In this review, we report worse developmental outcomes in both HIV+ and HEU children compared with HUU children.
Abstract
CONTEXT:
HIV-infected (HIV+) children have worse neurodevelopmental outcomes compared with HIV-uninfected children. However, little is known regarding the differences in neurodevelopment between young HIV+ children, HIV-exposed but uninfected (HEU) children, and HIV-unexposed and uninfected (HUU) children.
OBJECTIVE:
To systematically review and meta-analyze data on neurodevelopmental performance between young HIV+, HEU, and HUU children.
DATA SOURCES:
We systematically searched the following electronic bibliographic databases: Ovid Medline, Embase, PsycINFO, Education Resources Information Center, and the Cochrane Database of Systematic Reviews.
STUDY SELECTION:
Studies were selected on the basis of defined inclusion criteria. Titles, abstracts, and full texts were assessed by 2 independent reviewers.
DATA EXTRACTION:
Data were extracted by 2 independent reviewers and cross-checked by 2 additional reviewers.
RESULTS:
Forty-five studies were identified for inclusion in the systematic review, and of these, 11 were included in the meta-analysis on the basis of availability of Bayley Scales of Infant and Toddler Development scores. Within the meta-analysis, when compared with their HUU peers, HIV+ and HEU children had lower cognitive and motor scores. HIV+ and HEU children with antiretroviral (ARV) exposure had lower cognitive and motor scores compared with those without ARV exposure.
LIMITATIONS:
We were unable to control adequately for intravenous drug use, geographic location, or quality of the assessment independently.
CONCLUSIONS:
Both HIV+ and HEU children had worse developmental outcomes compared with HUU children. HIV+ and HEU children with ARV exposure also had worse developmental outcomes compared with those without exposure; however, these results should be interpreted with caution. More research is needed to identify the impact of ARV exposure on young children.
HIV-infected (HIV+) children have worse neurodevelopmental outcomes compared with their unexposed peers.1–3 Cognitive abilities have been a critical area of focus, and in a recent meta-analysis, researchers identified working memory, processing speed, and executive function as the domains most affected by HIV in older children and adolescents.2 Younger HIV+ children also have lower scores in various domains of development while still having the greatest potential for benefit from early interventions.1,3 Past reviews have generated important insights and understanding into developmental outcomes for young children born to HIV+ mothers; however, results were reported qualitatively and are limited by heterogeneity within each domain assessed.1,3
Furthermore, there is increasing global attention on children who are HIV-exposed but ultimately uninfected.4,5 Because of the global expansion of prevention of mother-to-child transmission of HIV treatment and services, the number of new pediatric HIV cases has decreased substantially, with annual rates of new infections in this population being cut in half from 2010 to 2015.6 As we strive for an AIDS-free generation, there is a growing population of HIV-uninfected children who are exposed to both HIV and antiretroviral (ARV) medications. Little is known about how these exposures may affect neurodevelopment. There are few studies in which researchers look at neurodevelopment in this population compared with unexposed children, and some researchers suggest that HIV-exposed but uninfected (HEU) children may also have poorer development compared with their unexposed peers.7,8
No aggregated, quantitative evidence exists in which neurodevelopment in young children who are HIV+ or HEU is compared with that of HIV-unexposed and uninfected (HUU) peers. Our objective with this systematic review and meta-analysis is to assess the literature on neurodevelopment in young children born to HIV+ mothers. By understanding how neurodevelopment is affected by HIV in early childhood, we may help inform therapies and treatment to address delays and take steps in elucidating the mechanisms of neuroimpairment in this young, vulnerable population.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols 2015 checklist was used when conducting this systematic review and meta-analysis.9 The Population-Intervention-Comparison-Outcome (PICO) question that guided our search strategy was, “What is the extent of developmental delays in young HIV+ and HEU children compared with HUU children?”
Eligibility
The following inclusion criteria were applied: (1) include at least 2 out of 3 populations (HIV+, HEU, or HUU), (2) only include children <8 years of age, and (3) use a standardized neuropsychological instrument with reported results. Children were limited to a younger age group to focus on studies in which researchers evaluated development when the brain is most rapidly growing and receptive to interventions. The United Nations International Children’s Emergency Fund considers early childhood to encompass birth to 8 years of age.10 Additionally, a widely used neurocognitive test, the Wechsler Preschool and Primary Scale of Intelligence, has an upper age limit of 7 years and 7 months.11 For these reasons, the upper age limit of the study was set at 8 years. Studies were excluded if their entire population had a significant confounding factor, such as hemophilia or congenital cytomegalovirus infection. Review articles, published abstracts without full-text publications, and case study reports containing <10 participants were excluded. Although our search strategy specified English language articles, a few non-English articles were included in our initial search results and they were included for evaluation. We did not exclude published theses that otherwise met inclusion criteria.
Search Strategy
We conducted a systematic search using a protocol designed by a medical librarian specifically for this study (Supplemental Table 4) in 4 electronic databases: Ovid Medline, PsycINFO, Embase, and Education Resources Information Center. We also searched Google Scholar, the Cochrane Database of Systematic Reviews, and the bibliographies of pertinent articles. The search encompassed articles published from January 1990 to January 12, 2017. The initial screening was performed by 2 independent reviewers (M.S.M. and C.I.M.), who assessed the titles and abstracts from the search. Records were managed by using the citation managing program Endnote (version X7; Clarivate Analytics, Philadelphia, PA). From this initial screen, articles were immediately excluded if they did not focus on neurodevelopment in an HIV-exposed population or if they were a review article. After the initial exclusion process, 2 authors (M.S.M. and C.I.M.) independently reviewed the full text of the remaining articles to determine whether articles met the predetermined eligibility criteria, as noted above. Disagreements between the 2 reviewers regarding the inclusion or exclusion of particular studies were settled by consultation with a third reviewer (R.C.V.).
