In this study, HSV infection was identified in 0.42% of 26 533 encounters in 0 to 60-day-old infants being evaluated by LP for CNS infection.
Abstract
BACKGROUND:
Although neonatal herpes simplex virus (HSV) is a potentially devastating infection requiring prompt evaluation and treatment, large-scale assessments of the frequency in potentially infected infants have not been performed.
METHODS:
We performed a retrospective cross-sectional study of infants ≤60 days old who had cerebrospinal fluid culture testing performed in 1 of 23 participating North American emergency departments. HSV infection was defined by a positive HSV polymerase chain reaction or viral culture. The primary outcome was the proportion of encounters in which HSV infection was identified. Secondary outcomes included frequency of central nervous system (CNS) and disseminated HSV, and HSV testing and treatment patterns.
RESULTS:
Of 26 533 eligible encounters, 112 infants had HSV identified (0.42%, 95% confidence interval [CI]: 0.35%–0.51%). Of these, 90 (80.4%) occurred in weeks 1 to 4, 10 (8.9%) in weeks 5 to 6, and 12 (10.7%) in weeks 7 to 9. The median age of HSV-infected infants was 14 days (interquartile range: 9–24 days). HSV infection was more common in 0 to 28-day-old infants compared with 29- to 60-day-old infants (odds ratio 3.9; 95% CI: 2.4–6.2). Sixty-eight (0.26%, 95% CI: 0.21%–0.33%) had CNS or disseminated HSV. The proportion of infants tested for HSV (35%; range 14%–72%) and to whom acyclovir was administered (23%; range 4%–53%) varied widely across sites.
CONCLUSIONS:
An HSV infection was uncommon in young infants evaluated for CNS infection, particularly in the second month of life. Evidence-based approaches to the evaluation for HSV in young infants are needed.
What’s Known on This Subject:
Herpes simplex virus (HSV) can present subtly yet have devastating outcomes. Fortunately, it is a relatively uncommon infection, yet empirical acyclovir therapy is commonly provided to at-risk infants. Accurate frequency data are needed to guide decision-making.
What This Study Adds:
Of 26 533 infants 0 to 60 days old who underwent lumbar puncture at 1 of 23 emergency departments, 0.42% (95% confidence interval: 0.35%–0.51%) had HSV infection. For every 1 infant with HSV infection, 237 infants needed to be treated. This knowledge could guide empiric acyclovir use.
Although herpes simplex virus (HSV) infection in young infants can have subtle initial clinical presentations, mortality rates as high as 30% have been reported.1,2 Published single-center experiences and studies with national-level data have reported case rates per 100 000 live births ranging from 1.7 (British Isles) to 3.3 (Australia) to 5.9 (Canada) and 13.4 (United States).3–9 A recently published global estimate indicated an incidence rate of 10 cases of HSV infection out of 100 000 live births, or ∼14 000 worldwide cases per year.10 In industrialized countries, neonatal HSV has been reported to cause 0.82 deaths per 100 000 live births.11
Symptoms in infants presenting to the emergency department (ED) with HSV infection vary from hypothermia to pyrexia and from poor feeding to convulsions. Because the symptoms in young infants overlap with those of bacterial infection, infants undergoing evaluation for bacterial infections also require the consideration of an HSV infection. The rarity of infection, coupled with its myriad of clinical features, has led to a variation in the ED management of at-risk infants, which has a direct impact on patient outcomes because the delayed administration of acyclovir is associated with increased mortality.12 However, HSV testing and empirical antiviral therapy in low-risk infants are associated with prolonged hospitalization, increased costs, and nephrotoxicity.13–15 Thus, the decision to test and treat infants for suspected HSV infection is of paramount importance, and requires an accurate knowledge of the risk of HSV infection to optimize decision-making.
Given the described limitations in the existing knowledge, we evaluated a large cross-sectional sample of infants ≤60 days old who were assessed for a possible central nervous system (CNS) infection in EDs located in the United States and Canada. The primary goal of this investigation was to accurately determine the proportion of at-risk infants in whom HSV infection was identified. Secondary goals were to quantify the frequency of HSV testing and empirical acyclovir treatment across study sites.
Methods
Study Setting
We performed a retrospective cross-sectional study at 23 tertiary-care pediatric EDs, each with university affiliations (Supplemental Table 4). Our study protocol was endorsed by the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Participating sites were located in 1 of 16 US states or 2 Canadian provinces. Institutional review board approval was obtained at each participating site.
