The positive effects of a 27-month home visiting parenting program on overweight and obesity among 2-year-old children living in lower-income urban homes.
Abstract
BACKGROUND:
Young children living in historically marginalized families are at risk for becoming adolescents with obesity and subsequently adults with increased obesity-related morbidities. These risks are particularly acute for Hispanic children. We hypothesized that the prevention-focused, socioecological approach of the “Minding the Baby” (MTB) home visiting program might decrease the rate of childhood overweight and obesity early in life.
METHODS:
This study is a prospective longitudinal cohort study in which we include data collected during 2 phases of the MTB randomized controlled trial. First-time, young mothers who lived in medically underserved communities were invited to participate in the MTB program. Data were collected on demographics, maternal mental health, and anthropometrics of 158 children from birth to 2 years.
RESULTS:
More children in the intervention group had a healthy BMI at 2 years. The rate of obesity was significantly higher (P < .01) in the control group (19.7%) compared with the intervention group (3.3%) at this age. Among Hispanic families, children in the MTB intervention were less likely to have overweight or obesity (odds ratio = 0.32; 95% confidence interval: 0.13–0.78).
CONCLUSIONS:
Using the MTB program, we significantly lowered the rate of obesity among 2-year-old children living in low-socioeconomic-status communities. In addition, children of Hispanic mothers were less likely to have overweight or obesity at 2 years. Given the high and disproportionate national prevalence of Hispanic young children with overweight and obesity and the increased costs of obesity-related morbidities, these findings have important clinical, research, and policy implications.
What’s Known on This Subject:
Although overall obesity rates have plateaued nationwide, there is a widening racial and/or ethnic disparity in childhood overweight and obesity, particularly among Hispanic children early in life. There are few programs that address obesity in this age group.
What This Study Adds:
Children living in families who received a 27-month parenting home visiting intervention were significantly less likely to be obese at 2 years of life. Hispanic children in the intervention families were also less likely to have a BMI >85%.
The current rate of children with obesity, 17.4%,1 remains two-and-a-half times higher than it was 25 years ago.2 Of further concern is the trend of widening racial and/or ethnic disparities3–5 in obesity rates. The rate of obesity among Hispanic and non-Hispanic African American children has consistently been higher than that of non-Hispanic white children.3,6 In addition, both groups are less likely to return to normal weight levels,7 raising concern that they will become adults with obesity and develop serious obesity-related health problems.8,9 Despite the fact that more than half of children with overweight or obesity between 2 and 20 years of age develop overweight before the age of 2,10 most obesity prevention interventions focus on school-aged children and adolescents.11,12 And yet early childhood is a time when behaviors are modifiable, physiologic development is adaptable,13,14 and interventions are often more cost-effective and feasible15; thus, interventions that successfully reduce rates of obesity are urgently needed before the child is 2 years old, an age referred to as the “tipping point” in obesity prevention.10
To date, most obesity prevention interventions in young children have been aimed at secondary prevention, namely detecting and treating preclinical weight changes (eg, obesity screening) as they occur16,17; researchers conducting these interventions have demonstrated only modest effects. By contrast, in approaches in which the social ecology of a child’s relationship to food is addressed (by supporting parent-child relationships and promoting a healthy family lifestyle and diet), a means to primary prevention is offered, reducing risk (eg, promoting nurturing relationships, altering behaviors, and addressing multigenerational patterns) by targeting the broader context of parent-child interactions and family systems.18 Home visiting programs, many of which are geared specifically toward the development of secure parent-child relationships and healthy family management and are well known to have a range of health benefits for mother and child,19 may be particularly effective in obesity prevention.20 In this article, we examine the rates of obesity among 2-year-old children of mothers who were followed in a 27-month randomized controlled trial (RCT) of a primary prevention and home visiting parenting intervention: “Minding the Baby” (MTB).
We hypothesized that MTB would have an impact on the rate of childhood overweight and obesity in the first 2 years of life (ie, address the Healthy People 2020 goal to reduce early childhood obesity).21 Because the participants in the program were predominantly Hispanic, and Hispanic children have the highest rates of obesity at age 2 years,1,3 we were also interested in examining whether the MTB intervention was a protective factor against overweight and obesity for Hispanic children at the age of 2 years.
