Bats exhibit persistent social foraging (producer-scrounger) ties.
Abstract
Social foraging theory suggests that group-living animals gain from persistent social bonds, which lead to increased tolerance in competitive foraging and information sharing. Bats are among the most social mammals, often living in colonies of tens to thousands of individuals for dozens of years, yet little is known about their social foraging dynamics. We observed three captive bat colonies for over a year, quantifying >13,000 social foraging interactions. We found that individuals consistently used one of two foraging strategies, either producing (collecting) food themselves or scrounging it directly from the mouth of other individuals. Individual foraging types were consistent over at least 16 months except during the lactation period when females shifted toward producing. Scroungers intentionally selected whom to interact with when socially foraging, thus generating persistent nonrandom social relationships with two to three specific producers. These persistent producer-scrounger relationships seem to reduce aggression over time. Finally, scrounging was highly correlated with vigilance, and we hypothesize that vigilant-prone individuals turn to scrounging in the wild to mitigate the risk of landing on a potentially unsafe fruit tree. We find the bat colony to be a rich and dynamic social system, which can serve as a model to study the role that social foraging plays in the evolution of mammalian sociality. Our results highlight the importance of considering individual tendencies when exploring social behavior patterns of group-living animals. These tendencies further emphasize the necessity of studying social networks over time.
INTRODUCTION
Social interactions among group-living animals are characterized by nonrandom associations, with phenotypic differences determining who will interact with whom and in what fashion (1–9). The social networks emerging from these individual choices lay the foundation for future interactions—the intrinsic properties of the networks will further shape specific interactions (10–12). This, in turn, sets the stage for individual behaviors, such as choosing mates and acquiring information, in addition to population-level processes, such as disease transfer and gene flow (13–16). Therefore, a major challenge in the study of sociality is revealing the rules governing the social choices of individuals (17). Social foraging, the way in which animals search and compete for food in groups, is an extremely important context for interactions between individuals, and it is considered a primary contributing factor to the evolution of sociality (18, 19). The behavioral diversity within socially foraging groups is often manifested in alternative foraging strategies used by animals to gain access to valued food sources, with exploitation of group mates being especially common (20). The factors underlying strategy choice by individuals, and how they influence the global social structure, are, however, largely unclear (21–25). Social foraging theory suggests that group-living animals can gain from persistent social relations, allowing both increased tolerance in competitive foraging and information sharing (20, 26). These benefits should be amplified in long-lived gregarious animals (such as bats), as implied by a handful of examples of social foraging primates (27, 28).
Here, we studied social foraging relationships between individuals within a complex social system of mammals: Egyptian fruit bats (Rousettus aegyptiacus). Accounting for more than a fifth of mammalian species, bats are among the most social of mammals, often living together in colonies of thousands of individuals for dozens of years, exhibiting a vast range of social behaviors (29–37). Despite this, many aspects of bat social behavior remain unclear. Some major open questions include the following: Do different social strategies exist in the bat colony? How persistent are they? How do they influence the colony’s social structure?
We studied three different captive Egyptian fruit bat colonies, observing more than 13,000 social foraging interactions over a period of up to 16 months. Bats in all three colonies exhibited clear individual preferences for one of two foraging strategies: either acquiring food on their own by directly producing it from a bowl or scrounging food from the mouth of another individual. These exploitation interactions were extremely common in all three colonies. In ~80% of foraging events, “producer” bats that had obtained food were approached by another individual that attempted to seize food from its mouth. We used these interactions to reveal how individual foraging strategies and social preferences of whom to interact with shape the social foraging structure of the colony and to examine how this structure changed over time. Specifically, we aimed to reveal whether individuals were consistent in their strategy choice, whether they exhibited consistent social preferences for whom to scrounge upon, and what was the underlying reason for the alternative strategies.
RESULTS
Study 1: Bats use two alternative foraging strategies
Our initial study was conducted on a colony of bats that were born in captivity (the “Established Colony”). We also used two additional colonies of wild-born, most likely nonrelated bats for comparison (see Methods). Bats in all three colonies exhibited a distinct individual preference toward one of the two alternative foraging strategies (that is, collecting food from a bowl or scrounging it from another individual), which we will refer to as producing or scrounging. This was reflected by a clear bimodal distribution of the producer indices (PIs; Eq. 1 and Fig. 1).
| (1) |
where PIi is the producer index of bat i, pi is the number of producing events of bat i, and si is the number of scrounging events of bat i. The PI ranges between −1 (representing a pure scrounger) and +1 (representing a full producer). It does not weigh the absolute number of foraging events per individual. However, our results are based on a large data set with a minimum of 110 foraging events per individual and thus are not likely to be biased by the variance of observations per individual.
Fig. 1. Bats exhibit two alternative foraging strategies—the Established Colony.
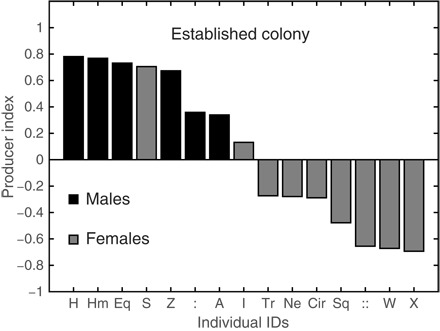
Individual PIs for all bats in the Established Colony with males in black (n = 6) and females (n = 9) in gray. Symbols on the x axis depict bats’ IDs. All PIs in this panel were calculated over the entire study period (January 2012 to April 2013).
Accordingly, a producer was defined as an individual with a positive PI and a scrounger was defined as an individual with a negative PI (this division was validated by a correspondence analysis of strategy use by ID; see Methods and tables S1 to S3). To account for the effects of prolonged captivity and genetic relatedness, we examined two additional control colonies of wild bats (Wild Control Colonies 1 and 2; see Methods). Bats in these colonies exhibited similar patterns (see fig. S1 and table S2 for Wild Control Colony 1 and fig. S2 and table S3 for Wild Control Colony 2), immediately adopting one of the two foraging strategies (fig. S3). The immediate display of strategy preference strongly suggests that these two alternative foraging strategies (producing and scrounging) are not an artifact of prolonged captivity (see more in Discussion).
Individual strategy preference in both the Established Colony and Wild Control Colony 2 was correlated with sex, with males using the producer strategy significantly more than females (Established Colony: male PI = 0.52 ± 0.28 versus female PI = −0.278 ± 0.45, mean ± SD, P < 0.003; Wild Control Colony 2: male PI = 0.49 ± 0.47 versus female PI = −0.12 ± 0.75, P < 0.009, permuted t test). A similar though nonsignificant pattern was observed in Wild Control Colony 1 (male PI = 0.27 ± 0.72 versus female PI = 0.146 ± 0.65, P = 0.13, permuted t test), suggesting that other factors besides sex play a role in strategy choice.
The tendency of males to produce more than females was consistent temporally across the entire study period in all three colonies. However, females altered their strategy preference according to the reproductive period of the colony (Fig. 2A and figs. S4A and S5A). Females in both the Established Colony and Wild Control Colony 2 exhibited a rise in producing, which started in the middle of the gestation period and peaked throughout lactation, reaching positive producing PI values. This increase was followed by a drop (back to negative scrounging PI values) in the postweaning phase. This was also observed in the following gestation period (12 months after the first one). This pattern was very clear in the Established Colony and in Wild Control Colony 2, and it was observed to a lesser extent in Wild Control Colony 1, probably because females in this colony did not reproduce during their first year in captivity (Fig. 2A and figs. S4A and S5A, respectively).
