Abstract
Reactive oxygen species (ROS) are important signaling molecules that mediate oxidative stress and cellular damage when improperly regulated. ROS and oxidative stress can activate autophagy, which generally serves as a cytoprotective negative feedback mechanism to selectively eliminate sources of ROS, including mitochondria and peroxisomes. In this review we describe the mechanisms by which ROS directly and indirectly activate autophagy, and conversely, how selective autophagy suppresses the formation of ROS. Furthermore, we highlight what appear to be contradictory examples in which ROS suppress, rather than activate, autophagy; and where selective autophagy promotes, rather than inhibits ROS production, thereby contributing to cell death. Given that ROS are implicated in cancer, diabetes, atherosclerosis, neurodegenerative diseases and ischemia/reperfusion injury, a deeper understanding of the connections linking ROS and autophagy is greatly needed.
Graphical Abstract

1. Introduction
Reactive oxygen species (ROS) are a group of highly reactive molecules important for intracellular and intercellular signaling including, but not limited to, the superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (HO•) [1]. The majority of endogenous ROS are produced as a byproduct of inefficient electron transfer during oxidative phosphorylation within mitochondria, where excessive ROS can oxidize and irreversibly damage proteins, lipids and DNA, creating a pathological condition known as oxidative stress that has been implicated in numerous human diseases [2]. However, specific ROS, often at lower levels, also participate in highly complex biological pathways that regulate cell growth, cell death and senescence, depending upon the specific context [3].
One important process regulated by ROS and oxidative stress is macroautophagy (hereafter referred to as autophagy), which is a mechanism that delivers intracellular material to lysosomes for degradation. Autophagy involves the envelopment of excess or dysfunctional organelles, specific proteins and protein aggregates, or non-specific portions of the cytoplasm into double-membrane vesicles called ‘autophagosomes’, which then fuse with lysosomes, exposing the sequestered material to degradative acid hydrolases. Autophagy can be activated by other stressors including hypoxia, nutrient or growth factor deprivation, and chemo- or radiotherapy—all of which, not coincidentally, can also trigger the accumulation of ROS. Indeed, some evidence suggests that ROS are essential for the efficient activation of stress-induced autophagy [4–6]. By eliminating intracellular sources of ROS, autophagy is generally thought to be cytoprotective; however, prolonged activation of autophagy or specific types of selective autophagy can also result in cell death [7–9].
2. ROS-mediated regulation of autophagy
2.1 mTORC1 Complex
The ULK1 complex, comprised of unc-51 like autophagy activating kinase 1 (ULK1), autophagy-related 13 (ATG13), RB1-inducible coiled coil 1 (RB1CC1/FIP200) and ATG101, integrates a variety of upstream signaling cascades and is responsible for initiating formation of the autophagosome precursor membrane called the ‘phagophore’. One major regulator of the ULK1 complex is the mechanistic target of rapamycin complex 1 (mTORC1), which normally suppresses complex activity by binding and phosphorylating ULK1 at Ser757 and ATG13 at Ser258 [10]. A variety of stressors, including numerous chemotherapeutic agents, reportedly generate ROS that suppress phosphatidylinositol 3-kinase (PI3K)/Akt/mTORC1 signaling and activate autophagy through ill-defined mechanisms [9,11]. In contrast, H202 has been shown to oxidize a specific fraction of phosphatase and tensin homolog (PTEN), forming a disulfide bond that reversibly inhibits its phosphatase activity and thus activates the PI3K/Akt pathway [12,13] (Fig 1A). Given these data, it is apparent that the specific type and extent of ROS generation can result in vastly differing effects on the upstream signaling pathways that control autophagy.
Figure 1. Regulation of autophagy by ROS.