Patients
We screened hospital records to identify infants ≤60 days old who presented to a participating study institution’s ED and had cerebrospinal fluid (CSF) bacterial cultures obtained within 24 hours of ED arrival. CSF bacterial culture collection was used as a proxy to indicate a clinical concern for CNS infection. Eligible infants were identified by querying either available electronic health records or laboratory databases from January 1, 2005, to December 31, 2013. Sites submitted data based on the start of their electronic health records. We excluded encounters in which a CSF culture was obtained before arrival at the study site ED. Encounters for the same infant were included more than once if CSF cultures were performed on different admissions; repeat encounters after an HSV diagnosis were excluded.
Data Sources
Discrete elements were extracted from available electronic databases or medical records. Each investigator received standardized data abstraction training. The manual of operations provided detailed instructions for the collection of the following data elements: demographics, triage temperature, disposition, length of stay, blood tests, viral culture, HSV typing, polymerase chain reaction (PCR) tests, and antiviral therapy.
Outcome Measures
The primary outcome was the proportion of ED encounters in which HSV infection was identified. We classified infants as having HSV infection if the virus was detected by PCR and/or viral culture from any of the following sites: CSF, blood, surface swabs (eg, skin, conjunctivae, mouth, or nasopharynx), or other body fluids. The PCR assays used are described in the Supplemental Information. HSV was classified as skin, eye, and mouth (SEM), CNS, or disseminated using the definitions as they appear in Red Book 2015: Report of the Committee on Infectious Diseases.16 SEM disease was defined as a PCR or viral culture from only skin, eyes, or mouth positive for HSV. CNS disease was defined as a CSF PCR (with or without skin findings) positive for HSV or viral culture in the absence of other (non-CNS) end-organ damage. Disseminated HSV was defined as presence of hepatitis (alanine aminotransferase >1.5 times the upper limit of normal and/or evidence of disseminated intravascular coagulation)17 or other end-organ damage other than isolated CNS manifestations (with or without skin findings).16,18 A blood PCR positive for HSV alone did not define disease as disseminated.16 HSV serologies were not used to define cases of HSV because serologies could reflect maternal exposure rather than neonatal infection.16 Infants who were not tested for HSV were assumed to be uninfected if HSV infection was not diagnosed during the inpatient hospital stay or through a repeat ED encounter, given the short incubation period and presumed progression to clinically evident disease-associated symptoms.
Secondary outcomes were the frequency of (1) HSV infection in infants in whom the virus was isolated from blood and/or CSF, not exclusively from surface sources, (2) encounters in which HSV testing was performed, and (3) encounters in which empirical acyclovir administration was initiated within 24 hours of ED arrival.
Statistical Analyses
The unit of analysis was the ED encounter. The relative frequency of HSV infection was compared across the following age groups: week of life and month of life (ie, 0–28 days vs 29–60 days and 0–42 days vs 43–60 days). Because infants who were not tested for HSV were assumed to be uninfected, we performed subgroup analyses to evaluate HSV frequency encounters in admitted infants and in infants in whom HSV testing was performed.
We subsequently compared the frequency of HSV testing, treatment, and infection across participating centers by using Pearson χ2 tests. For these analyses, we excluded ED encounters with missing data on acyclovir administration. Participating EDs were categorized into pentiles for HSV testing, treatment, and infection rates, to enable visual comparisons between EDs based on these parameters and then used Spearman correlation coefficients to further explore these relationships. We calculated the number needed to test and empirically treat as the inverse of the HSV disease prevalence, assuming that all empirical therapy in this scenario would be provided to all infants undergoing CSF evaluation for CNS infection. We used Stata 11 (StataCorp, College Station, TX) for all statistical analyses.
Results
Study Population
We identified 26 533 eligible ED encounters, of which 13 687 (51.6%; 95% confidence interval [CI]: 51.0%–52.2%) involved infants ≤28 days old. In total, 23 184 (87.4%; 95% CI: 87.0%–87.8%) encounters resulted in inpatient hospitalization (Table 1). There were 26 102 unique patients included in the study; repeat ED encounters in which CSF was obtained accounted for 521 visits (2.0% of encounters, 95% CI: 1.8%–2.1%).
TABLE 1.