Fig. 2. Temporal consistency of strategy use—the Established Colony.
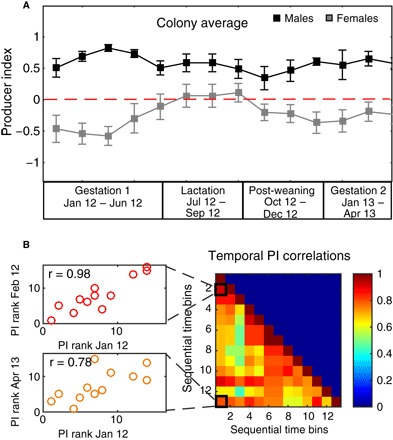
(A) Average PI + SE for males (black, n = 6) and females (gray, n = 9). The female reproductive periods are denoted along the x axis. Notice that the average female PI is positive in July-August-September. (B) There were high correlations between monthly PI ranks (n = 16), implying that even when bats changed strategy use, they maintained a relative order; that is, individuals that were stronger producers remained stronger and vice versa. Left: An example of the correlation between the first and second time bins (January and February 2012; r = 0.9, P = 0) and between the first and last time bins (r = 0.78, P = 0.001, January 2012 and April 2013). Right: Temporal correlation matrix. Each cell denotes the Spearman correlation coefficient between two time bins (1 to 2 months each). The color indicates the strength of the correlation. All correlations were significant [P < 0.05, following a false discovery rate (FDR) P value adjustment for multiple comparisons] except for those of bin 3 with 6, 7, 9, and 12 representing March 2012 and June 2012/July 2012/August 2012/February 2018) which had P values between 0.1 and 0.06.
The differences in females’ use of alternative foraging strategies between reproductive periods were significant: PIs during lactation were significantly higher than those in gestation (0.08 ± 0.48 versus −0.4 ± 0.51 in the Established Colony; P < 0.01, permuted t tests with a Bonferroni adjustment) and postweaning periods (0.08 ± 0.48 versus −0.26 ± 0.4 in the Established Colony; P < 0.005). However, no significant differences were found between gestation and postweaning PIs (−0.4 ± 0.51 versus −0.26 ± 0.4, respectively, in the Established Colony, P > 0.1; see figs. S4 and S5 for Wild Control Colonies 1 and 2). Each female shifted its strategy around its specific date of parturition (fig. S8). Females that were not pregnant and thus did not require more energy also shifted their strategy in synchrony with the other females in the Established Colony. The same trend was evident in Wild Control Colony 2 (table S4). Male PIs did not change significantly during different reproductive periods (for the Established Colony: Gestation-Lactation, P = 0.3; Lactation-Postweaning, P = 0.9; Postweaning-Gestation, P = 0.08; Fig. 2A, black curves; see figs. S4A and S5A for Wild Control Colonies 1 and 2). Although female strategy preference shifts temporally, individuals (males and females) maintained their relative PI ranking. To this end, significant correlations were found between the PI rankings of individuals in all three colonies across the study period (Fig. 2B and figs. S4B and S5B), meaning that the strongest scroungers and strongest producers remained the strongest across different periods.
Study 2: Bats maintain persistent social foraging bonds
Next, we examined whether scroungers intentionally chose whom to scrounge upon. When comparing our social foraging network to a completely random network (n = 1000 simulations), a total of 60% of the dyads in our colony significantly deviated from random, interacting either more or less than expected by chance. This is not surprising because this analysis ignores the influence of individual foraging tendencies (that is, producers versus scroungers) on the social foraging network. We had to control for the propensity of individuals to use alternative strategies to infer real social preferences independent of these tendencies. For example, in the case where individual “A” produced a food item 200 times and individual “B” produced an item only 10 times, if individual “C” was to approach individual “B” at all 10 events, then this would reflect a stronger social preference toward “B” even if it approached “A” 20 times. We thus estimated the full directional dyadic interaction matrix (that is, the absolute number of approaches for each dyad A→B and B→A) and compared this matrix to simulated social foraging networks (n = 1000 simulations) that were generated according to the tendency of individuals to produce or scrounge. In these simulated matrices, the number of times an individual scrounged a food item and the number of times an individual was scrounged upon were kept constant, but the identity of recipients was chosen at random (Methods). These networks thus represented the expected networks that would be observed if bats maintained their individual tendency to scrounge or produce, even if they had no specific preferences of whom to interact with. Note that our social networks are based on foraging interactions and not on physical proximity as is commonly done. We thus term them social foraging networks.
The distribution of the observed and simulated foraging networks significantly differed, suggesting that the decision of whom to scrounge upon was nonrandom (Kolmogorov-Smirnov test, P = 0.017). Almost one-quarter (24%) of all possible directional dyads significantly deviated from their expected relation, interacting either significantly more or significantly less than expected (Fig. 3A; significance was assessed by z scores following an FDR P value adjustment for multiple comparisons). This strongly implies that scroungers actively chose the producers they scrounged upon and the producers they avoided.
Fig. 3. Bats exhibit individually specific social preferences—the Established Colony.
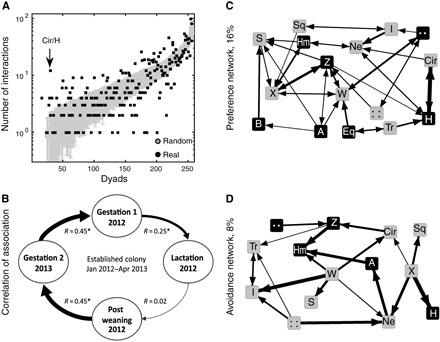
(A) The number of observed and expected dyadic interactions (y axis) for each of the 240 possible directional dyads (x axis). Expected interactions (mean + SD) are depicted by gray bars and are sorted by their values. The corresponding observed number of interactions is depicted by a black dot. Note that dyads are directional. (B) The social foraging network was stable over time. There were significant correlations between the temporal interaction matrices representing the different reproductive periods except between lactation and postweaning. Each circle represents a reproductive period, with thickness of the connective arrows representing the strength of the correlation coefficient between the social foraging networks of two reproductive periods. *P < 0.001 (Mantel test with a Bonferroni correction). (C and D) Preference and avoidance social foraging networks containing only those ties that significantly exceed (that is, preference) or fall below (that is, avoidance) their expected values. Black nodes, males; gray nodes, females. Networks are weighted and directed. Arrows indicate the directionality of the relationship with the arrowhead pointing toward the producer in the dyad and the thickness of the arrows representing the relative strength of the relationships (in the case of double-sided arrows, the thicker arrowhead represents the stronger of the directional ties). The numbers on the left of each network represent the proportion of dyads out of the total 240 possible dyads in the full network.
Similar individual social preferences were also evident in both Wild Control Colonies (Kolmogorov-Smirnov test, P < 0.001). Here, 18 and 19% of all possible directional dyads (in each colony, respectively) significantly deviated from their simulation-based expected relation, interacting either significantly more or significantly less than expected (figs. S6A and S7A, respectively). Moreover, in all three colonies, each scrounger typically had between two and three preferred producers whom it consistently approached significantly more often than by chance (Established Colony: mean, 2.4 ± 1 preferred producers; Wild Control Colony 1: mean, 2.7 ± 1 preferred producers; Wild Control Colony 2: mean = 2.6 ± 1 preferred producers; Fig. 3, C and D). Because producers might have been loyal to specific spatial locations within the colony, always returning to these locations with food, we validated that scroungers’ social preferences were a result of preferring a specific individual and not a specific location. To this end, we calculated the average distance traveled by scroungers between two scrounging events, and we compared this to the travel expected by a (simulated) bat that would have only stayed in the same zone or moved up to two zones away (Methods). All real bats moved significantly more than this simulated bat (P = 0 for all bats), proving that they did not just scrounge at a specific location.