The ULK1 complex integrates upstream mTORC1 and AMPK signaling pathways and ultimately activates the PIK3C3 complex to initiate phagophore formation (see text for details). (A) ROS reportedly activate and suppress PI3K/Akt/mTORC1 signaling through multiple poorly defined mechanisms. (B) ROS activate ATM, which activates an LKB1-AMPK pathway that inhibits the mTORC1 complex and induces autophagy. ROS may also directly oxidize and activate AMPK. (C) Anti-apoptotic Bcl-2 family members suppress autophagy by binding Beclin 1 and inhibiting PIK3C3 complex activity. Oxidation of HMGB1 and ASK1 results in disruption of the Bcl-2-Beclin 1 interaction through direct displacement or phosphorylation of key residues within the Bcl-2. (D) ROS activate Ca2+ channels, triggering Ca2+ influx that leads to translocation of TFEB and the expression of ATG and lysosomal genes. Ca2+ may also activate CAMKK2 and CAMK2, and in turn, positively or negatively regulate PIK3C3 complex activity. (E) Following the activation of the ULK1 and PIK3C3 complexes, LC3/GABARAP proteins are cleaved by ATG4 and conjugated to PE on the phagophore, resulting in its elongation and closure to form a mature autophagosome. ATG4 then deconjugates LC3/GABARAP proteins from the outer autophagosomal membrane for recycling (see text for details). Stress reportedly triggers formation of a TXNIP-REDD1 complex, which induces ROS that oxidize and inhibit ATG4 activity, thereby activating autophagy presumably by suppressing the deconjugation of LC3/GABARAP proteins during phagophore elongation and autophagosome maturation.
2.2 AMPK Complex
Another regulatory kinase, AMP-activated protein kinase (AMPK), directly phosphorylates multiple residues on ULK1 and activates its kinase activity [10]. Moreover, AMPK also phosphorylates and inhibits the mTORC1 complex, leading to de-repression of the ULK1 complex [14]. Thus, perhaps not surprisingly, ROS-mediated activation of AMPK is reportedly essential for starvation-induced autophagy [4]. Indeed, H202 has been shown to oxidize and directly activate AMPK [15], and is known to activate ataxia-telangiectasia mutated (ATM) [16], which can indirectly activate AMPK, inhibit mTORC1, and induce autophagy through activation of liver kinase B1 (LKB1) [17] (Fig. 1B). Therefore, ROS activates AMPK through multiple mechanisms, resulting in mTORC1 suppression and autophagy activation.
2.3 PIK3C3 Complex
The ULK1 complex mediates the recruitment and activation of the phosphatidylinositol 3-kinase class 3 (PIK3C3) complex that consists of a catalytic subunit, PIK3C3; a scaffold subunit, phosphoinositide-3-kinase regulatory subunit 4 (PIK3R4/VPS15); and multiple regulatory subunits such as ATG14, Beclin 1, and autophagy and Beclin 1 regulator 1 (AMBRA1) [18]. ULK1 phosphorylation of Beclin 1 and AMBRA1 results in recruitment of the PIK3C3 complex to sites of phagophore formation and stimulates its lipid kinase activity [19,20]. PIK3C3 then generates site-specific phosphatidylinositol 3-phosphate (PtdIns3P) that is essential for the formation of an endoplasmic reticulum (ER)/mitochondria-associated structure called the ‘omegasome’, which serves as a scaffold for the expanding phagophore [21]. It has been established for some time that anti-apoptotic B-cell lymphoma (Bcl-2) family members bind to Beclin 1, suppress PIK3C3 complex activity, and block autophagy [22,23]. More recently, however, starvation-induced ROS have been shown to oxidize high mobility group box 1 (HMGB1), triggering its translocation from the nucleus into the cytoplasm, where it interacts with Beclin 1, displaces Bcl-2, and induces autophagy [24,25] (Fig. 1C). Phosphorylation of multiple residues in Bcl-2 by c-Jun N-terminal protein kinase 1 (JNK1) similarly activates autophagy by disrupting its inhibitory interaction with Beclin 1 [26]. Notably, JNK1 is activated following the oxidation of its upstream redox-sensitive regulator, apoptosis signal-regulating kinase 1 (ASK1/MAP3K5) [27,28] (Fig. 1C). Thus, like the ULK1 complex, the PIK3C3 complex is indirectly regulated by ROS through multiple mechanisms.