Methods
MTB
The MTB program focuses on multiple aspects of families’ socioecological systems (the child’s development, primary relationships, culture, and community)22,23 to support the health, mental health, and development of mothers and children living in marginalized families.,24,25 In this interdisciplinary, relationship-based program, we aim to develop and enhance parent-child attachment and maternal reflective functioning (RF) and to promote a range of positive parenting behaviors.26,27 RF, the mother’s capacity to understand her child’s behavior in light of thoughts, feelings, and intentions, can be addressed in primary care pediatric settings as well as more intensive programs28 and has been linked with higher rates of secure attachment and more sensitive caregiving.26,29–34
Participants and Design
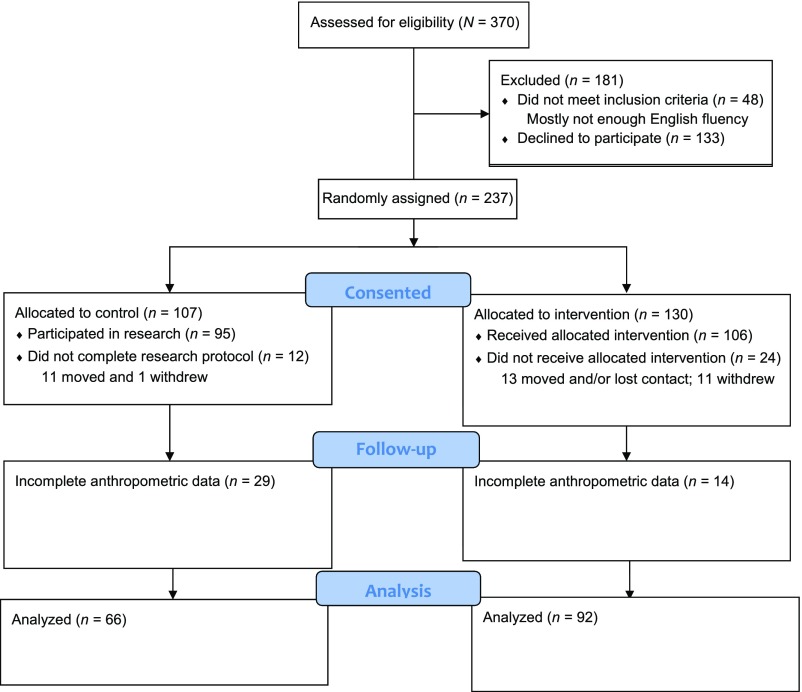
This study is a prospective longitudinal cohort study in which we include data collected during both phases of the MTB program RCT pilot testing from 2002 to 200832 and an efficacy trial from 2008 to 2016.24,25,33 First-time mothers who lived in medically underserved communities (where most families live at or below the poverty level) and received prenatal care at 2 inner-city community health clinics (CHCs) were invited to participate. The communities are culturally and ethnically diverse with a large population self-reporting as Hispanic. Seventy-eight percent of the visits took place in participants’ homes (versus an alternate place of convenience to the mother). In both phases of the RCTs, we used a 2-group experimental design with cluster randomization using a sealed envelope method to assign group status on the basis of due dates before recruitment.35 At the first CHC, prenatal care was delivered in groups (6 groups per year, organized according to women’s due dates). At the second CHC, women were seen individually and clustered artificially by matching the due dates used at the first CHC so that the same randomization scheme could be used at both sites. Women in the control group received standard group prenatal and primary care at both CHCs, and women in the intervention group received standard care in addition to the MTB program; hence, research personnel and study participants were not blinded to treatment. The inclusion criteria were (1) English-speaking, (2) 14 to 25 years of age, (3) having a first child, (4) no active drug use, (5) no serious mental illness, and (6) no major medical illness in the mother. In the current study cohort, we included 158 children (92 intervention and 66 control) from both phases of the RCT with complete anthropometric data collected at 24 months of age (see Fig 1). Additional details of design, recruitment, and retention procedures are available in the report on the pilot study findings32 and the efficacy trial.36
FIGURE 1.
MTB combined phase 1 and 2 Consolidated Standards of Reporting Trials flowchart.
Procedures
The MTB program provided home visiting by a master’s-prepared social worker and pediatric nurse weekly from the third trimester of pregnancy until the child’s first birthday and biweekly through the child’s second birthday. The home visits were typically 1 hour in duration, but this varied according to the dyad’s life circumstances. The clinician pairs were varied between families and CHCs to reduce threats to internal validity. They received weekly supervision and participated in team case presentations to ensure fidelity. During the 2 phases, there were 2 nurses and 5 part-time social workers at various points in time. Further details on the manualized MTB program have been published previously.24,32,37,38 Research ethics approval was obtained through the university and CHCs.
Measures
Main Exposure
The main exposures in this study were the group status (intervention or control) and race and/or ethnicity. At the time of consent, all participants were pregnant women who self-reported their race and/or ethnicity after random assignment.