Description of the Study Population (N = 26 533)
| Variable | Total Encounters (N = 26 533), No. (%) | HSV Uninfected (n = 26 421), No. (%) | HSV Infected (n = 112), No. (%) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, days, median | 28 (IQR 15–41) | 28 (IQR 15–41) | 14 (IQR 9–24) | <.001 |
| Female | 11 716/26 505 (44.2) | 11 669/26 393 (44.2) | 47 (42.0) | .63 |
| Triage temperaturea | ||||
| Hypothermic | 589/22 562 (2.6) | 584/22 465 (2.6) | 5/97 (5.2) | .63 |
| Normothermic | 13 599/22 562 (60.3) | 13 537/22 465 (60.3) | 62/97 (63.9) | .19 |
| Febrile | 8374/22 562 (37.1) | 8344/22 465 (37.1) | 30/97 (30.9) | .13 |
| Laboratory evaluation | ||||
| Any HSV testing obtained | 9185 (34.6) | 9073 (34.4) | 112 (100) | <.001 |
| HSV testing: HSV CSF PCR obtained | 8902 (33.6) | 8794 (33.3) | 108 (96.4) | <.001 |
| HSV testing: HSV blood PCR obtained | 1192 (4.5) | 1143 (4.3) | 49 (43.8) | <.001 |
| Empirical acyclovir therapy | ||||
| Acyclovir initiated within 24 h of ED presentation | 5938/25 358 (23.4) | 5845/25 247 (23.2) | 94 (83.9) | <.001 |
| Disposition | ||||
| Admitted, general ward | 19 636/25 543 (76.9) | 19 559/25 431 (76.9) | 77 (68.8) | <.001 |
| Admitted, ICU | 3548/25 543 (13.9) | 3516/25 431 (13.8) | 32 (28.6) | |
| Discharged | 2359/25 543 (9.2) | 2356/25 431 (9.3) | 3 (2.7) |
Missing data are as follows: sex was not specified in 28 (0.1%) children, triage temperature in 3971 (15.0%), disposition in 998 (3.8%), and acyclovir receipt in 1180 (4.4%). P values are for comparisons between HSV infected and uninfected infants.
Fever was defined as ≥38°C (100.4°F) and hypothermia as <36°C (96.8°F)
HSV Infection
Overall, HSV infection was identified in 0.42% of all encounters (112/26 533; 95% CI: 0.35%–0.51%) and 1.2% (112/9185; 95% CI: 1.0%–1.5%) of all infants tested for HSV. Forty-four infants had SEM disease (0.17%; 95% CI: 0.13%–0.23%), 36 CNS disease (0.14%; 95% CI: 0.10%–0.19%), and 32 disseminated disease (0.12%; 95% CI: 0.08%–0.17%). The frequency of HSV among infants from whom HSV was isolated only from CSF or blood PCR was 46 out of 26 465 (0.17%, 95% CI: 0.13%–0.23%) infants. The most common sites of HSV isolation are described in Table 2. Thirty infants (26.8%; 95% CI: 19.5%–35.7%) with HSV had testing obtained from surface, CSF, and blood sources.
TABLE 2.
Sites of HSV Disease and HSV Detection in 112 Infants
| HSV Classificationa | No. | Specimen Source | HSV Typeb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface PCR/Culture, No. +/No. Obtained (%) | CSF PCR/Culture, No. +/No. Obtained (%) | Blood HSV PCR, No. +/No. Obtained (%) | HSV Only Detected in Culture (%) | HSV-1 (%) | HSV-2 (%) | HSV-1 and HSV-2 (%) | HSV Untyped (%) | ||
| SEM | 44 | 44/44 (100) | 0/41 (0) | 8/16 (50) | 19 (43.2) | 29 (65.9) | 7 (15.9) | 0 | 8 (18.2) |
| CNS | 36 | 10/16 (62.5) | 36/36 (100) | 4/9 (44.4) | 0 (0) | 9 (25.0) | 22 (61.1) | 1 (2.8) | 4 (11.1) |
| Disseminated | 32 | 15/18 (83.3) | 19/31 (61.3) | 22/24 (91.7) | 2 (6.3) | 13 (40.6) | 14 (43.8) | 1 (3.1) | 4 (12.5) |
Percentages reflect within-row percentages and may not sum to 100% because of rounding.
Twelve infants >6 wk of age had HSV infection: 8 had SEM disease, and 2 each had CNS and disseminated disease.
Laboratory protocols varied in terms of HSV typing and some laboratories did not perform routine PCR viral typing. If HSV typing was unavailable from the PCR results, viral culture results were also included. Percentages for HSV typing are within-row percentages.