2.4 Calcium Signaling
It is increasingly clear that the crosstalk between ROS and calcium (Ca2+) signaling likewise plays an important role in regulating autophagy. For example, ROS activate the lysosomal Ca2+ channel, mucolipin-1 (MCOLN1/TRPML1), thereby triggering Ca2+ release and calcineurin-dependent translocation of transcription factor EB (TFEB) into the nucleus, where it promotes autophagy by activating autophagy-related (ATG) and lysosomal gene expression [29] (Fig. 1D). ROS, produced in response to hypoxia, reportedly also trigger re-localization of stromal interaction molecule 1 (STIM1) to the plasma membrane, where it activates Ca2+ release activated Ca2+ (CRAC) channels, resulting in Ca2+ influx and the activation of calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) [30]. Since CAMKK2 can activate AMPK, this in turn results in the suppression of mTORC1 and the induction of autophagy [31]. More recently, STIM1-dependent Ca2+ influx was similarly shown to activate CAMKK2, AMPK and autophagy in response to treatment with oxidized low-density lipoproteins (oxLDL) that are known to induce ROS [32,33]. In contrast, however, ROS-dependent activation of transient receptor potential cation channel subfamily M member 2 (TRPM2) (and the resulting Ca2+ influx) reportedly inhibits autophagy by activating calcium/calmodulin-dependent protein kinase II (CAMK2), which in turn phosphorylates Beclin 1 and disrupts its interaction with PIK3C3 [34] (Fig. 1D). It is unknown whether CAMKK2 activates CAMK2 as a negative feedback mechanism to prevent overactivation of autophagy following the influx of Ca2+. Further studies are needed to determine specifically how ROS/Ca2+ signaling regulates autophagy.
2.5 Redox Regulation of ATG4
While the ULK1 and PIK3C3 complexes control initiation of the phagophore, the Atg8 conjugation system mediates the recruitment of diverse cargo to the phagophore and promotes its elongation and closure to form the autophagosome [35]. Mammalian homologs of yeast Atg8, collectively known as the LC3/GABARAP family, are ubiquitin-like proteins that undergo a ubiquitin-like conjugation reaction requiring activation by the E1-like enzyme ATG7, transfer to the E2-like enzyme ATG3, and finally, conjugation to phosphatidylethanolamine (PE) directly on the phagophore membrane. The final conjugation step is catalyzed by the ATG12–ATG5-ATG16L1 complex, whose formation requires a second ubiquitin-like reaction involving ATG12, which is similarly activated by ATG7, but is instead transferred to the E2-like enzyme ATG10, before being conjugated to ATG5 [36]. Prior to PE conjugation, LC3/GABARAP family proteins require initial processing by one of four mammalian homologs of the yeast cysteine protease Atg4 (ATG4A-D). ATG4 cleaves the C-termini of LC3/GABARAP family proteins, exposing C-terminal glycine residues that are then conjugated to the amine head group of PE on the phagophore (Fig. 1E). Unlike yeast Atg8, which is reportedly cleaved from the outer autophagosomal membrane by Atg4 [37,38], at least a portion of LC3/GABARAP family proteins appear to remain conjugated to PE and participate in autophagosome-lysosome fusion [39,40].
Importantly, H202 treatment and starvation-induced ROS can oxidize and inhibit ATG4A and ATG4B through reversible conversion of the catalytic Cys77 to its sulfenic acid, or through formation of a disulfide bridge involving Cys77 and Cys81 [41]. Interestingly, despite its essential role in the initial processing of LC3/GABARAP family proteins, inhibition of ATG4 was shown to induce phagophore formation and autophagy, presumably by preventing the deconjugation of LC3/GABARAP family proteins during phagophore elongation and closure [41] (Fig. 1E). One potential explanation for this apparent discrepancy is that ATG4-dependent cleavage of pro-LC3 may be more efficient than LC3 deconjugation and thus less impacted by partial inhibition of the ATG4 pool. A subsequent in vitro study revealed that inhibitory oxidation of yeast Atg4 similarly generates a single disulfide bond involving its active-site cysteine, and reduction of the Cys-Cys disulfide bond by thioredoxin (Trx) was sufficient to restore Atg4 activity [42]. Notably, in mammals, thioredoxin interacting protein (TXNIP) binds TRX and forms an intermolecular disulfide bond that inhibits its antioxidant activity and thus might be expected to indirectly enhance the oxidation of ATG4 [43]. TXNIP reportedly also forms a complex with the mTORC1 inhibitor, regulated in development and DNA damage response 1 (REDD1/DDIT4), which triggers ROS formation in response to hypoxia, nutrient starvation and exercise [6,44] (Fig. 1E). Whether the so-called ROS ‘burst’ that accompanies TXNIP-REDD1 complex formation results from enhanced inhibition of TRX remains to be determined; however, the resulting oxidation of ATG4 was found to drive stress-induced activation of autophagy [6].