To examine whether specific preference/avoidance relationships were consistent over time, we divided the data into four periods corresponding to the reproductive periods (Fig. 2A). These periodic social foraging networks were correlated over time (Mantel test with Bonferroni correction, P < 10−5 for the Established Colony) with the exception of the lactation and postweaning foraging networks (Mantel test, P = 0.14; Fig. 3B). This indicates that scroungers persistently preferred to scrounge on specific producers and to avoid others, except when females shifted strategy during lactation. In this period, females displayed a more opportunistic approach, simultaneously increasing producing rates and being less selective when deciding on whom to scrounge. The social foraging network of the Established Colony was stable for more than a year, suggesting that scroungers returned to prefer and avoid the same producers after altering their foraging strategy during the lactation period (Fig. 3B).
Wild Control Colony 1 also showed preference consistency over time but had a slightly different trend. This colony also showed temporally stable social preferences (across a 10-month period; Mantel test, P < 0.05; fig. S6B). However, here, correlations gradually increased throughout the 10-month period from when the colony was initially established. This difference probably resulted from the fact that females in Wild Control Colony 1 did not undergo gestation, and accordingly, their social preferences were not destabilized during lactation (fig. S6B). Wild Control Colony 2, which underwent a gestation period, also exhibited relatively stable social relations, with only a slight drop in correlation values between lactation and postweaning periods. This colony had a low female-to-male ratio compared to the Established Colony (2.2:1 versus 0.6:1), which might have masked the effect of female reproduction on social relations (fig. S7B).
Producer-scrounger interactions in the Established Colony were characterized by significantly less aggressiveness than those in the two newly established Wild Control Colonies—47% versus 88% and 83% of the interactions were aggressive, respectively (Fisher’s exact test, P < 0.0001). Furthermore, when comparing the nature of interactions between scroungers and their preferred and avoided producers, we found that, in the Established Colony, preference relationships were significantly associated with sharing as opposed to avoidance relationships, which were associated with aggression (383 sharing versus 139 aggressive interactions with preferred individuals and 74 sharing versus 108 aggressive interactions with avoided individuals; Fisher’s exact test, P < 0.0001). We ruled out kinship as a confounding factor by demonstrating that mother/offspring pairs did not show less aggression than a random pair in the colony. In the Established Colony, mother/offspring pairs even exhibit significantly more aggression than sharing (Fisher’s exact test; Established Colony: P = 0.018).
This was not the case in Wild Control Colonies 1 and 2, which were dominated by aggressive interactions (76 sharing versus 344 aggressive interactions with preferred individuals in Wild Control Colony 1 and 334 versus 1155 in Wild Control Colony 2; Fisher’s exact test, P = 0.326 and P = 0.001, respectively). We did not find evidence that interactions with preferred group mates increased the probability of obtaining food from the producer. The probability for a successful scrounging event (that is, for gaining food) was 64% with a preferred group mate and 72% with an avoided one for the Established Colony; the same trend was observed in Wild Control Colonies 1 and 2 (permuted paired t test, P > 0.59 for all three colonies).
Study 3: Foraging strategies are frequency-dependent
We next tested whether strategy use by individuals is frequency-dependent (that is, depends on the ratio of producers to scroungers in the colony), a fundamental characteristic of the producer-scrounger game (26, 38). We examined bats’ strategy use in small groups of four that had different producer-to-scrounger ratios. These small groups might represent the situation that is often observed in foraging sites where a few individuals are foraging together on a single tree. Under these conditions, producer bats were found to remain loyal to their original foraging strategy, exhibiting consistently positive PIs, independent of the composition of the group (R = 0.14, P = 0.168; Fig. 4, black dashed line). In contrast, scroungers exhibited flexibility in strategy use, significantly increasing their use of producing as the producer-to-scrounger ratio decreased (R2 = 0.88; P < 0.0001, in a regression analysis with a slope of β = 0.93 ± 0.41; Fig. 4, gray dashed line). The relative increase in producing was immediate and evident from the first session (of five sessions) of the experiments. Moreover, three of the five scroungers that were tested completely switched their strategy from scrounging to producing (that is, from PI < 0 to PI > 0) when the producer-to-scrounger ratio was equal to or lower than 2:2, that is, when there were fewer producers than scroungers in the group. Individuals that showed a lower tendency to produce in the colony were also less likely to switch their strategy when the producer-to-scrounger ratio decreased in the small groups.
Fig. 4. Flexibility in strategy use in social bats.
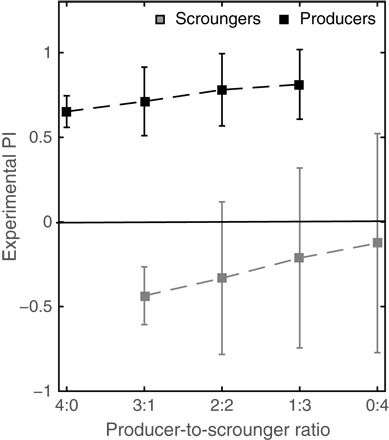
Experimental PI as a function of the producer-to-scrounger ratio. The average (+SD) for all producers (black) or scroungers (gray) at each ratio is shown. Notice how some of the scroungers start producing (PI > 0) when the ratio is above 2:2, as denoted by the fact that the SD crosses the 0 line. The overall average scrounger PI does not cross zero because the individual strategy diverges with some individuals maintaining their old scrounging tendency and others switching to producing.
Study 4: Scroungers are vigilant individuals
To reveal some of the possible underlying reasons for scrounging, we conducted another study in Wild Control Colony 2, in which we quantified the vigilance of the individuals. To this end, we placed six bowls of fruit in the center of the colony and monitored the collecting-to-landing ratio, that is, the proportion of landing events that ended in fruit collection (10 sessions of 2 hours each were quantified; Methods). We found a significant correlation between the tendency to produce (PI) and the collecting-to-landing ratio (Fig. 5; R = 0.83, P < 0.001, Pearson correlation). Producers typically landed on a bowl and immediately collected a food item, flying with it to the wall. Scroungers landed much less to begin with, and when they did land, they showed vigilant behavior typical of Rousettus and bird species (39–41), keeping their head high and scanning around with their ears. These events mostly did not end in collecting fruit items.
Fig. 5. Collecting-to-landing ratio as a factor of PI.
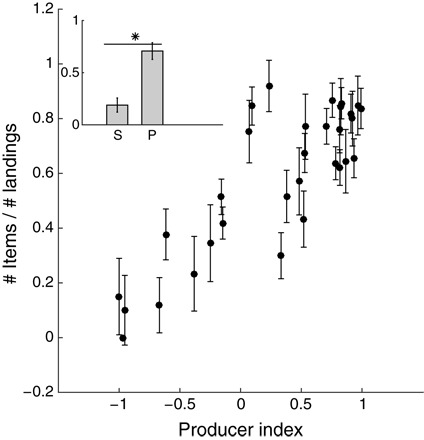
The y axis shows the average overall number of fruit items collected divided by the overall number of landings on the food source, and the PI of the bats in Wild Control Colony 2 (n = 32) is presented on the x axis. The average + SE collection-to-landing ratios for scroungers “S” and producers “P” are inserted in the upper left corner. The asterisk denotes significant differences (P < 0.01).