Data Extraction
Two reviewers (M.S.M. and C.I.M.) independently extracted data from the articles into an electronic table. All extracted data were then cross-checked independently by 2 other reviewers (C.B.B., A.R.D.). The following variables were extracted from the studies: study population (age, HIV status, country, exclusion criteria, and exposures), study design, neuropsychological testing and associated results, rates of developmental delays (with criteria for determining delay), and P values of developmental differences between groups. After the initial data were extracted, the Bayley Scales of Infant and Toddler Development (BSID) was identified as the assessment for which the meta-analysis would be focused. The primary outcomes of interest for the meta-analysis were the means and SDs of the Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant and Toddler Development, First Edition (BSID-I) or Bayley Scales of Infant and Toddler Development, Second Edition (BSID-II). For studies in which researchers used the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III), the Cognitive Scale score replaced the MDI, and the Motor Scale score replaced the PDI. When reporting the prevalence of severe delays within the results, standardized BSID scores of −2 SDs below the mean or a score of <70 were used.
Meta-analysis
In the analysis, we included studies in which neurodevelopment (BSID’s MDI and PDI) among HIV+, HEU, and HUU children was compared. To consider all combinations within a single analysis, we performed a network meta-analysis to augment evidence from the different study designs. Network meta-analysis was chosen because it allows for borrowing of strength from indirect evidence to bolster direct evidence.12,13
Group-specific sample sizes, means, and SDs were abstracted from articles considered in the systematic review. In cases in which SDs were not given, they were calculated by using the margin of error and sample size. If a study was longitudinal, summaries from the last encounter were used only if losses to follow-up did not exceed 50% of baseline sample size. Similar methods were used to determine the rates of neurodevelopmental delays in the systematic review. This was performed to maximize the ability of the results to reflect persisting delays in development by evaluating at an older age while being mindful of the limitations imposed by significant loss to follow-up. Studies in which researchers reported raw BSID scores were excluded from network meta-analysis.
Children not exposed to HIV were treated as the statistical reference group in the network meta-analysis. The resultant effect measures were interpreted as differences from the mean BSID score for the unexposed group.
Analyses were performed on the basis of complementation of available frequentist and Bayesian methods. In the final results, we reported Bayesian posterior mean estimates with their 95% credible intervals. The evidence base was summarized by using a network plot, with nodes representing comparison groups and edges representing all direct comparisons. Consistency within networks was assessed by comparing direct estimates to indirect estimates at the .05 α-level. Analyses were adjusted for statistical variation by using random effects. Systematic heterogeneity was assessed by using subgroup analyses and meta-regression. Confounders considered in subgroup analyses and meta-regression included study quality (low versus high) and record of ARV medication exposure (yes versus no). Vague normal priors were assumed for all regression coefficients in the Bayesian meta-regression. Statistical analyses were performed by using SAS version 9.4 (SAS Institute, Inc, Cary, NC), R version 3.2.2, and Stata version 15.0 (StataCorp, College Station, TX).
Quality Assessment
Quality was assessed 2 ways within this review: methodological quality and quality of the neuropsychological assessment. The Downs and Black14 checklist was used to assess the quality of the methods used within the included studies. Although this checklist is commonly used in assessing quality in observational and cross-sectional studies, it reflects only the standard of reporting for a given study. Because several studies included in this review were published before the development of quality checklists, these quality assessments were not a focus in the analysis. The checklist results are included to facilitate interpretation of the results (Supplemental Table 5).
Many of the studies included in this review were performed in settings where neuropsychological instruments have not been normed and validated, which may compromise the validity of the results within those studies. Therefore, we categorized studies on the basis of the degree to which the assessment was appropriate for the population within which it was used. In close consultation with a pediatric neuropsychologist (B.C.M.), we created criteria for the quality of the neuropsychological assessments (Supplemental Table 6). Briefly, “high quality” assessments were normed for use within a similar population as the study population; “moderate–high quality” assessments were not normed for use in the study population, but a citation of adaptation for use in the study population was included; “moderate–low quality” assessments were those in which the authors described adapting, translating, and/or piloting the assessment for use in the study population; “low quality” assessments mentioned none of the actions described previously. For the meta-analysis, all studies that were deemed high or moderate–high quality were considered high quality, and the remaining studies were considered low quality.
Results
Description of Search Results
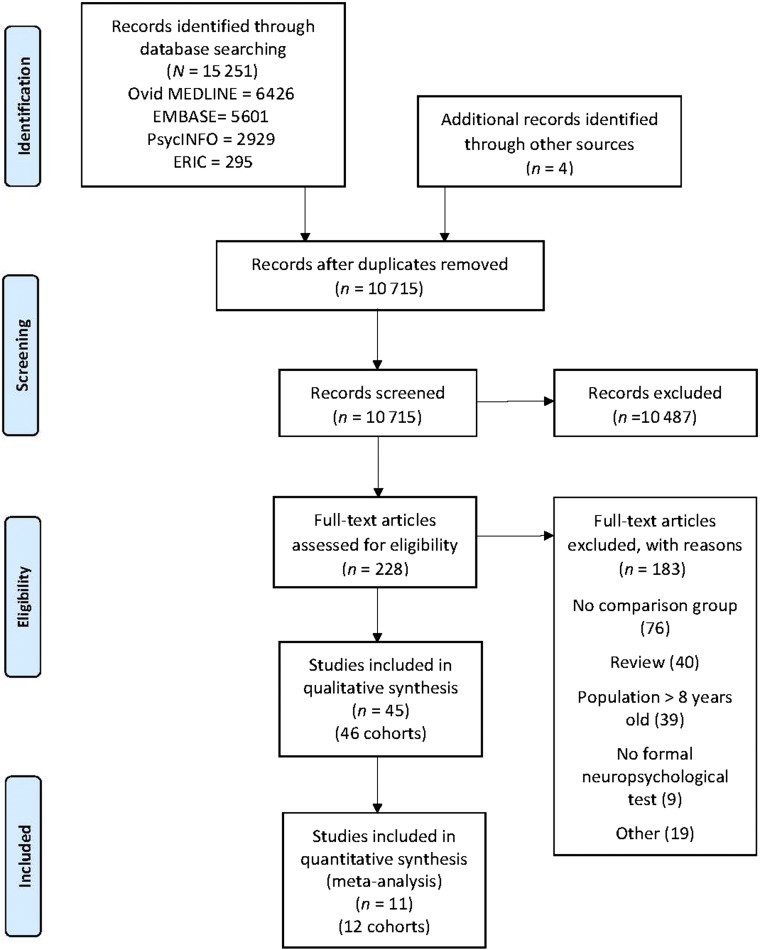
The search strategy yielded a total of 10 715 records (after duplicates were removed). After the initial screen, 228 studies remained for full-text article review. Forty-five publications met criteria for inclusion in our systematic review (Fig 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. ERIC, Education Resources Information Center.