As alluded to above, given that ATG4 cleavage of LC3/GABARAP family proteins is required for PE-conjugation, long-term oxidation and complete inhibition of ATG4 would be expected to block, rather than induce, LC3/GABARAP lipid conjugation and autophagy. Consequently, more investigation is needed to determine how both the processing and deconjugation activities of ATG4 are redox regulated during autophagosome formation and maturation. To date, ATG4 remains the only core ATG protein whose activity is known to be directly regulated by ROS. While multiple E1 and E2 enzymes involved in ubiquitin and small ubiquitin-like modifier (SUMO) conjugation reactions reportedly function as redox sensors [45,46], it is still unknown whether the active-site cysteines of ATG7, ATG10 or ATG3 are similarly redox-regulated.
3. Regulation of ROS by Selective Autophagy
3.1 Mitophagy
While ROS are normal byproducts of oxidative phosphorylation, localized ‘bursts’ of ROS have been shown to cause mitochondrial depolarization, triggering a selective form of autophagy known as ‘mitophagy’ [47,48] (Fig. 2A). The most studied mitophagy mechanism is initiated when mitochondrial depolarization disrupts the normal import, processing and degradation of PTEN-induced putative kinase 1 (PINK1), resulting in its accumulation on the outer mitochondrial membrane (OMM) [49]. Recent evidence suggests that PINK1 phosphorylates Ser65 on ubiquitin that is conjugated to OMM proteins. Phosphorylated ubiquitin in turn recruits the E3 ubiquitin ligase parkin (PARK2), resulting in its phosphorylation and activation by PINK1, allowing PARK2 to then catalyze the polyubiquitination of OMM proteins. As these newly conjugated ubiquitin moieties are substrates for PINK1, this initiates a feed-forward amplification loop that further augments mitophagy [50] (Fig. 2A). PINK1 also phosphorylates and activates TANK binding kinase 1 (TBK1) [51], which in turn phosphorylates the essential mitophagy receptors, calcium binding and coiled-coil domain 2 (NDP52/CALCOCO2) and optineurin (OPTN), and in some cases the non-essential receptors sequestosome-1 (SQSTM1/p62), neighbor of BRCA1 gene 1 (NBR1) and Tax1-binding protein 1 (TAX1BP1) [50]. This increases their affinities for ubiquitin, allowing them to recruit ubiquitinated mitochondria to PE-conjugated LC3/GABARAP proteins on existing phagophores via LC3 interacting regions (LIRs) that are shared by all autophagy receptors [49,51] (Fig. 2B). Alternatively, NDP52 and OPTN were also found to initiate phagophore formation at the OMM by recruiting ULK1 and other essential ATG proteins, therefore acting upstream of phagophore formation [50] (Fig. 2C). While much has been discovered, further investigation will be required to determine precisely how PINK1-mediated phosphorylation and PARK2-mediated ubiquitination coordinately initiate mitophagy, as well as how mitochondria are then delivered to lysosomes for degradation.
Figure 2. PINK1-mediated mitophagy.
(A) Localized bursts of ROS can trigger mitochondrial depolarization resulting in the accumulation of PINK1 on the OMM. PINK1 then initiates mitophagy by phosphorylating ubiquitin (Ub) that is conjugated to OMM proteins. The E3 ubiquitin ligase PARK2 binds to the phosphorylated Ub, which results in its phosphorylation and activation by PINK1. PARK2 then catalyzes the polyubiquitination of OMM proteins that are similarly phosphorylated by PINK1 and recognized by PARK2, thus amplifying the mitophagy signal. TBK1 phosphorylates mitophagy receptors NDP52 and OPTN (and possibly others), enhancing their affinity for phosphorylated Ub. (B) NDP52 and OPTN can recruit mitochondria to existing phagophores by binding PE-conjugated LC3/GABARAP proteins via LC3-interacting regions (LIRs), or alternatively, (C) may recruit the ULK1 complex and other ATG proteins to the OMM to induce the formation of phagophores. By eliminating mitochondria that serve as the major source of intracellular ROS, mitophagy is an important defense against oxidative stress.