The median age of HSV infection was the following: any site = 14 days (range: 2–56 days; interquartile range [IQR]: 9–24 days); surface source only = 14 days (range: 2–55 days; IQR: 10–35 days); and CSF or blood only = 15 days (range: 4–56 days; IQR: 9–24 days). Among infants with available HSV type, herpes simplex virus type 1 (HSV-1) accounted for 48 out of 87 (55.2%) cases in the first 6 weeks of life and 5 out of 9 (55.6%) cases in 7- to 9-week-old infants. The per-patient frequency of HSV infection, in which only the first ED visit was included, was 110 out of 26 102 (0.42%, 95% CI: 0.35%–0.51%). Typing was available for 96 (85.7%) infants, of whom 51 out of 96 (53.1%) had HSV-1 and 43 (44.8%) had herpes simplex virus type 2 (HSV-2); in 2 infants, both HSV-1 and HSV-2 were detected.
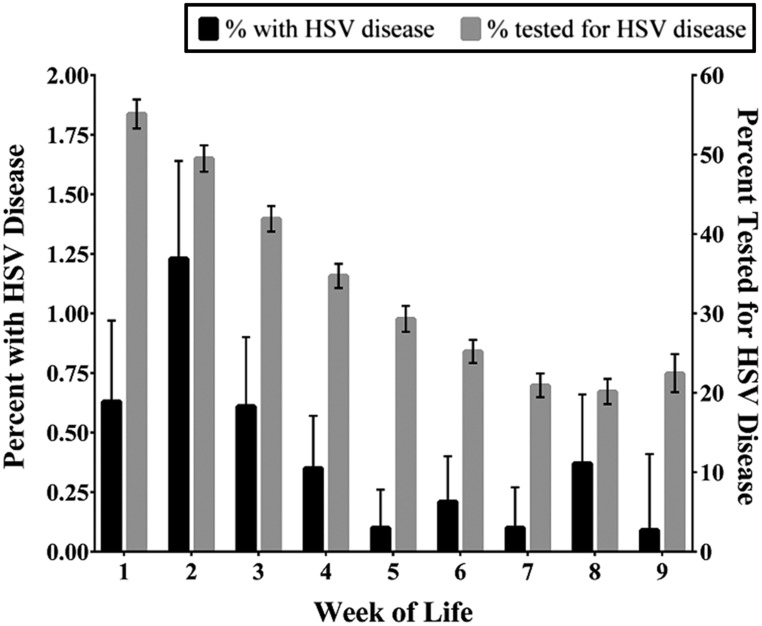
HSV identification from any specimen was more frequent in encounters of 0 to 28-day-old infants versus 29- to 60-day-old infants (odds ratio [OR] 3.9; 95% CI: 2.4–6.2) and in 0 to 42-day-old infants versus 43- to 60-day-old infants (OR: 2.70, 95% CI: 1.48–4.91). The highest frequency of HSV infections was in the second week of life (Fig 1). Twenty-two of 12 846 (0.17%, 95% CI: 0.11%–0.26%) encounters in infants 29 to 60 days of age had HSV infection. Fifteen of the 22 infants had SEM disease (68.2%, 95% CI: 47.3%–83.6%), 5 infants had CNS disease (22.7%, 95% CI: 10.1%–43.4%), and 2 infants had disseminated disease.
FIGURE 1.
HSV cases and testing by week of life.
HSV Testing
Of the 26 533 encounters, 9185 (34.6%; 95% CI: 34.1%–35.2%) had HSV testing performed (Table 1), with considerable variability among EDs (range: 14.4%–72.1%; P < .001) (Table 3). Infants in the first 28 days of life were more likely to be tested for HSV than 29- to 60-day-old infants (OR 2.6; 95% CI: 2.4–2.7). HSV testing was performed in 3040 out of 12 846 (23.7%; 95% CI: 22.9%–24.4%) encounters for 29- to 60-day-old infants. In 17 (73.9%) centers, all HSV PCRs were performed on-site; in the remainder, HSV PCRs were sent out for testing.
TABLE 3.
Variation in Testing and Diagnostic Yield for HSV Infection Across EDs
| Diagnostic Test | Site | No. Obtained | % Obtained (Range Across Centers, %) | % Positive (95% CI) |
|---|---|---|---|---|
| PCR | CSF | 8902 | 33.5 (12.5–70.9) | 0.6 (0.47–0.79) |
| Blood | 1192 | 4.5 (0–17.4) | 2.9 (2.1–4.1) | |
| Skina | 218 | 0.8 (0–4.6) | 14.4 (10.4–19.6) | |
| Eye | 158 | 0.6 (0–3.6) | 3.8 (1.8–8) | |
| Mouth | 110 | 0.4 (0–3.3) | 7.1 (3.4–14) | |
| Viral culture | CSF | 966 | 3.6 (0–47.1) | 0.5 (0.22–1.2) |
| Skinb | 420 | 1.6 (0–8.4) | 7.4 (5.2–10.2) | |
| Eye | 325 | 1.2 (0–16) | 2.8 (1.5–5.2) | |
| Mouth | 299 | 1.1 (0–14.6) | 4 (2.3–6.9) |
If surface cultures and PCRs were collected all on 1 swab, they were classified as “skin.”