Systematic Review
Forty-five studies (containing 46 study cohorts) were summarized within the systematic review, including those within the meta-analysis. Most of the study cohorts were from the United States or Canada (n = 20) and sub-Saharan Africa (n = 18). Four cohorts were evaluated in Europe, 3 studies were performed in South America, and 1 study was in Asia (Supplemental Table 7). Results for the systematic review were heterogeneous because of the number of neurodevelopmental assessments used. The most commonly used assessment was the BSID (n = 23). The next most commonly used assessments were the Denver Developmental Screening Test (DDST) (n = 5) and Clinical Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS) (n = 3). All remaining assessments were used in ≤2 studies.
In most studies (n = 28), researchers indicated HIV+ children had worse developmental outcomes relative to comparison group(s), and in a few studies (n = 3), researchers also described HEU children as having worse developmental outcomes compared with HUU children. An HIV-unexposed population was included in 25 studies (Supplemental Tables 8 and 9).
The rates of developmental delay varied considerably across studies (Supplemental Table 10). For HIV+ children, rates of severe cognitive delays determined using the BSID (typically defined as below −2 SDs from the mean) were as low as 3%15 or as high as 90.0%.8 In most articles researchers reported rates of severe cognitive delay between 21% and 35%.16–22 Rates of severe motor delays determined by using the BSID varied similarly, ranging from 14%20 to 81%.8 In most of the articles, researchers reported rates of severe motor delay between 21% and 66%.15–19,21–23
The range of rates of neurodevelopmental delays was slightly more confined for HEU children. The rates of severe cognitive delays determined by using the BSID ranged from 1% to 31%.8,15–22 The rates of severe motor delays ranged from 0% to 39%.8,15–22 For those studies in which researchers reported outcomes for HUU children, rates of severe cognitive delays ranged from 1% to 15%,8,15,23,24 and rates of severe motor delays ranged from 0% to 6%.8,15,23,24
Meta-analysis
On further review, the original BSID or its subsequent editions were used in 23 publications. Of these, researchers in 10 publications (11 study cohorts) reported the data qualifying their study for inclusion in the meta-analysis.7,16,18,19,22,24–28 The authors of the other 13 publications were contacted (by M.S.M.) regarding the availability of data needed to be included in the meta-analysis, and 1 additional study was added from this process,29 bringing the total number of publications in the meta-analysis to 11 (12 study cohorts).
Study Characteristics
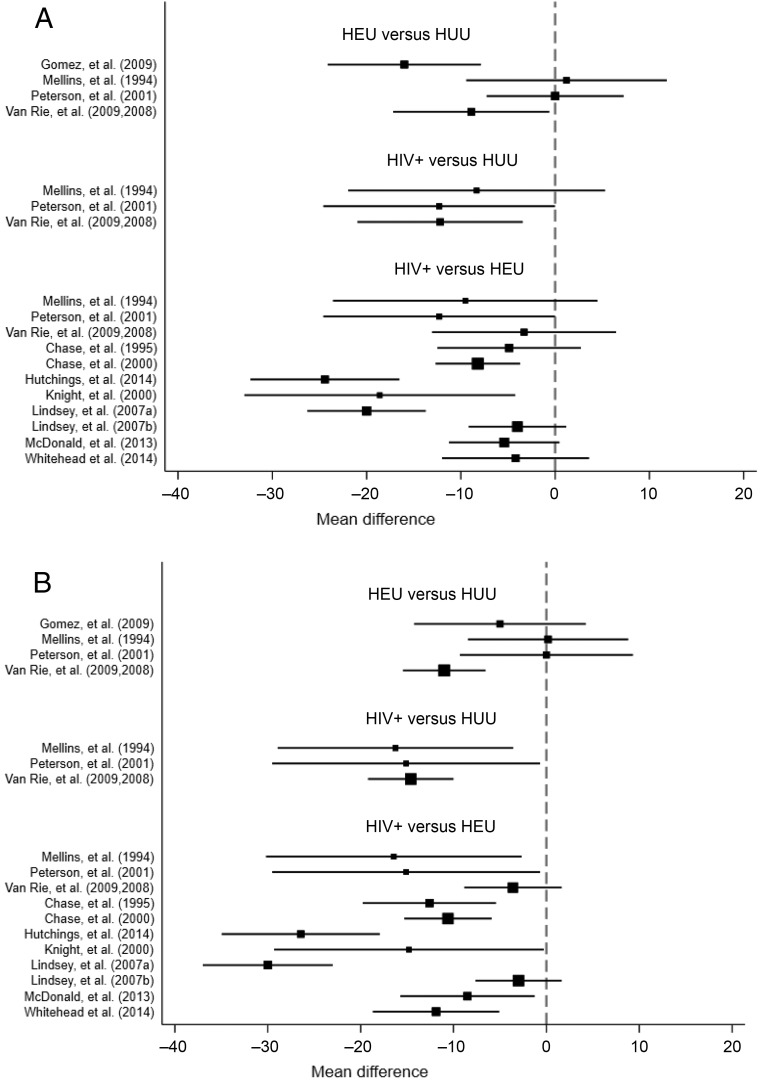
The MDI was assessed in 1751 children (367 HIV+, 1231 HEU, and 153 HUU), whereas the PDI was assessed in 1748 children (365 HIV+, 1230 HEU, and 153 HUU) (Table 1). Six study cohorts were from the United States, 5 study cohorts were from sub-Saharan Africa, and 1 study cohort was from Colombia. All assessments performed in the United States were high quality, and these study populations had a proportion of their sample exposed to intravenous (IV) drug use. The assessments performed outside the United States all received a rating of low quality, and in none of those studies did researchers indicate whether IV drug exposure occurred in their study population. Seven cohorts were assessed by using the BSID-I, 3 were assessed by using the BSID-II, and 2 were assessed by using the BSID-III. In 1 longitudinal study, researchers used both the BSID-I and BSID-II.19 Eight study cohorts had exposure to ARVs (mother, child, or both). Study cohort–specific differences in mean MDI and PDI scores are noted in Fig 2. Further details regarding the data pulled from these articles is in Supplemental Table 10.