Finally, since mitochondria serve as the major source of intracellular ROS, mitophagy is critically important for suppressing oxidative stress [1]. Cardiac deletion of Atg5 or Pink1 in mice results in age-related cardiomyopathies that are associated with mitochondrial dysfunction and oxidative stress [52,53]. ROS-mediated oxidative damage of genomic and mitochondrial DNA has also been implicated in genomic instability and tumorigenesis, while ROS-mediated signaling is associated with metabolic reprogramming and tumor growth in vivo [54]. Collectively, the aforementioned studies suggest that mitochondrial ROS can trigger depolarization and serve as a signal to initiate PINK1-dependent mitophagy, which functions to eliminate a major source of intracellular ROS that contribute to a variety of pathological conditions.
3.2 Pexophagy
In addition to oxidative phosphorylation in mitochondria, ROS are also generated as a byproduct of fatty acid β-oxidation in peroxisomes. Therefore, similar to mitophagy, ROS can trigger the selective degradation of peroxisomes in a process known as ‘pexophagy’ [55]. As previously mentioned, H202 was found to activate ATM, ultimately inducing autophagy by activating AMPK and inactivating mTORC1 [17] (Fig. 1B). Later, H202 was found to stimulate the association of ATM with peroxisomes, where it phosphorylated peroxisomal biogenesis factor 5 (PEX5), resulting in the monoubiquitination of PEX5 by PEX2 and subsequent recognition by p62, thereby targeting the peroxisome for autophagic degradation [55,56]. Neighbor of BRCA1 gene 1 (NBR1) reportedly also cooperates with p62 in mediating the recruitment of peroxisomes to the phagophore [57] (Fig. 3A). Thus, while the specific factors mediating mitophagy and pexophagy differ, both processes share fundamental mechanistic and functional similarities.
Figure 3. Mechanisms of pexophagy and ferritinophagy.
(A) ROS activate ATM and promote its localization to peroxisomes where it phosphorylates PEX5, resulting in its monoubiquitination by PEX2. p62 and NBR1 cooperatively bind the monoubiquitinated PEX5, thus recruiting the peroxisome to the phagophore. Since ROS are also produced in peroxisomes as a byproduct of fatty acid β-oxidation, pexophagy is another mechanism by which sources of ROS can be eliminated. (B) The selective degradation of ferritin, or ferritinophagy, results in increased levels of free iron (Fe2+) that can catalyze formation of the highly reactive HO• through Fenton chemistry. This process is mediated by NCOA4, which binds to ferritin and PE-conjugated LC3/GABARAP proteins on the phagophore. Excessive ferritinophagy and high free Fe2+ levels result in lipid peroxidation and ferroptosis.
3.3 Ferritinophagy
Redox metals like ferrous iron (Fe2+) catalyze the breakdown of H2O2 into hydroxyl anions (HO−) and hydroxyl radicals (HO•) in what is known as the Fenton reaction. The HO• is highly reactive and damages lipids, proteins and DNA. Therefore, to meet basic metabolic needs and avoid oxidative damage, Fe2+ is normally maintained at low levels in a ‘labile iron pool’ (LIP), while excess Fe2+ is sequestered in ferritin complexes and stored as ferric iron (Fe3+). Following depletion of the LIP, ferritin is targeted to lysosomes via nuclear receptor coactivator 4 (NCOA4) and degraded through ‘ferritinophagy’, which releases Fe2+ and restores the LIP [58]. Increased levels of free Fe2+ resulting from excessive ferritinophagy, however, can also catalyze the formation of HO• that stimulate lipid peroxidation, damage membranes, and induce a form of non-apoptotic, iron-dependent cell death known as ‘ferroptosis’ [8,59,60] (Fig. 3B). Therefore, unlike the previously discussed mechanisms that suppress ROS production and are generally considered cytoprotective, ferritinophagy can instead trigger formation of the highly reactive HO• and promote cell death.