DISCUSSION
Individual preference toward a foraging strategy
Bats in all three colonies exhibited strong individual preferences toward either the producer or scrounger foraging strategy. These two distinct strategies were immediately evident in newly caught control colonies of most likely unfamiliar and unrelated wild bats, demonstrating that they are not an artifact of long-term captivity.
In most producer-scrounger systems described in literature, producers are individuals that find food patches, which are then exploited by scroungers. In this respect, our system is different because food was collected from a bowl by producers rather than discovered. Our system can be considered a variation of the classical producing-scrounging case in which producers make the food available to scroungers by collecting it from the “risky” bowl and moving it to the “safe” ceiling. Moreover, strategy use was found to be frequency-dependent, a main criterion of the producer-scrounger game.
In the Established Colony and Wild Control Colony 2, producers were mostly males, but in Wild Control Colony 1, the association between sex and strategy was much less pronounced, suggesting that other factors play a role in strategy selection. In Wild Control Colony 2, we had five juvenile bats (ages 5 to 18 months), so we could test the effect of age on foraging strategy (adult bats are impossible to age). We found that juveniles were slightly more likely to act as scroungers than adults, but this pattern did not reach significance, and one juvenile was a pronounced producer during the entire research period [M = −0.18, SE = 0.25 and M = 0.39, SE = 13, respectively; analysis of variance (ANOVA), P = 0.06].
Other possible factors for foraging strategy choice include the colony female-to-male ratio, social familiarity, and individual body condition (21, 42–45). An interesting example was the case of individual “B” that shifted from the producer to the scrounger strategy following a wing injury (Fig. 6).
Fig. 6. Fine-scale temporal analysis of producing/scrounging.
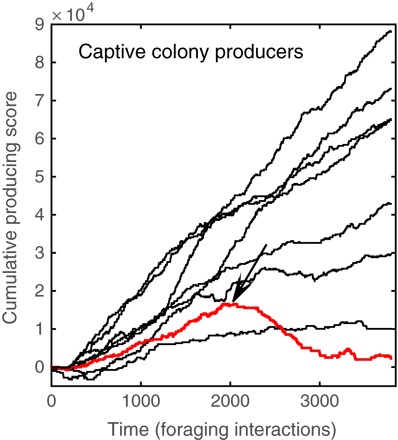
We used an Elo rating–inspired algorithm to examine the temporal dynamics of strategy use (84). The x axis represents time, with each bin representing one interaction event. The y axis represents the cumulative producing score, and each line represents one individual (notice that the graph only includes positive scores, that is, of producers). The analysis above shows cumulative producing scores of all male producers, which consistently increase, indicating the consistent use of the producer strategy. In red, the producer score of individual “B” initially increases as in the case of producers, until he was injured (the date of injury is denoted by an arrow), after which his score consistently drops, reflecting a change in strategy from producing to scrounging.
Persistent social relationships
Beyond their preference to produce or scrounge, bats in all three colonies also exhibited individual preferences of whom to interact with while foraging. Between 18 and 24% of all dyads were significantly stronger or weaker than chance in the three colonies. Accordingly, the social foraging network was composed of small cliques with each individual showing a strong preference toward two to three other individuals on average (and a tendency to avoid one to two individuals). Similar degrees of preference can be found in networks describing small primate and human social groups (46, 47).
Social relationships in the fruit bat colonies were persistent over time. Dyadic relationships in the Established Colony were maintained over a 16-month period despite being temporarily disassembled in the lactation period. It seems that, during lactation, because of high energetic demands, female scroungers become more opportunistic, both exhibiting higher levels of producing and being less selective when deciding on whom to scrounge. During the lactation period, only 6% of dyads exhibited social preference (versus 16% in other periods), and 0% exhibited avoidance (versus 8%). Similarly, in Wild Control Colony 2, social preference dropped during lactation from 13 to 6.5% and avoidance decreased from 6 to 2%. Such temporary disassembly of relationships was not observed in Wild Control Colony 1, where females did not undergo gestation. In this colony, we observed gradually increasing correlations in social relations across a 10-month period from when the colony was artificially assembled. The maintenance of persistent social relationships observed in the colonies suggests the ability to recognize and track colony mates, implying high social cognition (48).
What is the advantage of maintaining persistent foraging relationships? One commonly suggested direct advantage of persistent social bonds is information sharing (19, 26, 49–51), which does not seem to be relevant in our system because food was always offered at the same location. Another advantage often attributed to maintaining persistent relationships is a reduction in the levels of aggression, in this case, aggression of food owners to familiar scroungers (28, 52–54). In the Established Colony where individuals were familiar with each other for many years, significantly less aggression was observed than in the newly established Wild Control Colonies, and this was probably not a result of increased kinship because mother/pup pairs in this colony actually showed more aggression than average. Furthermore, in the Established Colony, interactions between scroungers and their preferred producers were characterized by sharing food as opposed to avoided relationships, which were characterized by aggression (Fisher’s exact test, P < 0.001). This relationship was not observed in the recently established Wild Control Colonies, which were dominated by aggressive interactions. Although our results indicate an advantage of persistent producer-scrounger relationships in the form of reduced aggression, we did not find evidence that interacting with preferred group mates increases profitability in terms of food consumption (permuted paired t tests, P > 0.59 for all three colonies).
One of the main remaining open questions is, Why do producers tolerate scrounging? It is possible that the fitness benefits that producers receive from avoiding the need to defend their food are greater than the cost of food loss, as suggested by tolerated theft and sharing under pressure models in both primates and humans (55–57).
Studies in primates further suggest that long-term relationships can promote forms of delayed reciprocity, where tolerating social costs imposed by group mates (that is, tolerating scrounging) can be paid back in a different commodity [for example, mating opportunity (58–60)]. Because strategy often corresponded to sex in our system, with high female-male preference, food production and tolerating costly scrounging may be an indicator of male quality (61). Tolerating scrounging might thus be paid back, for example, by an acceptance of a copulation attempt—a hypothesis that we plan to investigate in the future. Egyptian fruit bat females can be highly aggressive in response to mating attempts and can probably control with whom they copulate. Aside from mating success, recent work has demonstrated various other indirect fitness advantages of persistent social bonds (62–64). More work is necessary to determine whether any of these explanations drives social preferences in the fruit bat colony.
Finally, we asked what determines strategy use and why all individuals did not collect their food from the easily accessible bowl. We found a strong correlation between scrounging and vigilance. Scroungers landed on the fruit bowl less than producers, but even when they did land, they only collected fruit in 18% of the cases, whereas producers collected fruit in 70% of their landings. Moreover, the behavior of scroungers that landed on the bowl was markedly different from that of the producers. Scroungers exhibited a head-up posture and substantial ear movements that are typical for scanning the environment in this species. A similar trait was described in birds where scroungers were found to be more vigilant (65), exhibiting head movements aiming to scan their surroundings for predators and competitors (19, 48). Landing on a fruit tree in the wild is a dangerous event because predators are more likely to ambush a bat on the tree, and a stationary bat is an easier target for a flying predator. Egyptian fruit bats are known not to perch on trees with thin foliage such as date trees. When feeding on these trees, they will typically pick a fruit item and then fly to a nearby tall tree with thick foliage (66). Furthermore, when picking fruit from a tree, these bats will avoid the lower branches, which are associated with higher predation risk (67, 68). The bats will typically pick the fruit from the top branches gradually moving downward (over many nights) but never touching the lower branches (even when all other fruit has been depleted; see fig. S9 for a typical example of such a tree). We therefore hypothesize that scroungers are more vigilant individuals that exploit the less hesitant producers, which have already landed on a tree. Scroungers probably either land near the producer and pick fruit themselves or try to scrounge it from the producer’s mouth as has been shown, for example, in birds (41). Future experiments should focus on questions such as which individuals tend to be more vigilant and how does this relate to their life history and genes.