TABLE 1.
Characteristics of Studies Included in the Meta-analysis
| Study | Study Design | Country | Year | Development Scale | Quality | ARV | Domain | mHIV+ | seHIV+ | mHEU | seHEU | mHUU | seHUU | nHIV+ | nHEU | nHUU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chase et al16 | L | United States | 1995 | BSID-I | HQ | Child | MDI | 83.1 | 16.1 | 94.8 | 18.2 | — | — | 24 | 27 | — |
| PDI | 85.8 | 16.4 | 97.8 | 16.7 | — | — | 24 | 27 | — | |||||||
| Chase et al25 | L | United States | 2000 | BSID-I | HQ | Child | MDI | 83.1 | 23.0 | 94.4 | 16.1 | — | — | 57 | 220 | — |
| PDI | 83.5 | 23.8 | 101.2 | 16.8 | — | — | 57 | 213 | — | |||||||
| Gómez et al26 | L | Colombia | 2009 | BSID-II | LQ | Mother | MDI | — | — | 83 | 10.3 | 99 | 12.3 | — | 15 | 15 |
| PDI | — | — | 98 | 13.5 | 103 | 12.3 | — | 15 | 15 | |||||||
| Hutchings and Potterton18 | CS | Zimbabwe | 2014 | BSID-III | LQ | Both | MDI | 75.89 | 17.69 | 100.31 | 12.75 | — | — | 28 | 32 | — |
| PDI | 79.71 | 20.91 | 106.16 | 10.20 | — | — | 28 | 32 | — | |||||||
| Knight et al27 | L | United States | 2000 | BSID-I | HQ | None | MDI | 73.2 | 30.5 | 86.4 | 18.4 | — | — | 20 | 25 | — |
| PDI | 80.6 | 28.2 | 98.3 | 24.1 | — | — | 20 | 25 | — | |||||||
| Lindsey et al19 | L | United States | 2007 | BSID-I | HQ | Both | MDI | 85 | 22 | 105 | 17 | — | — | 54 | 221 | — |
| PDI | 77 | 25 | 107 | 16 | — | — | 54 | 221 | — | |||||||
| Lindsey et al19 | L | United States | 2007 | BSID-II | HQ | Both | MDI | 75 | 17 | 79 | 17 | — | — | 47 | 350 | — |
| PDI | 84 | 17 | 87 | 17 | — | — | 47 | 349 | — | |||||||
| McDonald et al29 | L | Tanzania | 2013 | BSID-II | LQ | None | MDI | 81.6 | 13.7 | 87 | 11.4 | — | — | 25 | 90 | — |
| PDI | 80.4 | 16.2 | 88.9 | 16.8 | — | — | 25 | 90 | — | |||||||
| Mellins et al7 | CS | United States | 1994 | BSID-I | HQ | None | MDI | 75.50 | 29.00 | 85.03 | 22.03 | 83.83 | 17.48 | 24 | 30 | 23 |
| PDI | 73.58 | 29.35 | 90.01 | 20.06 | 89.83 | 11.70 | 24 | 30 | 23 | |||||||
| Peterson et al28 | CS | Uganda | 2001 | BSID-I | LQ | None | MDI | 79.3 | 18.0 | 91.6 | 13.1 | 91.6 | 13.1 | 10 | 25 | 25 |
| PDI | 79.0 | 20.7 | 94.1 | 16.8 | 94.1 | 16.8 | 10 | 25 | 25 | |||||||
| Van Rie et al24 | L | DRC | 2009 | BSID-I | LQ | Child | MDI | 84.3 | 21.73 | 87.6 | 19.92 | 96.5 | 24.2 | 35 | 35 | 90 |
| PDI | 90.4 | 11.47 | 94.0 | 10.87 | 105.0 | 12.6 | 35 | 35 | 90 | |||||||
| Whitehead et al22 | L | South Africa | 2014 | BSID-III | LQ | Child | MDI | 94.75 | 14.09 | 98.95 | 10.61 | — | — | 20 | 19 | — |
| PDI | 93.7 | 10.89 | 105.58 | 10.88 | — | — | 20 | 19 | — |
ARV, antiretroviral medication exposure; CS, cross-sectional; DRC, Democratic Republic of Congo; HQ, high quality; L, longitudinal; LQ, low quality; mHEU, mean score for HIV-exposed but uninfected; mHIV+, mean score for HIV-infected; mHUU, mean score for HIV-unexposed and uninfected; nHEU, number of HIV-exposed but uninfected children included in the analyzed sample; nHIV+, number of HIV-infected children included in the analyzed sample; nHUU, number of HIV-unexposed and uninfected children included in the analyzed sample; seHEU, SE for HIV-exposed but uninfected; seHIV+, SE for HIV-infected; seHUU, SE for HIV-unexposed and uninfected; —, not applicable.
FIGURE 2.
Study cohort–specific differences in mean BSID scores by subtest and comparison of HIV exposure groups. The size of the box representing the point estimate of the mean is proportional to the inverse square of the SE. The second group in each comparison had the larger mean BSID score. A, MDI. B, PDI.
