance, with a C statistic of 0.526.
Table 1.
Patient and Hospital Factors According to Use of Anticoagulation During Sepsis
| Characteristic | Valuea | P Valueb | Standardized Differencec | |
|---|---|---|---|---|
| Anticoagulation (13 611 [35.3%]) | No Anticoagulation (24 971 [64.7%]) | |||
| Demographics | ||||
| Age, mean (SD), y | 73.2 (11.7) | 75.8 (11.7) | <.001 | −0.227 |
| Female sex | 6670 (49.0) | 12 936 (51.8) | <.001 | −0.056 |
| Race/ethnicity | ||||
| White | 10 340 (76.0) | 18 552 (74.3) | <.001 | 0.039 |
| Black | 960 (7.1) | 2221 (8.9) | −0.068 | |
| Hispanic | 112 (0.8) | 234 (0.9) | −0.012 | |
| Other | 2199 (16.2) | 3964 (15.9) | 0.008 | |
| Hospital characteristics | ||||
| Geographic location | ||||
| Northeast | 2085 (15.3) | 4511 (18.1) | .11 | −0.074 |
| Midwest | 2749 (20.2) | 4764 (19.1) | 0.028 | |
| South | 5897 (43.3) | 10 566 (42.3) | 0.021 | |
| West | 2880 (21.2) | 5130 (20.5) | 0.015 | |
| Teaching hospital | 5171 (38.0) | 9623 (38.5) | .78 | −0.011 |
| Comorbidities | ||||
| Prior bleeding | 930 (6.8) | 2845 (11.4) | <.001 | −0.159 |
| Prior ischemic stroke | 487 (3.6) | 829 (3.3) | .28 | 0.014 |
| Preexisting atrial fibrillation | 10 783 (79.2) | 20 277 (81) | .08 | −0.050 |
| Heart failure | 5712 (42.0) | 9792 (39.2) | <.001 | 0.056 |
| Type 1 or type 2 diabetes | 5130 (37.7) | 8734 (35.0) | .004 | 0.056 |
| Hypertension | 9561 (70.2) | 17 278 (69.2) | <.001 | 0.023 |
| Coronary heart disease or myocardial infarction | 4532 (33.3) | 7970 (31.9) | .07 | 0.029 |
| Chronic lung disease | 5862 (43.1) | 9268 (37.1) | <.001 | 0.122 |
| Chronic kidney disease | 3971 (29.2) | 8696 (34.8) | <.001 | −0.121 |
| Valvular heart disease | 2010 (14.8) | 3348 (13.4) | <.001 | 0.039 |
| Peripheral vascular disease | 1841 (13.5) | 3285 (13.2) | .28 | 0.011 |
| Cancer | 1540 (11.3) | 3786 (15.2) | <.001 | −0.114 |
| Dementia | 776 (5.7) | 1976 (7.9) | <.001 | −0.088 |
| CHA2DS2VASc score, mean (SD) | 3.4 (1.5) | 3.6 (1.5) | −0.083 | |
| Acute organ failure | ||||
| Total acute organ failures, No., mean (SD) | 1.9 (1.4) | 2.1 (1.4) | −0.146 | |
| Acute neurologic failure | 2046 (15.0) | 4256 (17.0) | .001 | −0.055 |
| Acute kidney failure | 7612 (55.9) | 15 814 (63.3) | <.001 | −0.151 |
| Acute respiratory failure | 5308 (39.0) | 9442 (37.8) | .08 | 0.024 |
| Acute circulatory failure | 5478 (40.2) | 10 895 (43.6) | <.001 | −0.067 |
| Acute hematologic failure | 1839 (13.5) | 4552 (18.2) | <.001 | −0.129 |
| Metabolic acidosis | 3296 (24.2) | 6756 (27.1) | .002 | −0.129 |
| Acute hepatic failure | 502 (3.7) | 1207 (4.8) | <.001 | −0.058 |
| Intensive care | 8692 (63.9) | 15 295 (61.3) | <.001 | 0.054 |
| Vasopressor use | 5084 (37.4) | 10 002 (40.1) | <.001 | −0.056 |
| Type of infection | ||||
| Pneumonia | 5537 (40.7) | 9012 (36.1) | <.001 | 0.095 |
| Gastrointestinal tract infection | 2040 (15.0) | 3892 (15.6) | .17 | −0.017 |
| Urinary tract infection | 4550 (33.4) | 9194 (36.8) | .46 | −0.071 |
| Skin or soft-tissue infection | 1315 (9.7) | 1766 (7.1) | <.001 | 0.094 |
| Primary bacteremia or fungemia | 153 (1.1) | 305 (1.2) | .52 | −0.009 |
| Attending specialty | ||||
| Internal medicine | 11 478 (84.3) | 21 257 (85.1) | <.001 | −0.022 |