Potential Covariates
We considered several early life risk factors known to be associated with childhood obesity as potential covariates: maternal mental health,39 rapid infant weight gain,40,41 and feeding other than exclusive breastfeeding.42 In this study, mothers in both groups met with research staff to complete questionnaires at 24 months and a semistructured interview prenatally and at 24 months to assess maternal mental health, including depressive symptoms (Center for Epidemiologic Studies Depression Scale),43 parenting stress (Parenting Stress Index),44 posttraumatic stress symptoms (Mississippi Scale),45 and maternal RF (Pregnancy Interview and Parent Development Interview).46,47 Details on the instruments, reliability, and validity have been reported elsewhere.32 Rapid infant weight gain was defined as a change in weight-for-age z score >0.67 SD on the basis of World Health Organization growth data (between birth and age 12 months), which is interpreted clinically as crossing centile lines on a growth chart.48 Data were collected on weeks of exclusive breastfeeding.42,49
Outcome Measures
The primary outcome is the prevalence of overweight (≥85th percentile) or obesity (≥95th percentile) in children at 2 years, which was assessed by using the Centers for Disease Control and Prevention reference data, adjusting for age and sex (z score).50 Weight and height data at birth, 12 months, and 24 months were collected via medical chart review.
Families Without Complete Data
There were 75 families excluded from this study because of a combination of dropout from MTB and incomplete anthropometric growth data in the children’s medical charts at 24 months. There was no difference in the number of families in the intervention and control groups among those excluded. There were no significant differences in any demographic variables between included and excluded families (see Supplemental Table 4).
Statistical Analysis
We compared demographic characteristics, maternal mental and physical health factors, and child health factors by treatment group assignment. We then created logistic regression models predicting child overweight and obesity with group status and any covariates not equivalent between groups. Because a large majority of the participants were Hispanic, we also examined the Hispanic subsample separately. Because of the cluster randomization strategy, we tested interclass correlations (ICCs) to determine if multilevel modeling would be appropriate. Between the CHCs, the ICCs were <0.01 for each model, and we determined that multilevel modeling was not necessary. We considered clustering at the prenatal group level, but the groups were too small for multilevel analysis to be conducted (average group size = 1.9; range: 1–5).51 We compared participant baseline characteristics between CHC sites and found no significant differences between participants in the 2 sites. All analyses were intent to treat.
Results
Demographic Characteristics
There were no differences between treatment groups in maternal age, education, marital status, children’s gestational age, or sex (see Table 1). About one-third of the mothers were teenagers and the majority were single with a high school education.
TABLE 1.
Descriptive Statistics
| Intervention (n = 92), % or Mean (SD) | Control (n = 66), % or Mean (SD) | |
|---|---|---|
| Home visits (91 visits planned over 27-mo program) | 72 (39) | n/a |
| Maternal demographic characteristics | ||
| Age at consent (y) | 19.6 (2.8) | 19.4 (2.6) |
| Under 19 y old | 33.7 | 33.3 |
| Education (y) | 12.4 (2.9) | 13.0 (2.8) |
| Race and/or ethnicity | ||
| White | 5.4 | 7.6 |
| Hispanic and/or Latino* | 77.2 | 59.1 |
| African American | 14.1 | 33.3 |
| Native Hawaiian and/or Pacific Islander | 1.1 | 0 |
| Marital status | ||
| Never married | 79.3 | 94.0 |
| Cohabitating and/or common law | 1.1 | 0 |
| Married | 8.7 | 4.5 |
| Divorced and/or separated | 3.3 | 0 |
| Engaged | 7.6 | 1.5 |
| Maternal psychopathology | ||
| Depressive symptoms at 24 mo (CESD: Nint = 74; Nctr = 63) | 11.0 (8.5) | 11.4 (7.7) |
| Parenting stress at 24 mo (PSI: Nint = 73; Nctr = 63) | 65.3 (18.4) | 64.1 (16.1) |
| Posttraumatic stress symptoms at 24 mo (Nint = 69; Nctr = 63) | 74.9 (17.8) | 75.4 (15.0) |
| Pregnancy RF (PI: Nint = 84; Nctr = 65) | 3.2 (0.80) | 3.2 (0.67) |
| Parental RF at 24 mo (PDI: Nint = 74; Nint = 61) | 3.7 (0.90) | 3.6 (1.2) |
| Maternal health factors | ||
| History of smoking | 33.7 | 33.3 |
| No. weeks of exclusive breastfeeding | 11.8 (15.4) | 11.6 (16.6) |
| Child characteristics | ||
| Gestational age (wk) | 38.5 (2.9) | 39.3 (1.5) |
| Male | 50 | 58 |
| Birth wt (g)** | 3014.3 (655.8) | 3240.8 (436.8) |
| Wt at 12 mo (kg)* | 9.8 (1.2) | 10.6 (1.6) |
| Wt gain (kg) from birth to 12 mo** | 6.8 (1.1) | 7.4 (1.5) |
| Outcomes | ||
| BMI at 24 mo* | 16.8 (1.3) | 17.7 (2.3) |
| BMI-for-age z score at 24 mo* | 0.19 (1.00) | 0.68 (1.12) |
| Overweight at 24 mo | 16.3 | 13.6 |
| Obese at 24 mo* | 3.3 | 19.7 |
CESD, Center for Epidemiologic Studies Depression Scale; Nctr, Control group sample size; Nint, Intervention group sample size; n/a, not applicable; PDI, Parental Development Interview; PI, Pregnancy Interview; PSI, Parenting Stress Index.