Network Meta-analysis
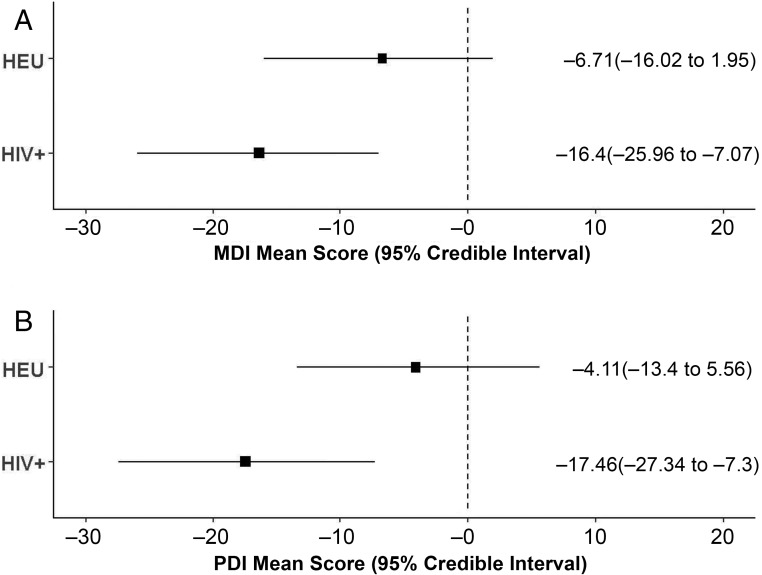
Tests for consistency between direct and indirect MDI comparisons suggested no evidence of inconsistency (χ2(2) = 3.83, P value = .147). Similarly, no evidence of inconsistency was observed in direct and indirect comparisons of the PDI (χ2(2) = 0.67, P value = .714). The estimated means for the MDI and PDI in each HIV exposure group is described in Table 2. Group differences were seen in all study cohorts except 1,7 with HIV+ children having worse scores compared with both HEU and HUU children and HEU children having worse scores compared with HUU children. The summarized Bayesian estimates of the MDI and PDI reveal a similar pattern (Fig 3).
TABLE 2.
Estimated Means and 95% Credible Intervals of the BSID by HIV Exposure Group and Subtest
| Outcome | Group | Estimate | 95% Credible Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| MDI | HUU | 89.79 | 78.78 | 101.07 |
| HEU | 83.03 | 75.73 | 90.99 | |
| HIV+ | 73.38 | 66.87 | 80.29 | |
| PDI | HUU | 99.01 | 87.21 | 111.06 |
| HEU | 94.85 | 87.16 | 103.23 | |
| HIV+ | 81.53 | 74.66 | 88.70 | |
The normative group for the BSID has a mean score of 100 and an SD of 15.
FIGURE 3.
Summarized Bayesian estimates of mean scores and 95% credible intervals relative to the HIV-unexposed group by HIV exposure group and BSID subtest. A, MDI. B, PDI.
Sensitivity Analysis
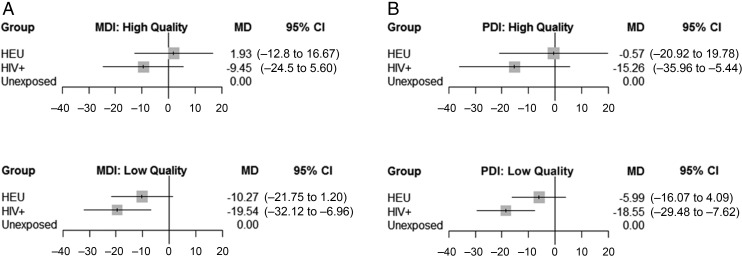
In studies with high-quality assessments, HEU children had similar scores to HUU children on the MDI and PDI (Fig 4). This was not the case for HEU children in studies with low-quality assessments, in which their mean scores were 10.27 and 5.99 points lower than HUU children for the MDI and PDI, respectively. HIV+ children had lower MDI and PDI scores compared with HUU children in high- and low-quality assessment studies. Of note, the difference in mean MDI scores between HIV+ and HUU children was much greater when low-quality assessments were used (mean difference: −9.45 with high-quality assessments versus −19.54 with low-quality assessments).
FIGURE 4.
Comparison of high- versus low-quality assessments by HIV exposure group and BSID subtest. A, MDI. B, PDI. CI, confidence interval; MD, mean difference.
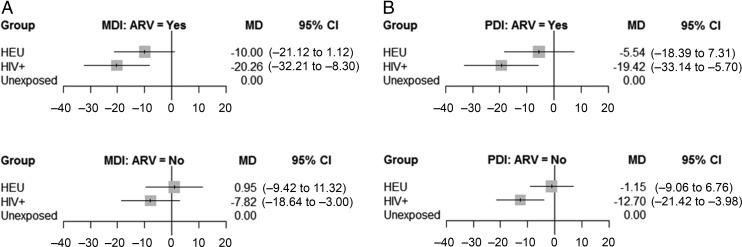
When looking at ARV exposure, HEU children with no ARV medication exposure had similar MDI and PDI scores to the HUU children (Fig 5). HIV+ children with no ARV medication exposure had lower MDI and PDI scores compared with HUU children. However, the magnitude of this difference was smaller than that seen in the summarized Bayesian estimate. For the MDI and PDI, HIV+ and HEU children exposed to ARV medications had lower mean difference scores compared with those who were not exposed.
FIGURE 5.
Comparison of ARV medication exposure versus none by HIV exposure group and BSID subtest. A, MDI. B, PDI. CI, confidence interval; MD, mean difference.
Further exploration of systematic heterogeneity using meta-regression showed that the covariates we considered explained little of the heterogeneity in both the MDI and PDI models. In the PDI model, inclusion of covariates reduced between-study heterogeneity from 4.51 to 4.5. Heterogeneity in the MDI model did not change after inclusion of covariates, and it remained at a posterior median of 3.82. Moreover, in both the MDI and PDI models, the point estimates of the mean differences did not change after inclusion of potential confounders (Table 3).
TABLE 3.