| Surgery | 788 (5.8) | 1208 (4.8) | 0.042 | |
| Pulmonary or critical care | 1058 (7.8) | 2143 (8.6) | −0.030 | |
| Cardiology | 287 (2.1) | 363 (1.5) | 0.050 | |
| Year of service | −0.066 | |||
| 2010 | 2085 (15.3) | 3561 (14.3) | <.001 | |
| 2011 | 4789 (35.2) | 8122 (32.5) | ||
| 2012 | 4506 (33.1) | 8834 (35.4) | ||
| 2013 | 2231 (16.4) | 4454 (17.8) | ||
Abbreviation: CHA2DS2VASc, congestive heart failure, hypertension, age ≥75 y (doubled), type 1 or type 2 diabetes, stroke or transient ischemic attack or thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–75 y, sex category (female).
Data are presented as number (percentage) of patients unless otherwise indicated.
P values derived from multivariable-adjusted models with all covariates except CHA2DS2VASc score and number of acute organ failures.
Standardized differences are measures of effect size calculated as the ratio between group difference and SD. A standardized difference greater than 0.1 is often considered to represent a potentially important imbalance between treatment groups.
Anticoagulation and Outcomes
Unadjusted rates of ischemic stroke among patients who did not receive intravenous or subcutaneous anticoagulation (342 [1.4%]) and did receive anticoagulation (174 [1.3%]) did not differ significantly (relative risk [RR], 0.94; 95% CI, 0.78–1.12). Unadjusted risk of bleeding was higher among patients who received parenteral anticoagulation (1174 [8.6%]) compared with those who did not receive anticoagulation (1773 [7.1%]) (RR, 1.24; 95% CI, 1.13–1.36) (Table 2).
Table 2.
Outcomes Associated With Parenteral Anticoagulation of Atrial Fibrillation During Sepsis
| Analysis | No. (%) | RR (95% CI) | |
|---|---|---|---|
| Anticoagulation | No Anticoagulation | ||
| Unadjusted | |||
| Ischemic stroke | 174/13 611 (1.3) | 341/24 971 (1.4) | 0.94 (0.78–1.12) |
| Bleeding | 1174/13 611 (8.6) | 1773/24 971 (7.1) | 1.24 (1.13–1.36) |
| Primary analysis: Propensity score matcheda | |||
| Ischemic stroke | 174/13 505 (1.3) | 185/13 505 (1.4) | 0.94 (0.77–1.15) |
| Bleeding | 1163/13 505 (8.6) | 979/13 505 (7.2) | 1.21 (1.10–1.32) |
Abbreviation: RR, relative risk.
Propensity score models used to match patients or produce inverse probability of treatment weights included the following variables: age, sex, race, hospital teaching status, hospital geographical location, attending physician specialty, presence of preexisting or newly diagnosed atrial fibrillation, prior stroke, prior bleeding, intensive care, history of heart failure, diabetes, hypertension, coronary artery disease, chronic kidney disease, chronic lung disease, valvular heart disease, peripheral vascular disease, cancer, dementia, acute respiratory failure, acute renal failure, acute circulatory failure, acute neurologic failure, acute hematologic failure, acute hepatic failure, acidosis, source of infection, use of vasopressors, and year of hospitalization. Propensity scores matched 13 505 of 13 611 (99.2%) patients who received anticoagulation, including all patients receiving anticoagulation who experienced stroke, with 13 505 of 24 971 (54.1%) patients who did not receive anticoagulation.