3.4 Selective KEAP1 degradation
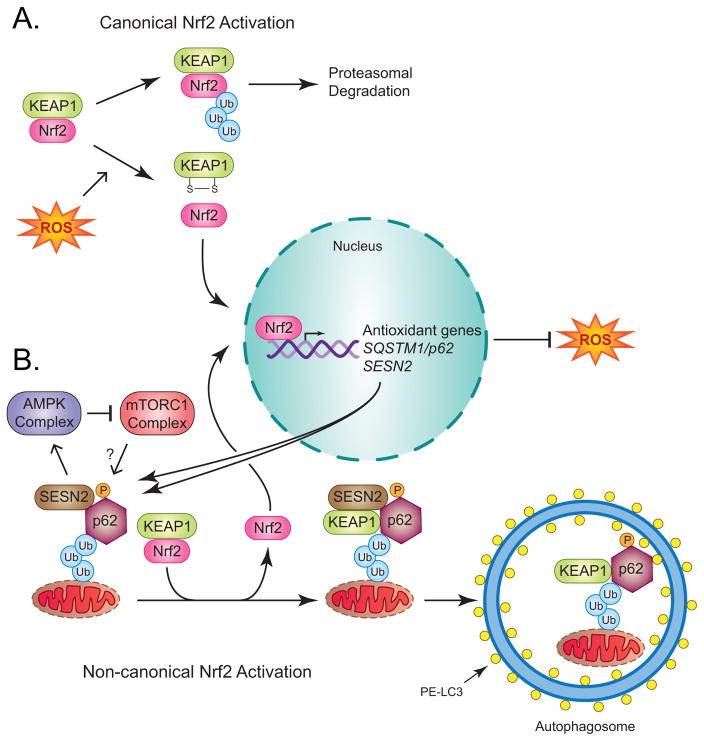
Kelch like ECH associated protein 1 (KEAP1) normally functions as a substrate adaptor protein within a larger E3 ubiquitin ligase complex containing cullin 3 (CUL3) and ring-box 1 (RBX1). Thus, it facilitates the ubiquitination and proteasomal degradation of protein substrates, including the transcription factor, nuclear factor erythroid 2 like 2 (Nrf2). Canonical activation of Nrf2 occurs when KEAP1 is oxidized, triggering its dissociation and allowing stabilized Nrf2 to translocate into the nucleus and activate the expression of numerous antioxidant and detoxification genes [61] (Fig. 4A). Interestingly, two of these gene products, p62 and sestrin 2 (SESN2), activate a non-canonical pathway leading to further activation of Nrf2 and more robust suppression of ROS [62] (Fig. 4B). In this pathway, ubiquitin-bound p62 is phosphorylated in an mTORC1-dependent manner, which enhances its affinity for KEAP1. Phosphorylated p62, together with SESN2, then mediate the selective autophagic degradation of KEAP1, resulting in the stabilization of Nrf2 [63–65]. Notably, SESN2 can also activate AMPK and inhibit mTORC1 [66], which might serve as a negative feedback loop to suppress p62 phosphorylation and selective KEAP1 degradation during autophagy activation (Fig. 4B). While the relationship between mTORC1 and the selective autophagic degradation of KEAP1 is complex and incompletely understood, it is clear that autophagy can indirectly regulate ROS by inducing transcriptional upregulation of antioxidant genes through non-canonical activation of Nrf2.
Figure 4. Canonical and non-canonical mechanisms of Nrf2 activation.
(A) KEAP1 functions as an adaptor protein that mediates Nrf2 polyubiquitination and proteasomal degradation. Canonical activation of Nrf2 occurs when ROS oxidize KEAP1, forming a disulfide bond that liberates Nrf2 and allows it to translocate into the nucleus and transcriptionally activate numerous antioxidant genes whose gene products in turn suppress ROS. (B) Nrf2 promotes SQSTM1/p62 and SESN2 expression, whose gene products mediate non-canonical activation of Nrf2, thus forming a positive feedback loop. Non-canonical Nrf2 activation involves phosphorylation of ubiquitin-bound p62 by an unidentified kinase in a mTORC1- dependent manner, which enhances its affinity for KEAP1. SESN2 binds to p62 and KEAP1, resulting in Nrf2 displacement and the selective degradation of KEAP1. Nrf2 can then translocate into nucleus and activate further expression of SQSTM1/p62, SESN2 and other antioxidant genes.