Unadjusted and Adjusted Meta-regression Results of Mean Differences Between HIV Exposure Groups Compared With HIV-Unexposed Children by BSID Subtest
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| 95% Credible Interval | 95% Credible Interval | ||||||
| Measure | Group | Estimate | Lower | Upper | Estimate | Lower | Upper |
| MDI | HEU | −6.71 | −16.02 | 1.95 | −6.61 | −15.45 | 2.54 |
| HIV+ | −16.40 | −25.96 | −7.07 | −16.32 | −26.180 | −7.08 | |
| PDI | HEU | −4.11 | −13.40 | 5.56 | −4.25 | −13.71 | 5.12 |
| HIV+ | −17.46 | −27.34 | −7.30 | −17.62 | −27.28 | −7.59 | |
The normative group for the BSID has a mean score of 100 and an SD of 15.
Discussion
In this systematic review, we assessed the current state of evidence on neurodevelopmental delays in children born to HIV+ mothers. We used a Bayesian meta-regression to quantify the mean differences in mental and motor scores among HIV+, HEU, and HUU children. HIV+ children had much lower mental and motor scores compared with HUU. HEU children were also found to have lower mental and motor scores compared with HUU, although not to the degree of HIV+ children. In our sensitivity analyses, we also found differences in developmental scores on the basis of the quality of the assessment and ARV exposure. These results help inform clinical practice as well as future directions to investigate how we can optimize neurodevelopment for this vulnerable population.
To our knowledge, this is the first aggregated, quantitative analysis of the neurodevelopment of young HIV-exposed children. In addition, by separating the HIV-negative groups as HEU and HUU, we could compare the results of all 3 groups in a network meta-analysis so that a more comprehensive analysis of HEU children could be performed. Because we used a Bayesian model, statistical significance cannot be determined. However, the mean differences and 95% credible intervals suggest HEU children have worse development compared with HUU children. HEU children are of particular interest globally as prevention of mother-to-child transmission access expands and the world seeks to eliminate perinatal infections. Although child HIV infections may be averted, it is critical for both clinicians and researchers to know if there are potential adverse consequences of ARV exposure or HIV exposure. Individually, researchers in many studies looking at both HEU and HUU children could not answer these questions, with mixed results on the impact of HIV exposure on neurodevelopment.7,15,24,26,30,31 Their conclusions have been limited by the small sample sizes of their studies and the varying domains assessed, affecting the generalizability of their results. By grouping studies in which researchers measure the same outcomes, we could generate more compelling evidence that the HEU population may have lower mental and motor scores compared with HUU children, even when controlling for quality of the assessment and ARV exposure.
When evaluating neurodevelopment in children, many factors could have confounding effects on the results, such as nutritional status, prematurity, maternal education, socioeconomic level, and maternal mental health.32,33 Although the reporting in the studies included within this review was too heterogeneous to control for all factors, we adjusted our model for the confounding variables for which we had data. It was possible to control for the quality of the assessment and ARV medication exposure. Other variables considered to be included in the model were the location of the study (United States versus non–United States) and exposure to IV drug use. However, by coincidence, when we separated our meta-analysis studies into groups on the basis of the use of high-quality versus low-quality assessments, the same groups held true for location and IV drug exposure. All high-quality assessments were performed in the United States, and all US studies had a portion of children exposed to IV drugs. This was not the case for the classification of the studies within the systematic review, only for the subset that qualified for the meta-analysis. Therefore, we cannot tease out the impact that each of the 3 factors might have had on the results, leaving several potential hypotheses. First, it is possible that when appropriate, high-quality neurodevelopmental assessment tools are used, no detectable difference in neurodevelopment exists in HEU children compared with HUU children. Second, it is also possible that children in resource-limited settings face multiple risk factors for poor neurodevelopment that are compounded by HIV exposure. However, with these data and the current published literature, we cannot draw any definitive conclusions regarding these hypotheses.
These same factors may affect the results related to ARV exposure. In this study, we found that when exposed to ARV medications, both HIV+ and HEU children had greater decrement in their mean MDI and PDI scores compared with HUU children. As noted above, there are multiple confounding factors we could not account for in this analysis that may influence the results. Although attention should be given to these limitations, the results should not be dismissed. Determining if and how either ARV or HIV exposure adversely affects neurodevelopment in HIV-negative children emerges as a key priority. When reviewing the limited literature on this topic, ARV exposure has not definitively been shown to have negative effects on neurodevelopment.34,35 The evidence is mixed on whether HIV exposure itself may have negative effects on neurodevelopment.15,36,37 These mixed findings may be due in part to the research challenge of finding HIV-exposed young children not exposed to ARV medications as perinatal ARV therapy use expands. However, with our findings, we support continued efforts by researchers to explore avenues of translational research to investigate the potential impact of ARV and HIV exposure in young HIV-negative individuals using culturally adapted and appropriate assessments.
The actual neurobiological mechanism of dysfunction is another important consideration for the neurodevelopment of HIV-exposed children. HIV+ children have been found to have differences in their brain structure and function, including alterations in cortical thickness, subcortical volumes, regional connectivity, and neurometabolites.38,39 These changes have been associated with lower cognitive functioning.38,39 Although HIV exposure having negative impacts on HIV+ and HEU children is supported in the results of our study, the assessments used throughout the review were general in nature. The BSID, which was used for our meta-analysis, separated its main developmental domains into “mental” and “motor” in its first 2 editions. The other 2 most commonly used assessments, DDST and CAT/CLAMS, are also considered general assessments. By using more targeted neuropsychological assessments, we may understand better the specific domains affected and learn more about the region and timing of the neurologic insults. However, targeted cognitive assessments can be challenging to administer and interpret in young children because these areas of the brain are rapidly developing during the first few years of life. Thus, targeted assessments are more commonly used in older populations. For example, in a recent meta-analysis of older HIV+ children and adolescents, researchers found working memory, processing speed, and executive function were most affected by HIV.2 These skills are more formally developed and easier to test in older children; however, these children also have more time and opportunities to experience positive or negative external factors that may affect development, such as social stimulation and supportive learning environments. By assessing more specific domains early, with measures such as those found within the National Institutes of Health Toolbox,40 we could have a clearer understanding of the direct impact that HIV exposure has on neurodevelopment. Researchers conducting future studies should consider using more targeted neuropsychological assessments to evaluate young children.