After matching patients on propensity scores, measured covariates were well balanced between patients who did and did not receive intravenous or subcutaneous anticoagulation (eTable 4 and eFigure 1 in the Supplement). In the propensity score–matched analysis, ischemic stroke events did not differ significantly based on receipt of intravenous or subcutaneous anti-coagulation, occurring among 174 of 13 505 patients (1.3%) who received anticoagulation and 185 of 13 505 (1.4%) who did not receive anticoagulation (RR, 0.94; 95% CI, 0.77–1.15). Bleeding events were increased among patients who received intravenous or subcutaneous anticoagulation (1163 of 13 505 [8.6%]) compared with patients who did not receive anticoagulation (979 of 13 505 [7.2%]) (RR, 1.21; 95% CI, 1.10–1.32).
Outcomes Stratified by Newly Diagnosed and Preexisting AF
Rates of parenteral anticoagulation did not differ significantly between patients with newly diagnosed and preexisting AF (Table 1). Although patients with newly diagnosed AF had significantly higher rates of ischemic stroke (104 of 5585 [1.9%]) than did patients with preexisting AF (255 of 21 425 [1.2%]), the RR of ischemic stroke associated with anticoagulation did not differ significantly between patients with preexisting AF (1.12; 95% CI, 0.86–1.44) or newly diagnosed AF (0.85; 95% CI, 0.57–1.27; P = .31 for interaction). The risk of bleeding was higher among patients with newly diagnosed AF (703 of 5585 [12.6%]) than patients with preexisting AF (1439 of 21 425 [6.7%]), but the RR of bleeding associated with parenteral anticoagulation was lower among patients with newly diagnosed AF (0.97; 95% CI, 0.83–1.14) than patients with preexisting AF (1.23; 95% CI, 1.10–1.36; P = .008 for interaction).
Sensitivity analyses using different specifications for cohort definition, stroke and bleeding outcomes, and analytic methods to adjust for confounding either did not identify differences in risk of stroke or bleeding based on receipt of intravenous or subcutaneous anticoagulants during AF and sepsis or showed results generally similar to those of the primary analysis (Table 3).
Table 3.
Sensitivity Analyses
| Sensitivity Analysis | RR (95% CI) |
|---|---|
| Cohort including patients with AF during sepsis regardless of rate or rhythm control medication, propensity score-matched cohorta | |
| Ischemic stroke | 0.90 (0.74–1.10) |
| Bleeding | 1.25 (1.16–1.35) |
| Primary propensity-matched cohortb | |
| Ischemic stroke, requiring brain imaging after receipt of anticoagulation | 0.90 (0.72–1.11) |
| Bleeding, requiring blood transfusion after receipt of anticoagulation | 0.99 (0.87–1.12) |
| Inverse probability of treatment-weighted analysisc | |
| Ischemic stroke | 0.96 (0.79–1.15) |
| Bleeding | 1.19 (1.08–1.30) |
| Ecologic exposure instrument: hospital rate of anticoagulation for AF during sepsisc | |
| Ischemic stroke | 1.08 (0.62–1.90) |
| Bleeding | 1.23 (0.88–1.72) |
Abbreviations: AF, atrial fibrillation; RR, relative risk.
n = 44 330.
n = 27 010.
n = 38 582.
Oral Anticoagulants
Exploratory analyses demonstrated that patients receiving oral anticoagulants as the initial anticoagulant during hospitalization were more likely to have a history of preexisting AF than newly diagnosed AF (adjusted RR, 3.17; 95% CI, 2.89–3.49), as well as more frequent cardiovascular comorbidity and lower incidence of acute organ failures (eTable 5 in the Supplement). In propensity score–matched analysis among patients with preexisting AF, those who received initial oral anticoagulants experienced lower rates of stroke (44 of 8364 [0.5%]) and bleeding (434 of 8364 [5.2%]) than did matched patients who did not receive anticoagulants (stroke, 236 of 18661 [1.3%]; bleeding, 1121 of 18 661 [6.0%]) (RR for stroke, 0.46; 95% CI, 0.32–0.66; RR for bleeding, 0.85; 95% CI, 0.74–0.97). However, results were not robust to instrumental variable approach using rates of oral anticoagulation in the hospital; risk for stroke (highest hospital quartile vs lowest: adjusted odds ratio, 0.85; 95% CI, 0.60–1.20) and bleeding (highest hospital quartile vs lowest: adjusted odds ratio, 1.08; 95% CI, 0.88–1.34) were similar at hospitals with high and low rates of oral anticoagulant use.