A total of 9185 infants received any diagnostic test for HSV.
Acyclovir Therapy
We abstracted data on acyclovir administration for 25 358 (95.5%) encounters. Within 24 hours of ED presentation, 23.4% (5938 out of 25 358 encounters; 95% CI: 22.9%–24.0%) of infants received acyclovir, with considerable variation among EDs in empirical treatment rates (range 4.2%–53.0%; P < .001). Compared to encounters for infants in whom acyclovir therapy was not provided, infants who received acyclovir were younger (mean age 22 ± 14 days vs 31 ± 16 days, P < .001) and were more likely to be tested for HSV (5467 out of 5934 encounters, 92.1% vs 3273 out of 19 419 encounters, 16.9%; OR 57.7; 95% CI: 52.2–63.9). Ninety-four infants with HSV infection received empirical acyclovir (83.9%; 95% CI: 76.0%–89.6%). Of the 18 infants infected with HSV who did not receive empirical acyclovir, all had HSV testing obtained within 24 hours of the initial ED encounter. Three infants who were initially tested but not treated for HSV were discharged from the hospital, then later diagnosed with the following HSV infections when they returned within 48 hours to the ED: (1) 24-day-old infant with SEM disease, (2) 49-day-old infant with SEM disease, and (3) 11-day-old infant with disseminated HSV who later died with multiorgan system failure.
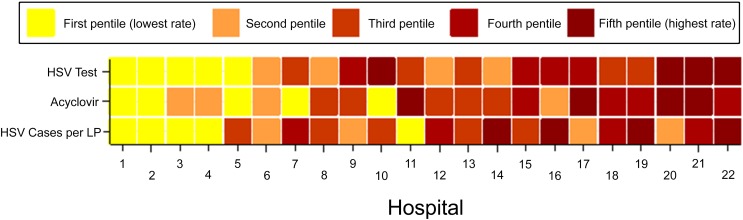
The frequency of HSV infection by institution ranged from 0.0% to 2.8% (median 0.44%; IQR: 0.24%–0.67%). However, the frequency of HSV infection was not associated with the frequency of HSV testing (Spearman’s coefficient: 0.097) or the frequency of empirical treatment (Spearman’s coefficient 0.001) by hospital site (Fig 2). The frequencies of HSV testing and empirical acyclovir administration per site were weakly associated (Spearman’s coefficient 0.23).
FIGURE 2.
Association between HSV testing, empirical acyclovir usage, and HSV frequency for the 22 study sites that contributed data on acyclovir usage for each variable (HSV testing, acyclovir usage, and HSV burden); hospitals were categorized into pentiles. Hospitals were assigned an aggregate ranking based on the sum of the 3 pentile rankings and are ordered in the figure according to their aggregate ranking. Therefore, hospital 1 had the lowest aggregate rank for HSV testing, acyclovir use, and HSV burden.
Number Needed to Treat
The number needed to treat (NNT) with empirical acyclovir to ensure all cases of HSV were treated initially for potential infection was 237 across the entire cohort of young infants undergoing evaluation for CNS infection. Initiation of acyclovir therapy would be required in 152 (95% CI: 123–185) 0 to 28-day-old infants and 583 (95% CI: 384–909) 29- to 60-day-old infants to treat all young infants with HSV infection. The NNT for infants without SEM findings was 588 (95% CI: 435–769). If we limit the analysis to those 9185 infants in whom HSV testing was obtained, the NNT was 82 (95% CI: 68–98).
Discussion
We conducted a multicenter retrospective review of 26 533 encounters for infants evaluated in an ED for CNS infection to determine the proportion of encounters in which infants had HSV infection. Overall, HSV infections were uncommon, identified in only 0.42% of all eligible encounters. In other words, only 1 in 237 infants evaluated for potential CNS infection had HSV infection. Frequency of HSV infection was highest in the second week and declined substantially in the second month of life, although clinician index of suspicion for HSV remained high based on HSV testing frequency in this age group. We identified substantial variation across participating sites in the frequency of HSV testing and empirical treatment; this variation did not correlate with the frequency of HSV infection.