One limitation of this study was the quality of the included studies. All studies included in this review exhibited at least 1 methodological flaw, as determined by the Downs and Black14 quality rating checklist (Supplemental Table 4). Furthermore, most assessments used in the included studies were developed and normed in populations different from the ones in which they were used, with no indication of substantial attempts to culturally adapt and validate the assessment before its administration. We attempted to control for this by evaluating the quality of the neurodevelopmental assessment and by requiring at least 2 populations to be evaluated in the study to serve as a form of reference (ie, HEU or HUU groups), but the quality may affect the results.
The generalizability of this study is also somewhat limited by the study locations. Nearly 70% of the world’s HIV+ individuals live in sub-Saharan Africa.41 Thirty-eight percent of the studies in our systematic review and 46% in our meta-analysis had study cohorts based in sub-Saharan Africa. We also only had 1 study from southeast Asia, which has the second-highest number of HIV+ individuals.41
Our study was limited by the heterogeneity of the assessments used across studies. HIV-exposed children live all over the world, and they are evaluated by using a variety of assessment tools. Research in this area would benefit from having an international standard for developmental assessments in young children that has been validated across cultures and can be used in multiple settings. Additionally, this study was limited by the heterogeneity of the ARV regimens and exposures across studies. Because of this heterogeneity and the small number of studies included within the meta-analysis, we were unable to determine how change in HIV treatments over time may have impacted neurodevelopmental outcomes for HIV-exposed and HIV+ children.
Conclusions
With this review, we found that both HIV+ and HEU children show worse cognitive and motor outcomes compared with HUU children. Both HIV+ and HEU children exposed to ARV medications may also have worse cognitive development compared with those who were unexposed, but these results should be interpreted with caution, and further research is needed in this area.
Acknowledgments
We thank Elaine Skopelja, MALS, AHIP, at Indiana University School of Medicine’s Ruth Lilly Medical Library for helping us create our search strategy. We thank Christine McDonald, ScD, and Christopher Duggan, MD, MPH, for sharing their study’s data for inclusion in the meta-analysis.
Glossary
- ARV
antiretroviral
- BSID
Bayley Scales of Infant and Toddler Development
- BSID-I
Bayley Scales of Infant and Toddler Development, First Edition
- BSID-II
Bayley Scales of Infant and Toddler Development, Second Edition
- BSID-III
Bayley Scales of Infant and Toddler Development, Third Edition
- CAT/CLAMS
Clinical Adaptive Test/Clinical Linguistic and Auditory Milestone Scale
- DDST
Denver Developmental Screening Test
- HEU
HIV-exposed but uninfected
- HIV+
HIV-infected
- HUU
HIV-unexposed and uninfected
- IV
intravenous
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
Footnotes
Dr McHenry conceptualized and designed the study, consulted with the medical librarian regarding search terms, ran the searches, reviewed all titles, abstracts, and full-text articles included in the review, extracted data from the included articles and cross-checked the remaining articles, coordinated and supervised statistical efforts for the meta-analysis, wrote the initial draft of the manuscript, and reviewed and revised the manuscript; Ms McAteer reviewed all titles, abstracts, and full-text articles included in the review, extracted data from the included articles and cross-checked the remaining articles, assessed quality of the assessments and the study design, and reviewed and revised the manuscript; Dr Oyungu helped to conceptualize and design the study, advocated for a meta-analysis of our initial systematic review, and reviewed and provided input for the manuscript; Dr McDonald helped to create the criteria for the quality of the assessment in a given population, settled disputes or uncertainty regarding the categorization of an assessment on the basis of their quality, and reviewed and revised the manuscript; Mr Bosma helped to cross-check the data collected, reformatted the data into the supplementary tables, and reviewed and revised the manuscript; Mr Mpofu planned and executed the statistical design of the meta-analysis, created the forest plots within the results, and reviewed and revised the manuscript; Mr Deathe helped to obtain full-text articles through the medical library for the review, cross-checked the data collected within the reformatted tables, and reviewed and provided input for the manuscript; Dr Vreeman consulted on the design of the study and was consulted if there were disagreements between the 2 primary reviewers regarding the inclusion or exclusion of particular studies, and she also reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by a training grant entitled “Training in STIs and Other Infections of Global Health Significance” (T32AI007637; principal investigator: Dr Wools Kaloustian) from the National Institute of Allergy and Infectious Diseases (Bethesda, MD). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Abubakar A, Van Baar A, Van de Vijver FJ, Holding P, Newton CR. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2008;13(7):880–887 [DOI] [PubMed] [Google Scholar]
- 2.Phillips N, Amos T, Kuo C, et al. . HIV-associated cognitive impairment in perinatally infected children: a meta-analysis. Pediatrics. 2016;138(5):e20160893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherr L, Croome N, Parra Castaneda K, Bradshaw K, Romero RH. Developmental challenges in HIV infected children—an updated systematic review. Child Youth Serv Rev. 2014;45:74–89 [Google Scholar]
- 4.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14(3):276–287 [DOI] [PubMed] [Google Scholar]
- 5.Powis KM, Slogrove AL, Mofenson L. Protecting the health of our AIDS-free generation: beyond prevention of mother-to-child HIV transmission. AIDS. 2017;31(2):315–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS Prevention gap report. 2016. Available at: www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Accessed June 20, 2017