What is known about scrounging outdoors? It is hard to observe the bats’ behavior within the thick foliage, but we have witnessed scrounging many times in the wild when a bat returns to its colony with a piece of fruit in its mouth and is approached by individuals trying to scrounge the food. We do not have data on persistent producer-scrounger ties outdoors. However, an inspection of 24 scrounging attempts on bats that returned from foraging with food in their mouth to a wild monitored colony revealed two scroungers that scrounged at least four times on different nights and four additional scroungers that scrounged at least twice on different nights (movie S1).
Egyptian fruit bats live in mixed-sex colonies year-round, allowing persistent relationships to form (69, 70). This species is always found in groups at foraging sites often emitting social vocalizations, indicating that social interactions occur throughout foraging. They exhibit periodic loyalty to foraging sites, returning to them night after night (71). Following our results, it will be interesting to test whether, in the wild, scroungers persistently join closely affiliated producers to foraging sites.
METHODS
Study site, study species, and colonies
Established Colony
The Established Colony consisted of 16 mature Egyptian fruit bats [7 males and 9 females and their respective young (n = 7)], which were born during the period of the study in May/June 2012. The colony has been in captivity at the Zoological Garden of Tel Aviv University for approximately 10 years before our study. During these 10 years, bats were introduced from the wild periodically to increase genetic variation. There was, unfortunately, no documentation of the pedigree of the colony. Housing and observations were carried out within an indoor aviary simulating a natural cave (2.5 m × 4 m × 2.5 m) with a reversed 12-hour day/night cycle. The observers were sitting behind a tinted glass window while the bats were active within this enclosure.
Wild Control Colony 1
This colony consisted of 17 mature individuals (10 males and 7 females). The bats were all caught at the same roost (32°10′5.41″N, 34°48′51.33″E, ca. 1 year apart). This is a huge Rousettus colony with at least 6000 individuals spread within multiple compartments. At each capture, we made sure to distribute the capture in time and space; namely, the bats were collected along 1 hour from different locations in the cave. Moreover, once we entered the cave, a commotion started, with bats flying all over the place, so the probability of collecting two related bats or two bats from the same original cluster was very low. Genetic analysis confirms that this strategy results in capturing unrelated adults (see the next paragraph). The colony was housed and observed in a semi-indoor aviary (2 m × 4 m × 3 m) with a naturally fluctuating day/night cycle and access to a semi-outdoor mesh aviary via a single open window.
Wild Control Colony 2
A second control colony consisted of 25 mature individuals (14 males and 11 females) and 5 juveniles. The bats were caught in an identical manner to Wild Control Colony 1 and housed identically to the Established Colony.
All bats were visually identifiable either via unique symbols on a necklace or via bleach marking on the head and back (72). In addition, each bat was tagged with a unique radio-frequency identification chip (Trovan).
Genetic analysis
Tissue sample collection
We sampled two 3-mm-diameter wing punches from each individual in “Wild Control Colony 2.” Two punches per individual were preserved in molecular grade 100% ethanol and frozen at −80°. Wing tissues were obtained using sterile, disposable 3-mm skin biopsy punches. One biopsy punch was used per individual, and the samples were taken from regions of the wing that were far enough from major blood vessels and the edge of the wing to avoid tearing.
Molecular methods and genetic analyses
Genomic DNA was extracted for all adults in the colony using DNeasy tissue Extraction kit (Qiagen). Samples were genotyped at 10 microsatellite marker loci developed for Rousettus madagascariensis or Rousettus leschenaulti using previously described conditions (73, 74). Amplified products were visualized on an ABI 3100 genetic analyzer. Allele size scoring was performed using GeneMarker v2.6.7 (SoftGenetics, LLC), verified, and amended by eye. We examined the deviation from Hardy-Weinberg equilibrium (HWE) and the presence of null alleles using the software Cervus v3.0.7 (75). Pairwise relatedness among adults was calculated using the package “related” in R (76), using several available estimators (77–81). Microsatellite markers were polymorphic (mean allele number per locus, 6.8; range, 5 to 9), did not deviate from HWE, and had a low level of null alleles (<15%).
Genetic results
Relatedness estimates among adults were qualitatively similar across the various estimators used. Using Wang’s estimator (81), the relatedness estimate was r = −0.047 ± 0.012 (mean ± SE), confirming that individuals were not related. Because bats in Wild Control Colony 1 were captured using the exact same method, we assumed that their relatedness was similar. Unfortunately, we could not repeat this analysis for the captive colony, because these bats were released.
Study 1: Determining individual social foraging strategy use
Data collection and experimental procedure
Two observers carried out observations of foraging bouts. Observations began once all bats had dispersed from the sleeping cluster. Before each observation bout, a bowl containing slices of banana (ca. 400 g) was placed on a podium (ca. 1 m high) in the center of the enclosure. Each observation session lasted approximately 40 min because this was the period needed for the bats to deplete the bowl. All foraging events, defined as any food acquisition by an individual, were scored. Foraging events were then categorized as either producing or scrounging events, with food collection from the bowl noted as a producing event and approaching behaviors to the producer’s mouth denoted as scrounging events (these occurred on average within 0.8 min from the producing event). Scrounging events were further divided into two distinct types: food sharing and aggressive interactions over food. The type of interaction was categorized on the basis of the response of the food owner to an approaching individual. A “sharing” event was noted when the owner shared the food, allowing the approaching bat to eat directly from its mouth. An “aggressive” interaction was recorded when the food owner responded to an approaching individual with physically (82) aggressive behavior. The overall success rate was defined as the probability of gaining food by scrounging and was calculated per dyad.
Observations were collected from January 2012 to June 2016. The Established Colony was observed for 140 days from April 2012 to January 2013. Wild Control Colony 1 was observed for 85 days from April 2012 to January 2013, and Wild Control Colony 2 was observed for 104 days from March 2015 to June 2016. A 40-min observation session was conducted on each of these days (reaching a total of 220 hours of observation). Juveniles (5 to 18 months) were excluded from all sex-related analyses of foraging behavior, because their sexual maturity was reached throughout the study period. Pups (0 to 4 months) that were born during the study period were excluded from all analyses. Overall, 3551, 2873, and 7401 foraging records were collected for the Established Colony, Wild Control Colony 1, and Wild Control Colony 2, respectively. We confirmed that individuals did not change their strategies after the first 40 min by continuing observation for 3 hours on 10 occasions.
Data analysis
Permutation tests
Because of the non-normal distribution of some of the data, all statistical analyses of study 1 were carried out using permutation t tests. For each test (for example, PI differences between males and females), we performed 10,000 random assignments of the measured PIs to individuals while holding the parameter (for example, sex) constant. The actual observed t statistic was then compared to the distribution of these 10,000 t values.