The frequency of HSV infection was similar to previous studies. In a single-center ED study of 5817 neonates admitted to the hospital from an ED for any indication, only 0.2% (95% CI: 0.1%–0.3%) of eligible infants had HSV infection identified from any site.19 Our study cohort differs significantly in that only 45% of neonates included in the aforementioned single-center study had a CSF culture obtained, which may have underestimated the prevalence of CNS HSV infection. In another single-center study, with a similar population (ie, all 570 neonates who had a lumbar puncture [LP] performed in the ED), the HSV infection rate was 0.5% (95% CI: 0.2%–1.5%).20 However, our study differs from previous reports in several ways. First, we included a large number of centers from across North America, making our results highly generalizable. Second, we queried laboratory databases to identify eligible ED encounters rather than either discharge diagnostic codes or surveillance networks which are less reliable and comprehensive. Finally, our large cohort size provides a precise HSV infection point estimate in young infants, thereby increasing pertinence to clinical decision-making.
Nationally, ED providers do not have a standardized approach to the diagnosis and management of HSV infection in febrile infants.21,22 We observed a wide variation in the rates of HSV testing and empirical treatment across participating institutions which did not correlate with the local HSV frequency. The similarities between included EDs precluded analyses to determine if hospital-level characteristics (eg, urban versus rural hospital location) are associated with testing and treatment trends. Delays in acyclovir initiation for infants with HSV infection have been associated with increased in-hospital mortality.9 However, given the rarity of HSV infections, the appropriate approach to evaluation and empirical treatment of at-risk infants has been challenging.
In deciding whether to test and to treat an at-risk infant for possible HSV infection, clinicians must balance the risk of delayed therapy in an infant with HSV infection with the costs and potential iatrogenic complications of acyclovir therapy in uninfected infants. HSV testing and acyclovir treatment of uninfected infants have been associated with a 30% increase in length of stay and a 40% increase in charges.12 Between 1999 and 2012, the use of acyclovir in US children’s hospitals for children with suspected HSV encephalitis increased from 18% to 29% in 0 to 28-day-old infants and from 10% to 19% in 29- to 60-day-old infants.23 Although acyclovir has a relatively favorable safety profile in young infants and nephrotoxicity is uncommon in well-hydrated infants receiving short-course therapy,24 acyclovir use has been associated with increases in serum creatinine in up to one-third of children receiving acyclovir for proven HSV meningoencephalitis,13,14 and 45% of infants treated at least 14 days with acyclovir had at least 1 adverse event, including electrolyte disturbances, myelosuppression, hypotension, and seizures.25 Fortunately, the increased availability of real-time HSV PCR assays26,27 in clinical laboratories can provide clinicians with results rapidly, and thus help guide clinical decision-making regarding acyclovir use. Decreasing the turn-around times for viral diagnostics may lessen the impact of HSV testing on resource utilization and length of hospital stay. Nonetheless, the possibility of expanded HSV testing has the potential to improve outcomes among at-risk infants because of fewer delayed diagnoses of HSV infection.
An optimal approach to the evaluation of and empirical treatment of HSV infection in the young infant could reduce costly testing and empirical treatment of low-risk infants, while ensuring rapid identification and initiation of acyclovir for infants with HSV infection. One cost analysis28 evaluated diagnostic and treatment strategies for the young febrile infant with undifferentiated fever. The authors concluded that for infants with fever and CSF pleocytosis, HSV CSF PCR testing and empirical treatment with acyclovir was cost-effective and would lead to reductions in morbidity and mortality. However, universal testing and treatment was not cost-effective if applied to all febrile neonates. More extensive HSV testing (eg, HSV blood PCR or viral cultures) is indicated in certain populations (eg, infants with meningitis or coagulopathies).
Our study had several limitations. First, in only 34% of all encounters did infants have HSV CSF PCR obtained. To ensure that the other eligible infants did not have HSV, we made assumptions about HSV infection: (1) untreated hospitalized infants would develop signs of HSV infection while hospitalized given the fulminant clinical course29 and (2) discharged infants who developed signs of HSV infection would re-present to the same ED or be transferred back to the tertiary care center. The latter is based on knowledge that almost every participating center serves as the regional source of pediatric care,30 suggesting subsequent presentation with HSV infection to an alternate institution would be exceedingly rare. However, when the analysis was restricted to encounters in which HSV testing was performed, HSV remained rare. Second, HSV testing (including typing and PCR modality) was variable and not all tested infants had uniform laboratory evaluation; thus, a negative HSV test did not exclude all types of HSV disease. Third, HSV testing platforms varied across the study sites and over the study period which limited the availability of HSV viral type data. Fourth, limited clinical data were available for the included infants and the reason the LP was performed (eg, fever before triage) was unavailable. Fifth, performance of LPs among 29- to 60-day-old infants is variable,31 thereby potentially leading to the selection of more acutely ill febrile infants for inclusion in our cohort, which suggests that the point estimate cannot be extrapolated to all febrile 29- to 60-day-olds. Lastly, our findings may not be generalizable to community EDs and primary care office settings.