- 7.Mellins CA, Levenson RL Jr, Zawadzki R, Kairam R, Weston M. Effects of pediatric HIV infection and prenatal drug exposure on mental and psychomotor development. J Pediatr Psychol. 1994;19(5):617–627 [DOI] [PubMed] [Google Scholar]
- 8.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNICEF The formative years: UNICEF’s work on measuring early childhood development. 2014. Available at: https://data.unicef.org/resources/the-formative-years-unicefs-work-on-measuring-ecd/. Accessed October 2, 2017
- 11.Pearson Wechsler preschool and primary scale of intelligence - fourth edition. 2012. Available at: https://www.pearsonclinical.com/psychology/products/100000102/wechsler-preschool-and-primary-scale-of-intelligence–fourth-edition-wppsi-iv.html. Accessed October 2, 2017
- 12.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. [DOI] [PubMed] [Google Scholar]
- 13.Scott DA, Boye KS, Timlin L, Clark JF, Best JH. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab. 2013;15(3):213–223 [DOI] [PubMed] [Google Scholar]
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drotar D, Olness K, Wiznitzer M, et al. . Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100(1). Available at: www.pediatrics.org/cgi/content/full/100/1/e5 [DOI] [PubMed] [Google Scholar]
- 16.Chase C, Vibbert M, Pelton SI, Coulter DL, Cabral H. Early neurodevelopmental growth in children with vertically transmitted human immunodeficiency virus infection. Arch Pediatr Adolesc Med. 1995;149(8):850–855 [DOI] [PubMed] [Google Scholar]
- 17.Gay CL, Armstrong FD, Cohen D, et al. . The effects of HIV on cognitive and motor development in children born to HIV-seropositive women with no reported drug use: birth to 24 months. Pediatrics. 1995;96(6):1078–1082 [PubMed] [Google Scholar]
- 18.Hutchings J, Potterton J. Developmental delay in HIV-exposed infants in Harare, Zimbabwe. Vulnerable Child Youth Stud. 2014;9(1):43–55 [Google Scholar]
- 19.Lindsey JC, Malee KM, Brouwers P, Hughes MD; PACTG 219C Study Team . Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119(3). Available at: www.pediatrics.org/cgi/content/full/119/3/e681 [DOI] [PubMed] [Google Scholar]
- 20.Macmillan C, Magder LS, Brouwers P, et al. . Head growth and neurodevelopment of infants born to HIV-1-infected drug-using women. Neurology. 2001;57(8):1402–1411 [DOI] [PubMed] [Google Scholar]
- 21.Pollack H, Kuchuk A, Cowan L, et al. . Neurodevelopment, growth, and viral load in HIV-infected infants. Brain Behav Immun. 1996;10(3):298–312 [DOI] [PubMed] [Google Scholar]
- 22.Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26(4):497–504 [DOI] [PubMed] [Google Scholar]
- 23.Ferguson G, Jelsma J. The prevalence of motor delay among HIV infected children living in Cape Town, South Africa. Int J Rehabil Res. 2009;32(2):108–114 [DOI] [PubMed] [Google Scholar]
- 24.Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2009;52(5):636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chase C, Ware J, Hittelman J, et al. ; Women and Infants Transmission Study Group . Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Pediatrics. 2000;106(2). Available at: www.pediatrics.org/cgi/content/full/106/2/e25 [DOI] [PubMed] [Google Scholar]
- 26.Gómez C, Archila ME, Rugeles C, Carrizosa J, Rugeles MT, Cornejo JW. A prospective study of neurodevelopment of uninfected children born to human immunodeficiency virus type 1 positive mothers [in Spanish]. Rev Neurol. 2009;48(6):287–291 [PubMed] [Google Scholar]
- 27.Knight WG, Mellins CA, Levenson RL Jr, Arpadi SM, Kairam R. Brief report: effects of pediatric HIV infection on mental and psychomotor development. J Pediatr Psychol. 2000;25(8):583–587 [DOI] [PubMed] [Google Scholar]
- 28.Peterson NJ, Drotar D, Olness K, Guay L, Kiziri-Mayengo R. The relationship of maternal and child HIV infection to security of attachment among Ugandan infants. Child Psychiatry Hum Dev. 2001;32(1):3–17 [DOI] [PubMed] [Google Scholar]
- 29.McDonald CM, Manji KP, Kupka R, et al. . Stunting and wasting are associated with poorer psychomotor and mental development in HIV-exposed Tanzanian infants. J Nutr. 2013;143(2):204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condini A, Axia G, Cattelan C, et al. . Early language development in 40 uninfected children born to HIV-positive mothers. Giorn Neuropsi Evol. 1997;17(2):105–111 [Google Scholar]
- 31.Wachtel RC, Tepper VJ, Houck DL, Nair P, Thompson C, Johnson JP. Neurodevelopment in pediatric HIV-1 infection: a prospective study. Pediatr AIDS HIV Infect. 1993;4(4):198–203 [Google Scholar]
- 32.Ali SS. A brief review of risk-factors for growth and developmental delay among preschool children in developing countries. Adv Biomed Res. 2013;2:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker SP, Wachs TD, Grantham-McGregor S, et al. . Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–1338 [DOI] [PubMed] [Google Scholar]
- 34.Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM; PACTG 219C Team . Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nozyce ML, Huo Y, Williams PL, et al. ; Pediatric HIVAIDS Cohort Study . Safety of in utero and neonatal antiretroviral exposure: cognitive and academic outcomes in HIV-exposed, uninfected children 5-13 years of age. Pediatr Infect Dis J. 2014;33(11):1128–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14(1):13–21 [DOI] [PubMed] [Google Scholar]
- 37.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de Perre P, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993;92(6):843–848 [PubMed] [Google Scholar]
- 38.Yadav SK, Gupta RK, Garg RK, et al. . Altered structural brain changes and neurocognitive performance in pediatric HIV. Neuroimage Clin. 2017;14:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller MA, Venkatraman TN, Thomas A, et al. . Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62(10):1810–1817 [DOI] [PubMed] [Google Scholar]
- 40.Health Measures NIH toolbox. 2017. Available at: www.healthmeasures.net/explore-measurement-systems/nih-toolbox. Accessed June 30, 2017
- 41.AVERT Global HIV and AIDS statistics. 2015. Available at: https://www.avert.org/global-hiv-and-aids-statistics. Accessed June 30, 2017