Testing the consistency of individuals’ foraging strategy
Data were initially divided into monthly bins. Months with fewer than 100 records were merged with the following month to ensure sufficient data for further analysis, resulting in 13 bins with between 120 and 567 records per bin in the Established Colony, 10 bins with between 244 and 388 records per bin in Wild Control Colony 1, and 16 bins with between 210 and 910 records per bin in Wild Control Colony 2. PIs were calculated (according to Eq. 1) for each individual at each time bin.
To explore the influence of the females’ reproductive state on the foraging strategy, male and female PIs were separately averaged per reproductive condition, that is, gestation, lactation, and postweaning (table S6). We conducted a permuted t test (10,000 randomizations) comparing the difference in male and female PIs between reproductive periods. For each pair of reproductive periods, the same-sex observed t statistic (for example, gestating females versus lactating females) was compared to that of the 10,000 simulated t values. A Bonferroni correction was used to correct for multiple comparisons.
Study 2: Persistent social foraging bonds
We used social network analysis to explore the role of individual social biases (preference or avoidance) toward specific group mates when deciding on whom to scrounge upon.
Data analysis
To explore whether bats prefer to scrounge from specific individuals, we had to control for the propensity of individuals to use alternative strategies to infer real social preferences independent of these tendencies. We compared the number of observed interactions per dyad with the expected number of interaction for that dyad assuming that they each behave according to their individual foraging tendency (probability to produce and scrounge) but with an equal probability of targeting each individual (that is, with no individual preference). The expected probability of interactions per dyad “ij” was thus calculated as the probability of individual “i” to collect food multiplied by the probability of individual “j” to scrounge. Note that this analysis is directional, meaning that each pair of individuals constructs two dyads—where “i” is the producer and “j” is the scrounger and vice versa. We ran 1000 stochastic simulations in which interacting dyads were drawn according to these probabilities to generate 1000 full interaction matrices of all dyads. Each of these networks thus represented the expected networks that would be observed if bats had no specific preferences of whom to scrounge upon.
To explore whether dyadic foraging relationships were maintained on a persistent basis, we created sequential interaction matrices for each of the reproductive periods. In each interaction matrix, every cell (ij) represented the interaction rate, that is, the number of times individual “i” scrounged upon individual “j” divided by the number of times individual “j” had food. This calculation accounts for the bias of individuals toward foraging strategies. A Mantel test was used for identifying correlations between sequential social foraging interaction matrices (83). The commonly used lagged association analysis was not suitable in our case because it assumes stationary relations, which was not the case in our system (because the ties are broken during the lactation period; see Results).
To validate the idea that scroungers preferred individual producers and did not simply stay in specific spatial zones in the colony (and scrounged upon whoever was there), we estimated their traveled distance per interaction and compared this to a simulated bat that has a zone preference. The simulated bat would either stay in its zone or hop up to two zones away (that is, ca. one-third of the colony’s length) with equal probability at each interaction. Ten thousand such bats were simulated, and the observed distance was then compared to the expected distances of these bats. Note that this simulated bat moved quite a bit, so if the real bats moved more (as they did; see Results), then this means that they clearly had no regional preference.
Study 3: Frequency-dependent social foraging strategies
Experimental group construction
To explore the frequency-dependent nature of alternative strategy use, eight small foraging groups of four individuals with various producer-to-scrounger ratios were constructed and tested separately. Producer-to-scrounger ratios ranged from pure producer groups (denoted 4p; table S5) to pure scrounger groups (denoted 4s; table S5), with three intermediate conditions (3p:1s, 2p:2s, and 1p:3s). Each intermediate condition had two groups (with different individuals), and the two extreme conditions (4p and 4s) had one group each. A total of 10 individuals took part in these experiments.
Experimental apparatus and procedure
The experimental apparatus consisted of an indoor cage (1.5 m × 2 m × 2.5 m) with six evenly spaced opaque cardboard boxes placed on the floor. Each box had an entry hole (10 cm in diameter) with a mesh ladder leading to a food source consisting of 15 pieces of banana. The use of numerous boxes in this setup increased the difficulty of food finding to emphasize the difference between producers and scroungers. Each group was released in the apparatus while two experimenters observed the bats’ behavior and video-recorded it. The total number of producing events—defined as entering the box to obtain food—and the total number of scrounging events—defined as approaching a food owner in an attempt to gain access to its food—were recorded. Each bat was tested in at least three group composition conditions (for example, 4p:0s, 3p:1s, 2p:2s, 1p:3s, and 0p:4s). Each group was tested five times over a period of 75 days. A PI for each individual in each trial was calculated in an identical manner to that in the baseline colony condition described above (Eq. 1).
Data analysis
To examine the effect of the producer-to-scrounger ratio on flexibility in individual strategy use, PIs (Eq. 1) were averaged over the five trials per individual and compared across conditions. In cases where an individual participated in two groups with the same producer-to-scrounger ratio, its experimental PIs were averaged to obtain one value per bat per condition. To test the effect of the producer-to-scrounger ratio on foraging strategy, the linear regression between this ratio and the bats’ PIs in each condition was estimated.
Study 4: The determinant of the scrounging strategy
We hypothesized that scrounging might be related to vigilance. To explore this, we observed 10 feeding sessions of 2 hours each. We scored the overall number of landings and food items collected per individual across 10 observation days. We used these observations to calculate an individual “vigilance index” by dividing the overall number of food items collected by the overall number of landings. To explore the relation between strategy use and vigilance, we then correlated the average hesitance (or vigilance) index of individuals with their PI.
Supplementary Material
Acknowledgments
We thank M. Taub for assistance with data collection and L. Neigev for technical support in the laboratory. We are grateful to G. Carter, E. Gefen, and A. Illany for helpful comments on an earlier version of the manuscript. All experiments were performed with permission from the Tel Aviv University Institutional Animal Care and Use Committee (number L-11-043). Funding: This study was partially supported by a research grant to Y.Y. from the European Research Council (ERC-GPSBAT). Author contributions: L.H. and Y.Y. conceived and designed the experiment. L.H. designed and constructed the setup. L.H., Y.M., N.G., and H.N. conducted the experiments. R.D. carried out the genetic analysis, and L.H. and Y.Y. created the analysis tools and performed the analysis. Y.Y. supervised the study. L.H. and Y.Y. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/e1603293/DC1
fig. S1. Wild Control Colony 1.
fig. S2. Wild Control Colony 2.
fig. S3. The cumulative producing rates over time for the Established Colony and Wild Control Colony 1.
fig. S4. Temporal consistency of strategy use—Wild Control Colony 2.
fig. S5. Temporal consistency of strategy use—Wild Control Colony 1.
fig. S6. Bats exhibit individually specific social preferences—Wild Control Colony 1.
fig. S7. Bats exhibit individually specific social preferences—Wild Control Colony 2.
fig. S8. Females’ rise in producing corresponded to parturition.
fig. S9. Bats avoid foraging on the lower branches of trees.
table S1. Strategy indices (PIs) across individuals within the Established Colony (n = 16).
table S2. Strategy indices (PIs) across individuals within Wild Control Colony 1 (n = 17).
table S3. PIs across individuals within Wild Control Colony 2 (n = 31).
table S4. Results of permutation-based t tests between female PIs across reproductive periods.
table S5. Reproductive periods.
table S6. Study 3 experimental groups.
movie S1. An example of an aggressive scrounging attempt on a bat returning to its colony with food.