Discussion
We investigated the practice patterns and outcomes associated with receipt of systemic anticoagulation during hospitalization with AF and sepsis. Hospital practices for the use of parenteral anticoagulation in patients with AF during sepsis varied widely, with one-third of hospitals deviating significantly from mean rates of anticoagulation use. Patients with AF during sepsis who received parenteral anticoagulation generally had fewer comorbid and acute conditions associated with bleeding risk compared with patients who did not receive anticoagulation. Rates of ischemic stroke during sepsis were generally low and did not differ based on initial receipt of parenteral anticoagulation; however, risk of bleeding was greater among patients receiving parenteral anticoagulation during sepsis. Evaluations of initial parenteral anticoagulation were robust to multiple sensitivity analyses, including evaluation in cohorts defined by broader inclusion criteria and use of different methods to address measured and unmeasured confounding, demonstrating no reduction in risk of ischemic stroke with use of parenteral anticoagulants during sepsis. Although exploratory analyses of patients with preexisting AF showed potentially lower rates of stroke with initial use of oral anticoagulants, the findings on use of oral anticoagulants should be viewed in the context of increased risk of bias from unmeasured confounding. Our findings demonstrate large variation in practice patterns, suggesting clinical equipoise for use of parenteral anticoagulation for AF during sepsis; however, we did not find consistent evidence that the potentially increased risk of bleeding associated with parenteral anticoagulation during sepsis was offset by significantly lower rates of stroke.
To our knowledge, few studies have investigated outcomes associated with anticoagulation use among patients with AF during sepsis. A retrospective, single-center study by Darwish et al9 evaluated 115 patients with preexisting AF and sepsis; no ischemic stroke events were recorded and an increased risk of bleeding was observed among the 30% of patients who received any anticoagulation during sepsis. The results of this study were similar to our results among an approximate 20% sample of patients in the United States: about one-third of patients with AF and sepsis received parenteral anticoagulation, rates of ischemic stroke during hospitalization were low, and risks of bleeding risks higher among patients receiving anticoagulation.
Parenteral anticoagulation for AF during hospitalization for sepsis may be considered analogous to using intravenous anticoagulants to bridge to oral anticoagulation for AF during a perioperative period. Similar to our findings, a recent randomized trial demonstrated an increase in bleeding without significant reductions in rates of ischemic stroke with the use of perioperative parenteral anticoagulation for AF.23 Given relatively low in-hospital rates of ischemic stroke (1%-2% in our study, 2.5% reported previously5 during sepsis with acute organ dysfunction and AF), moderate rates of bleeding complications, and poor performance of CHA2DS2-VASc scores to stratify risk of stroke during sepsis, current evidence does not support a benefit associated with use of parenteral anticoagulation to reduce AF-associated thromboembolic complications during sepsis.24
Exploratory analyses of initial oral anticoagulant use among patients with preexisting AF suggested a benefit for lower risk of stroke during hospitalization for sepsis, a finding supported by studies investigating continuation of oral anticoagulants for AF in clinical settings that do not involve sepsis (eg, during placement of a pacemaker).25 Given the lower rates of bleeding among patients given oral anticoagulants compared with those not receiving anticoagulants (potentially related to prevalent user bias),26 and the lack of supporting results from instrumental variable analysis, we urge caution in interpreting the analysis of oral anticoagulants. In addition, because we did not have access to patients’ medication history before admission, we could not reliably ascertain new vs continued use of oral anticoagulants. Furthermore, our study did not examine postdischarge outcomes among patients prescribed an oral anticoagulant at discharge. Our findings do not address, nor do we advocate, changes to chronic anticoagulant therapy for preexisting AF following a resolution of sepsis or discharge from hospitalization for sepsis. Further study is necessary to evaluate the optimal timing of reinitiating oral anticoagulants for patients receiving chronic therapy and the risk-to-benefit ratio of longer-term anticoagulation after sepsis among patients with newly diagnosed AF during a sepsis-related hospitalization.