4. Concluding Remarks
Crosstalk between ROS and autophagy occurs through multiple unique and seemingly conflicting means, making the characterization of their individual functional effects a significant challenge. Evidence increasingly suggests that redox regulation of both upstream regulators and the core autophagic machinery plays a major role in stress-induced autophagy. In most cases, ROS activate autophagy as a cytoprotective negative-feedback mechanism that eliminates the sources of ROS production and prevents oxidative damage. However, we have provided several examples wherein either ROS suppress autophagy or autophagy promotes ROS production, both of which can result in cellular injury or death. Consequently, the specific mechanisms of this complex relationship have important implications for numerous human diseases associated with ROS and autophagy.
Highlights.
ROS regulate autophagy through activation of multiple upstream kinase signaling pathways and direct inhibition of ATG4.
Depolarization of injured ROS-producing mitochondria triggers PINK1 and PARK2-dependent mitophagy.
Activation of ATM by ROS results in its re-localization to peroxisomes where it stimulates pexophagy and limits further ROS production.
Excessive ferritinophagy liberates iron that in turn catalyzes HO•-dependent lipid peroxidation and ferroptosis.
Oxidation and selective autophagy of KEAP1 leads to canonical and non-canonical activation of the Nrf2 antioxidant signaling pathway, respectively.
Acknowledgments
This work was supported by a NIH grant RO1 GM116024 and a University of Texas MD Anderson Cancer Center Institutional Research Grant (IRG) to SBB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013;25:50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- ••6.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, et al. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun. 2015;6:7014. doi: 10.1038/ncomms8014. Demonstrated that cellular stress triggers formation of a novel REDD1/TXNIP complex that results in ROS-mediated oxidization of ATG4B and autophagy activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 8.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leng ZG, Lin SJ, Wu ZR, Guo YH, Cai L, Shang HB, Tang H, Xue YJ, Lou MQ, Zhao W, et al. Activation of DRD5 (Dopamine Receptor D5) Inhibits Tumor Growth by Autophagic Cell Death. Autophagy. 2017 doi: 10.1080/15548627.2017.1328347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61–68. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pant K, Saraya A, Venugopal SK. Oxidative stress plays a key role in butyrate-mediated autophagy via Akt/mTOR pathway in hepatoma cells. Chem Biol Interact. 2017;273:99–106. doi: 10.1016/j.cbi.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 13.Shen SM, Guo M, Xiong Z, Yu Y, Zhao XY, Zhang FF, Chen GQ. AIF inhibits tumor metastasis by protecting PTEN from oxidation. EMBO Rep. 2015;16:1563–1580. doi: 10.15252/embr.201540536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop EA, Tee AR. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem Soc Trans. 2013;41:939–943. doi: 10.1042/BST20130030. [DOI] [PubMed] [Google Scholar]
- 15.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •16.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. Identified and characterized the redox regulation of ATM. [DOI] [PubMed] [Google Scholar]
- 17.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley JH, Young LN. Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(Suppl 1):S137–148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal. 2011;15:2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. doi: 10.1038/ncomms12109. Linked ROS to lysosomal calcium channel activation and TFEB-mediated expression of autophagy-related genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Zabirnyk O, Liu W, Khalil S, Sharma A, Phang JM. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis. 2010;31:446–454. doi: 10.1093/carcin/bgp299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Yu J, Li D, Yu S, Ke J, Wang L, Wang Y, Qiu Y, Gao X, Zhang J, et al. Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL exposure. Autophagy. 2017;13:82–98. doi: 10.1080/15548627.2016.1245261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••34.Wang Q, Guo W, Hao B, Shi X, Lu Y, Wong CW, Ma VW, Yip TT, Au JS, Hao Q, et al. Mechanistic study of TRPM2-Ca(2+)-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition. Autophagy. 2016;12:1340–1354. doi: 10.1080/15548627.2016.1187365. Discovered that ROS-induced Ca2+ influx inhibits autophagy through CAMK2-dependent phosphorylation of Beclin 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaaf MB, Keulers TG, Vooijs MA, Rouschop KM. LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J. 2016;30:3961–3978. doi: 10.1096/fj.201600698R. [DOI] [PubMed] [Google Scholar]