REFERENCES AND NOTES
- 1.Croft D. P., James R., Ward A. J. W., Botham M. S., Mawdsley D., Krause J., Assortative interactions and social networks in fish. Oecologia 143, 211–219 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Hirsch B. T., Stanton M. A., Maldonado J. E., Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLOS ONE 7, e37301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause J., James R., Croft D. P., Personality in the context of social networks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 4099–4106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wey T. W., Blumstein D. T., Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim. Behav. 79, 1343–1352 (2010). [Google Scholar]
- 5.Wey T., Blumstein D. T., Shen W., Jordán F., Social network analysis of animal behaviour: A promising tool for the study of sociality. Anim. Behav. 75, 333–344 (2008). [Google Scholar]
- 6.Whitehead H. A. L., Analysing animal social structure. Anim. Behav. 1053–1067 (1997).9398362 [Google Scholar]
- 7.D. P. Croft, R. James, J. Krause, Exploring Animal Social Networks (Princeton Univ. Press, 2008). [Google Scholar]
- 8.Flack J. C., Girvan M., de Waal F. B. M., Krakauer D. C., Policing stabilizes construction of social niches in primates. Nature 439, 426–429 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Voelkl B., Kasper C., Social structure of primate interaction networks facilitates the emergence of cooperation. Biol. Lett. 5, 462–464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leskovec J., Lang K. J., Dasgupta A., Mahoney M. W., Community structure in large networks: Natural cluster sizes and the absence of large well-defined clusters. Internet Math. 6, 29–123 (2009). [Google Scholar]
- 11.Watts D. J., Strogatz S. H., Collective dynamics of ‘small-world’ networks. Nature 393, 440–442 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Barabási A.-L., Albert R., Emergence of scaling in random networks. Science 286, 509–512 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Klovdahl A. S., Potterat J. J., Woodhouse D. E., Muth J. B., Muth S. Q., Darrow W. W., Social networks and infectious disease: The Colorado Springs study. Soc. Sci. Med. 38, 79–88 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Ross K. G., Molecular ecology of social behaviour: Analyses of breeding systems and genetic structure. Mol. Ecol. 10, 265–284 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Sugg D. W., Chesser R. K., Stephen Dobson F., Hoogland J. L., Population genetics meets behavioral ecology. Trends Ecol. Evol. 11, 338–342 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Kurvers R. H. J. M., Krause J., Croft D. P., Wilson A. D. M., Wolf M., The evolutionary and ecological consequences of animal social networks: Emerging issues. Trends Ecol. Evol. 29, 326–335 (2014). [DOI] [PubMed] [Google Scholar]
- 17.W. J. Sutherland, From Individual Behaviour to Population Ecology (Oxford Series in Ecology and Evolution, Oxford Univ. Press, 1996). [Google Scholar]
- 18.Dall S. R. X., Giraldeau L.-A., Olsson O., McNamara J. M., Stephens D. W., Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (2005). [DOI] [PubMed] [Google Scholar]
- 19.G. S. Wilkinson, J. W. Boughman, Social influences on foraging in bats, in Mammalian Social Learning: Comparative and Ecological Perspectives, H. O. Box, K. R. Gibson, Eds. (Cambridge Univ. Press, 1999), pp. 188–204. [Google Scholar]
- 20.Galef B. G. Jr, Giraldeau L.-A., Social influences on foraging in vertebrates: Causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Lendvai Á. Z., Barta Z., Liker A., Bókony V., The effect of energy reserves on social foraging: Hungry sparrows scrounge more. Proc. Biol. Sci. 271, 2467–2472 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croft D. P., Darden S. K., Wey T. W., Current directions in animal social networks. Curr. Opin. Behav. Sci. 12, 52–58 (2016). [Google Scholar]
- 23.Pruitt J. N., Keiser C. N., The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Anim. Behav. 93, 87–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keiser C. N., Pinter-Wollman N., Augustine D. A., Ziemba M. J., Hao L., Lawrence J. G., Pruitt J. N., Individual differences in boldness influence patterns of social interactions and the transmission of cuticular bacteria among group-mates. Proc. R. Soc. B 283, 20160457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones T. B., Aplin L. M., Devost I., Morand-Ferron J., Individual and ecological determinants of social information transmission in the wild. Anim. Behav. 129, 93–101 (2017). [Google Scholar]
- 26.Vickery W. L., Giraldeau L.-A., Templeton J. J., Kramer D. L., Chapman C. A., Producers, scroungers, and group foraging. Am. Nat. 137, 847–863 (1991). [Google Scholar]
- 27.King A. J., Clark F. E., Cowlishaw G., The dining etiquette of desert baboons: The roles of social bonds, kinship, and dominance in co-feeding networks. Am. J. Primatol. 73, 768–774 (2011). [DOI] [PubMed] [Google Scholar]
- 28.King A. J., Isaac N. J. B., Cowlishaw G., Ecological, social, and reproductive factors shape producer-scrounger dynamics in baboons. Behav. Ecol. 20, 1039–1049 (2009). [Google Scholar]
- 29.Wilkinson G. S., Wenrick Boughman J., Social calls coordinate foraging in greater spear-nosed bats. Anim. Behav. 55, 337–350 (1998). [DOI] [PubMed] [Google Scholar]
- 30.G. S. Wilkinson, Social and vocal complexity in bats, in Animal Social Complexity: Intelligence, Culture, and Individualized Societies, F. B. M. de Waal, P. L Tyack, Eds. (Harvard Univ. Press, 2003), pp. 322–341. [Google Scholar]
- 31.Kerth G., Causes and consequences of sociality in bats. Bioscience 58, 737–746 (2008). [Google Scholar]
- 32.Kerth G., Animal sociality: Bat colonies are founded by relatives. Curr. Biol. 18, R740–R742 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Kerth G., Van Schaik J., Causes and consequences of living in closed societies: Lessons from a long-term socio-genetic study on Bechstein’s bats. Mol. Ecol. 21, 633–646 (2012). [DOI] [PubMed] [Google Scholar]
- 34.A. Zubaid, G. F. McCracken, T. H. Kunz, Functional and Evolutionary Ecology of Bats (Oxford Univ. Press, 2005. [Google Scholar]
- 35.Knörnschild M., Nagy M., Metz M., Learned vocal group signatures in the polygynous bat Saccopteryx bilineata. Anim. Behav. 84, 761–769 (2012). [Google Scholar]
- 36.Kerth G., Reckardt K., Information transfer about roosts in female Bechstein’s bats: An experimental field study. Proc. Biol. Sci. 270, 511–515 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter G. G., Wilkinson G. S., Food sharing in vampire bats: Reciprocal help predicts donations more than relatedness or harassment. Proc. Biol. Sci. 280, 20122573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mottley K., Giraldeau L.-A., Experimental evidence that group foragers can converge on predicted producer–scrounger equilibria. Anim. Behav. 60, 341–350 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Coolen I., Giraldeau L.-A., Lavoie M., Head position as an indicator of producer and scrounger tactics in a ground-feeding bird. Anim. Behav. 61, 895–903 (2001). [Google Scholar]
- 40.Glück E., An experimental study of feeding, vigilance and predator avoidance in a single bird. Oecologia 71, 268–272 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Pöysä H., Group foraging, distance to cover and vigilance in the teal, Anas crecca. Anim. Behav. 48, 921–928 (1994). [Google Scholar]
- 42.Brockmann H. J., The evolution of alternative strategies and tactics. Adv. Study Behav. 30, 1–51 (2001). [Google Scholar]
- 43.Strodl M. A., Schausberger P., Social familiarity modulates group living and foraging behaviour of juvenile predatory mites. Naturwissenschaften 99, 303–311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David M., Giraldeau L.-A., Zebra finches in poor condition produce more and consume more food in a producer–scrounger game. Behav. Ecol. 23, 174–180 (2012). [Google Scholar]