P < .01; ** P = .03.
Main Exposure
The main exposure variable in the statistical analysis was group status, which was included in the model as the independent variable. The overall sample was largely Hispanic (68%). However, there were significantly more Hispanic mothers in the intervention compared with the control group (P = .02). Therefore, mothers’ self-identification as Hispanic was included as a covariate in the models.
Covariates
There were no differences between the groups with respect to mothers’ self-report of depressive symptoms, parenting stress, posttraumatic symptoms, or RF when their children were 2 years old (Table 1). Hence, the maternal mental health variables were not included as covariates in the statistical models.
There was a significant group difference in the children’s weight at birth, 12 months, and 24 months. The children in the control group gained significantly more weight in the first 12 months of life (P = .03). However, accounting for birth weight and the sex of the child, the difference between the groups with respect to rapid weight gain in the first 12 months was not significant. Finally, there were no significant group differences in the duration of exclusive breastfeeding. Both groups breastfed for <3 months, which is half the amount of time considered to be protective against obesity.42,49 Birth weight was the only early life risk factor found to be statistically different between groups and was therefore included as a covariate in the analysis.
Primary Outcome
There were significantly more (P = .03) children in the MTB program within the normal BMI range at 2 years (78.3%) than there were in the control group (63.6%). And although there were more children in the intervention group (16.3%) with overweight than in the control group (13.6%) at 2 years, the percentage of children with obesity at 24 months was significantly higher (P < .01) in the control group (19.7%) compared with the intervention group (3.3%).
In our first logistic regression model, we examined the effect of the MTB program participation on the development of early childhood obesity at 2 years (Table 2). The results reveal that children in the intervention group were 88% less likely to have obesity than children in the control group, controlling for Hispanic ethnicity and birth weight (odds ratio [OR] = 0.12; 95% confidence interval [CI]: 0.03–0.47). When only Hispanic children were included, the magnitude and significance of the finding remained similar (OR = 0.14; 95% CI: 0.03–0.53).
TABLE 2.
Logistic Regression Models Predicting Obesity at 24 Months
| Full Sample (n = 158) | Hispanic Only (n = 110) | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Treatment arma | ||
| Control group | Reference | Reference |
| Home visiting group | 0.12 (0.03–0.47)* | 0.14 (0.03–0.53)* |
| Ethnicityb | ||
| Non-Hispanic | Reference | n/a |
| Hispanic | 2.96 (0.75–11.6) | n/a |
| Birth wtc | 1.43 (0.80–2.53) | 1.24 (0.67–2.28) |
n/a, not available.
Assignment to the intervention group receiving MTB home visitation versus the control group.
Self-reported Hispanic ethnicity.
Birth wt from medical records, converted to z scores on the basis of national growth chart data from the Centers for Disease Control and Prevention and included as a continuous variable.
P < .05.
We then examined the effect of MTB participation on the development of combined overweight and obese weight status at 2 years of age. It was indicated in the results that the intervention was not associated with overweight and obesity when the full sample was examined (Table 3). However, when only the Hispanic children were included, children in the intervention group were 68% less likely to be overweight or obese than children in the control group (OR = 0.32; 95% CI: 0.13–0.78).
TABLE 3.
Logistic Regression Models Predicting Overweight and Obesity at 24 Months
| Full Sample (n = 158) | Hispanic Only (n = 110) | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Treatment arma | ||
| Control group | Reference | Reference |
| Home visiting group | 0.52 (0.24–1.11) | 0.32 (0.13–0.78)* |
| Ethnicityb | ||
| Non-Hispanic | Reference | n/a |
| Hispanic | 1.10 (0.48–2.50) | n/a |
| Birth wtc | 1.26 (0.91–1.74) | 1.39 (0.90–2.16) |
n/a, not available.