Multiple factors complicate the use and evaluation of therapeutic anticoagulation during sepsis as prophylaxis against thromboembolic complications of AF. The dysregulated immune response of sepsis may produce both prothrombotic and antithrombotic states that predispose patients to both thrombotic and bleeding complications27 unrelated to prior cardiovascular risk, potentially explaining the poor predictive ability of CHA2DS2-VASc scores for stroke during sepsis. In addition, complications and treatments of sepsis may result in seizures, encephalopathy, and delirium that produce transient stroke-like symptoms unrelated to thromboembolism. Furthermore, cerebral hypoperfusion during sepsis may cause watershed ischemia, which is difficult to distinguish from cardioembolic infarcts related to AF. Finally, emergency procedures for critically ill patients with sepsis (eg, placement of a central catheter) may also increase risk for bleeding among patients receiving anticoagulants. Our findings suggest that the physiological changes and clinical demands of sepsis may attenuate benefits and increase risks of anticoagulation for AF in the short term during hospitalization with sepsis.
Some limitations should be considered in evaluation of our findings. Claims data may insufficiently characterize comorbidities or acute severity of illness, although prior studies have suggested that predictive ability of claims data approaches that of severity of illness scores such as Acute Physiology and Chronic Health Evaluation II.28 Subgroup analysis may be underpowered to show interaction between groups with preexisting and newly diagnosed AF for stroke and bleeding outcomes. We could not compare among intravenous or subcutaneous anticoagulation regimens, type of anticoagulation, or patients with supratherapeutic or subtherapeutic levels of anticoagulation. We acknowledge that individuals with newly diagnosed AF may have actually had AF that was previously clinically unrecognized; however, patients with newly diagnosed AF were unlikely to have had prior anticoagulant treatment for AF. In addition, because we did not have access to medication history before admission, we could not analyze new vs continued use of oral anticoagulants. Although we stratified analyses by preexisting and newly diagnosed AF during sepsis, we lacked sufficient granularity of data to distinguish between paroxysmal or permanent AF. Finally, the observational nature of our study design limits etiologic inference. Although large randomized trials would eliminate unmeasured residual confounding that may be present in our observational study, trials that could achieve adequate power to detect relatively rare outcomes of stroke associated with AF during sepsis would be difficult to conduct.
Conclusions
We investigated practice patterns and outcomes associated with use of therapeutic anticoagulation for AF that occurs during sepsis. After adjusting for differences in measured patient characteristics, large variation among hospitals remained in the use of parenteral anticoagulation for AF during sepsis. Younger patients with fewer risk factors for bleeding were more likely to receive parenteral anticoagulation. In multiple analyses adjusting for measured and unmeasured confounding, use of therapeutic parenteral anticoagulation during hospitalization with sepsis and AF was not associated with reduction in ischemic stroke, and showed a potentially higher bleeding risk. Whereas current evidence suggests that benefits may not outweigh risks of parenteral anticoagulation for AF during sepsis, further study is warranted to determine optimal timing for restarting treatment with oral anticoagulants among patients with preexisting AF and long-term anticoagulation strategies after hospitalization for patients with newly diagnosed AF during sepsis.
Supplementary Material
Key Points.
Question
What are the risks of ischemic stroke and bleeding associated with use of parenteral anticoagulants for prophylaxis of arterial thromboembolism for patients with atrial fibrillation during sepsis?
Findings
In a cohort study of 38 582 hospitalized patients with atrial fibrillation and sepsis, receipt of parenteral anticoagulants during sepsis was not associated with a significantly reduced risk of in-hospital ischemic stroke (1.3%) compared with patients who did not receive anticoagulants (1.4%). However, rates of clinically significant bleeding were markedly higher among patients receiving anticoagulation (8.6%) than those not receiving anticoagulation (7.2%) of atrial fibrillation during sepsis.
Meaning
Use of parenteral anticoagulation for prophylaxis of arterial thromboembolism in patients with atrial fibrillation during sepsis may be associated with greater risk than benefit.
Acknowledgments
Funding/Support: This study was supported by grants K01HL116768 (Dr Walkey); KL2RR031981, 5R01HL126911-02, 1R15HL121761-01A1, 1UH2TR000921-02 (Dr McManus); 2R01HL092577, and 1R01HL128914 (Dr Benjamin) from the National Institutes of Health.