- 36.Noda NN, Inagaki F. Mechanisms of Autophagy. Annu Rev Biophys. 2015;44:101–122. doi: 10.1146/annurev-biophys-060414-034248. [DOI] [PubMed] [Google Scholar]
- 37.Yu ZQ, Ni T, Hong B, Wang HY, Jiang FJ, Zou S, Chen Y, Zheng XL, Klionsky DJ, Liang Y, et al. Dual roles of Atg8-PE deconjugation by Atg4 in autophagy. Autophagy. 2012;8:883–892. doi: 10.4161/auto.19652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair U, Yen WL, Mari M, Cao Y, Xie Z, Baba M, Reggiori F, Klionsky DJ. A role for Atg8-PE deconjugation in autophagosome biogenesis. Autophagy. 2012;8:780–793. doi: 10.4161/auto.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Sun HQ, Zhu X, Zhang L, Albanesi J, Levine B, Yin H. GABARAPs regulate PI4P-dependent autophagosome:lysosome fusion. Proc Natl Acad Sci U S A. 2015;112:7015–7020. doi: 10.1073/pnas.1507263112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •40.guyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol. 2016;215:857–874. doi: 10.1083/jcb.201607039. LC3/GABARAP proteins were found to be nonessential for cargo recruitment or autophagosome formation but rquired for phagophore elongation and autophagosome-lysosome fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •41.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. First example of redox regulation of core autophagy machinery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Perez ME, Zaffagnini M, Marchand CH, Crespo JL, Lemaire SD. The yeast autophagy protease Atg4 is regulated by thioredoxin. Autophagy. 2014;10:1953–1964. doi: 10.4161/auto.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang J, Suh HW, Jeon YH, Hwang E, Nguyen LT, Yeom J, Lee SG, Lee C, Kim KJ, Kang BS, et al. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat Commun. 2014;5:2958. doi: 10.1038/ncomms3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellisen LW. Growth control under stress: mTOR regulation through the REDD1-TSC pathway. Cell Cycle. 2005;4:1500–1502. doi: 10.4161/cc.4.11.2139. [DOI] [PubMed] [Google Scholar]
- 45.Stewart MD, Ritterhoff T, Klevit RE, Brzovic PS. E2 enzymes: more than just middle men. Cell Res. 2016;26:423–440. doi: 10.1038/cr.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- •47.Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8:1462–1476. doi: 10.4161/auto.21211. Confirmed a role for ROS in mitophagy induction through utilization of a novel photosensitizer that generates mitochondrial-specific bursts of ROS. [DOI] [PubMed] [Google Scholar]
- 48.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •49.Yamano K, Matsuda N, Tanaka K. The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation. EMBO Rep. 2016;17:300–316. doi: 10.15252/embr.201541486. Excellent review on mechanisms of mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••50.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. Landmark study that challenged multiple aspects of the accepted models for mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol Cell. 2015;60:7–20. doi: 10.1016/j.molcel.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 54.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••55.Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 2015;17:1259–1269. doi: 10.1038/ncb3230. Linked ATM to peroxisomes and ROS-induced pexophagy in mammalian cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sargent G, van Zutphen T, Shatseva T, Zhang L, Di Giovanni V, Bandsma R, Kim PK. PEX2 is the E3 ubiquitin ligase required for pexophagy during starvation. J Cell Biol. 2016;214:677–690. doi: 10.1083/jcb.201511034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deosaran E, Larsen KB, Hua R, Sargent G, Wang Y, Kim S, Lamark T, Jauregui M, Law K, Lippincott-Schwartz J, et al. NBR1 acts as an autophagy receptor for peroxisomes. J Cell Sci. 2013;126:939–952. doi: 10.1242/jcs.114819. [DOI] [PubMed] [Google Scholar]
- •58.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. An unbiased proteomics approach identified an essential mediator of ferritinophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhee SG, Bae SH. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- ••64.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. Identified KEAP1 as a target for selective autophagy and non-conanical Nrf2 activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]