Assignment to the intervention group receiving MTB home visitation versus the control group.
Self-reported Hispanic ethnicity.
Birth wt from medical records, converted to z scores on the basis of national growth chart data from the Centers for Disease Control and Prevention and included as a continuous variable.
P < .05.
Post hoc calculations indicated that we had >0.95 power to detect the ORs estimated for both obesity models. For overweight and obesity models, the power was 0.73 for the Hispanic sample, which had a significant finding, but only 0.43 for the full sample, which did not; if the full sample had the same effect size as the Hispanic sample, we would have had sufficient power to detect it (0.88).
Discussion
In the results of this RCT, we suggest that children who received services from the MTB program were significantly less likely to have obesity at 2 years of life compared with children in the control group. Additionally, children in the intervention were significantly more likely to have weights in the normal range at 2 years. When rates of overweight and obesity were combined, children in the MTB intervention who were Hispanic were significantly less likely to have overweight and obesity at 2 years of age. This study took place in Connecticut, ranked 12th in the nation for the highest obesity rates among low-income children.52 The 3.3% rate of obesity for MTB participants was considerably lower than the state average (15.3%) and below the Healthy People 2020 goal (Nutrition and Weight Status objective 10) of a 9.4% obesity rate among 2- to 5-year-old children.21
Given our previous success in promoting a range of positive health outcomes in a largely Hispanic sample,32,53 we hypothesized that MTB’s socioecological approach to enhance parent-child relationships and support family management in the first 2 years of life might lower the rate of overweight and obesity among children in the intervention group. Our findings support this hypothesis. But we also raise a critical question: What elements of the MTB intervention might have contributed to the greater likelihood of normal weight and the diminished likelihood of obesity in our intervention group? Given that secondary prevention programs have been relatively unsuccessful in lowering rates of obesity,17,54 we believe that MTB’s comprehensive approach and primary-prevention design likely contributed to its success in this area. By focusing broadly on attachment, health, mental health, parenting, and life course outcomes, we aimed to prevent difficulties rather than interrupt them once begun. Home visitors in the MTB program focused generally on the development of a secure attachment relationship between mother and child, one in which the child felt safe both in seeking comfort and in exploring the world. They also encouraged mothers to be curious about their child’s feelings, thoughts, and needs. Although obesity prevention was not a primary aim, the clinicians continually worked with mothers to recognize children’s hunger cues and to take time to pause and engage with their children during feedings. They also regularly addressed issues commonly related to obesity prevention in young children (eg, sleep, early introduction of solids, etc), and would often discuss nutrition, food preparation, and choices. Finally, they made every effort to understand the socioecological influences on feeding practices, used family-centered approaches to address the mothers’ fears and beliefs about food, and made efforts to consider, and in some cases “soften,” cultural and community influences. For example, clinicians remained sensitive to the fact that in Hispanic families, a “well-fed” baby may be considered a sign of good nurturing and success as a parent.55,56
Our findings dovetail with recent work by Taveras et al,13 in which a number of modifiable prenatal and early childhood risk factors were linked to racial and/or ethnic disparities in childhood rates of overweight and obesity. These include the following: rapid infant weight gain, feeding other than exclusive breastfeeding, early introduction of solids, insufficient sleep, presence of a television in the bedroom, and intake of sugar-sweetened beverages and fast-food.13 The MTB curriculum57 embeds education on these factors within the framework of the program. We hypothesize that the ongoing “whole family” focus on healthy patterns of eating, sleeping, and general wellness within a relationship-based intervention had a significant impact on BMI outcomes.
Strengths of our study include the use of prospective data collected prenatally through age 2 years in a program that was guided by the socioecological framework of the MTB program. Given that rates of obesity are particularly problematic in Hispanic families, we were also fortunate in having a largely Hispanic sample. At the same time, the sample’s relative homogeneity limits the generalizability of our findings to non-Hispanic African American and non-Hispanic white families. The generalizability of our findings is also limited by the fact that despite an attrition rate (21%) lower than in similar studies,58 there were incomplete growth data in the children’s medical records in both groups. Future analyses should include the effect of family-centered home visiting on other diverse ethnicities known to have disparate rates of obesity as well as an examination of the impact of cultural differences (language spoken in the home, specific cultural identity) within Hispanic homes. Finally, we did not collect data on other obesity risk factors (namely, gestational weight gain, the timing of the introduction of solids, and sleep patterns).