- 45.Mathot K. J., Wright J., Kempenaers B., Dingemanse N. J., Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos 121, 1009–1020 (2012). [Google Scholar]
- 46.Lehmann J., Boesch C., Sociality of the dispersing sex: The nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim. Behav. 77, 377–387 (2009). [Google Scholar]
- 47.Apicella C. L., Marlowe F. W., Fowler J. H., Christakis N. A., Social networks and cooperation in hunter-gatherers. Nature 481, 497–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunbar R. I. M., Shultz S., Evolution in the social brain. Science 317, 1344–1347 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Safi K., Kerth G., Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am. Nat. 170, 465–472 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Ratcliffe J. M., ter Hofstede H. M., Roosts as information centres: Social learning of food preferences in bats. Biol. Lett. 1, 72–74 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giraldeau L.-A., Dubois F., Social foraging and the study of exploitative behavior. Adv. Study Behav. 38, 59–104 (2008). [Google Scholar]
- 52.Hamilton W. D., The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 (1964). [DOI] [PubMed] [Google Scholar]
- 53.Trivers R., The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (1971). [Google Scholar]
- 54.Mathot K. J., Giraldeau L.-A., Family-related differences in social foraging tactic use in the zebra finch (Taeniopygia guttata). Behav. Ecol. Sociobiol. 64, 1805–1811 (2010). [Google Scholar]
- 55.Blurton Jones N. G., A selfish origin for human food sharing: Tolerated theft. Ethol. Sociobiol. 5, 1–3 (1984). [Google Scholar]
- 56.Stevens J. R., The selfish nature of generosity: Harassment and food sharing in primates. Proc. Biol. Sci. 271, 451–456 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silk J. B., Brosnan S. F., Henrich J., Lambeth S. P., Shapiro S., Chimpanzees share food for many reasons: The role of kinship, reciprocity, social bonds and harassment on food transfers. Anim. Behav. 85, 941–947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes C. M., Boesch C., Wild chimpanzees exchange meat for sex on a long-term basis. PLOS ONE 4, e5116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith E. A., Why do good hunters have higher reproductive success? Hum. Nat. 15, 343–364 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Clutton-Brock T., Cooperation between non-kin in animal societies. Nature 462, 51–57 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Zahavi A., Mate selection—A selection for a handicap. J. Theor. Biol. 53, 205–214 (1975). [DOI] [PubMed] [Google Scholar]
- 62.Crockford C., Wittig R. M., Whitten P. L., Seyfarth R. M., Cheney D. L., Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm. Behav. 53, 254–265 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Silk J. B., Beehner J. C., Bergman T. J., Crockford C., Engh A. L., Moscovice L. R., Wittig R. M., Seyfarth R. M., Cheney D. L., The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc. Biol. Sci. 276, 3099–3104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wittig R. M., Crockford C., Lehmann J., Whitten P. L., Seyfarth R. M., Cheney D. L., Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 54, 170–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathot K. J., Giraldeau L.-A., Increasing vulnerability to predation increases preference for the scrounger foraging tactic. Behav. Ecol. 19, 131–138 (2007). [Google Scholar]
- 66.Izhaki I., Korine C., Arad Z., The effect of bat (Rousettus aegyptiacus) dispersal on seed germination in eastern Mediterranean habitats. Oecologia 101, 335–342 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Riginos C., Grace J. B., Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology 89, 2228–2238 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Suhonen J., Predation risk influences the use of foraging sites by tits. Ecology 74, 1197–1203 (1993). [Google Scholar]
- 69.Kwiecinski G. G., Griffiths T. A., Rousettus egyptiacus. Mamm. Species 611, 1–9 (1999). [Google Scholar]
- 70.Korine C., Izhaki I., Makin D., Population structure and emergence order in the fruit bat (Rousettus aegyptiacus: Mammalia, Chiroptera). J. Zool. 232, 163–174 (1994). [Google Scholar]
- 71.Geva-Sagiv M., Las L., Yovel Y., Ulanovsky N., Spatial cognition in bats and rats: From sensory acquisition to multiscale maps and navigation. Nat. Rev. Neurosci. 16, 94–108 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Ceballos-Vasquez A., Caldwell J. R., Faure P. A., A device for restraining bats. Acta Chiropterol. 16, 255–260 (2014). [Google Scholar]
- 73.Hua P. Y., Chen J. P., Sun M., Liang B., Zhang S. Y., Wu D. H., Characterization of microsatellite loci in fulvous fruit bat Rousettus leschenaulti. Mol. Ecol. Notes 6, 939–941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andrianaivoarivelo A. R., Shore G. D., McGuire S. M., Jenkins R. K. B., Ramilijaona O., Louis E. E. Jr, Brenneman R. A., Characterization of 22 microsatellite marker loci in the Madagascar rousette (Rousettus madagascariensis). Conserv. Genet. 10, 1025–1028 (2009). [Google Scholar]
- 75.Kalinowski S. T., Taper M. L., Marshall T. C., Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Pew J., Muir P. H., Wang J., Frasier T. R., related: An R package for analysing pairwise relatedness from codominant molecular markers. Mol. Ecol. Resour. 15, 557–561 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Li C. C., Weeks D. E., Chakravarti A., Similarity of DNA fingerprints due to chance and relatedness. Hum. Hered. 43, 45–52 (1993). [DOI] [PubMed] [Google Scholar]
- 78.Lynch M., Ritland K., Estimation of pairwise relatedness with molecular markers. Genetics 152, 1753–1766 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Queller D. C., Goodnight K. F., Estimating relatedness using genetic markers. Evolution 43, 258–275 (1989). [DOI] [PubMed] [Google Scholar]
- 80.Ritland K., Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. 67, 175–185 (1996). [Google Scholar]
- 81.Wang J., An estimator for pairwise relatedness using molecular markers. Genetics. 160, 1203–1215 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prat Y., Taub M., Yovel Y., Everyday bat vocalizations contain information about emitter, addressee, context, and behavior. Sci. Rep. 6, 39419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mantel N., The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220 (1967). [PubMed] [Google Scholar]
- 84.Albers P. C. H., de Vries H., Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 61, 489–495 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/e1603293/DC1
fig. S1. Wild Control Colony 1.
fig. S2. Wild Control Colony 2.
fig. S3. The cumulative producing rates over time for the Established Colony and Wild Control Colony 1.
fig. S4. Temporal consistency of strategy use—Wild Control Colony 2.
fig. S5. Temporal consistency of strategy use—Wild Control Colony 1.
fig. S6. Bats exhibit individually specific social preferences—Wild Control Colony 1.
fig. S7. Bats exhibit individually specific social preferences—Wild Control Colony 2.
fig. S8. Females’ rise in producing corresponded to parturition.
fig. S9. Bats avoid foraging on the lower branches of trees.
table S1. Strategy indices (PIs) across individuals within the Established Colony (n = 16).
table S2. Strategy indices (PIs) across individuals within Wild Control Colony 1 (n = 17).
table S3. PIs across individuals within Wild Control Colony 2 (n = 31).
table S4. Results of permutation-based t tests between female PIs across reproductive periods.
table S5. Reproductive periods.
table S6. Study 3 experimental groups.
movie S1. An example of an aggressive scrounging attempt on a bat returning to its colony with food.