Role of the Funder/Sponsor: The funding source had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Walkey had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Walkey.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Walkey, Quinn, Winter.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Walkey, Quinn, Winter.
Obtained funding: Walkey.
Administrative, technical, or material support: Walkey, McManus.
Study supervision: Walkey.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Contributor Information
Allan J. Walkey, Division of Pulmonary and Critical Care Medicine, The Pulmonary Center, Boston University School of Medicine, Boston, Massachusetts; Center of Implementation and Improvement Sciences, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts.
Emily K. Quinn, Data Coordinating Center, Boston University School of Public Health, Boston, Massachusetts.
Michael R. Winter, Data Coordinating Center, Boston University School of Public Health, Boston, Massachusetts.
David D. McManus, Section of Cardiac Pacing and Electrophysiology, Division of Cardiovascular Medicine, University of Massachusetts Medical School, Worcester.
Emelia J. Benjamin, Section of Cardiovascular Medicine, Boston University School of Medicine, Boston, Massachusetts; Section of Preventive Medicine, Boston University School of Medicine, Boston, Massachusetts; Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torio CM, Andrews RM. Healthcare Cost and Utilization Project Statistical Brief #160. Rockville, MD: Agency for Healthcare Research and Quality; National inpatient hospital costs: the most expensive conditions by payer, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf. Published August 2013. Accessed June 17, 2015. [PubMed] [Google Scholar]
- 3.Annane D, Sébille V, Duboc D, et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178(1):20–25. doi: 10.1164/rccm.200701-031OC. [DOI] [PubMed] [Google Scholar]
- 4.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949–955.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 7.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 8.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864–2875. doi: 10.1378/chest.128.4.2864. [DOI] [PubMed] [Google Scholar]
- 9.Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother. 2013;47(10):1266–1271. doi: 10.1177/1060028013500938. [DOI] [PubMed] [Google Scholar]
- 10.Walkey AJ, Evans SR, Winter MR, Benjamin EJ. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest. 2016;149(1):74–83. doi: 10.1378/chest.15-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Safavi KC, Dharmarajan K, Kim N, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127(8):923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark S, Costantino T, Rudnitsky G, Camargo CA., Jr Observational study of intravenous versus oral corticosteroids for acute asthma: an example of confounding by severity. Acad Emerg Med. 2005;12(5):439–445. doi: 10.1197/j.aem.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J, editor. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hilldale, NJ: Lawrence Ehrlbaum Associates; 1988. [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 20.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SC. Combining ecological and individual variables to reduce confounding by indication: case study–subarachnoid hemorrhage treatment. J Clin Epidemiol. 2000;53(12):1236–1241. doi: 10.1016/s0895-4356(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 22.Johnston SC, Henneman T, McCulloch CE, van der Laan M. Modeling treatment effects on binary outcomes with grouped-treatment variables and individual covariates. Am J Epidemiol. 2002;156(8):753–760. doi: 10.1093/aje/kwf095. [DOI] [PubMed] [Google Scholar]
- 23.Douketis JD, Spyropoulos AC, Kaatz S, et al. BRIDGE Investigators Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh AY, McManus DD. A bridge too far? findings of bridging anticoagulation use and outcomes in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Circulation. 2015;131(5):448–450. doi: 10.1161/CIRCULATIONAHA.114.014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birnie DH, Healey JS, Wells GA, et al. BRUISE CONTROL Investigators Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084–2093. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]
- 26.van Rein N, Cannegieter SC, le Cessie S, et al. Statins and risk of bleeding: an analysis to evaluate possible bias due to prevalent users and healthy user aspects. Am J Epidemiol. 2016;183(10):930–936. doi: 10.1093/aje/kwv255. [DOI] [PubMed] [Google Scholar]
- 27.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Hemost. 2015;41(6):650–658. doi: 10.1055/s-0035-1556730. [DOI] [PubMed] [Google Scholar]
- 28.Lagu T, Lindenauer PK, Rothberg MB, et al. Development and validation of a model that uses enhanced administrative data to predict mortality in patients with sepsis. Crit Care Med. 2011;39(11):2425–2430. doi: 10.1097/CCM.0b013e31822572e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