The MTB intervention is an intensive intervention that has been reviewed by the US Department of Health and Human Services and listed as 1 of 19 home visiting programs to demonstrate effectiveness and is therefore eligible for funding from the Health Resources and Services Administration’s Maternal, Infant, and Early Childhood Home Visiting Program state funding. Because the intensity of the program may be a limitation to addressing obesity on a large scale, further examination of the mechanisms that contributed to the results in this study are needed. Cohort effects were limited, as evidenced by the low ICC and absence of any major historical or community events that would have affected the results. Additionally, we built strong relationships with our community partners at the CHCs to determine that there was consistency in terms of the socioeconomic makeup and health care protocols at both CHCs before recruitment.
Conclusions
Young children living in low-income and ethnic-minority communities are at risk for having obesity in adolescence7,41 and subsequently in adulthood, with increased obesity-related morbidities. These risks are particularly acute for Hispanic children. The reasons for the disparity between poor and/or minority children and their more privileged peers are complex and likely include influences of culture, poverty, community access to healthy food options, food insecurity, and the like. Nevertheless, considering the growing population of Hispanic children (predicted to be 1 in 3 children by 2030), addressing this health disparity will help ensure the well-being of numerous individuals and families and curtail the ever-rising US health care spending59 on obesity-related morbidities.60,61
Recently, in the White House Task Force Report, “Solving the Problem of Childhood Obesity Within a Generation,”62 as well as in 2 subsequent Institute of Medicine reports,63,64 the need for interventions early in life to prevent obesity has been emphasized. On the basis of our findings, we suggest that home visiting programs that focus on the whole child and on the early mother-child relationship using a socioecological approach may be in the best position to build the foundation for healthy development. Much more empirical evidence is required to confirm this hypothesis, but with our results, we suggest that this approach may be highly beneficial in lowering rates of obesity in at-risk populations.
Acknowledgments
We thank Denise Webb and Tanika Simpson for their thoughtful review and editorial assistance. We also thank the CHCs and families who generously provided their time and trust in our program.
Glossary
- CHC
community health clinic
- CI
confidence interval
- ICC
interclass correlation
- MTB
Minding the Baby
- OR
odds ratio
- RCT
randomized controlled trial
- RF
reflective functioning
Footnotes
Dr Ordway conceptualized and designed the cohort study, conducted the initial analyses, and drafted the initial manuscript; Drs Sadler, Slade, Close, and Mayes codesigned and directed the “Minding the Baby” program and the 2 phases of the randomized clinical trial and reviewed and revised the manuscript; Dr Holland reviewed the initial analyses, conducted the final analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01458145).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by The FAR Fund, the Irving B. Harris Foundation, the Pritzker Early Childhood Foundation, the Seedlings Foundation, the Child Welfare Fund, the Stavros Niarchos Foundation, The Patrick and Catherine Weldon Donaghue Foundation, The Edlow Family Fund, the Schneider Family, The New York Community Trust, the National Institute of Nursing Research (P30NR08999, K23NR16277, T32NR008346), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21HD048591, RO1HD057947), the Jonas Center for Nursing and Veterans Healthcare, and the National Center for Advancing Translational Sciences (KL2 TR000140). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999-2014. Obesity (Silver Spring). 2016;24(5):1116–1123 [DOI] [PubMed] [Google Scholar]
- 2.Hadley A, Hair E, Dreisbach N. What Works for the Prevention and Treatment of Obesity Among Children: Lessons From Experimental Evaluations of Programs and Interventions. Washington, DC:Child Trends; 2010 [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falbe J, Cotterman C, Linchey J, Madsen KA. Ethnic disparities in trends in high BMI among California adolescents, 2003-2012. Am J Prev Med. 2016;51(2):e45–e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci USA. 2014;111(4):1338–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendall MS, Weden MM, Fernandes M, Vaynman I. Hispanic and black US children’s paths to high adolescent obesity prevalence. Pediatr Obes. 2012;7(6):423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115(1):22–27 [DOI] [PubMed] [Google Scholar]