Conclusions
HSV infection was diagnosed in 0.42% of 26 533 encounters in infants ≤60 days of age who had a CSF culture obtained within 24 hours of an ED encounter. HSV prevalence peaked in the second week of life and declined substantially in the second month of life. Diagnostic testing and empirical treatment of HSV varied widely across sites and neither was related to the frequency of HSV infection. Our data emphasize the need for improved management strategies focused on the early identification of infants at both high and low risk of HSV infection. Future work should identify clinical and laboratory factors that can assist with HSV infection risk stratification, enabling the accurate identification of infants at high risk and in need of empirical HSV testing and treatment while minimizing the testing and treatment of low-risk infants.
Acknowledgments
We would like to thank Elizabeth R. Alpern, MD, MPH (Lurie Children's Hospital), A. Chantal Caviness, MD, PhD (Baylor College of Medicine), Nathan Kuppermann, MD, MPH (University of California-Davis), and Michael Monuteaux, ScD (Boston Children's Hospital) for help with study design and execution. We would also like to thank Jennifer L. Jones, MS, and Victor M. Gonzalez, MD, MPH, from the Baylor Data Center.
Glossary
- CI
confidence interval
- CNS
central nervous system
- CSF
cerebrospinal fluid
- ED
emergency department
- HSV
herpes simplex virus
- HSV-1
herpes simplex virus type 1
- HSV-2
herpes simplex virus type 2
- IQR
interquartile range
- LP
lumbar puncture
- NNT
number needed to treat
- OR
odds ratio
- PCR
polymerase chain reaction
- SEM
skin, eye, and mouth
Footnotes
Dr Cruz participated in study design, collected local data, coordinated data transfer from other sites, performed data analyses, drafted the manuscript, and incorporated revisions, and takes responsibility for the manuscript as a whole; Dr Freedman conceptualized and designed the study, supervised data collection locally and nationally, and reviewed and revised the manuscript; Dr Kulik helped conceptualize the study, collected local data, and reviewed and revised the manuscript; Drs Okada, Fleming, Mistry, Thomson, Schnadower, Arms, Mahajan, Garro, Pruitt, Balamuth, Uspal, Aronson, Lyons, Thomson, Curtis, Ishimine, Schmidt, Bradin, Grether-Jones, Miller, and Louie collected data at their sites and reviewed and revised the manuscript; Dr Shah contributed to study design and reviewed and revised the manuscript; Dr Nigrovic conceptualized and designed the study, supervised data collection locally and nationally, and drafted the manuscript; Drs Freedman and Nigrovic supervised data analysis; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Section of Emergency Medicine of the American Academy of Pediatrics and Baylor College of Medicine. Dr Freedman is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness. This work was supported, in part, by a Clinical and Translational Science Award grant (KL2 TR001862 for Dr Aronson) from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH). Fran Balamuth was supported in part by a career development award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD082368). Article contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2017-3647.
References
- 1.Kimberlin DW, Lin CY, Jacobs RF, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108(2):230–238 [DOI] [PubMed] [Google Scholar]
- 2.Kimberlin DW, Lin C-Y, Jacobs RF, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229 [DOI] [PubMed] [Google Scholar]
- 3.Tookey P, Peckham CS. Neonatal herpes simplex virus infection in the British Isles. Paediatr Perinat Epidemiol. 1996;10(4):432–442 [DOI] [PubMed] [Google Scholar]
- 4.Jones CA, Raynes-Greenow C, Isaacs D; Neonatal HSV Study Investigators and Contributors to the Australian Paediatric Surveillance Unit . Population-based surveillance of neonatal herpes simplex virus infection in Australia, 1997-2011. Clin Infect Dis. 2014;59(4):525–531 [DOI] [PubMed] [Google Scholar]
- 5.Kropp RY, Wong T, Cormier L, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117(6):1955–1962 [DOI] [PubMed] [Google Scholar]
- 6.Mark KE, Kim HN, Wald A, Gardella C, Reed SD. Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate? Am J Obstet Gynecol. 2006;194(2):408–414 [DOI] [PubMed] [Google Scholar]
- 7.Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e1 [DOI] [PubMed] [Google Scholar]