- 9.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17.e2 [DOI] [PubMed] [Google Scholar]
- 10.Harrington JW, Nguyen VQ, Paulson JF, Garland R, Pasquinelli L, Lewis D. Identifying the “tipping point” age for overweight pediatric patients. Clin Pediatr (Phila). 2010;49(7):638–643 [DOI] [PubMed] [Google Scholar]
- 11.Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. 2016;50(6):780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50(6):761–779 [DOI] [PubMed] [Google Scholar]
- 13.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167(8):731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring). 2008;16(7):1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karoly LA, Kilburn MR, Cannon JS. Early Childhood Interventions: Proven Results, Future Promise. Santa Monica, CA: RAND Corporation; 2005 [Google Scholar]
- 16.Birch LL, Anzman-Frasca S, Paul IM. Starting early: obesity prevention during infancy. Nestle Nutr Inst Workshop Ser. 2012;73:81–94 [DOI] [PubMed] [Google Scholar]
- 17.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr. 2016;170(8):742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JD, Montaño Z, Dishion TJ, Shaw DS, Wilson MN. Preventing weight gain and obesity: indirect effects of the family check-up in early childhood. Prev Sci. 2015;16(3):408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olds DL, Sadler L, Kitzman H. Programs for parents of infants and toddlers: recent evidence from randomized trials. J Child Psychol Psychiatry. 2007;48(3–4):355–391 [DOI] [PubMed] [Google Scholar]
- 20.Garner AS. Home visiting and the biology of toxic stress: opportunities to address early childhood adversity. Pediatrics. 2013;132(suppl 2):S65–S73 [DOI] [PubMed] [Google Scholar]
- 21.Office of Disease Prevention and Health Promotion Nutrition and weight status. 2016. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/nutrition-and-weight-status. Accessed February 1, 2016
- 22.Centers for Disease Control and Prevention The social-ecological model: a framework for prevention. 2007. Available at: www.cdc.gov/violenceprevention/overview/social-ecologicalmodel.html. Accessed January 24, 2015
- 23.Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychol Rev. 1994;101(4):568–586 [DOI] [PubMed] [Google Scholar]
- 24.Sadler LS, Slade A, Mayes LC. Minding the baby: a mentalization-based parenting program In: Allen JG, Fonagy P, eds. Handbook of Mentalization-Based Treatment. Hoboken, NJ: John Wiley and Sons, Ltd; 2006:271–288 [Google Scholar]
- 25.Slade A, Sadler L, Dios-Kenn CD, Webb D, Currier-Ezepchick J, Mayes L In: King RA, Neubauer PB, Abrams S, Dowling AS, eds. The Psychoanalytic Study of the Child. New Haven, CT: Yale University Press; 2005:74–100 [DOI] [PubMed] [Google Scholar]
- 26.Slade A. Parental reflective functioning: an introduction. Attach Hum Dev. 2005;7(3):269–281 [DOI] [PubMed] [Google Scholar]
- 27.Ordway MR, Sadler LS, Dixon J, Slade A. Parental reflective functioning: analysis and promotion of the concept for paediatric nursing. J Clin Nurs. 2014;23(23–24):3490–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ordway MR, Webb D, Sadler LS, Slade A. Parental reflective functioning: an approach to enhancing parent-child relationships in pediatric primary care. J Pediatr Health Care. 2015;29(4):325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherford HJV, Goldberg B, Luyten P, Bridgett DJ, Mayes LC. Parental reflective functioning is associated with tolerance of infant distress but not general distress: evidence for a specific relationship using a simulated baby paradigm. Infant Behav Dev. 2013;36(4):635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suchman NE, DeCoste C, Leigh D, Borelli J. Reflective functioning in mothers with drug use disorders: implications for dyadic interactions with infants and toddlers. Attach Hum Dev. 2010;12(6):567–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenblum KL, McDonough SC, Sameroff AJ, Muzik M. Reflection in thought and action: maternal parenting reflectivity predicts mind-minded comments and interactive behavior. Infant Ment Health J. 2008;29(4):362–376 [DOI] [PubMed] [Google Scholar]
- 32.Sadler LS, Slade A, Close N, et al. . Minding the baby: enhancing reflectiveness to improve early health and relationship outcomes in an interdisciplinary home visiting program. Infant Ment Health J. 2013;34(5):391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slade A. Keeping the baby in mind: a critical factor in perinatal mental health In: Slade A, Mayes L, Epperson N, eds. Special Issue on Perinatal Mental Health. Washington, DC: Zero to Three; 2002:10–16 [Google Scholar]
- 34.Slade A. Reflective parenting programs: theory and development. Psychoanal Inq. 2007;26(4):640–657 [Google Scholar]
- 35.Hauck WW, Gilliss CL, Donner A, Gortner S. Randomization by cluster. Nurs Res. 1991;40(6):356–358 [PubMed] [Google Scholar]