- 8.Morris SR, Bauer HM, Samuel MC, Gallagher D, Bolan G. Neonatal herpes morbidity and mortality in California, 1995-2003. Sex Transm Dis. 2008;35(1):14–18 [PubMed] [Google Scholar]
- 9.Handel S, Klingler EJ, Washburn K, Blank S, Schillinger JA. Population-based surveillance for neonatal herpes in New York City, April 2006-September 2010. Sex Transm Dis. 2011;38(8):705–711 [DOI] [PubMed] [Google Scholar]
- 10.Looker KJ, Magaret AS, May MT, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5(3):e300–e309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampath A, Maduro G, Schillinger JA. Infant deaths due to herpes simplex virus, congenital syphilis, and HIV in New York City. Pediatrics. 2016;137(4):e20152387. [DOI] [PubMed] [Google Scholar]
- 12.Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SS, Volk J, Mohamad Z, Hodinka RL, Zorc JJ. Herpes simplex virus testing and hospital length of stay in neonates and young infants. J Pediatr. 2010;156(5):738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao S, Abzug MJ, Carosone-Link P, et al. Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr. 2015;166(6):1462–8.e1–4 [DOI] [PubMed] [Google Scholar]
- 15.Schreiber R, Wolpin J, Koren G. Determinants of aciclovir-induced nephrotoxicity in children. Paediatr Drugs. 2008;10(2):135–139 [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Herpes simplex In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book 2015: Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015:432–436 [Google Scholar]
- 17.McGoogan KE, Haafiz AB, González Peralta RP. Herpes simplex virus hepatitis in infants: clinical outcomes and correlates of disease severity. J Pediatr. 2011;159(4):608–611 [DOI] [PubMed] [Google Scholar]
- 18.Kotzbauer D, Frank G, Dong W, Shore S. Clinical and laboratory characteristics of disseminated herpes simplex virus infection in neonates. Hosp Pediatr. 2014;4(3):167–171 [DOI] [PubMed] [Google Scholar]
- 19.Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153(2):164–169 [DOI] [PubMed] [Google Scholar]
- 20.McGuire JL, Zorc J, Licht D, Hodinka RL, Shah SS. Herpes simplex testing in neonates in the emergency department. Pediatr Emerg Care. 2012;28(10):949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108–111 [DOI] [PubMed] [Google Scholar]
- 22.Benidir A, Lim R, Salvadori M, Sangha G, Poonai N. Current practice patterns regarding diagnostic investigations and empiric use of acyclovir by Canadian pediatric emergency physicians in febrile neonates. Pediatr Emerg Care. 2013;29(3):273–278 [DOI] [PubMed] [Google Scholar]
- 23.Gaensbauer JT, Birkholz M, Pfannenstein K, Todd JK. Herpes PCR testing and empiric acyclovir use beyond the neonatal period. Pediatrics. 2014;134(3). Available at: www.pediatrics.org/cgi/content/full/134/3/e651 [DOI] [PubMed] [Google Scholar]
- 24.Jones CA, Walker KS, Badawi N. Antiviral agents for treatment of herpes simplex virus infection in neonates. Cochrane Database Syst Rev. 2009;(3):CD004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ericson JE, Gostelow M, Autmizguine J, et al. ; Pediatric Trials Network Executive Committee and Investigators . Safety of high-dose acyclovir in infants with suspected and confirmed neonatal herpes simplex virus infections. Pediatr Infect Dis J. 2017;36(4):369–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong AA, Pabbaraju K, Wong S, Tellier R. Development of a multiplex real-time PCR for the simultaneous detection of herpes simplex and varicella zoster viruses in cerebrospinal fluid and lesion swab specimens. J Virol Methods. 2016;229:16–23 [DOI] [PubMed] [Google Scholar]
- 27.Kuypers J, Boughton G, Chung J, et al. Comparison of the Simplexa HSV1 & 2 Direct kit and laboratory-developed real-time PCR assays for herpes simplex virus detection. J Clin Virol. 2015;62:103–105 [DOI] [PubMed] [Google Scholar]
- 28.Caviness AC, Demmler GJ, Swint JM, Cantor SB. Cost-effectiveness analysis of herpes simplex virus testing and treatment strategies in febrile neonates. Arch Pediatr Adolesc Med. 2008;162(7):665–674 [DOI] [PubMed] [Google Scholar]
- 29.Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Pediatr Clin North Am. 2013;60(2):351–365 [DOI] [PubMed] [Google Scholar]
- 30.Freedman SB, Thakkar VA. Easing the strain on a pediatric tertiary care center: use of a redistribution system. Arch Pediatr Adolesc Med. 2007;161(9):870–876 [DOI] [PubMed] [Google Scholar]
- 31.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative . Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667–677 [DOI] [PubMed] [Google Scholar]