- 36.Slade A, Sadler LS, Close N, et al. . Minding the Baby: An Evidence-Based Intervention for Mothers, Infants, and Their Families. Branford, CT: Connecticut Association for Infant Mental Health; 2016 [Google Scholar]
- 37.Slade A, Sadler LS. Minding the baby: complex trauma and home visiting. Int J Birth Parent Educ. 2013;1:50–53 [Google Scholar]
- 38.Slade A, Sadler L, Close N, Fitzpatrick S, Simpson T, Webb D. Minding the baby: the impact of threat on the mother-baby and mother-clinician relationship In: Gojman de Millan S, Herreman C, Sroufe LA, eds. Attachment Across Cultural and Clinical Contexts. London, United Kingdom: Routledge; 2017:182–205 [Google Scholar]
- 39.Benton PM, Skouteris H, Hayden M. Does maternal psychopathology increase the risk of pre-schooler obesity? A systematic review. Appetite. 2015;87:259–282 [DOI] [PubMed] [Google Scholar]
- 40.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–908 [DOI] [PubMed] [Google Scholar]
- 41.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring). 2006;14(3):491–499 [DOI] [PubMed] [Google Scholar]
- 42.Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes. 2009;4(4):196–204 [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401 [Google Scholar]
- 44.Abidin RR. The Parenting Stress Index: Short Form. Charlottesville, VA: Pediatric Psychology Press; 1995 [Google Scholar]
- 45.Keane TM, Caddell JM, Taylor KL. Mississippi scale for combat-related posttraumatic stress disorder: three studies in reliability and validity. J Consult Clin Psychol. 1988;56(1):85–90 [DOI] [PubMed] [Google Scholar]
- 46.Slade A. The Pregnancy Interview-Revised. New Haven, CT: Yale Child Study Center; 2003 [Google Scholar]
- 47.Slade A, Aber JL, Berger B, Bresgi I, Kaplan M. The Parent Development Interview: Revised. New York, NY: The City University of New York; 2003 [Google Scholar]
- 48.Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, De Groh M, Morrison H. Perinatal and early childhood factors for overweight and obesity in young Canadian children. Can J Public Health. 2013;104(1):e69–e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention Defining childhood obesity. 2016. Available at: https://www.cdc.gov/obesity/childhood/defining.html. Accessed December 15, 2016
- 51.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan L, Freedman D, Sharma A, et al. . Trends in obesity among participants aged 2–4 years in the special supplemental nutrition program for women, infants, and children–United States, 2000–2014. Morb Mortal Wkly Rep. 2016;65(45):1256-1260 [DOI] [PubMed] [Google Scholar]
- 53.Ordway MR, Sadler LS, Dixon J, Close N, Mayes L, Slade A. Lasting effects of an interdisciplinary home visiting program on child behavior: preliminary follow-up results of a randomized trial. J Pediatr Nurs. 2014;29(1):3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birch LL, Ventura AK. Preventing childhood obesity: what works? Int J Obes (Lond). 2009;33(suppl 1):S74–S81 [DOI] [PubMed] [Google Scholar]
- 55.Sherry B, McDivitt J, Birch LL, et al. . Attitudes, practices, and concerns about child feeding and child weight status among socioeconomically diverse white, Hispanic, and African-American mothers. J Am Diet Assoc. 2004;104(2):215–221 [DOI] [PubMed] [Google Scholar]
- 56.Kumanyika SK. Environmental influences on childhood obesity: ethnic and cultural influences in context. Physiol Behav. 2008;94(1):61–70 [DOI] [PubMed] [Google Scholar]
- 57.Slade A, Sadler LS, Close N, et al. . Minding the Baby Treatment Manual: Intervention and Training Guide. New Haven, CT: Yale University; 2014 [Google Scholar]
- 58.O’Brien RA, Moritz P, Luckey DW, McClatchey MW, Ingoldsby EM, Olds DL. Mixed methods analysis of participant attrition in the nurse-family partnership. Prev Sci. 2012;13(3):219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trust for America’s Health and Robert Wood Johnson Foundation Obesity in Latino communities. Special report: racial and ethnic disparities in obesity. 2014. Available at: https://stateofobesity.org/disparities/latinos/. Accessed August 5, 2016
- 60.Cawley J. The economics of childhood obesity. Health Aff (Millwood). 2010;29(3):364–371 [DOI] [PubMed] [Google Scholar]
- 61.Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics. 2014;133(5):854–862 [DOI] [PubMed] [Google Scholar]
- 62.White House Task Force on Childhood Obesity Solving the Problem of Childhood Obesity Within a Generation: White House Task Force on Childhood Obesity Report to the President. Washington, DC: Executive Office of the President of the United States; 2010 [DOI] [PubMed] [Google Scholar]
- 63.Institute of Medicine Early Childhood Obesity Prevention Policies. Washington, DC: National Academies Press; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Institute of Medicine Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. Washington, DC: National Academy of Sciences; 2012 [Google Scholar]